Abstract
Estrogen is involved in promoting lung cancer cell division and metastasis. MICA and MICB function as ligands for NKG2D, an important immunoreceptor expressed on natural killer (NK) cells. However, whether estrogen regulates MICA/B expression and affects tumor immune escape remains unknown. In this study, we measured the mRNA levels of MICA, MICB and ADAM17in non-small cell lung cancer (NSCLC) cell lines treated with estrogen. Surface expression of MICA/B on LTEP-a2 and A549 was detected using flow cytometry. We demonstrate that both mRNA and secretory protein levels of MICA/B in lung adenocarcinoma cell lines were upregulated by estradiol. Estradiol enhanced the expression of ADAM17, which was associated with the secretion of MICA/B. This secretion of MICA/B downregulated the NKG2D receptor on the surface of NK92 cells and impaired the cytotoxic activity of NK cells. Estradiol enhanced the expression of ADAM17, which was associated with the secretion of MICA/B. Furthermore, a significant correlation between the concentration of estradiol and the expression of MICA was found in tumor tissues of NSCLC patients. Therefore, we conclude that estrogen can regulate the expression and secretion of MICA/B through ADAM17, which helps lung cancer cells escape NKG2D-mediated immune surveillance.
Keywords: ADAM17, estradiol, MICA, MICB, tumor immune escape
Introduction
Lung cancer is the leading cause of cancer death worldwide.1 While most lung cancers develop as a result of smoking,2 some do not. Women appear to have a higher risk of non-small cell lung cancer (NSCLC) than men among the non-smoking population.3 The reason for this phenomenon is still unclear; however, some evidence has shown that estrogen and the estrogen signaling pathway play a critical role in the development and pathogenesis of lung cancer.4 Estrogen receptors (ERα and ERβ) are widely expressed in normal lung tissue and NSCLC tissue.5,6,7 17β-estradiol can activate p44/p42 mitogen activated protein kinase and increase the proliferation of NSCLC cells.8,9 Moreover, aromatase, an estrogen synthetase, is also detected in NSCLC tissues, and lower levels of aromatase are predictive of a greater chance of survival in women with NSCLC.10 Our previous study indicated that ERβ was highly expressed on the tumors of NSCLC patients and silencing the expression of ERβ could inhibit the growth of NSCLC cells in vivo.11 However, the role estrogen plays in the development of lung cancers, especially in the immune surveillance of tumors, is still unclear.
As ligands for NKG2D, MICA and MICB are essential immunoreceptors expressed on natural killer (NK) cells. The interaction between NKG2D and its ligands plays an important role in immune surveillance.12 MICA and MICB are widely expressed on cancer cells, and the engagement of MICA and MICB by NKG2D triggers the antitumor responses of NK cells, CD8+ T cells and γδ T cells.13 Clinical data have demonstrated a high level of secretory MICA and MICB in the serum of cancer patients.14,15 Cancer cells can evade NKG2D-mediated immunity by the shedding of MICA and MICB, which results in impaired NKG2D function on NK cells and CTLs.16,17 Previous reports have suggested that estradiol may regulate MICA expression in human endometrial cells.18 Therefore, we put forward a hypothesis that estrogen may regulate MICA/B expression and affect immune surveillance during the development of lung cancer.
Material and methods
Cell culture and treatment
A549 andLTEP-a2 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). NK-92 cells (a gift from Zhigang Tian, University of Science and Technology of China, China) were maintained in α-MEM (Hyclone, Logan, Utah, USA) containing 100 U/ml IL-2 (Peptech, Burlington, MA, USA). The cells were maintained at 37 °C in a water-saturated atmosphere with 5% CO2.As described previously,19 the cells were placed in phenol red-free media supplemented with 5% dextran-coated and charcoal-stripped fetal bovine serum for 72–96 h. EtOH was used as control for all experiments. The cells were treated with various concentrations of estradiol. For the indicated experiments, the cells were pre-treated with 1 µM tamoxifen for 6 h before estradiol treatment.
Clinical samples
The tumor tissues were collected from 61 NSCLC patients during surgery at the Nanjing Chest Hospital (Nanjing, China). The pathological data of these 61 patients with NSCLC are summarized in Table 1. The sera collected from61patientswerekept at −80 °C before the assay. All of the patients consent to the study, and the protocol was approved by the institutional ethics committee of the Medical School of Nanjing University.
Table 1. Clinical variables and tumor histological features.
| Gender | |
|---|---|
| Males | 42 (68.9%) |
| Females | 19 (31.1%) |
| Age (years) | |
| Median | 60.71 |
| Range | 29–77 |
| Smoking history | |
| Active | 25 (41%) |
| Never | 18 (29.5%) |
| Undetermined | 18 (29.5%) |
| Histology | |
| Adenocarcinoma | 30 (49.2%) |
| Squamous cell carcinoma | 21 (34.4%) |
| Others | 10 (19.4%) |
| Tumor stage | |
| IA/IB | 24 (39.3%) |
| IIA/IIB | 13 (21.3%) |
| IIIA/IIIB | 22 (36.1%) |
| IV | 1 (1.6%) |
| Undetermined | 1 (1.6%) |
RNA isolation and quantitative real-time PCR analysis
Total RNA was isolated using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer's recommendations. One microgram total RNA was reverse-transcribed to synthesize cDNA according to manufacturer's protocols. Quantitative PCR analysis was carried out to quantify the mRNA levels of MICA, MICB and ADAM17, and expression of these candidate genes was normalized against the internal control β-actin of the same sample. The primer sequences of MICA, MICB, ADAM17 and β-actin are shown in Table 2. For mRNA quantification, the relative expression of these genes was obtained using the 2−ΔΔCt method.
Table 2. Primers used in this study.
| Gene | Primer (5′–3′) |
|---|---|
| MICA-F | CCTTGGCCATGAACGTCAGG |
| MICA-R | CCTCTGAGGCCTCGCTGCG |
| MICB-F | ACCTTGGCTATGAACGTCACA |
| MICB-R | CCCTCTGAGACCTCGCTGCA |
| ADAM17-F | CCGCTGTGTGCCCTATGT |
| ADAM17-R | CCAGGACAGACCCAA |
| β-actin-F | CCACGAAACTACCTTCAACTC |
| β-actin-R | TCATACTCCTGCTGCTTGCTGATCC |
Enzyme-linked immunosorbent assay (ELISA)
Estrogen, MICA and MICB contents in sera and tumor culture supernatants were quantified using the E2 ELISA assay kit (Assay Design & Stressgen, Ann Arbor, Michigan, USA ), MICA ELISA assay kit (Abcam, Cambridge, MA, USA) and MICB ELISA assay kit (Abcam, Cambridge, MA, USA), respectively. All assays were performed in duplicate.
Immunohistochemistry assay
Immunohistochemistry was performed by the National Engineering Center for Biochip at Shanghai (China). The anti-human MICA/B antibodies (Biolegend, San Diego, California, USA ) or anti-human aromatase antibodies (Epitomics Inc., Burlingame, California, USA) were used for immunohistochemistry. Staining with1% BSA instead of primary antibodies was used as negative control sections. Cells from five different microscope fields of each stained tissue were used to quantitate positive cells.
Flow cytometry analysis
LTEP-a2 and A549 were collected and washed with PBS. 1.5×106 cells were incubated under saturating PE anti-human MICA/B (eBioscience, San Diego, California, USA ) at 4 C for 30 min following the standard procedure. Labeled cells were analyzed on a FACSCalibur flow cytometer using the Cell Quest software (Becton Dickinson, Franklin Lakes, New Jersey USA ) or FlowJo software (Tree star, Ashland, North Carolina, USA).
Western blot analysis
LTEP-a2 and A549 cells were lysed in an ice-cold buffer (150 mM NaCl, 0.02% NaN3, 0.1% SDS, 50 mM TrisCl, pH 8.0, 100 µg/ml phenylmethylsufonyl fluoride, 1 µg/ml Aprotinin, 1% Triton) for 30 min. The cells lysates were collected, and the concentrations were measured using the BCA Protein Assay Reagent Kit (Beyotime, Shanghai, China). Fifty micrograms of lysate were then separated by 12% SDS–PAGE gel and transferred onto PVDF membranes (Roche, Penzberg, Upper Bavaria, Germany).
After blocking in TBST-containing 2% BSA for 1 h, antibodies specific for ADAM17 (1∶500; Santa Cruz, Dallas, Texas, USA), GAPDH (1∶2000; Cell Signaling Technology, Inc., Danvers, MA, USA) and HRP-conjugated goat anti-rabbit secondary antibodies (1∶1000; Promab, Richmond, CA , USA ) were used. The protein bands were detected by the enhanced chemiluminescence reaction (Kibbutz Beit Haemek, D.N. Oshrat, Israel), and the intensity of each band was quantified by Quantity One software.
Small interfering RNAs transfection targeting ADAM17
Approximately 3×106 cells/well were cultured in 12-well plates and transfected with either ADAM17-siRNA (100 nM) or negative control siRNA (100 nM) using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. The specific RNAi sequence for human ADAM17 was synthesized by RiboBio (Co Ltd, Guangzhou, China). Forty-eight hours after transfection, the cells were collected and treated with estrogen. The cells and supernatants were harvested at appropriate times with estrogen for flow analysis, quantitative real-time PCR and ELISA.
NK cell cytotoxicity assay
Two million CD56-selected NK effector cells were isolated from the peripheral blood of a healthy donor. Primary NK cells and NK92 cells were treated with estradiol with and without MICA/B neutralizing antibodies at 37 °C for 24 h. K562 cells were labeled using 5.0 µM CFSE for 8 min at 37 °C. The NK cytotoxicity assay used an NK cells/K562 cells ratio of 5∶1 and was incubated for 4 h at 37 °C in 5% CO2.
Statistical analysis
All of the results are presented as the means±s.e.m. of at least three independent experiments. The Student's t-test was used to assess differences between two groups. A value of P<0.05 was considered statistically significant.
Results
The transcription of MICA and MICB is upregulated by estradiol
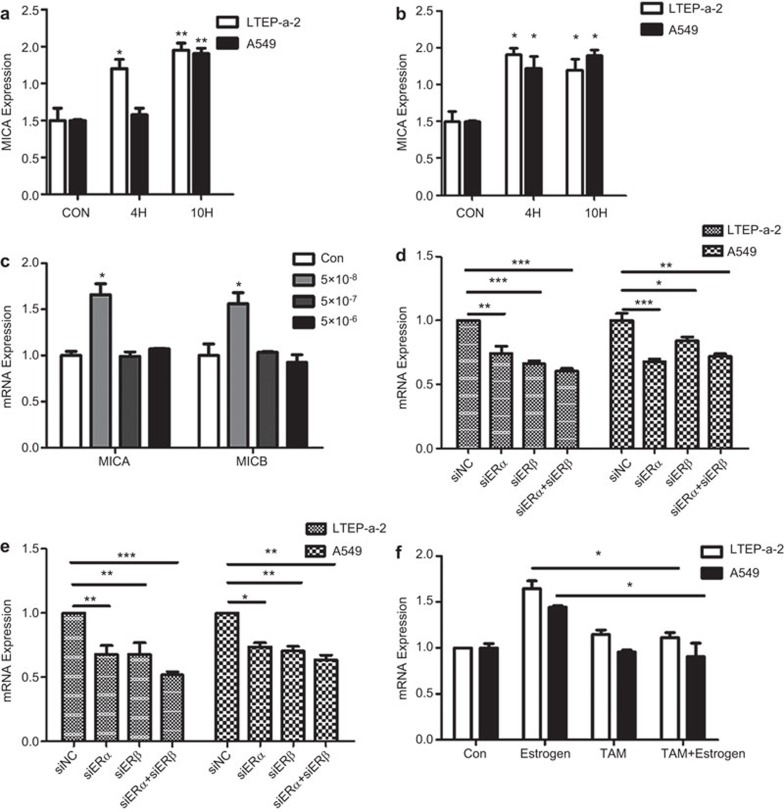
To determine whether estradiol regulates the transcription of MICA and MICB in NSCLC cells, A549 and LTEP-a2 cells were exposed to physiologically relevant estradiol concentrations (5×10−8 M) for 4 h and 10 h. MICA and MICB mRNA expression was significantly upregulated by estradiol in both cell lines (Figure 1a and b). This increase in MICA and MICB mRNA was only observed at the concentration of 5×10−8 M estradiol, while 5×10−7 M and 5×10−6 M estradiol had no effect on the expression of MICA/B (Figure 1c). We also determined the MICA genotype in these cell lines. Both MICA*001 and MICB*008 were detected in A549 cells and LTEP-a2 cells (Supplementary Figure 1a). To determine whether the estradiol-induced increase of MICA and MICB was mediated directly by estrogen receptors, small interfering RNAs targeting ERα and ERβ were designed and validated (Supplementary Figure 1b). As shown in Figure 1d and e, both ERα and ERβ were involved in the activation of MICA/B. We also pre-treated LTEP-a2 cells with tamoxifen for 6 h before estradiol treatment. As shown in Figure 1f, tamoxifen could block the estradiol-induced increase in MICA mRNA expression, while it had no effect on MICB expression (data not shown).
Figure 1.
Estrogen regulates the transcription of MICA and MICB. The mRNA levels of MICA (a) and MICB (b) were detected in LTEP-a2 and A549 cells treated with estradiol (5×10−8 M) using qPCR. (c) The mRNA levels of MICA and MICB after treatment with different estradiol concentrations 5×10−8 M, 5×10−7 M and 5×10−6 M. The mRNA levels of MICA (d) and MICB (e) in LTEP-a2 and A549 cells treated with inhibitor of estrogen receptor. The mRNA levels of MICA (f) in lung cancer cells treated with TAM. The data represent three independent experiments. All of the error bars indicate mean±s.e.m. *P<0.05, **P<0.01, *** P<0.001.
The secretory protein of MICA is promoted by estradiol
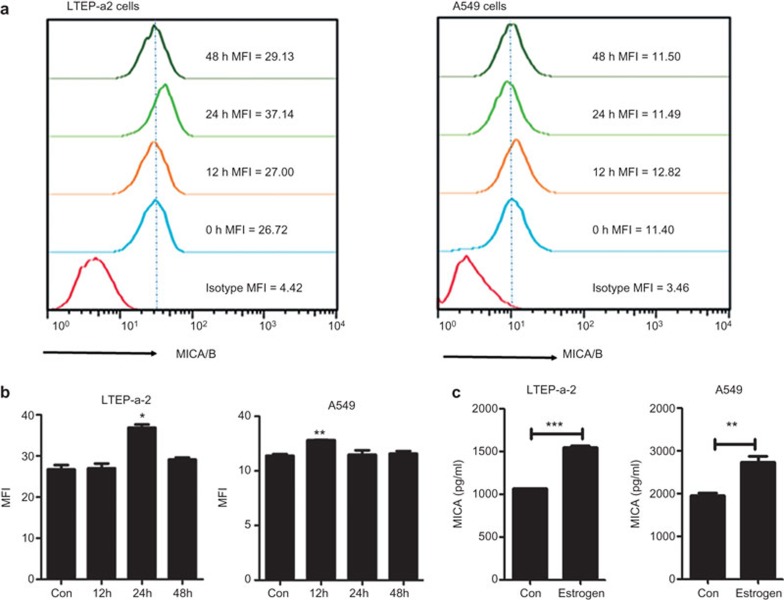
Previous research has shown that MICA and MICB proteins can exist in two forms: surface protein and secretory protein. These forms have different functions in the immune response. Surface MICA and MICB can lead to the activation of NK cells and γδ T cells that help to eliminate cancer cells, while secretory MICA and MICB proteins can help tumor cells escape the cytotoxicity of NK cells.20 In our study, after treatment of estradiol (5×10−8 M), surface proteins of MICA/B were transiently increased and returned to normal levels after 48 h (Figure 2a and b). We then detected the secretory protein of MICA and MICB using ELISA. While LTEP-a2 and A549 cells produced significant amounts of MICA secretory protein (Figure 2c), MICB secretory protein was not detected in the supernatants (data not shown).
Figure 2.
The shedding of MICA is accelerated by estradiol. (a, b) Flow cytometer analyzes the surface expression of MICA/B on LTEP-a2 and A549 after treatment of estrogen. (c) ELISA analyzes the concentration of MICA in the supernatants of cells treated with estradiol after 48 h. The data represent three independent experiments. All of the error bars indicate mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001.
Estradiol increases the expression of ADAM17
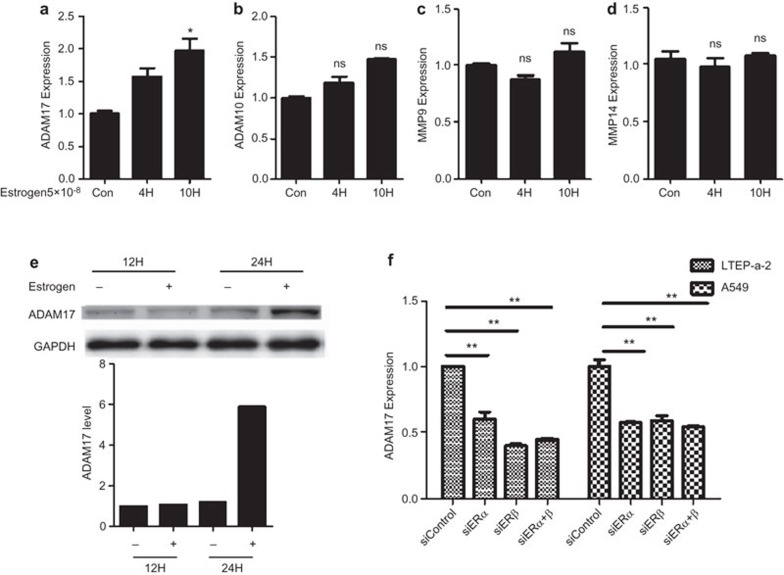
Previous studies have shown that ADAMs (including ADAM10 and ADAM17) and MMPs were the main proteases mediating the secretion of MICA and MICB.21,22 Therefore, we investigated the effect of estradiol on the expression of ADAM10, ADAM17, MMP9 and MMP14. The mRNA expression of ADAM17 was significantly increased after treatment with estradiol (Figure 3a). However, estradiol had no effect on the expression of ADAM10, MMP9 and MMP14 (Figure 3b–d). The protein level of ADAM17 was also significantly increased after estradiol treatment (Figure 3e). We also found that silencing ERα and ERβ could downregulate ADAM17 expression (Figure 3f).
Figure 3.
Estrogen increased the expression of ADAM17. The mRNA expression levels of ADAM17 (a), ADAM10 (b), MMP9 (c) and MMP14 (d) were detected in cells treated with estrogen. (e) Western blot analyses of the protein expression of ADAM17 inLTEP-a2 cells after treatment with estradiol. The data represent three independent experiments (average and s.e.m. of triplicate samples). (f) The mRNA levels of ADAM17 in LTEP-a2 and A549 cells treated with an inhibitor of the estrogen receptor. *P<0.05, **P<0.01.
ADAM17 is involved in the estradiol-induced increase of MICA/B
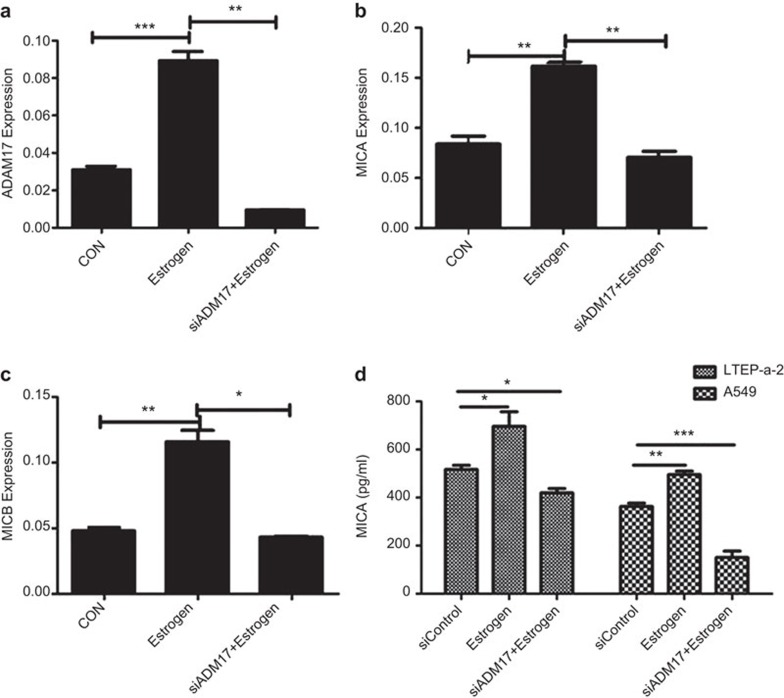
To verify whether ADAM17 was involved in the estradiol-induced increase of MICA/B, siRNA was used to knock down ADAM17 in LTEP-a2 cells (Figure 4a). Interestingly, the mRNA levels of MICA decreased 56.4% and the expression of MICB decreased 62.8% in the siADAM17+estradiol group compared with the estradiol group (Figure 4b and c). The secretory protein level of MICA in LTEP-a2 cells was also significantly decreased after infection with siADAM17 (Figure 4d).These data suggested that ADAM17 could regulate both the transcription and secretion of MICA/B.
Figure 4.
ADAM17 was involved in estradiol-induced increase of MICA/B. (a) The mRNAs levels of ADAM17 in LTEP-a2 cells transfected with si-ADAM17 μsing Q-PCR. The mRNA levels of MICA (b) and MICB (c) in LTEP-a2 cells transfected with si-ADAM17. (d) The protein levels of MICA were detected in the supernatant of LTEP-a2 cells transfected with siADAM17 using ELISA. The data represent three independent experiments. All of the error bars indicate mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001.
Estradiol decreases NKG2D expression and NK cell cytotoxicity through the regulation of MICA/B
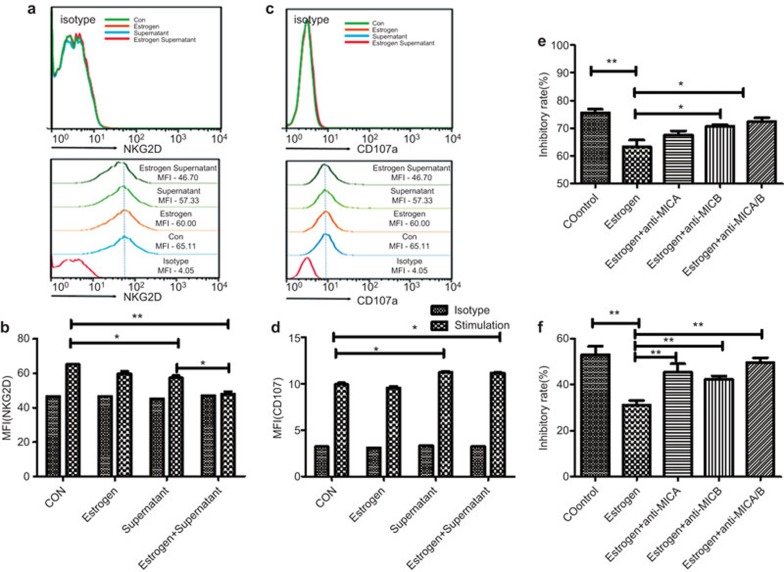
Secretory MICA and MICB could bind to NKG2D on NK cells and downregulate the surface expression of NKG2D.23,24,25 To validate whether estradiol-induced secretion of MICA could also affect the expression of NKG2D, NK-92 cells were cocultured with tumor supernatants with or without estradiol pre-treatment. The surface protein level of NKG2D on NK-92 cells was significantly decreased in NK-92 cells cocultured with tumor supernatants. Notably, estradiol pre-treated supernatants revealed an augmented decrease in the protein level of NKG2D in NK-92 cells (Figure 5a and b). Although estradiol alone showed little influence on CD107 expression, estradiol pre-treated supernatants could increase the levels of CD107 on the surface of NK-92 cells (Figure 5c and d).
Figure 5.
The tumor supernatant treated with estrogen can inhibit the NKG2D expression on NK92 cells. The expression of NKG2D (a) and CD107a (c) on NK92 cells was detected using flow cytometry. (b, d) The mean fluorescence intensity are represented and statistically analyzed. The cytotoxic activity of NK92 cells (e) and primary NK cells (f) against K562 cells were measured. The data represent three independent experiments. All of the error bars indicate mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001.
The cytotoxicity of the NK92 cells cultured with the supernatants was further confirmed. As shown in Figure 5e, the cytotoxicity of NK92 cells pre-treated with estradiol was significantly decreased. In addition, MICA/B neutralizing antibodies reversed these inhibitory effects. Consistent with NK cell lines, primary NK cells also showed decreased cytotoxicity when pre-treated with estradiol (Figure 5f).</tpb -4pt?>
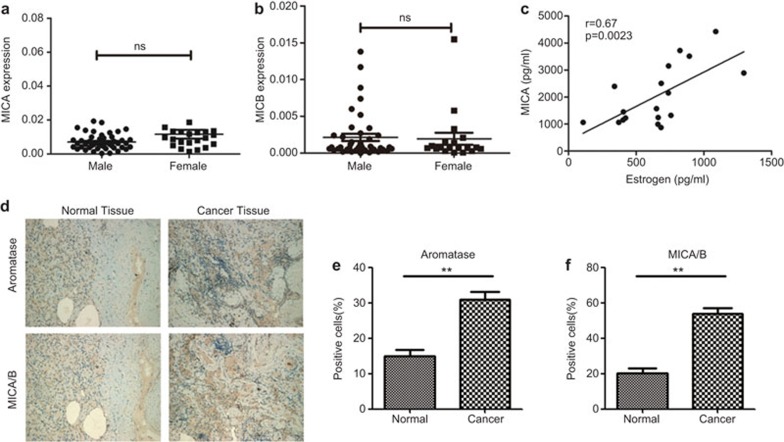
Correlation between estradiol and MICA/B expression in NSCLC patients
MICA and MICB expression levels in the tumor tissues from 61 NSCLC samples were detected to extend our analysis to clinical patients. No significant differences were found in MICA and MICB mRNA expression between females and males (Figure 6a and b). There was a significant correlation between the concentration of estradiol and the expression of MICA (r=0.67, P=0.0023) (Figure 6c) in the female group; however, there was no correlation between estradiol and MICB expression (r=0.28, P=0.25). To further identify the correlation between estradiol and MICA/B, we performed immunohistochemical analysis on NSCLC samples and normal tissue. As shown in Figure 6d–f, the expression levels of aromatase and MICA/B in NSCLC tissue were much higher than those in normal tissue.
Figure 6.
Correlation between estradiol and MICA/B expression in NSCLC patients. The tumor samples were classified into male (n=42) and female (n=19) groups. The mRNA expression levels of MICA (a) and MICB (b) in cancer tissue were detected using qPCR. (c) The correlation between estradiol and MICA in the serum of NSCLC patients was analyzed using ELISA. (d) Immunochemistry analyses of aromatase and MICA/B expression in normal and cancer tissue; (scale bars, 100 µm). Aromatase- (e) and MICA/B- (f) positive cells in tumor and normal tissues were statistically analyzed. All of the error bars indicate mean±s.e.m. **P<0.01.
Discussion
Estrogen and estrogen signaling pathways have been reported to induce epithelial–mesenchymal transition and play a critical role in the development and pathogenesis of lung cancer.4 In this study, our data have important consequences for the role of estrogen in the immune surveillance of the tumor microenvironment. First, we found that both mRNA and secretory protein levels of MICA/B in lung adenocarcinoma cell lines were upregulated by estradiol. We further proved that the regulatory role of estradiol on MICA/B is mediated through the enhanced expression of ADAM17. This secretion of MICA/B downregulated the NKG2D receptor on the surface of NK92 cells, and impaired the cytotoxic activity of NK cells. In addition, we extended our findings to the clinical setting: there was a significant correlation between the concentration of estradiol and the expression of MICA in tumor tissues of NSCLC patients.
The protein forms of MICA/B, surface protein and shedding protein perform difference functions in the immune response. These molecules are absent from most cells and tissues, but can be induced by viral and bacterial infections and are frequently expressed in epithelial tumors.26,27,28,29 Surface MICA/B can lead to the activation of NK cells and γδ T cells that help to eliminate the cancer cells, while the shedding of MICA and MICB can help cancer cells escape the cytotoxicity of NK cells and γδ T cells.20 In our study, we found that both mRNA and shedding protein levels of MICA/B in lung adenocarcinoma cell lines were upregulated by estradiol. However, estradiol has little effect on the surface protein of MICA/B. We also analyzed the effects of estrogen receptors on the estradiol-induced increase in MICA and MICB. We found that tamoxifen can block the estradiol-induced increase in MICA mRNA expression but has no effect on MICB expression. These differing effects of tamoxifen may be due to its interaction with the estrogen receptor. Tamoxifen could combine with the estrogen receptor to form a complex, which could upregulate the expression of ERBB2,30 and ERBB2 signaling could increase the expression of MICB.31
Given that MMPs and ADAMs are involved in the shedding of MICA and MICB,21,22 we investigated the effect of estradiol on the expression of ADAM10, ADAM17, MMP9 and MMP14 in lung cancer cells. Our data showed that only ADAM17 was significantly increased after estradiol treatment. Moreover, both mRNAs levels and shedding proteins of MICA/B in ADAM17-siRNA-transfected cells were significantly decreased. Based on these data, we conclude that ADAM17 is a specific protein involved in the estradiol-induced increase in MICA/B.
17β-estradiol can suppress the cytotoxicity and proliferative capacity of murine splenic NK1.1 in vitro.32 The interaction between NKG2DL and NKG2D plays an important role in immune surveillance, and MICA/B are ligands for NKG2D.11 To validate whether the increased shedding of MICA can also downregulate the expression of NKG2D, NK-92 cells were cocultured with tumor supernatants with or without estradiol pre-treatment. Our data indicated that estradiol pre-treated supernatants downregulated the expression of NKG2D on NK-92 cells. Moreover, CD107 is a sensitive marker of NK cell activity on NK-92 cells.33 We found that the expression of CD107 on NK-92 cells was also increased when cocultured with tumor supernatants, but not when pre-treated with estradiol. It is reasonable to predict that treatment with estradiol could promote the expression and secretion of NKG2D. Thus, the secretion of MICA/B downregulated the NKG2D receptor on the surface of NK92 cells, thereby impairing the cytotoxicity of NK92 cells that is involved in immune surveillance.
Taken together, our observations have important consequences. Specifically, estradiol can regulate the expression and secretion of MICA/B through ADAM17, impair the cytotoxic activity of NK cells and eventually lead to the escape of tumor cells from NKG2D-mediated immune surveillance.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81101552 and 81201598), and the Natural Science Foundation of Jiangsu Province (BK2011571).
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- 1Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61: 212–236. [DOI] [PubMed] [Google Scholar]
- 2Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer 2004; 45(Suppl 2): 3–9. [DOI] [PubMed] [Google Scholar]
- 3Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer 2007; 7: 778–790. [DOI] [PubMed] [Google Scholar]
- 4Carey MA, Card JW, Voltz JW, Arbes SJJr, Germolec DR, Korach KS et al. It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 2007; 18: 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 1997; 138: 4613–4621. [DOI] [PubMed] [Google Scholar]
- 6Raso MG, Behrens C, Herynk MH, Liu S, Prudkin L, Ozburn NC et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res 2009; 15: 5359–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Abe K, Miki Y, Ono K, Mori M, Kakinuma H, Kou Y et al. Highly concordant coexpression of aromatase and estrogen receptor beta in non-small cell lung cancer. Hum Pathol 2010; 41: 190–198. [DOI] [PubMed] [Google Scholar]
- 8Hershberger PA, Stabile LP, Kanterewicz B, Rothstein ME, Gubish CT, Land S et al. Estrogen receptor beta (ERbeta) subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J Steroid Biochem Mol Biol 2009; 116: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res 2005; 65: 1459–1470. [DOI] [PubMed] [Google Scholar]
- 10Mah V, Seligson DB, Li A, Márquez DC, Wistuba II, Elshimali Y et al. Aromatase expression predicts survival in women with early-stage non-small cell lung cancer. Cancer Res 2007; 67: 10484–10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Zhao G, Zhao S, Wang T, Zhang S, Lu K, Yu L et al. Estrogen receptor β signaling regulates the progression of Chinese non-small cell lung cancer. J Steroid Biochem Mol Biol 2011; 124: 47–57. [DOI] [PubMed] [Google Scholar]
- 12Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 2003; 3: 781–790. [DOI] [PubMed] [Google Scholar]
- 13Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999; 285: 727–729. [DOI] [PubMed] [Google Scholar]
- 14Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Invest 2004; 114: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O'Reilly RJ, Dupont B et al. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol 2003; 171: 6891–6899. [DOI] [PubMed] [Google Scholar]
- 16Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002; 419: 734–738. [DOI] [PubMed] [Google Scholar]
- 17Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S et al. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol 2005; 43: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 18Basu S, Pioli PA, Conejo-Garcia J, Wira CR, Sentman CL. Estradiol regulates MICA expression in human endometrial cells. Clin Immunol 2008; 129: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Zhao G, Nie Y, Lv M, He L, Wang T, Hou Y. ERβ-mediated estradiol enhances epithelial mesenchymal transition of lung adenocarcinoma through increasing transcription of midkine. Mol Endocrinol 2012; 26: 1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol 2013; 78: 120–129. [DOI] [PubMed] [Google Scholar]
- 21Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol 2002; 169: 4098–4102. [DOI] [PubMed] [Google Scholar]
- 22Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A et al. Tumor-associated MICA is shed by ADAM proteases. Cancer Res 2008; 68: 6368–6376. [DOI] [PubMed] [Google Scholar]
- 23Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res 2006; 66: 2520–2526. [DOI] [PubMed] [Google Scholar]
- 24Holdenrieder S, Stieber P, Peter WA, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant diseases. Int J Cancer 2006; 118: 684–687. [DOI] [PubMed] [Google Scholar]
- 25Rebmann V, Schutt P, Brandhorst D, Opalka B, Moritz T, Nowrousian MR et al. Soluble MICA as an independent prognostic factor for the overall survival and progression-free survival of multiple myeloma patients. Clin Immunol 2007; 123: 114–120. [DOI] [PubMed] [Google Scholar]
- 26Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumour-associated expression and recognition by tumour-derived γδT cells of MICA and MICB. Proc Natl Acad Sci USA 1999; 96: 6879–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T et al. MICA engagement by human T cells enhances their antigen-dependent effector function. Immunity 2001; 15: 83–93. [DOI] [PubMed] [Google Scholar]
- 28Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8 αβT cells by NKG2D via engagement by MIC induced on virus infected cells. Nat Immunol 2001; 2: 255–260. [DOI] [PubMed] [Google Scholar]
- 29Tieng V, Le Bouguénec C, du Merle L, Bertheau P, Desreumaux P, Janin A et al. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc Natl Acad Sci USA 2002; 99: 2977–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA et al. Role of the estogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 2003; 95: 353–361. [DOI] [PubMed] [Google Scholar]
- 31Okita R, Mougiakakos D, Ando T, Mao Y, Sarhan D, Wennerberg E et al. HER2/HER3 signaling regulates NK cell-mediated cytotoxicity via MHC class I chain-related molecule A and B expression in human breast cancer cell lines. J Immunol 2012; 188: 2136–2145. [DOI] [PubMed] [Google Scholar]
- 32Hao S, Li P, Zhao J, Hu Y, Hou Y. 17β-Estradiol suppresses cytotoxicity and proliferative capacity of murine splenic NK1.1+ cells. Cell Mol Immunol 2008; 5: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Alter G1, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 2004; 294: 15–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.