Abstract
Objective
To investigate the relationship of skeletal muscle FNDC5 mRNA expression and circulating irisin to the GH/IGF-I axis and to skeletal muscle mitochondrial function and mitochondria-related gene expression in obese men.
Design
Fifteen abdominally obese men with reduced growth hormone received 12 weeks of recombinant human GH (rhGH). Before and after treatment, they underwent 31P-magnetic resonance spectroscopy to evaluate phosphocreatine (PCr) recovery as a measure of mitochondrial function and skeletal muscle biopsy to assess expression of mitochondrial-related genes. Serum irisin and IGF-I and skeletal muscle FNDC5 and IGF-I mRNA were measured.
Results
At baseline, skeletal muscle FNDC5 mRNA was significantly and positively associated with IGF-I mRNA (ρ = 0.81, P=0.005) and rate of PCr recovery (ρ=0.79, P=0.006). Similar relationships of circulating irisin to IGF-I mRNA (ρ=0.63, P=0.05) and rate of PCr recovery (ρ = 0.48, P=0.08) were demonstrated, but were not as robust as those with muscle FNDC5 expression. Both serum irisin and skeletal muscle FNDC5 mRNA were significantly associated with PPARγ (ρ=0.73, P=0.02 and ρ=0.85, P=0.002), respectively. In addition, FNDC5 mRNA was correlated with skeletal muscle PGC-1α (ρ=0.68, P=0.03), NRF1 (ρ=0.66, P=0.04) and TFAM (ρ=0.79, P=0.007) mRNA. Neither serum irisin nor muscle mRNA expression of FNDC5 changed with rhGH treatment.
Conclusion
These novel data in skeletal muscle demonstrate that local expression of FNDC5 is associated with mRNA expression of IGF-I and mitochondrial function and mitochondria-related gene expression in obese subjects with reduced growth hormone and suggest a potential role for FNDC5 acting locally in muscle in a low GH state. Further studies are needed to clarify the relationship between the GH/IGF-I axis and irisin.
Keywords: Irisin, FNDC5, growth hormone, IGF-I, skeletal muscle, mitochondrial, obesity
INTRODUCTION
Obesity is a state of reduced growth hormone (GH) secretion. Although obesity related complications may contribute to reduced GH, low GH itself can exacerbate further metabolic disease. Reduced GH has been linked to cardiovascular disease in obesity [1–3]. Furthermore, GH is essential to the maintenance of body composition, and low GH results in reduced lean body mass and visceral fat accumulation. Data also suggest that GH and IGF-I may promote mitochondrial biogenesis and function [4, 5], such that relatively decreased GH secretion may contribute to mitochondrial dysfunction in obese individuals.
We have previously shown that GH and IGF-I are directly associated with a non-invasive assessment of mitochondrial function via phosphocreatinine (PCr) recovery [6]. Furthermore, skeletal muscle IGF-I expression is significantly and positively associated with both PCr recovery and mRNA expression of genes related to mitochondrial biogenesis and function, including nuclear respiratory factor-1 (NRF1), mitochondrial transcription factor A (TFAM), and peroxisome proliferator-activated receptor (PPAR)γ [7]. The exact mechanisms by which the GH/IGF-I axis is associated with mitochondrial biogenesis and function remain unclear.
Irisin is a myokine secreted into the circulation after proteolytic cleavage from the fibronectin type III domain-containing protein 5 (FNDC5) gene through peroxisome proliferator-activated receptor coactivator 1 alpha (PGC-1α) regulation [8]. Irisin stimulates uncoupling protein 1 (UCP-1) expression in brown adipose tissue, which in turn uncouples mitochondrial oxidative phosphorylation and dissipates energy as heat [8, 9]. Some studies highlight irisin as a compensatory myokine to promote mitochondrial biogenesis and reduce metabolic risk [10–12]. In vitro studies demonstrate that treatment of myocytes with irisin results in increased oxidative metabolism, mitochondrial uncoupling, and expression of genes relevant to mitochondrial biogenesis and function, including NRF1, TFAM, and PGC-1α [10]. Limited data from human studies vary and demonstrate that metabolic disease in obesity may be related to irisin deficiency or resistance [13, 14].
Given the data linking both GH and irisin to mitochondrial biogenesis and function, we utilized a cohort of 15 obese men with reduced GH who received 12 weeks of recombinant human GH (rhGH) in order to investigate the relationships between irisin, the GH/IGF-I axis, and mitochondrial function [7]. We hypothesized that irisin would be linked to mitochondrial function and related gene expression in obese subjects with reduced GH and that treatment with rhGH may increase irisin or FNDC5 mRNA in skeletal muscle.
METHODS
Study participants
Fifteen abdominally obese male subjects with reduced GH secretion between the ages of 18 and 60 years were recruited at the Massachusetts General Hospital as previously described [7]. Abdominal obesity was defined as BMI ≥ 30 kg/m2 and waist circumference ≥ 102 cm. Only men with peak-stimulated GH ≤ 4.2 μg/L during GHRH-arginine stimulation test were included. Subjects were healthy with no known endocrine disorders or severe illness. Any subjects using hormone therapy, including testosterone, glucocorticoids, anabolic steroids, GHRH, or GH within 3 months of enrollment were excluded. All subjects had no known history of diabetes mellitus or use of antidiabetic medications. Additionally, subjects with creatinine > 1.5 mg/dl or AST> 2.5 U/dL times the upper limit of normal were excluded. The study was approved by the Partners Institutional Review Board. Written informed consent was obtained from all participants.
Interventional Study Design
All subjects received rhGH at an initial daily dose of 0.4 mg subcutaneously. The dose was subsequently titrated every 4 weeks to 0.6 mg follow by 0.8mg to achieve a serum IGF-I level in the upper-normal range according to age. Procedures were performed prior to commencement and after 12 weeks intervention with rhGH. At the 12 week visit, the last dose of rhGH was administered on the morning of procedures and blood sampling. The exercise challenge for the MRS procedure was performed on an alternate day.
31P-MRS protocol
31P-magnetic resonance spectroscopy (31P-MRS) was used to assess in vivo skeletal muscle mitochondrial function after submaximal exercise based on phosphocreatine (PCr) recovery [15]. Details of the procedure are reported elsewhere [7]. In brief, the image acquisition was performed after an overnight fast on a 3.0 T whole body MRS scanner (Trio, Siemens Medical Systems). A 13 cm diameter custom-built single-tuned 31P surface coil was placed in contact with the right posterior calf muscle. 31P spectra were acquired every 2 seconds over a 10 minute period during which time subjects underwent 2 minutes of rest, 3 minutes of plantar flexion exercise based on 40% maximal voluntary contraction (MVC) at 0.5Hz, followed by 5 minutes of recovery. MVC was determined prior to imaging using a hand held dynamometer (microFet2, Hoggan Health Industries), and the mean MVC was calculated from maximum plantar flexion force measured in triplicate.
Concentrations of PCr, inorganic phosphate (Pi), and adenosine triphosphate (ATP) resonances were fitted in the frequency domain using an in-house MATLAB-based software. Intracellular pH was estimated based on the chemical shift difference between PCr and Pi resonances. Mitochondrial function was determined by plotting the PCr peak integrated area vs. time during exercise recovery and fitting the recovery curve to a mono-exponential function to determine the recovery time constant (τPCr). The initial rate of PCr recovery (ViPCr) was determined from τPcr, and PCr depletion using the equation ViPCR = (60/τPcr) x PCr depletion [16]. ViPCr was chosen as the primary end point as it normalizes PCr recovery based on participant effort and is insensitive to end-of-exercise metabolic condition such as intracellular acidosis [16]. Greater ViPCr represents relatively better mitochondrial function.
Muscle biopsy
Biopsy of the right gastrocnemius muscle was performed according to standard procedure after an overnight fast on a day separate from the MRS imaging [17]. Subjects were instructed to lie in the prone position. Local anesthesia was provided using 1% lidocaine administered intradermally and subcutaneously. A 2cm stab incision was made in the skin and soft tissue overlying the lateral gastrocnemius muscle. A Bergstrom core biopsy needle [18] was inserted into the muscle at a 90° angle and manual suction was used to obtain approximately 50–100 mg of muscle tissue from each subject [19]. Samples were immediately flash frozen in liquid nitrogen and stored at −80°C until ready for use.
Gene expression studies
Total mRNA was extracted from the muscle biopsy samples using TRIzol (Invitrogen) as per manufacturer’s protocol. SuperScript® III First-Strand Synthesis SuperMix (Invitrogen) was used for the generation of cDNA for all samples. Expression of IGF-I, PGC-1α, PPARγ, PPARα, NRF1, TFAM and FNDC5 mRNA were determined by quantitative real time PCR using a Mastercyclerrealplex machine (Eppendorf) with iQ SYBR Green Supermix Kit (BIO-RAD) as per standard protocol. The primer sequences used for amplifying target-gene mRNA are shown in Table 1. For each sample, real-time PCR reactions were performed in triplicate and the average threshold cycle (Ct) was calculated. Expression of target-gene mRNA was normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression. The GAPDH primers were: 5′-GCGAGATCCCTCCAAAATCAA-3′ and 5′-GTTCACACCCATGACGAACAT-3′. Expression levels were calculated using the ΔΔCt method after correcting for differences in PCR efficiencies and were expressed relative to control levels. Data on FNDC5 were not previously analyzed.
Table 1.
Primer sequences used for amplifying target-gene mRNA
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
|
| ||
| IGF-I | CTCTTCAGTTCGTGTGTGGAGAC | CAGCCTCCTTAGATCACAGCTC |
| PGC1α | CCAAAGGATGCGCTCTCGTTCA | CGGTGTCTGTAGTGGCTTGACT |
| PPARγ | AGCCTGCGAAAGCCTTTTGGTG | GGCTTCACATTCAGCAAACCTGG |
| PPARα | TCGGCGAGGATAGTTCTGGAAG | GACCACAGGATAAGTCACCGAG |
| NRF-1 | GGCAACAGTAGCCACATTGGCT | GTCGTCTGGATGGTCATCTCAC |
| TFAM | GTGGTTTTCATCTGTCTTGGCAAG | TTCCCTCCAACGCTGGGCAATT |
| FNDC5 | AGCGAGCCTGTGCTCTTCAAGA | GAACAGGACCACGACGATGATC |
| GAPDH | GCGAGATCCCTCCAAAATCAA | GTTCACACCCATGACGAACAT |
Measurement of irisin
Serum samples were obtained from previously unthawed blood aliquoted into 2 mL Sarstedt microtubes and stored at −80°C. Samples were obtained in the fasting and rested state prior to any procedures or exertional activity. Data on irisin were not previously analyzed. Irisin was measured by competitive ELISA (Adipogen, intraassay variability 6.9%).
Body composition assessment
Waist circumference (WC) was reported as the circumferential measurement of the waist at the level of the top of the iliac crest around the umbilicus. Total body lean body mass and fat mass were determined by dual-energy x-ray absorptiometry (Discovery A; Hologic, Inc).
Indirect calorimetry
Resting energy expenditure (REE) was assessed by determining oxygen consumption (VO2) and carbon dioxide production (VCO2) using a calibrated calorimeter (VMAX29N, Sensormedics) after a 20 minute rest.
Physical activity assessment
Physical activity was evaluated through self-reported regular structured exercise during direct interview by the modifiable activity questionnaire and expressed as total hours of activity per week (metabolic equivalents) and hours of TV watching per day.
Statistical analysis
Normality of distribution was determined using the Shapiro-Wilk test. Data are presented as mean ± standard error of the mean or median [interquartile range (IQR)], depending on normality of the distribution. Categorical variables are reported as proportions. Linear regression was performed using Spearman’s correlation coefficient among all subjects. For mitochondrial function parameters and gene expression, the Bonferroni P values for significance, adjusted for the number of comparisons made were 0.025 and 0.008, respectively. All statistical analysis was performed using SAS JMP (version 9.0). Statistical significance was defined as P<0.05 unless adjusted for multiple comparisons as above.
RESULTS
Demographic and clinical characteristics
Baseline characteristics are presented in Table 2. The average age of subjects was 48±2 years. BMI and WC were consistent with abdominal obesity at 38±2 kg/m2 and 124±3 cm, respectively. As per study design, mean peak-stimulated GH on GHRH-arginine testing was reduced, 2.7±0.4 ug/L. Mean irisin level were 6.29±0.36 μg/mL among all subjects.
Table 2.
Baseline demographic and clinical characteristics
|
Demographics
| |
| Age (years) | 48±2 |
| Race (%) | |
| Caucasian | 67 |
|
| |
|
Body Composition Parameters
| |
| BMI (kg/m2) | 38±2 |
| Iliac waist (cm) | 124±3 |
| Total fat mass (kg) | 44.4±3.1 |
| Total lean mass (kg) | 70.3±1.8 |
|
| |
|
Metabolic Parameters
| |
| Peak stimulated GH on GHRH-arginine (μg/L) | 2.7±0.4 |
| IGF-I (μg/L) | 134 [115,150] |
| Irisin (μg/mL) | 6.29±0.36 |
|
| |
|
Energy Parameters
| |
| REE (kcal/day) | 1757±65 |
| REE/total lean mass (kcal/day*kg) | 24.98±0.71 |
| RQ | 0.88±0.01 |
| VO2 (L/min) | 0.25±0.01 |
| VCO2 (L/min) | 0.22±0.01 |
Data reported as mean ± standard error mean, percentage, or median [interquartile range].
Abbreviations: BMI, body mass index; GHRH, growth hormone releasing hormone; REE, resting energy expenditure; RQ, respiratory quotient, VO2, maximal oxygen consumption; VCO2, carbon dioxide production
Relationship of irisin and skeletal muscle FNDC5 mRNA to mitochondrial function
FNDC5 mRNA was strongly associated with ViPCr (ρ=0.79, P=0.006) (Table 3). Irisin tended to be related to ViPCr (ρ=0.48, P=0.08) as well as PCr at rest (ρ=0.48, P=0.08) and the beginning of recovery (ρ=0.48, P=0.08). Irisin was significantly and negatively correlated with adenosine diphosphate (ADP) at rest (ρ=−0.64, P=0.01), beginning of recovery (ρ=−0.75, P=0.002), and end of recovery (ρ=−0.58, P=0.03).
Table 3.
Univariate correlations with irisin and mRNA expression of skeletal muscle FNDC5
| Baseline | After 3 months Treatment with rhGH | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Serum Irisin | Skeletal Muscle FNDC5 mRNA | Serum Irisin | Skeletal Muscle FNDC5 mRNA | |||||
|
| ||||||||
| ρ | P Value | ρ | P Value | ρ | P Value | ρ | P Value | |
| Body Composition Parameters | ||||||||
|
| ||||||||
| BMI (kg/m2) | 0.38 | .17 | −0.15 | .68 | 0.21 | .46 | 0.09 | .80 |
| Iliac Waist (cm) | 0.30 | .28 | −0.24 | .51 | 0.11 | .70 | 0.26 | .47 |
| Total fat mass (kg) | 0.39 | .16 | −0.16 | .65 | 0.09 | .76 | −0.02 | .96 |
| Total lean mass (kg) | −0.14 | .62 | 0.04 | .91 | 0.05 | .85 | −0.09 | .80 |
|
| ||||||||
| Metabolic Parameters | ||||||||
|
| ||||||||
| Peak stimulated GH on GHRH-arginine (μg/L) | −0.20 | .49 | −0.45 | .19 | -- | -- | -- | -- |
| IGF-I (μg/L) | −0.38 | .17 | 0.08 | .83 | 0.20 | .47 | −0.22 | .53 |
|
| ||||||||
| Energy Parameters | ||||||||
|
| ||||||||
| REE (kcal/day) | 0.30 | .28 | 0.32 | .37 | 0.10 | .73 | 0.16 | .65 |
| REE/total lean mass (kcal/day*kg) | 0.30 | .27 | 0.26 | .47 | 0.24 | .40 | 0.31 | .38 |
| RQ | −0.18 | .52 | −0.49 | .15 | −0.15 | .58 | −0.19 | .60 |
| VO2 (L/min) | 0.31 | .27 | 0.37 | .29 | 0.19 | .50 | 0.19 | .60 |
| VCO2 (L/min) | 0.19 | .50 | 0.16 | .65 | 0.06 | .84 | 0.03 | .93 |
|
| ||||||||
| Mitochondrial Function | ||||||||
|
| ||||||||
| τPC | −0.10 | .74 | −0.28 | .43 | −0.02 | .95 | 0.19 | .60 |
| ViPCr (mM/min) | 0.48 | .08 | 0.79 | .006 | −0.02 | .96 | 0.22 | .53 |
| PCr (mM) | ||||||||
| Rest | 0.48 | .08 | 0.22 | .53 | 0.27 | .36 | −0.20 | .58 |
| Beginning of recovery | 0.48 | .08 | 0.13 | .73 | 0.05 | .88 | −0.30 | .40 |
| End of recovery | 0.38 | .18 | 0.04 | .91 | 0.24 | .41 | 0.02 | .96 |
| ADP (uM) | ||||||||
| Rest | −0.64 | .01 | −0.35 | .33 | 0.03 | .93 | 0.13 | .71 |
| Beginning of recovery | −0.75 | .002 | −0.56 | .09 | 0.31 | .29 | −0.05 | .88 |
| End of recovery | −0.58 | .03 | −0.25 | .49 | −0.09 | .76 | −0.01 | .99 |
|
| ||||||||
| mRNA Expression | ||||||||
|
| ||||||||
| IGF-I | 0.63 | .05 | 0.81 | .005 | 0.05 | .88 | 0.66 | .04 |
| PGC-1α | 0.44 | .21 | 0.68 | .03 | 0.19 | .60 | 0.39 | .26 |
| PPARγ | 0.73 | .02 | 0.85 | .002 | 0.01 | .99 | 0.10 | .78 |
| PPARα | 0.37 | .29 | 0.62 | .05 | 0.03 | .93 | 0.25 | .49 |
| NRF1 | 0.54 | .11 | 0.66 | .04 | −0.07 | .85 | 0.58 | .08 |
| TFAM | 0.48 | .16 | 0.79 | .007 | 0.03 | .93 | 0.38 | .28 |
Relationships were assessed by Spearman’s Correlation Coefficient.
Gene expression values are normalized to GAPDH, results are expressed as ratios in arbitrary units
Abbreviations: rhGH, recombinant human growth hormone; FNDC5, fibronectin type III domain-containing protein 5; BMI, body mass index; GHRH, growth hormone releasing hormone; REE, resting energy expenditure; RQ, respiratory quotient, VO2, maximal oxygen consumption; VCO2, carbon dioxide production; PCr, phosphocreatine; τ, recovery time constant; Vi, initial rate; ADP, adenosine diphosphate; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α; PPAR, peroxisome proliferator-activated receptor; NRF1, nuclear respiratory factor; TFAM, mitochondrial transfer factor A
Relationship of irisin and skeletal muscle FNDC5 mRNA to gene expression
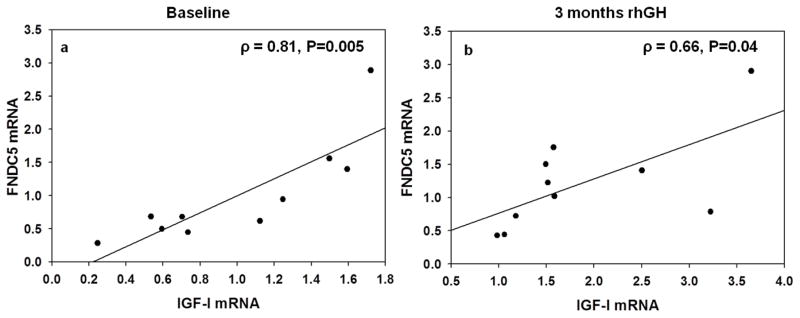
Both serum irisin and skeletal muscle FNDC5 mRNA were significantly associated with skeletal muscle IGF-I (ρ=0.63, P=0.05 and ρ=0.81, P=0.005) and PPARγ (ρ=0.73, P=0.02 and ρ=0.85, P=0.002), respectively (Figure 1a). In addition, mRNA expression of FNDC5 was correlated with mRNA expression of PGC-1α (ρ=0.68, P=0.03), NRF1 (ρ=0.66, P=0.04) and TFAM (ρ=0.79, P=0.007) in the skeletal muscle (Table 3).
Figure 1.
Relationship between IGF-I and FNDC5 mRNA expression (A.U) in the skeletal muscle at (a) baseline and (b) after treatment with rhGH for 3 months. Data were analyzed using Spearman’s correlation coefficient and are represented here as linear regression for purposes of illustrating the relationship.
Irisin and skeletal muscle FNDC5 mRNA expression after rhGH treatment
Neither serum irisin (6.29±0.36 vs.6.54±0.32, P=0.29) nor skeletal muscle mRNA expression of FNDC5 (0.68 [0.48, 1.44] vs. 1.12 [0.65, 1.56] A.U., P=0.32) changed after rhGH treatment, but FNDC5 and skeletal muscle mRNA expression of IGF-I remained associated after 12 weeks of treatment (ρ = 0.66, P=0.04) (Figure 1b).
DISCUSSION
Our findings demonstrate novel data that FNDC5 mRNA expression in skeletal muscle and irisin levels associated positively with parameters of mitochondrial function and gene expression in obese men with reduced GH. In addition, skeletal muscle FNDC5 mRNA expression was associated with IGF-I mRNA expression both before and after treatment with rhGH. Neither circulating irisin nor FNDC5 mRNA expression significantly increased following rhGH treatment, and the association between FNDC5 and IGF-I mRNA in skeletal muscle remained weak. This may suggest a potential physiological relationship between irisin and the GH/IGF-I axis such that FNDC5 is compensatory for metabolic dysrgulation during states of low GH secretion, or alternatively, regulation of IGF-I gene expression could be largely FNDC5-independent. Because this current study is small and was not powered to detect changes in irisin or FNDC5, we cannot exclude the possibility of type 2 error, i.e., the possibility that GH or IGF-I may have modest effects to increase FNDC5 expression. However, irisin treatment of human myocytes has been shown to increase IGF-I [20], and a causal relationship whereby muscle irisin stimulates local IGF-I expression may underlie the significant association seen in the present study. In that regard, neither serum IGF-I nor peak stimulated GH was associated with either circulating irisin or FNDC5 expression, unlike muscle IGF-I mRNA. Finally, there could be common mediators of both FNDC5 and IGF-I, an evaluation beyond the scope of the current study.
In addition to the relationship between FNDC5 and IGF-I, our study highlights associations between muscle FNDC5 mRNA expression and measures of mitochondrial function and gene expression of PPARγ, NRF1, and TFAM. Prior studies have shown that there is cross-talk between the muscle and adipose depot, such that irisin secreted from the muscle exerts its action specifically in brown adipose tissue through upregulation of UCP-1 [8]. The specific role of irisin in the human muscle depot has been less clear and poses the question of its autocrine/paracrine, rather than endocrine function. In vitro data suggest that skeletal myocytes treated with irisin demonstrate increased mitochondrial biogenesis, energy expenditure and oxidative metabolism, and expression of mitochondrial related genes, NRF1 and TFAM [10]. NRF1 is known to be a downstream target of PGC-1α, and TFAM a downstream target of NRF1. Our study complements these data and shows that FNDC5 was associated with both NRF1 and TFAM from the skeletal muscle. These data suggest that irisin has target effects in the muscle. In addition, muscle tissue has a significant role in glucose homeostasis, and insulin resistance is correlated with impaired mitochondrial biogenesis and function. Lee et al. recently proposed that irisin influences glucose uptake in the skeletal muscle through mediation of the AMPK pathway [21]. In the present study, we demonstrate that irisin and FNDC5 are also related to expression of PPARγ, a regulator of insulin sensitivity, in the muscle.
The significant associations of FNDC5 and irisin to muscle mitochondrial gene expression may be relevant to improved mitochondrial function. PCr recovery provides a measure of the oxidative capacity of the mitochondria. We have previously utilized 31P-MRS to assess ViPCr after submaximal exercise in obese men and demonstrated for the first time a significant relationship of ViPCr to both IGF-I and peak GH on standard stimulation testing [6]. Furthermore, in a subset of obese men with reduced GH, skeletal muscle IGF-I mRNA was correlated with PCr recovery as well as mitochondrial gene expression in skeletal muscle [7]. These findings suggest a unique relationship between GH regulation and mitochondrial function even in the presence of GH insufficiency. Our study is consistent with prior preclinical data demonstrating that treatment of human myocytes with irisin increases IGF-I expression [20] and provides further information, as we demonstrated that both irisin and FNDC5 expression were correlated with ViPCr, as well as IGF-I expression in the skeletal muscle. In addition, irisin was inversely correlated with ADP during recovery, which may represent reduced utilization of ADP in accordance with the uncoupling of oxidative phosphorylation. In this regard, the effects of irisin may persist despite overall presumed chronic loss of muscle mass in low GH states.
The relationships of mitochondrial parameters with circulating irisin were less robust compared to relationships between mitochondrial parameters and FNDC5 mRNA. Recent studies suggest that circulating irisin levels are detectable acutely after increased exercise workload within minutes to hours [22, 23]. We may not have detected a strong relationship to irisin in this study since subjects did not participate in a strenuous exercise program and levels were obtained prior to MVC. In this regard, local autocrine/paracrine action of the irisin precursor or irisin itself, rather than systemic expression of the cleaved product may be more closely correlated with mitochondrial indices in the muscle.
This study has some limitations. A small sample size was included. With a larger sample size, it may have been possible to detect stronger relationships to circulating irisin. Future studies can also be designed to evaluate relationships between FNDC5 and irisin and the GH/IGF-I axis over a longer treatment course. A number of comparisons were made in this study. The main findings that FNDC5 mRNA correlates with ViPCr and IGF-I mRNA remained significant at baseline when accounting for multiple comparisons, but significance was lost after 3 months of rhGH. In addition, the study included male subjects with evidence of low GH secretion. Future comparisons should include GH sufficient subjects and lean controls, as well as females. Based on recognized cut-offs for stimulation testing used in this protocol, reduced GH secretion is prevalent in approximately 20% of obese patients [2]. In this regard, subjects with reduced GH secretion are an interesting population to study and provide unique information regarding muscle composition and other metabolic complications.
In sum, these data provide novel information for the role of irisin and FNDC5 in the skeletal muscle depot with regards to mitochondrial parameters and highlight potential autocrine/paracrine function for this myokine. Additionally, our data show an association between FNDC5 expression and IGF-I expression, although we cannot determine the causal direction of this relationship. Finally, our data suggest that FNDC5 and irisin may have a protective role in low GH states to enhance mitochondrial biogenesis and function, and future studies should be designed to assess this further.
Highlights.
FNDC5 mRNA and circulating irisin are associated with mRNA expression of IGF-I.
FNDC5 mRNA correlates with rate of phosphocreatine recovery after exercise. FNDC5
mRNA and irisin do not change after 12 weeks of growth hormone replacement.
Acknowledgments
Funding: Funding was provided by NIH K23DK087857 (HM). The project described was supported by NIH 1UL1RR025758-04, 8UL1TR000170-05, M01RR01066, and UL1 TR001102 to the Harvard Clinical and Translational Science Center, from the National Center for Research Resources and National Center for Advancing Translational Sciences.
The investigators would like to thank the nursing staff on the MGH CRC for their dedicated patient care as well as the volunteers who participated in this study.
ABBREVIATIONS
- GH
growth hormone
- IGF-I
insulin-like growth factor I
- rhGH
recombinant human GH
- GHRH
growth hormone releasing hormone
- PCr
phosphocreatine
- NRF-1
nuclear respiratory factor 1
- TFAM
mitochondrial transcription factor A
- PPAR
peroxisome proliferator-activated receptor
- FNDC5
fibronectin type III domain-containing protein 5 gene
- PGC-1α
peroxisome proliferator-activated receptor coactivator 1 alpha
- UCP-1
uncoupling protein 1
- GADPH
glyceraldehyde 3-phosphate dehydrogenase
- 31P-MRS
31P-magnetic resonance spectroscopy
- MVC
maximal voluntary contraction
- Pi
inorganic phosphate
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- τPCr
recovery time constant
- ViPCr
initial rate of PCr recovery
- WC
waist circumference
- REE
resting energy expenditure
- VO2
oxygen consumption
- VCO2
carbon dioxide production
- AMPK
AMP-activated protein kinase
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Clinical Trial Registration: NCT 01421589
Disclosure Statement: SS, CS, JM, SRH, JEI, WF, MT, TS have nothing to declare. This study was supported by an investigator initiated research grant from Pfizer Inc to HM.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Makimura H, Stanley T, Mun D, You SM, Grinspoon S. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab. 2008;93:4254–4260. doi: 10.1210/jc.2008-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makimura H, Stanley T, Mun D, Chen C, Wei J, Connelly JM, et al. Reduced growth hormone secretion is associated with increased carotid intima-media thickness in obesity. J Clin Endocrinol Metab. 2009;94:5131–5138. doi: 10.1210/jc.2009-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Utz AL, Yamamoto A, Hemphill L, Miller KK. Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab. 2008;93:2507–2514. doi: 10.1210/jc.2008-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Short KR, Moller N, Bigelow ML, Coenen-Schimke J, Nair KS. Enhancement of muscle mitochondrial function by growth hormone. J Clin Endocrinol Metab. 2008;93:597–604. doi: 10.1210/jc.2007-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unterluggauer H, Hutter E, Viertler HP, Jansen-Durr P. Insulin-like growth factor-induced signals activate mitochondrial respiration. Biotechnol J. 2008;3:813–816. doi: 10.1002/biot.200700254. [DOI] [PubMed] [Google Scholar]
- 6.Makimura H, Stanley TL, Sun N, Hrovat MI, Systrom DM, Grinspoon SK. The association of growth hormone parameters with skeletal muscle phosphocreatine recovery in adult men. J Clin Endocrinol Metab. 2011;96:817–823. doi: 10.1210/jc.2010-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamarneh SR, Murphy CA, Shih CW, Frontera W, Torriani M, Irazoqui JE, et al. Relationship between serum IGF-I and skeletal muscle IGF-I mRNA expression to phosphocreatine recovery after exercise in obese men with reduced GH. J Clin Endocrinol Metab. 2015;100:617–625. doi: 10.1210/jc.2014-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaughan RA, Gannon NP, Barberena MA, Garcia-Smith R, Bisoffi M, Mermier CM, et al. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes Metab. 2014;16:711–718. doi: 10.1111/dom.12268. [DOI] [PubMed] [Google Scholar]
- 11.Pardo M, Crujeiras AB, Amil M, Aguera Z, Jimenez-Murcia S, Banos R, et al. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int J Endocrinol. 2014;2014:857270. doi: 10.1155/2014/857270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98:E769–778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 14.Park KH, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98:4899–4907. doi: 10.1210/jc.2013-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosseini Ghomi R, Bredella MA, Thomas BJ, Miller KK, Torriani M. Modular MR-compatible lower leg exercise device for whole-body scanners. Skeletal Radiol. 2011;40:1349–1354. doi: 10.1007/s00256-011-1098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roussel M, Bendahan D, Mattei JP, Le Fur Y, Cozzone PJ. 31P magnetic resonance spectroscopy study of phosphocreatine recovery kinetics in skeletal muscle: the issue of intersubject variability. Biochim Biophys Acta. 2000;1457:18–26. doi: 10.1016/s0005-2728(99)00111-5. [DOI] [PubMed] [Google Scholar]
- 17.Dietrichson P, Coakley J, Smith PE, Griffiths RD, Helliwell TR, Edwards RH. Conchotome and needle percutaneous biopsy of skeletal muscle. J Neurol Neurosurg Psychiatry. 1987;50:1461–1467. doi: 10.1136/jnnp.50.11.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- 19.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14:101–102. [PubMed] [Google Scholar]
- 20.Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond) 2014;38:1538–1544. doi: 10.1038/ijo.2014.42. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ, Lee JO, Kim N, Kim JK, Kim HI, Lee YW, et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol Endocrinol. 2015;29:873–881. doi: 10.1210/me.2014-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daskalopoulou SS, Cooke AB, Gomez YH, Mutter AF, Filippaios A, Mesfum ET, et al. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur J Endocrinol. 2014;171:343–352. doi: 10.1530/EJE-14-0204. [DOI] [PubMed] [Google Scholar]
- 23.Loffler D, Muller U, Scheuermann K, Friebe D, Gesing J, Bielitz J, et al. Serum irisin levels are regulated by acute strenuous exercise. J Clin Endocrinol Metab. 2015;100:1289–1299. doi: 10.1210/jc.2014-2932. [DOI] [PubMed] [Google Scholar]