Abstract
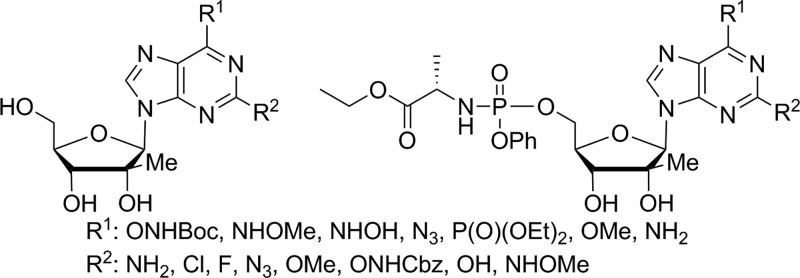
A variety of 2,6-modified purine 2′-C-methylribonucleosides and their phosphoramidate prodrugs were synthesized and evaluated for inhibition of HCV RNA replication in Huh-7 cells and for cytotoxicity in various cell lines. Cellular pharmacology and HCV polymerase incorporation studies on the most potent and selective compound are reported.
Keywords: HCV, antiviral, phosphoramidate prodrug, purine, nucleoside
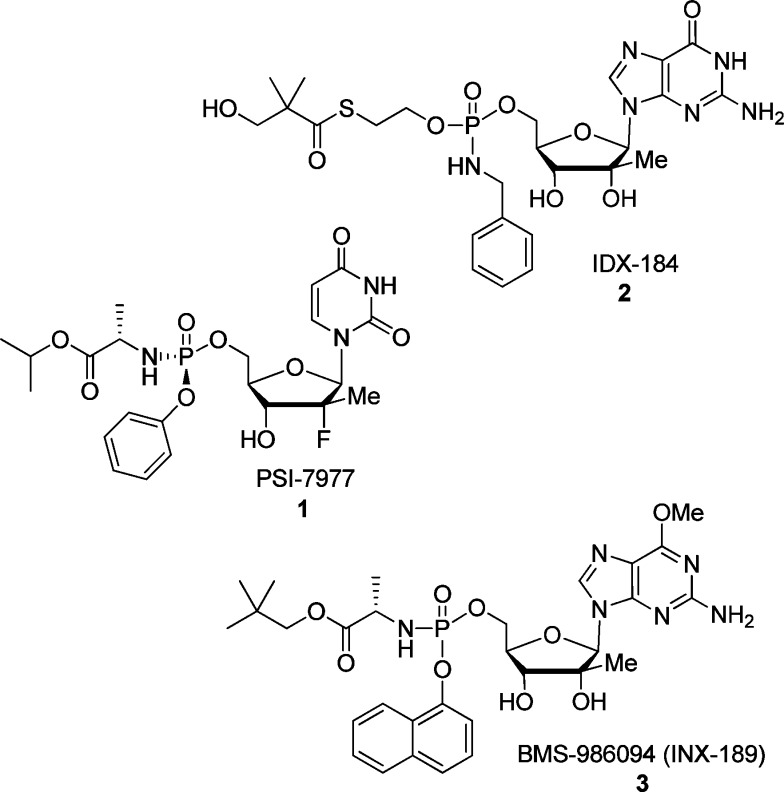
Hepatitis C virus (HCV) is a global health problem affecting an estimated 170 million individuals worldwide, and it is a leading cause of liver cirrhosis and hepatocellular carcinoma.1,2 Several curative options are now available for HCV infections, but they all require at least two direct acting antiviral agents to result in cure rates of 90 to 100%. Nucleoside inhibitors of HCV NS5B polymerase are favored since they generally have a high genetic barrier to drug resistance and are pan-genotypic activity.3 Sofosbuvir (PSI/GS-7977) 1,4 a 2′-deoxy-2′-α-fluoro-2′-β-C-methyl nucleoside monophosphate prodrug, was approved by the FDA in December 2013 as a safe and effective anti-HCV agent (Figure 1).5 IDX-184 2 and BMS-986094 (INX-189) 3, two related nucleoside prodrugs, precursors of the same active 2′-β-C-methyl guanosine triphosphate, also showed high potency in vitro and promising results in early clinical studies, but their development was terminated after low effectiveness in humans for IDX-184, and severe cardiac effects observed during a phase 2b study with BMS-986094. Based on the potential of these 2′-C-methyl nucleosides, we present, herein, the synthesis of new 2,6-modified purine 2′-C-methyl ribonucleosides that may offer potential alternatives to BMS-986094 and IDX-184 or maybe used in combination with Sofosbuvir.
Figure 1.
Selected clinical anti-HCV nucleoside analogues.
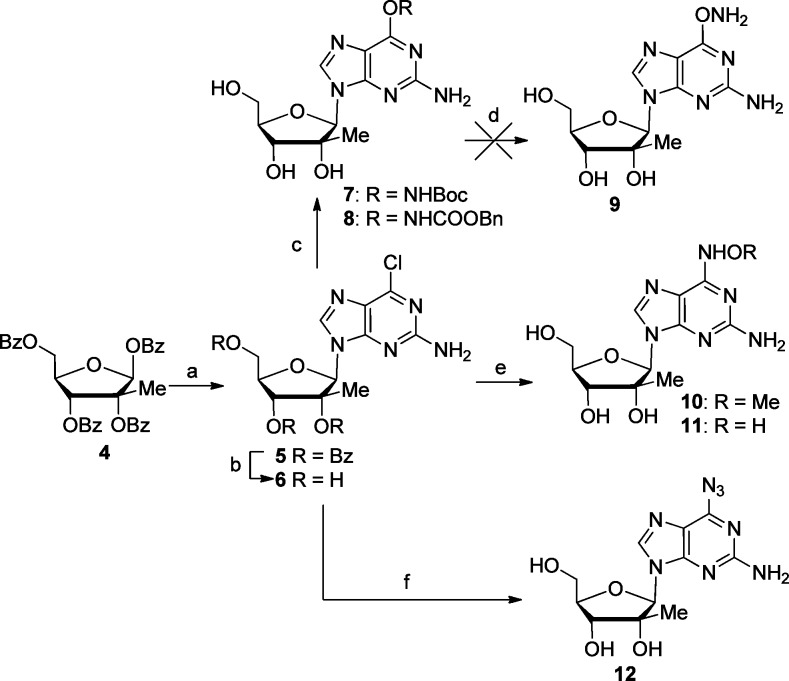
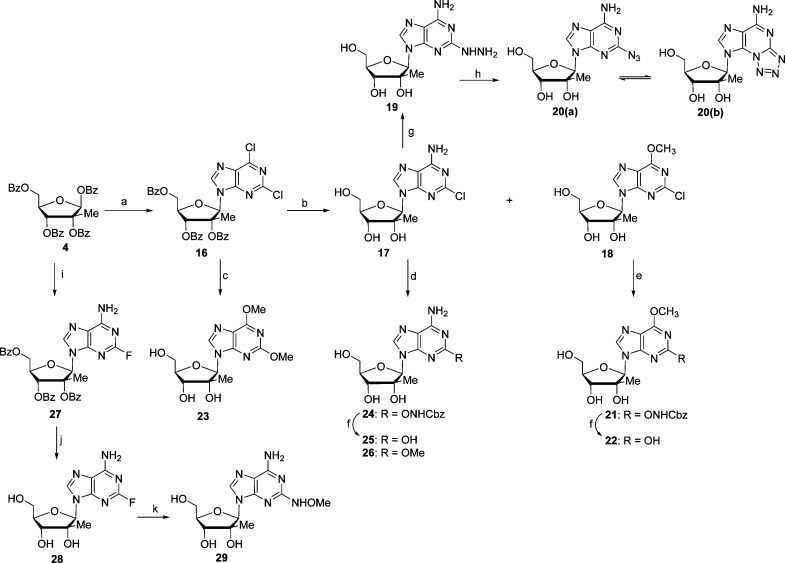
In the design of 6-position modifications, we strived to maintain groups that retained hydrogen bond accepting characteristics as is present in the 6-position of natural guanosine. The key to the synthesis of 2-amino, 6-modified 2′-C-methyl nucleosides was intermediate 5 that was prepared by known methods.6 Targeted 6-N3 and 6-NH-O-substituted purines 10–12 were easily prepared from debenzoylated nucleoside 6 by reaction with NaN3, methoxyamine, and hydroxylamine (Scheme 1). Interestingly, attempts to prepare the 6-ONH2 compound 9 from N-Boc-hydroxylamino and N-(benzyloxycarbonyl)hydroxylamino derivatives 7 and 8 were unsuccessful as all attempts to deprotect intermediates 7 and 8, using acidity (compound 7) or transition metal catalyzed hydrogenation (compound 8), lead exclusively to the formation of 2′-C-methyl guanosine.
Scheme 1.
Reagents and conditions: (a) 2-amino-6-chloropurine, DBU, TMSOTf, −40 to 80 °C, 5 h, 92%; (b) sat. NH3/MeOH, rt, overnight, 90%; (c) for 7, HONHBoc, NaH, THF, rt, 3 h, 69%; for 8, HONHCbz, NaH, THF, rt, 3 h, 88%; (d) for 7, 80% TFA, H2O, rt, overnight; for 8, H2, Pd/C 10%, MeOH, rt, overnight; (e) for 10, MeONH2, Et3N, EtOH/H2O, 65 °C, 24 h, 79%; for 11, NH2OH, EtOH/H2O, 35 °C, 24 h, 51%; (f) NaN3, DMF, 95 °C, 2 h, 62%.
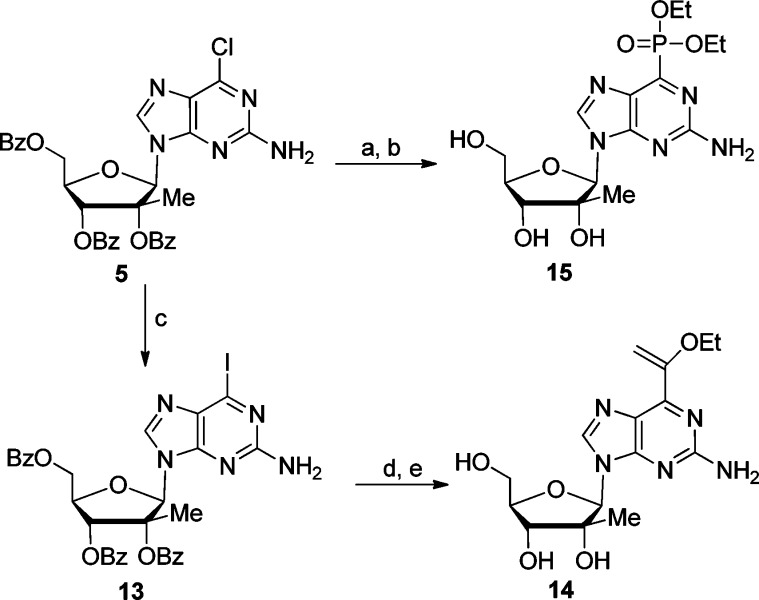
Other, more uncommon functionalities such as a phosphonate and an ethoxyvinyl group were also introduced at the 6-position (Scheme 2). Thus, 6-diethylphosphonate derivative 15 was prepared by reaction of 6-chloropurine nucleoside 5 with triethyl phosphite (Scheme 2) at 130 °C and subsequent deprotection in a saturated solution of ammonia in ethanol. Compound 5 was also reacted with TMSI to generate the more reactive iodo intermediate 13, which was coupled with tributyl(1-ethoxyvinyl)stannane under palladium catalyzed Stille coupling conditions. Final deprotection using a catalytic amount of sodium methoxide in methanol afforded final compound 14.
Scheme 2.
Reagents and conditions: (a) P(OEt)3, 130 °C, overnight, 78%; (b) sat. NH3/EtOH, rt, 4 d, 44%; (c) TMSI, CH2Cl2, rt, 9 h, 63%; (d) tributyl (1-ethoxyvinyl) stannane, Pd(PhP3)2Cl2, THF, 80 °C, 24 h, 54%; (e) Cat. NaOMe, CH3OH, rt, 12 h, 79%.
With the knowledge that the 2-position of adenosine is not involved in the hydrogen bonding of base pairing while the 2-amino group of guanosine is, we next turned our attention to the synthesis of 2-modified purine nucleosides. 2-Hydroxylamino-, 2-fluoro-, 2-methoxy-, and 2-azido-purine nucleosides were targeted as they potentially offer a variety of steric, electronic, and hydrogen bonding interactions, which may enhance recognition by HCV NS5B polymerase in their 5′-triphospate forms. The key tribenzoylated 2,6-dichloropurine-2′-Me nucleoside 16 was prepared in a manner similar to 6 (Scheme 1) with 2,6-dichloropurine, TMSOTf, and DBU. Treatment of the 2,6-dichloropurine nucleoside 16 with a saturated solution of ammonia in methanol lead to concomitant formation of 6-amino compound 17 and 6-methoxy compound 18 (Scheme 3). This reaction was preformed at room temperature to avoid displacement of the 2-chloro group and appeared to proceed predominantly by first formation of the 6-methoxy compound, which is then converted to the 6-amino via ammonia displacement. The benzoyl group deprotection occurred quite rapidly and was followed by a slow partial conversion of the 6-methoxy nucleoside 18 to the 6-amino nucleoside 17 over the three-day reaction. With 17 and 18 in hand, our initial goal was to prepare the corresponding 2-O-NH2 derivatives. While the reaction of 17 and 18 with N-Cbz hydroxylamine worked well, the subsequent palladium catalyzed hydrogenation of the CBz group generated only 2-hydroxy purine products 22 and 25. However, 2,6-dimethoxypurine 23 and 6-amino-2-methoxy purine derivative 26 were successfully synthesized by simple treatment of compounds 16 and 17 with sodium methoxide. 2-Azidopurine 20 was prepared in two steps by the reaction of 2-chloro purine derivative 17 with hydrazine hydrate followed by treatment of the resulting hydrazine compound 19 with sodium nitrite in acetic acid.7,8 As expected, 1H NMR showed that compound 20 exists as an equilibrium of azido (20a) and 1-N-tetrazole (20b) tautomeric forms.9−11 It is worth noting that a two-step sequence was used because direct treatment of 17 with NaN3 failed to provide 2-azido nucleoside 20.
Scheme 3.
Reagents and conditions: (a) 2,6-dichloropurine, DBU, TMSOTf, −40 to 80 °C, 4 h, 80%; (b) sat. NH3/MeOH, rt, 3 days; for 17, 46%; for 18, 21%; (c) K2CO3, MeOH, rt, 24 h, 87%; (d) for 24, HONHCbz, NaH, THF, 50 °C, 24 h, 81%; for 26, MeONa, MeOH, 65 °C, 24 h, 92%; (e) HONHCbz, NaH, THF, 50 °C, 24 h, 78%; (f) Pd/C, H2, MeOH, rt, 15 h; for 22, 83%; for 25, 97%; (g) NH2NH2, MeOCH2CH2OH, 110 °C, 5 h, 40%; (h) NaNO2, HOAc, 1 h, 77%; (i) 2-fluoroadenine, DBU, TMSOTf, −40 to 65 °C, 5 h; (j) sat. NH3/MeOH, rt, 2 d, 72% for two steps; (k) MeONH2, Et3N, EtOH/H2O, 110 °C, 15 h, 66%.
Vorbruggen-type coupling between tetrabenzoylated sugar 4 and 2-fluoro-6-aminopurine in the presence of TMSOTf and DBU afforded compound 27 in 80% (Scheme 3). Deprotection of the three benzyl groups using a saturated solution of ammonia in methanol gave access to 2-fluoro-6-aminopurine nucleoside 28, which was subsequently treated with O-methyl hydroxylamine to give the desired 2-N-methoxylamine purine derivative 29.
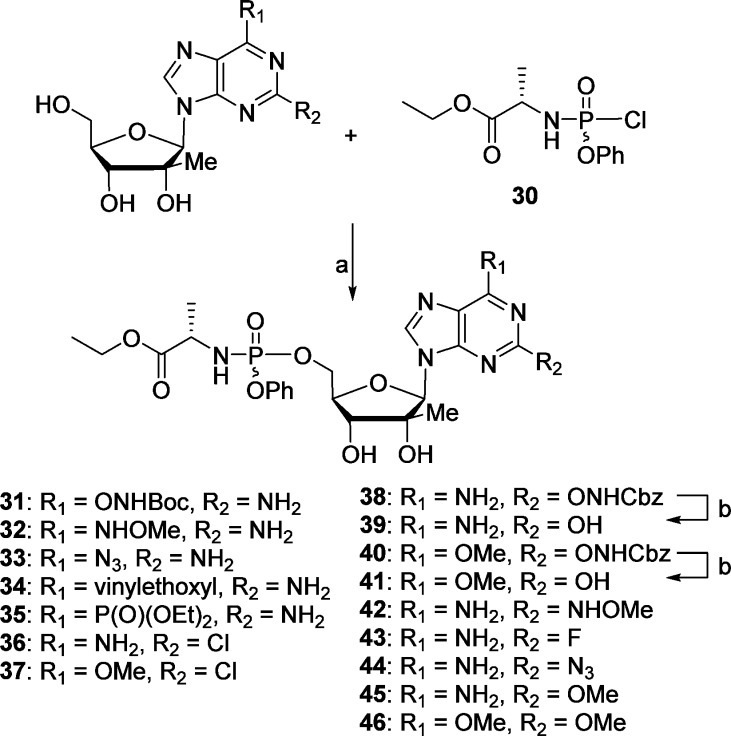
It has been now well established that nucleoside analogues are oftentimes unable to be intracellularly metabolized to their corresponding nucleoside triphosphates. Therefore, in order to overcome the often rate-limiting first phosphorylation step and improve the antiviral activity of our nucleoside analogues, we prepared their corresponding monophosphate McGuigan-type prodrugs.12 The synthesis of phosphoramidates 31–46 was performed following the Uchiyama procedure by reacting the nucleosides 10–12, 14, 15, 17, 18, 20, 22, 23, 25, 26, 28, and 29 with chlorophosphoramidate 30(13) in the presence of N-methylimidazole (Scheme 4).14,15 It is noteworthy that the use of acetonitrile as a cosolvent improved the solubility of certain nucleosides leading to better overall yields. Attempts to prepare the 2-aminooxypurine nucleoside prodrugs by Cbz removal of compounds 38 and 40 were not successful and instead afforded the isoguanosine derivatives 39 and 41.
Scheme 4.
Reagents and conditions: (a) NMI, THF/CH3CN, rt, 2–3 h; (b) Pd/C, H2, MeOH, 15 h.
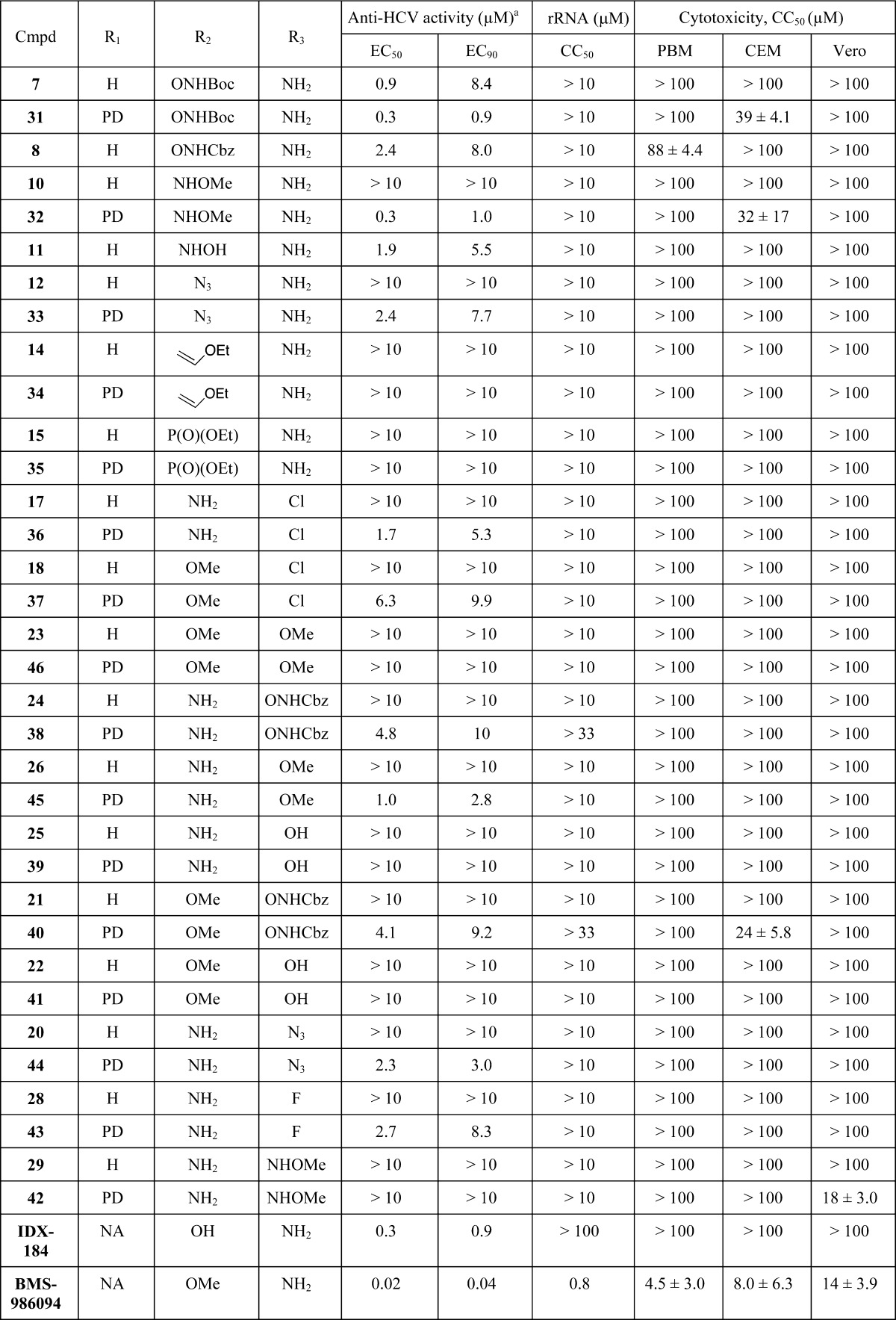
The nucleosides and phosphoramidate prodrugs were evaluated for inhibition of HCV RNA replication in Huh7 cells using a subgenomic HCV replicon system.16 Cytotoxicity in Huh7 cells was determined simultaneously with anti-HCV activity by extraction and amplification of both HCV RNA and cellular rRNA (rRNA).17 In addition cytotoxicity was determined in primary human peripheral blood mononuclear (PBM) cells, human lymphoblastoid CEM, and African Green monkey Vero cells (Table 1).18,19 In an initial set of compounds, unusual 6-modifications such as introduction of a phosphonate ester or an ethoxy vinyl group were counterproductive and lead to inactive nucleosides and monophosphate prodrugs 14, 15, 34, and 35. Similarly, purine derivatives 25 (2,6-diMeO), 22 (2-OH, 6-NH2), 23 (2-OH, 6-MeO), and 29 (2-NHOMe, 6-NH2) and their corresponding phosphoramidate prodrugs 39, 41, 46, and 42 showed to be inactive against HCV up to 10 μM. In contrast, 6-substituted purines derivatives 7 (−ONHBoc), 8 (−ONHCbz), and 11 (−NHOH) displayed EC50 values against HCV of 0.9, 2.4, and 0.3 μM, respectively, without apparent toxicity up to 10 μM in Huh7 cells and up to 100 μM in human PBM, CEM, and Vero cells. Preparation of 31, the phosphoramidate prodrug of 6-NHBoc substituted compound 7, even decreased the EC90 of 7 by a factor of 10 (8.4 compared to 0.9 μM for 7). However, it has been well established that 6-modified nucleosides can be substrate of deaminases,20 and therefore, we studied the fate of such compounds intracellularly. Thus, compound 7 and its prodrug 31 were incubated in Huh7 cells at 50 μM for 4 h at 37 °C. The cells were washed with phosphate-buffered saline and the intracellular metabolites were extracted with 70% ice-cold methanol in water and identified by LC–MS/MS. In this particular case, the only NTP metabolite observed in vitro was a 2′-β-C-methylguanosine-5′-triphosphate, a known inhibitor of HCV NS5B polymerase.3
Table 1. In Vitro Anti-HCV Activity and Cytotoxicity of Nucleosides and Phosphoramidate Prodrugsa.
The HCV replicon data is run in triplicate and the interwell variability must be less than ±0.5ΔCt. NA: not available.
Interestingly, compounds 10 (2-NH2 6-NHOMe), 12 (2-NH2, 6-N3), 17 (2-Cl, 6-NH2), 18 (2-Cl, 6-OMe), 24 (2-ONHCbz, 6-NH2), 26 (2-OMe, 6-NH2), 21 (2-ONHCBz, 6-OMe), 20 (2-N3, 6-NH2), and 28 (2-F, 6-NH2) did not show any activity against HCV in the replicon system when tested up to 10 μM, while their corresponding phosphoramidate prodrugs 32, 33, 36, 37, 38, 45, 40, 44, and 43 revealed their potency and exhibited median effective concentrations (EC50) between 0.3 and 6.3 μM (Table 1). As with compounds 7 and 31, a cellular pharmacology study of some of the most potent inhibitors 45 (2-OMe, 6-NH2, 1 μM), 44 (2-N3, 6-NH2, 2.3 μM), and 43 (2-F, 6-NH2, 2.7 μM) was undertaken and intracellular levels of nucleoside-MP, -DP, and -TP formed in Huh7 cells were quantified. All phosphoramidate derivatives 45, 44, and 43 produced high levels of their corresponding NTP along with considerable amounts of NMP and NDP derivatives. No other NTP was observed, which implies that 45, 44, and 43 NTPs are responsible for the anti-HCV activity observed in the replicon system and that 2-position modification of purines, unlike 6-modification, are quite stable in vitro.
As a first step toward understanding the difference of antiviral activity observed between inactive nucleoside 28 and its phosphoramidate prodrug 43 (EC50 = 2.7 μM), a comparative pharmacology study in Huh7 cells using the LC–MS/MS method described above was performed. While both compounds were found to deliver 28-TP, prodrug 43 produces ∼20 times more 5′-triphosphate than 28. This discrepancy directly correlates with the anti-HCV activity of these two compounds. Interestingly, we also noted that levels of metabolites produced by 23 were very low in Huh7 cells, even for the nucleoside itself. This seems to imply that, not only can the monophosphate prodrug 43 bypass the first phosphorylation step, but it also allows for a better intracellular penetration of the compound.
To further characterize compound 43, we decided to study the in vitro incorporation of 28-TP (active metabolite of phosphoramidate 43) by HCV NS5B polymerase (see Figure 2 in the Supporting Information section). RNA synthesis by HCV NS5B polymerase was monitored in the presence of increasing concentrations of 28-TP up to 100 μM. Incorporation of 28-TP was marked by the appearance of pausing sites opposite uridine residues at positions +11, +13, and +15 of the 20mer RNA template, confirming that 28-TP behaves as an A analogue. Finally, as expected, increased incorporation of 28-TP correlated with inhibition of full-length 20mer RNA product formation (Ki = 32 ± 0.07 μM). Finally, since off-target effects can be an issue with nucleoside analogues, we assessed the selectivity of our compound by testing 28-TP against host RNA polymerase II and human mitochondrial RNA polymerase (POLRMT). At concentrations up to 100 μM, 28-TP did not inhibit host RNA polymerase II (while α-amanitin, used as positive control, had an IC50 of 2.5 ± 1.7 nM) and was not significantly incorporated by POLRMT (11% incorporation as normalized to ATP).21
Despite the fact that base modifications are often not accepted by polymerases and can lead to undesired toxicity, we identified several substitutions to the purine base that allow their NTP to be recognized by HCV polymerase. Thus, we discovered phosphoramidates 32, 33, 36, 37, 38, 45, 40, 44, and 43, which displayed EC50 values in the low micromolar range against HCV without apparent toxicity in Huh7, PBM, CEM, and Vero cells up to 100 μM. Phosphoramidate 43 (2-F, 6-NH2), one of the most potent compounds, was further characterized. Interestingly, we found that 43, unlike its corresponding nucleoside 28, produced high levels of 28-TP in Huh7 cells. The 28-TP was shown to be a substrate of HCV NS5B polymerase and was incorporated as an adenosine analogue. Further preclinical profiling of compound 43 and exploration of its prodrug portion is in progress. We thus envisage comparing this compound or a related prodrug to Sofosbuvir, INX-189, and IDX-184 in different cellular and animal assays and evaluate the potential therapeutic benefit of such 2-position modification in purine nucleosides and nucleotides.
Glossary
ABBREVIATIONS
- RNA
ribo nucleic acid
- HCV
hepatitis C
- FDA
Food and Drug Administration
- DBU
1,8-diazabicyclo[5.4.0]undec-7-ene
- TMSOTf
trimethylsilyl trifluoromethanesulfonate
- Boc
tert-Butyloxycarbonyl
- CBz
benzyloxy carbamate
- TFA
trifluoroacetic acid
- TMSI
trimethylsilyl iodide
- HOAc
acetic acid
- NMI
1-methylimidazole
- LC
liquid chromatography
- MS
mass spectrometry
- NTP
nucleoside triphosphate
- NMP
nucleoside monophosphate
- NDP
nucleoside diphosphate
- TP
triphosphate
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00402.
Biological assays and complete experimental section with full characterization of all new compounds (PDF)
This work was supported in part by NIH grant 5P30-AI-50409 (CFAR) and by the Department of Veterans Affairs.
The authors declare the following competing financial interest: Dr. Schinazi is the Chairman and a major shareholder of CoCrystal Pharma, Inc. Emory received no funding from CoCrystal Pharma, Inc. to perform this work and vice versa.
Supplementary Material
References
- De Francesco R.; Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature 2005, 436, 953–960. 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- Sheldon J.; Barreiro P.; Soriano V. Novel protease and polymerase inhibitors for the treatment of hepatitis C virus infection. Expert Opin. Invest. Drugs 2007, 16, 1171–1181. 10.1517/13543784.16.8.1171. [DOI] [PubMed] [Google Scholar]
- Coats S. J.; Garnier-Amblard E. C.; Amblard F.; Ehteshami M.; Amiralaei S.; Zhang H.; Zhou L.; Boucle S. R.; Lu X.; Bondada L.; Shelton J. R.; Li H.; Liu P.; Li C.; Cho J. H.; Chavre S. N.; Zhou S.; Mathew J.; Schinazi R. F. Chutes and ladders in hepatitis C nucleoside drug development. Antiviral Res. 2014, 102, 119–147. 10.1016/j.antiviral.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia M. J.; Bao D.; Chang W.; Du J.; Nagarathnam D.; Rachakonda S.; Reddy P. G.; Ross B. S.; Wang P.; Zhang H.-R; Bansal S.; Espiritu C.; Keilman M.; Lam A. M.; Micolochick Steuer H. M.; Niu C.; Otto M. J.; Furman P. A. Discovery of a β-D-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem. 2010, 53, 7202–7218. 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]
- For a review of NS5B anti-HCV agents, see:Sofia M. J.; Chang W.; Furman P. A.; Mosley R. T.; Ross B. S. Nucleoside, nucleotide, and non-nucleoside inhibitors of hepatitis C virus NS5B RNA-dependent RNA-polymerase. J. Med. Chem. 2012, 55, 2481–2531. 10.1021/jm201384j. [DOI] [PubMed] [Google Scholar]
- Eldrup A. B.; Allerson C. R.; Bennett C. F.; Bera S.; Bhat B.; Bhat N.; Bosserman M. R.; Brooks J.; Burlein C.; Carroll S. S.; Cook P. D.; Getty K. L.; MacCoss M.; McMasters D. R.; Olsen D. B.; Prakash T. P.; Prhavc M.; Song Q.; Tomassini J. E.; Xia J. Structure-activity relationship of purine ribonucleosides for inhibition of hepatitis C virus RNA-dependent RNA polymerase. J. Med. Chem. 2004, 47, 2283–2294. 10.1021/jm030424e. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Ikeda R.; Sugiyama H. 8-Methylguanosine: a powerful Z-DNA stabilizer. J. Am. Chem. Soc. 2003, 125, 13519–13524. 10.1021/ja036233i. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J. US Pat. 19804199574, 1980.
- Lioux T.; Gosselin G.; Mathé C. Azido/tetrazole tautomerism in 2-azidoadenine β-D-pentofuranonucleoside derivatives. Eur. J. Org. Chem. 2003, 20, 3997–4002. 10.1002/ejoc.200300274. [DOI] [Google Scholar]
- Sodum R. S.; Fiala E. S. N2-amination of guanine to 2-hydrazinohypoxanthine, a novel in vivo nucleic acid modification produced by the hepatocarcinogen 2-nitropropane. Chem. Res. Toxicol. 1998, 11, 1453–1459. 10.1021/tx980160c. [DOI] [PubMed] [Google Scholar]
- Elzein E.; Kalla R.; Li X.-F.; Perry T.; Marquart T.; Micklatcher M.; Li Y.; Wu Y.-Z.; Zeng D.; Zablocki J. N6-Cycloalkyl-2-substituted adenosine derivatives as selective, high affinity adenosine A1 receptor agonists. Bioorg. Med. Chem. Lett. 2007, 17, 161–166. 10.1016/j.bmcl.2006.09.065. [DOI] [PubMed] [Google Scholar]
- Pradere U.; Garnier-Amblard E. C.; Coats S. J.; Amblard F.; Schinazi R. F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan C.; Pathirana N.; Balzarini J.; De Clercq E. Intracellular delivery of bioactive AZT nucleotides by aryl phosphate derivatives of AZT. J. Med. Chem. 1993, 36, 1048–1052. 10.1021/jm00060a013. [DOI] [PubMed] [Google Scholar]
- Uchiyama M.; Aso Y.; Noyori R.; Hayakawa Y. O-selective phosphorylation of nucleosides without N-protection. J. Org. Chem. 1993, 58, 373–379. 10.1021/jo00054a020. [DOI] [Google Scholar]
- McGuigan C.; Hassan-Abdallah A.; Srinivasan S.; Wang Y.; Siddiqui A.; Daluge S. M.; Gudmundsson K. S.; Zhou H.; McLean E. W.; Peckham J. P.; Burnette T. C.; Marr H.; Hazen R.; Condreay L. D.; Johnson L.; Balzarini J. Application of phosphoramidate ProTide technology significantly improves antiviral potency of carbocyclic adenosine derivatives. J. Med. Chem. 2006, 49, 7215–7226. 10.1021/jm060776w. [DOI] [PubMed] [Google Scholar]
- Rondla R.; Coats S. J.; McBrayer T. R.; Grier J.; Johns M.; Tharnish P. M.; Whitaker T.; Zhou L.-H.; Schinazi R. F. Anti-hepatitis C virus activity of novel beta-D-2′-C-methyl-4′-azido pyrimidine nucleoside phosphoramidate prodrugs. Antivir. Chem. Chemother. 2009, 20, 99–106. 10.3851/IMP1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuyver L. J.; Whitaker T.; McBrayer T. R.; Hernandez-Santiago B. I.; Lostia S.; Tharnish P. M.; Ramesh M.; Chu C. K.; Jordan R.; Shi J.; Rachakonda S.; Watanabe K. A.; Otto M. J.; Schinazi R. F. Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture. Antimicrob. Agents Chemother. 2003, 47, 244–254. 10.1128/AAC.47.1.244-254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F.; Sommadossi J. P.; Saalmann V.; Cannon D. L.; Xie M.-W.; Hart G. C.; Smith G. A.; Hahn E. F. Activities of 3′-azido-3′-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 1990, 34, 1061–1067. 10.1128/AAC.34.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuyver L. J.; Lostia S.; Adams M.; Mathew J.; Pai B. S.; Grier J.; Tharnish P.; Choi Y.; Chong Y.; Choo H.; Chu C. K.; Otto M. J.; Schinazi R. F. Antiviral activities and cellular toxicities of modified 2′,3′-dideoxy-2′,3′-didehydrocytidine analogues. Antimicrob. Agents Chemother. 2002, 46, 3854–3860. 10.1128/AAC.46.12.3854-3860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami E.; Bao H.; Mosley R. T.; Du J.; Sofia M. J.; Furman P. A. Adenosine deaminase-like protein 1 (ADAL1): Characterization and substrate specificity in the hydrolysis of N6- or O6-substituted purine or 2-aminopurine nucleoside monophosphates. J. Med. Chem. 2011, 54, 5902–5914. 10.1021/jm200650j. [DOI] [PubMed] [Google Scholar]
- Arnold J. J.; Sharma S. D.; Feng J. Y.; Ray A. S.; Smidansky E. D.; Kireeva M. L.; Cho A.; Perry J.; Vela J. E.; Park Y.; Xu Y.; Tian Y.; Babusis D.; Barauskus O.; Peterson B. R.; Gnatt A.; Kashlev M.; Zhong W.; Cameron C. E. Sensitivity of mitochondrial transcription and resistance of RNA polymerase II dependent nuclear transcription to antiviral ribonucleosides. PLoS Pathog. 2012, 8, e1003030. 10.1371/journal.ppat.1003030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.