Abstract
Response gene to complement 32 (RGC-32) is a cell cycle regulator involved in the proliferation, differentiation and migration of cells and has also been implicated in angiogenesis. Here we show that RGC-32 expression in macrophages is induced by IL-4 and reduced by LPS, indicating a link between RGC-32 expression and M2 polarization. We demonstrated that the increased expression of RGC-32 is characteristic of alternatively activated macrophages, in which this protein suppresses the production of pro-inflammatory cytokine IL-6 and promotes the production of the anti-inflammatory mediator TGF-β. Consistent with in vitro data, tumor-associated macrophages (TAMs) express high levels of RGC-32, and this expression is induced by tumor-derived ascitic fluid in an M-CSF- and/or IL-4-dependent manner. Collectively, these results establish RGC-32 as a marker for M2 macrophage polarization and indicate that this protein is a potential target for cancer immunotherapy, targeting tumor-associated macrophages.
Keywords: macrophage polarization, monocyte, response gene to complement 32
Introduction
Macrophages play indispensable roles in the innate and adaptive immune response to pathogens and tissue homeostasis.1,2 Peripheral blood monocytes originally derived from CD34+ myeloid progenitor cells in the bone marrow circulate in the bloodstream and migrate into tissues or body cavities to complete differentiation into resident macrophages through the coordinated expression of numerous genes. In response to specific requirements, macrophages express specialized and polarized functional properties.3 Mirroring the Th1/Th2 nomenclature, macrophages are divided schematically into two main classes.4 Classically activated M1 macrophages are induced by LPS and IFN-γ. These effector cells mediate resistance against intracellular parasites and tumors by producing pro-inflammatory cytokines, such as IL-6, TNF-α and IL-1β. In contrast, M2 macrophages are alternatively activated by distinct stimuli and can be subdivided into three subgroups: M2a, M2b and M2c. M2a macrophages are elicited by IL-4 or IL-13, and M2b macrophages are polarized by immune complexes, TLRs and IL-1Ra. Finally, M2c macrophages are stimulated by IL-10, TGF-β and glucocorticoids.5,6 These cells play an important role in tumor progression, tissue repair and remodeling.7,8
Tumor-associated macrophages (TAMs) derived from circulating monocytes represent a distinct type of M2 macrophage.9 TAMs carry out several M2-associated pro-tumoral functions, including the promotion of tumor cell survival, matrix remodeling and the suppression of adaptive immunity.10,11 Thus, TAMs represent a promising and effective target for cancer therapy.12 The heterogeneous tumor microenvironment differentially influences tumor-associated macrophages, suggesting that it will be necessary to identify common TAM targets for the synthesis of new therapeutic molecules. Therefore, the best target would be a protein that is expressed or overexpressed only in TAMs and not in the resident macrophages of normal tissues or in M1 macrophages.13
Response gene to complement 32 (RGC-32) primarily acts as a cell cycle regulator and plays an important role in cell proliferation. This protein forms complexes with cyclin-dependent kinase p34CDC2 to enhance kinase activity and induce S-phase entry and mitosis.14,15 Moreover, RGC-32 promotes cell migration, inhibits angiogenesis and induces smooth muscle cell differentiation.16,17,18,19 Interestingly, RGC-32 is abnormally expressed in the peripheral blood mononuclear cells of patients with hyper-immunoglobulin E syndrome and multiple sclerosis.20,21 Little is known about the role of RGC-32 in immunity.
In the present study, we show that RGC-32 is expressed at high levels in M2 macrophages and TAMs and that tumors induce RGC-32 expression in an M-CSF- and/or IL-4-dependent manner. Improved understanding of the impact of RGC-32 on macrophage functions has the potential to help in the development of new RGC-32-based antitumor strategies.
Materials and methods
Cytokines and reagents
M-CSF, IFN-γ and IL-4 were purchased from R&D Systems (Minneapolis, MN, USA) and used at 100, 20 and 20 ng/ml, respectively. LPS and phorbol 12-myristate 13-acetate (PMA) were obtained from Sigma (Sigma-Aldrich, St. Louis, MO, USA). Monoclonal anti-human M-CSF and anti-IL-4 blocking and isotype-matched antibodies (Abs) were obtained from R&D Systems (R&D Systems, Abingdon, UK), and both were used at 2.5 µg/ml. The phosphoinositide 3-kinase (PI3K) inhibitor LY294002 was purchased from Cell Signaling Technology (Cell Signaling Technology, Danvers, MA, USA) and used at 10 µM/ml.
Cell culture and experimental treatments
To generate PMA-treated macrophages, 1×106 THP-1 cells were seeded into complete growth medium supplemented with 100 ng/ml PMA for 48 h. To generate M1-polarized THP-1 macrophages, THP-1 cells were cultured with 100 ng/ml PMA for 6 h and then treated with PMA plus 100 ng/ml LPS and 20 ng/mL IFN-γ for 42 h. To generate M2-polarized THP-1 macrophages, THP-1 cells were cultured with 100 ng/ml PMA for 6 h and then treated with PMA plus 20 ng/ml IL-4 for 42 h.22
Human peripheral blood mononuclear cells were isolated from leukocyte-enriched buffy coats from healthy donors using density gradient centrifugation and were positively selected by MACS CD14 microbeads (Miltenyi Biotec, Auburn, CA, USA). The purity of the isolated CD14+ monocytes was >98%, as determined by flow cytometry. Monocytes were differentiated into macrophages (M0) in RPMI using 10% FBS containing 100 ng/ml human rhM-CSF for 7 days. M1 and M2 macrophages were obtained by culturing M0 cells for an additional 48 h with 100 ng/ml LPS containing 20 ng/ml IFN-γ (for M1 polarization) or 20 ng/ml IL-4 (for M2 polarization), respectively.23 Human TAMs were obtained from the ascetic fluid of colon adenocarcinoma as previously described.24 CD14+ macrophages were isolated through Ficoll gradient cell separation and subsequent magnetic cell sorting using CD14 microbeads. Informed consent was obtained from all patients, and the study protocol was approved by the Ethics Review Board of Qingdao Central Hospital.
For experiments using signaling kinase inhibitors, THP-1 cells were pre-incubated with the PI3K inhibitor LY294002 for 60 min. Subsequently, THP-1 cells were differentiated into macrophages.
RNA interference
Transient transfection with siRNA against human RGC-32 (cat. # sc-106499) or two negative siRNA controls (cat. # sc-37007 and sc-44230) was performed using HiPerFect Transfection Reagent (Qiagen, Milano, Italy) according to the manufacturer's instructions. Briefly, a suspension of THP-1 cells (2×106/ml) was diluted with an equal volume of serum-free RPMI containing HiPerFect Transfection Reagent (30 µl/ml) and scrambled or RGC-32 siRNA (Santa Cruz, CA, USA). After 12 h, the cells were induced to differentiate by the addition of PMA (100 ng/ml). Transfected cells were maintained in culture for 48 h, and subsequently used for the of protein expression.
Retroviral transfection
pBMN-GFP and pBMN-GFP-RGC-32 vectors were kind gifts from Dr Jian Li (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA). Supernatants containing pBMN-GFP or pBMN-GFP-RGC-32 were collected, filtered and subsequently used to infect THP-1 cells as previously described.16
Cytokine measurement
Culture supernatants were collected from PMA-treated THP-1 macrophages and M1/M2-polarized THP-1 macrophages at 48 h. Cytokine levels were measured using TNF-α, IL-1β, IL-6 and TGF-β ELISA kits (R&D Systems), and the assays were conducted according to the manufacturer's instructions. Briefly, microplates were coated with mouse mAbs specific to TNF-α, IL-1β, IL-6 and TGF-β. Standards and samples were added to each well and incubated for 2 h at room temperature. After washing to remove unbound proteins, enzyme-linked polyclonal antibodies specific to TNF-α, IL-1β, IL-6 and TGF-β were added to the wells and incubated for 2 h at room temperature. After washing to remove the unbound antibody–enzyme reagent, the substrate solution was added to the wells and incubated for 30 min at room temperature in the dark. The optical density (450 nm) of each sample was determined using a microplate reader, and the mean concentration of TNF-α, IL-1β, IL-6 and TGF-β was calculated.
Immunofluorescence staining
For immunofluorescence analysis, THP-1 cells were seeded onto poly-L-lysine-coated glass coverslips or plated onto glass coverslips prior to treatment with 100 ng/ml PMA for 48 h. The cells were then fixed in 4% formaldehyde in PBS for 15 min and permeabilized using 0.1% Triton X-100 in PBS for 5 min. After preblocking with 5% normal goat serum (Sigma-Aldrich, St Louis, MO, USA) in PBS for 30 min, the cells were incubated with RGC-32 antibody (Santa Cruz Biotechnology), washed with PBS and incubated with TRITC-conjugated secondary antibody for 1 h at 37 °C. DAPI (Dojindo, Kumamoto, Japan) was used for nuclear counterstaining. Between each step, the coverslips were washed three times for 5 min with PBS. The fluorescence was monitored using a ×40 Plan Fluor objective on a Nikon Eclipse E600 microscope. Images were acquired using a SPOT camera and adjusted using Adobe Photoshop software.
Quantitative RT-PCR
Total RNA from THP-1 and human primary cells was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), and the cDNA was synthesized by reverse transcription. Quantitative RT-PCR was performed in duplicate on a LightCycler 2.0 Instrument (Roche Diagnostic, Mannheim, Germany) and analyzed using the comparative 2−ΔCt method as previously described.25 GAPDH was used as an internal control. The primers for RGC-32, TNF-α, IL-1β, IL-6, TGF-β1 and GAPDH are listed in Supplementary Table 1.
Western blot
THP-1 cells were lysed in RIPA buffer containing a protease inhibitor cocktail. Cell lysates were subjected to 12% SDS–PAGE and transferred onto a nitrocellulose membrane. After blocking with TBST (Tris-buffered saline, 0.1% Tween 20) containing 5% non-fat dried milk, the membranes were incubated with the indicated antibodies, including anti-RGC32 (a kind gift from Professor Jian Li, Beth Israel Deaconess Medical Center, Harvard Medical School), anti-β-actin, anti-AKT and anti-pAKT (Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C, followed by washing with TBST and incubation with HRP anti-mouse or anti-rabbit IgG for 1 h. The protein bands were detected with enhanced chemiluminescence.
Coimmunoprecipitation (Co-IP) assays
Immunoprecipitation was carried out according to previously described methods.19,26 After treatment with 0 or 100 ng/ml PMA for 48 h, the cells were collected and suspended in Co-IP assay lysis buffer (Pierce, Rockford, USA) on ice for 30 min. Following rapid centrifugation, the resulting supernatants were incubated with IgG and AKT antibodies at 4 °C for 2 h, followed by incubation with 30 µl of a 1∶1 slurry of protein A/G Plus-agarose at 4 °C overnight. The precipitates were washed four times with Co-IP buffer and resuspended and boiled in SDS loading buffer. Western blotting was performed using an anti-RGC-32 antibody.
Statistical analysis
The statistical analysis was performed using SPSS software version 13.0 (SPSS, Chicago, IL, USA). The data are presented as the mean±s.d. based on triplet experiments. The data were analyze dusing analysis of variance and Student's t-test (two-tailed). Statistical significance was considered as P<0.05.
Results
High expression of RGC-32 in M2 macrophages
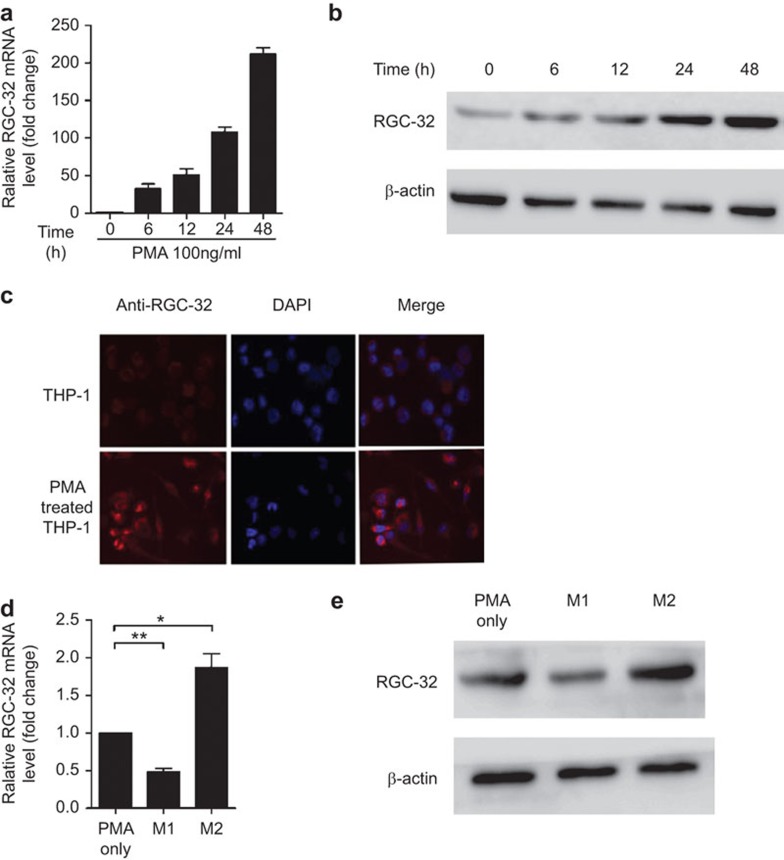
THP-1 cells can be activated and differentiated into macrophages by PMA, and this model has been used for studies on monocyte/macrophage differentiation.27,28 To clarify the role of RGC-32 in macrophages, we initially examined the expression of RGC-32. Treatment of THP-1 cells with PMA significantly upregulated RGC-32 mRNA expression in a time-dependent manner (Figure 1a). The expression of RGC-32 protein was also evaluated through western blotting and immunofluorescence staining experiments (Figure 1b and c). As shown in Figure 1b, RGC-32 protein was greatly increased in cells treated with PMA. Consistent with these data, RGC-32 was barely detectable in THP-1 cells, whereas PMA-treated THP-1 cells contained high levels of cytoplasmic RGC-32 (Figure 1c).
Figure 1.
RGC-32 is expressed at high levels in M2 macrophages. (a) RGC-32 expression was assayed using quantitative RT-PCR in THP-1 cells after induction for the indicated hours. (b) RGC-32 protein was detected using western blotting. (c) THP-1 cells and PMA-treated macrophages were stained with DAPI (blue) and TRITC-conjugated mAb (red) against RGC-32 and examined using a fluorescence microscope. (d) Quantitative RT-PCR analysis of RGC-32 mRNA in M1- and M2-polarized THP-1 macrophages. (e) RGC-32 expression in M1- and M2-polarized THP-1 macrophages was confirmed through western blotting. The graphs are representative of three separate experiments (*P<0.05, **P<0.01). RGC-32, response gene to complement 32; TAM, tumor-associated macrophage.
The M1- or M2-polarized THP-1 macrophage phenotype is induced by LPS and IFN-γ or IL-4, respectively (Supplementary Figure 1a).22 RGC-32 mRNA expression was considerably higher in PMA-treated and M2-polarized THP-1 macrophages than in M1-polarized THP-1 macrophages (Figure 1d). Consistent with RGC-32 mRNA expression, RGC-32 protein was increased in PMA-treated and M2-polarized THP-1 macrophages (Figure 1e). To further analyze RGC-32 mRNA expression in human primary cells, M1 and M2 macrophages were generated as described in the section on ‘Materials and methods'. As expected, the results indicated that RGC-32 was expressed at high levels in M2 macrophages (Supplementary Figure 1b). These results suggest that there is a regulatory mechanism for RGC-32 expression during macrophage polarization.
LPS and IL-4 modulated RGC-32 expression during macrophage polarization
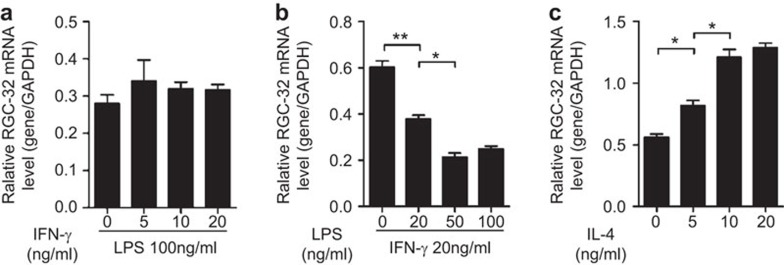
Four different doses of LPS or IFN-γ in combination with a standard dose of the other cytokine counterpart were applied to identify the contributions of these cytokines to the regulation of RGC-32 expression. As shown in Figure 2b, LPS significantly decreased RGC-32 mRNA expression in a dose-dependent manner, whereas IFN-γ alone did not show any effect (Figure 2a). In addition, the results also showed that RGC-32 mRNA expression was dependent on the dose of IL-4 in PMA- and IL-4-treated THP-1 cells (Figure 2c). Therefore, these results suggest that LPS and IL-4, but not IFN-γ, influence the expression of RGC-32 during macrophage polarization.
Figure 2.
RGC-32 expression during macrophage polarization was induced by IL-4 or LPS. (a) PMA-treated macrophages were polarized to M1 using a standard dose of LPS (100 ng/ml) in combination with graded doses of IFN-γ (0, 5, 10 and 20 ng/ml) for 42 h. (b) PMA-treated macrophages were stimulated using a standard dose of IFN-γ (20 ng/ml) in combination with graded doses of LPS (0, 25, 50 and 100 ng/ml) for 42 h. (c) PMA-treated macrophages were polarized to M2 using graded doses of IL-4 (0, 5, 10 and 20 ng/ml) for 42 h. PMA, phorbol 12-myristate 13-acetate; RGC-32, response gene to complement 32.
RGC-32 modulated cytokine production in macrophages
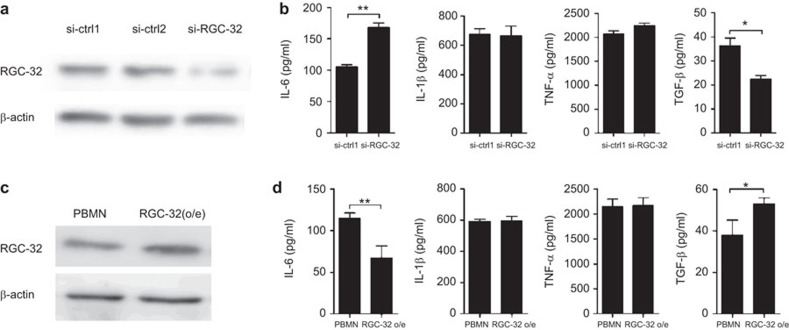
M2-polarized THP-1 macrophages are generally characterized by low production of pro-inflammatory cytokines (IL-6, TNF-α and IL-1β) and high production of TGF-β, in contrast to the pattern seen in M1-polarized THP-1 macrophages.6,22 Based on differences in RGC-32 expression in M1 and M2 macrophages, we next investigated whether RGC-32 influences the production of inflammatory cytokines. THP-1 cells were transfected with control or RGC-32 siRNA before being differentiated with PMA for 48 h. RGC-32 expression was effectively reduced using the specific siRNA (Figure 3a). As shown in Figure 3b, we observed increased production of the key pro-inflammatory cytokine IL-6 and decreased production of the anti-inflammatory cytokine TGF-β in RGC-32-silenced THP-1 macrophages. Complementary experiments in which RGC-32 was overexpressed in THP-1 macrophages resulted in significant inhibition of IL-6 and an increase in TGF-β (Figure 3c and d). Together, these results suggest that RGC-32 influences the production of inflammatory cytokines that define macrophage polarization.
Figure 3.
Effect of RGC-32 on inflammatory cytokines. THP-1 cells were transfected with RGC-32 siRNA or two siRNA controls (a) and PBMN-GFP or PBMN-GFP-RGC-32 (c). The transfected cells were treated with PMA (100 ng/ml) for 48 h. The culture supernatants were collected from PMA-treated THP-1 macrophages after 48 h. (b and d) The cell-free supernatants obtained from different cultures were collected and analyzed for IL-6, IL-1β, TNF-α and TGF-β using ELISA. *P<0.05, **P<0.01. PMA, phorbol 12-myristate 13-acetate; RGC-32, response gene to complement 32.
A crucial role for the PI3K signaling pathway in RGC-32-mediated IL-6 inhibition
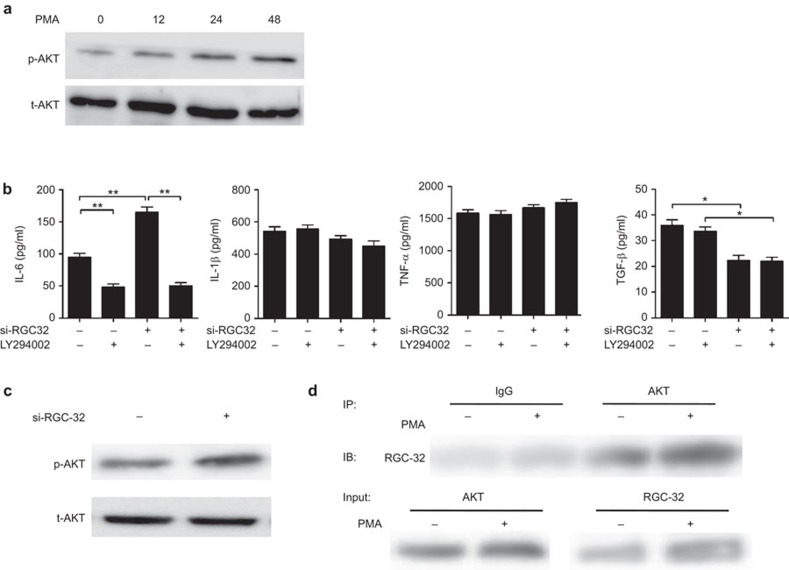
PI3K is involved in the positive regulation of IL-6 production.29 To determine whether the PI3K-mediated pathway is activated in PMA-treated THP-1 cells, we analyzed the phosphorylation of AKT. Western blot analysis showed that AKT phosphorylation gradually increased and peaked at 48 h after treatment with PMA (Figure 4a). To determine the mechanisms underlying RGC-32-regulated IL-6 production, we examined the effects of LY294002, a PI3K inhibitor, in RGC-32-silenced macrophages on the upregulation of IL-6 production. The pre-treatment of THP-1 cells with 10 µM LY294002 significantly inhibited PMA-induced IL-6 production (Figure 4b). Clearly, the positive effect of RGC-32 silencing on IL-6 production was completely blocked by LY294002 (Figure 4b). By contrast, the production of IL-1β, TNF-α and TGF-β was not affected in LY294002-pretreated macrophages. In addition, western blot analysis revealed that phosphorylated AKT was upregulated after 48 h in RGC-32-silenced macrophages, whereas the total amount of AKT was unaltered (Figure 4c). To test whether RGC-32 binds to AKT in THP-1 cells and PMA-treated macrophages, we performed coimmunoprecipitation. As shown in Figure 4d, RGC-32 physically interacts with AKT. Taken together, these results suggest that the PI3K pathway is critically involved in the regulation of IL-6 production by RGC-32 in macrophages.
Figure 4.
PI3K signaling is important for the RGC-32-mediated induction of IL-6 production. (a) THP-1 cells were exposed to 100 ng/ml PMA for the indicated times. Whole-cell lysates were prepared and subjected to western blot analysis to detect phosphorylated Akt. (b) RGC-32-silenced or control THP-1 cells were treated with LY294002 for 1 h prior to further stimulation with PMA. After 48 h, the production of IL-1β, IL-6, TNF-α and TGF-β in the cell-free supernatants was assayed by ELISA. (c) RGC-32-silenced or control THP-1 cells were exposed to PMA for 48 h. Whole-cell lysates were subjected to western blot analysis to detect phosphorylated Akt. (d) RGC-32 physically interacts with AKT in THP-1 cells and PMA-treated macrophages. THP-1 cells were treated with 0 or 100 ng/ml PMA for 48 h, and subsequently coimmunoprecipitation was performed. Control IgG and AKT antibodies were used for coimmunoprecipitation, and anti-RGC-32 was used for immunoblotting. *P<0.05, **P<0.01. PI3K, phosphoinositide 3-kinase; PMA, phorbol 12-myristate 13-acetate; RGC-32, response gene to complement 32.
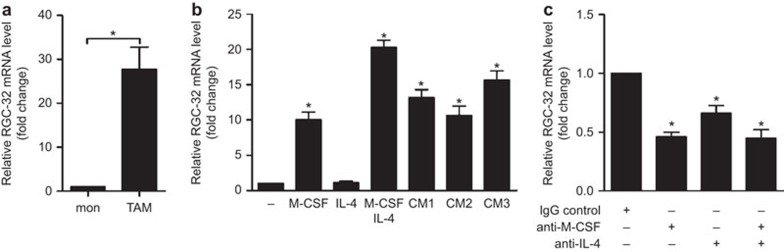
Increased expression of RGC-32 in TAMs
TAMs originate from blood monocytes recruited to the tumor site by molecules produced by tumors and stromal cells.30 CD14+ TAMs isolated ex vivo from colon adenocarcinoma ascitic fluid expressed higher levels of RGC-32 than did CD14+ monocytes isolated from human peripheral blood (Figure 5a). The increased expression of RGC-32 mRNA in TAMs prompted us to examine whether tumor cells influence the expression of this protein in monocytes. As shown in Figure 5b, colon adenocarcinoma ascitic fluid promoted strong upregulation of RGC-32 mRNA, confirming that tumor cells release factors that upregulate RGC-32 expression in monocytes.
Figure 5.
Induction of RGC-32 by tumor-conditioned medium is dependent on M-CSF and IL-4. (a) RGC-32 mRNA expression in monocytes obtained from peripheral blood and TAMs from ascitic fluid was assayed by quantitative RT-PCR (n=12). (b) RGC-32 mRNA expression in monocytes exposed for 72 h to M-CSF, IL-4 and conditioned medium from the ascitic fluid of three colon carcinomas (CM1, CM2 and CM3), as determined by quantitative RT-PCR. (c) Inhibitory effect of anti–M-CSF and anti-IL-4 on RGC-32 mRNA levels induced by ascitic fluid obtained from metastatic colon carcinoma. The results are depicted as the RGC-32 mRNA levels detected in the presence of the anti-M-CSF and anti-IL-4 antibody relative to the levels observed in control cells treated with an isotype-matched control Ab. *P<0.05, **P<0.01. Ab, antibody; RGC-32, response gene to complement 32.
Given that RGC-32 expression is induced by M-CSF and IL-4 (Figure 5b), we tested whether both cytokines contributed to RGC-32 induction in monocytes by tumor-derived ascetic fluids. As shown in Figure 5c, the blocking Abs anti-M-CSF and anti-IL-4 reduced the induction of RGC-32 by colon adenocarcinoma ascitic fluid by 46% and 66%, respectively. However, a synergistic effect was not observed when neutralizing Abs against M-CSF and IL-4 were added to the conditioned medium. Therefore, these results suggest that the increased expression of RGC-32 in TAMs is dependent on tumor-associated M-CSF and IL-4.
Discussion
RGC-32 has been reported to have pleiotropic effects in diverse cellular processes such as cell proliferation, differentiation and migration.18,31,32 However, a role for RGC-32 in macrophages has not been reported. In the present study, we investigated the expression and regulation of RGC-32 during macrophage polarization and demonstrated that RGC-32 was specifically expressed in M2 macrophages with regulatory or immunosuppressive effector functions.
THP-1 cells are widely used as a cell model to study macrophage differentiation and polarization. In response to LPS plus IFN-γ or Th2 cytokines (IL-4 or/and IL-13), macrophages express specialized and polarized functional properties. Our results indicate that IL-4 induces RGC-32 expression in differentiated THP-1 cells and monocyte-derived macrophages. In contrast, LPS negatively regulated RGC-32 expression. Taken together, this evidence indicates that M1 macrophages are devoid of RGC-32 expression, whereas RGC-32 is specific to M2 macrophages, which have anti-inflammatory and regulatory properties. Consistent with the presence of RGC-32 in M2 macrophages, this protein has been detected in CD68+ macrophages in multiple sclerosis plaques, and the expression of this cell marker is regulated by TGF-β.21 Therefore, RGC-32 expression marks a wider range of alternatively activated macrophages.
Macrophages are the primary source of pro-and anti-inflammatory cytokines, such as IL-6, TNF-α, IL-1β and TGF-β.33,34 In the present study, we investigated whether RGC-32 expression in macrophages influenced the expression of inflammatory cytokines. As expected, increased RGC-32 expression in macrophages significantly inhibited the expression of IL-6 and increased the expression of TGF-β, suggesting that RGC-32 influences macrophage polarization by inducing an IL-6lowTGF-βhigh cytokine profile.
The induction of IL-6 by LPS, IL-1β and lysophosphatidic acid is PI3k-dependent.29,35 In the present study, we provided evidence that the induction of IL-6 production in PMA-treated THP-1 macrophages was dependent on PI3K activation (Figure 5b). Interestingly, we observed that the increased production of IL-6 in RGC-32-silenced macrophages was inhibited by a PI3K inhibitor (Figure 5b), whereas the production of TGF-β was not altered. Silencing of RGC-32 stimulated a significant increase in AKT phosphorylation, demonstrating that RGC-32 negatively regulated the PI3K pathway (Figure 5c). The coimmunoprecipitation data demonstrated that RGC-32 physically interacts with AKT in THP-1 cells and PMA-treated macrophages. These results are in contrast with previously reported data on the RGC-32-mediated activation of AKT in endothelial cells.36 In addition, An et al.16 reported that RGC-32 had no significant effect on the phosphorylation of Akt in human umbilical vein endothelial cells.16 The disparities among these reports might reflect differences in RGC-32 functions in different cell types. Thus, these data suggest that RGC-32 can modulate IL-6 production through negative regulation of the PI3K pathway.
When circulating monocytes are recruited to tumor sites, they are surrounded by signals (such as M-CSF and IL-13) that promote their differentiation to macrophages and to other cells that are needed by the tumors (IL-4, IL-10 and TGF-β).13,37 We observed that TAMs express higher levels of RGC-32 than do circulating monocytes, suggesting that tumor-derived cytokines modulate RGC-32 expression in macrophage precursors or infiltrating macrophages. These results indicated that M-CSF induces RGC-32 mRNA expression in monocytes and is the key cytokine for RGC-32 acquisition during the differentiation of monocytes into macrophages. Tumor-derived IL-4, which induces macrophage polarization, further enhances RGC-32 expression in macrophages. Therefore, these data, in part, support the idea that increased RGC-32 mRNA expression in TAMs is induced by tumor-derived M-CSF and IL-4.
In conclusion, these results establish RGC-32 as a molecule that marks macrophages that have been polarized by tumor cells. However, whether the acquisition of RGC-32 expression in TAMs is detrimental to or favors tumor cell growth should be further investigated. Accordingly, RGC-32 might constitute a potential target for antitumor strategies aimed at eliminating tumor-polarized myeloid cells.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Nos. 31270971, 81072406 and 31100650), the China Postdoctoral Science Foundation (Nos. 2013M541922) and the Independent Innovation Foundation of Shandong University (No. 2012TS143). We would like to thank Professor Jian Li (Beth Israel Deaconess Medical Center, Harvard Medical School) for providing the RGC-32 overexpression vector and the RGC-32 antibody.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- 1Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 2009; 27: 669–692. [DOI] [PubMed] [Google Scholar]
- 2Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 2010; 327: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Cohen HB, Mosser DM. Extrinsic and intrinsic control of macrophage inflammatory responses. J Leukoc Biol 2013; 94: 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 2006; 177: 7303–7311. [DOI] [PubMed] [Google Scholar]
- 5Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol 2013; 120: 163–184. [DOI] [PubMed] [Google Scholar]
- 6Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677–686. [DOI] [PubMed] [Google Scholar]
- 7Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000; 164: 6166–6173. [DOI] [PubMed] [Google Scholar]
- 8Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012; 2012: 948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Obeid E, Nanda R, Fu YX, Olopade OI. The role of tumor-associated macrophages in breast cancer progression (review). Int J Oncol 2013; 43: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Menen RS, Hassanein MK, Momiyama M, Suetsugu A, Moossa AR, Hoffman RM et al. Tumor-educated macrophages promote tumor growth and peritoneal metastasis in an orthotopic nude mouse model of human pancreatic cancer. In Vivo 2012; 26: 565–569. [PubMed] [Google Scholar]
- 11Allavena P, Sica A, Garlanda C, Mantovani A. The Yin–Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev 2008; 222: 155–161. [DOI] [PubMed] [Google Scholar]
- 12Santoni M, Massari F, Amantini C, Nabissi M, Maines F, Burattini L et al. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2013; 62: 1757–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009; 86: 1065–1073. [DOI] [PubMed] [Google Scholar]
- 14Badea T, Niculescu F, Soane L, Fosbrink M, Sorana H, Rus V et al. RGC-32 increases p34CDC2 kinase activity and entry of aortic smooth muscle cells into S-phase. J Biol Chem 2002; 277: 502–508. [DOI] [PubMed] [Google Scholar]
- 15Schlick SN, Wood CD, Gunnell A, Webb HM, Khasnis S, Schepers A et al. Upregulation of the cell-cycle regulator RGC-32 in Epstein–Barr virus-immortalized cells. PLoS One 2011; 6: e28638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16An X, Jin Y, Guo H, Foo SY, Cully BL, Wu J et al. Response gene to complement 32, a novel hypoxia-regulated angiogenic inhibitor. Circulation 2009; 120: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Huang WY, Li ZG, Rus H, Wang X, Jose PA, Chen SY. RGC-32 mediates transforming growth factor-beta-induced epithelial–mesenchymal transition in human renal proximal tubular cells. J Biol Chem 2009; 284: 9426–9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Wang JN, Shi N, Xie WB, Guo X, Chen SY. Response gene to complement 32 promotes vascular lesion formation through stimulation of smooth muscle cell proliferation and migration. Arterioscler Thromb Vasc Biol 2011; 31: e19–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Guo X, Jose PA, Chen SY. Response gene to complement 32 interacts with Smad3 to promote epithelial–mesenchymal transition of human renal tubular cells. Am J Physiol Cell Physiol 2011; 300: C1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Tanaka T, Takada H, Nomura A, Ohga S, Shibata R, Hara T. Distinct gene expression patterns of peripheral blood cells in hyper-IgE syndrome. Clin Exp Immunol 2005; 140: 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Tegla CA, Cudrici CD, Azimzadeh P, Singh AK, Trippe R3rd, Khan A et al. Dual role of Response gene to complement-32 in multiple sclerosis. Exp Mol Pathol 2012; 94: 17–28. [DOI] [PubMed] [Google Scholar]
- 22Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol 2009; 129: 1016–1025. [DOI] [PubMed] [Google Scholar]
- 23Bellora F, Castriconi R, Dondero A, Reggiardo G, Moretta L, Mantovani A et al. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proc Natl Acad Sci USA 2010; 107: 21659–21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Puig-Kroger A, Sierra-Filardi E, Dominguez-Soto A, Samaniego R, Corcuera MT, Gomez-Aguado F et al. Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res 2009; 69: 9395–9403. [DOI] [PubMed] [Google Scholar]
- 25Shao Q, Ning H, Lv J, Liu Y, Zhao X, Ren G et al. Regulation of Th1/Th2 polarization by tissue inhibitor of metalloproteinase-3 via modulating dendritic cells. Blood 2012; 119: 4636–4644. [DOI] [PubMed] [Google Scholar]
- 26Zhang S, Yin J, Li X, Zhang J, Yue R, Diao Y et al. Jacarel hyperol A induced apoptosis in leukaemia cancer cell through inhibition the activity of Bcl-2 proteins. BMC Cancer 2014; 14: 689. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One 2010; 5: e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T et al. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res 1982; 42: 1530–1536. [PubMed] [Google Scholar]
- 29Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem 2008; 283: 25900–25912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol 2013; 35: 585–600. [DOI] [PubMed] [Google Scholar]
- 31Saigusa K, Imoto I, Tanikawa C, Aoyagi M, Ohno K, Nakamura Y et al. RGC32, a novel p53-inducible gene, is located on centrosomes during mitosis and results in G2/M arrest. Oncogene 2007; 26: 1110–1121. [DOI] [PubMed] [Google Scholar]
- 32Huang WY, Xie W, Guo X, Li F, Jose PA, Chen SY. Smad2 and PEA3 cooperatively regulate transcription of response gene to complement 32 in TGF-beta-induced smooth muscle cell differentiation of neural crest cells. Am J Physiol Cell Physiol 2011; 301: C499–C506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Hiraiwa K, van Eeden SF. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm 2013; 2013: 619523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol 2004; 76: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Dahle MK, Overland G, Myhre AE, Stuestol JF, Hartung T, Krohn CD et al. The phosphatidylinositol 3-kinase/protein kinase B signaling pathway is activated by lipoteichoic acid and plays a role in Kupffer cell production of interleukin-6 (IL-6) and IL-10. Infect Immun 2004; 72: 5704–5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Fosbrink M, Cudrici C, Tegla CA, Soloviova K, Ito T, Vlaicu S et al. Response gene to complement 32 is required for C5b-9 induced cell cycle activation in endothelial cells. Exp Mol Pathol 2009; 86: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev 2010; 24: 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.