Abstract
Platelet activation is associated with multiple immune responses and the pathogenesis of various immune-related diseases. However, the exact role and the underlying mechanism of platelets in the progression of allergic asthma remain largely unclear. In this study, we demonstrate that during antigen sensitization, platelets can be activated by ovalbumin (OVA) aerosol via the upregulation of CD154 (CD40L) expression. Platelet transfer promoted allergic asthma progression by inducing more severe leukocyte infiltration and lung inflammation, elevated IgE production and strengthened T helper 2 (Th2) responses in asthma-induced mice. Accordingly, platelet depletion compromised allergic asthma progression. Cd154-deficient platelets failed to promote asthma development, indicating the requirement of CD154 for platelets to promote asthma progression. The mechanistic study showed that platelets inhibited the induction of Foxp3+ regulatory T cells both in vivo and in vitro at least partially through CD154, providing an explanation for the increase of Th2 responses by platelet transfer. Our study reveals the previously unknown role of platelet CD154 in the promotion of asthma progression by polarizing Th2 responses and inhibiting regulatory T-cell generation and thus provides a potential clue for allergic disease interventions.
Keywords: allergic asthma, CD154, platelet, regulatory T cells, Th2 response
Introduction
Platelets are small anucleate fragments derived from bone marrow megakaryocytes and have traditionally been viewed as crucial mediators of coagulation and thrombosis. However, increasing evidence suggests that platelets also have important roles in regulating immunity and inflammation through various mechanisms.1 Platelets express a variety of sensors, including pattern recognition receptors, through which they can rapidly recognize and capture invading bacteria.2 In addition, they are equipped with a large number of intracellular bioactive granules (including δ-, α- and λ-granules), which are rapidly mobilized and released upon platelet activation. These released granules contain a variety of inflammatory cytokines, chemokines and adhesive molecules that can then recruit and activate neutrophils and monocytes, as well as other leukocytes, thereby amplifying local antimicrobial defense. Moreover, platelets can physically interact with adjacent monocytes and dendritic cells (DCs) via receptor-ligand interactions, particularly CD154/CD40 crosstalk, contributing to enhanced antigen presentation and adaptive immune responses.3,4
CD154 (CD40 ligand, CD40L) is a membrane bound protein belonging to the TNF superfamily. Engagement of CD40 by its ligand CD154 plays a central role in mediating the interaction between antigen-presenting cells (APCs) and lymphocytes.5 Particularly, CD154 derived from activated platelets is involved in multiple immune processes, including endothelial cell reactions,6 germinal center formation,7 T-helper cell priming8,9 and cytotoxic T-cell activation.10,11 However, the detailed mechanism how platelet CD154 influences the development of different T-cell subsets and consequently shapes the outcome of adaptive immune responses remains unclear.
By using different animal models, studies have shown the indispensable role of platelets in the initiation and progression of inflammatory and autoimmune diseases, including sepsis,12 atherosclerosis,13 autoimmune myocarditis,14 systemic lupus erythematosus,15 rheumatoid arthritis16,17,18,19 and so on. Allergic asthma is a chronic inflammatory pathological condition with critical involvement of T helper 2 (Th2) cells that is harmful to human health throughout the world.20 Identifying the role of platelets in the development of allergic asthma will not only benefit our understanding of the molecular and cellular mechanisms of allergic asthma but may also provide potential targets for therapy of this disease. Although previous studies showed evidence of platelet activation,21,22,23 as well as of the involvement of CD15424 in asthma, the exact role of platelet activation and the underlying mechanisms in the progression of allergic asthma remain elusive.
In this study, we found that allergens can directly activate platelets and upregulate the expression of CD154 by platelets. By platelet transfer or depletion experiments, we showed that platelets promote the progression of allergic asthma. Using Cd154-deficient (Cd154−/−) mice, we found that CD154 is required for the function of platelets in promoting asthma. Finally, we demonstrate that platelets inhibit the differentiation of regulatory T cells via CD154 and consequently polarize the Th2 response, adding new insights into the pathogenesis of allergic asthma.
Materials and methods
Mice
BALB/c and C57BL/6 mice were from Joint Ventures Sipper BK Experimental Animal Co. (Shanghai, China). Cd154−/− mice were from the Jackson Laboratory (Bar Harbor, ME, USA). All mice were maintained under pathogen-free conditions and used at 6–8 weeks of age. All animal experiments were carried out according to National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Second Military Medical University (Shanghai, China).
Reagents
RPMI medium 1640 and fetal bovine serum were purchased from PAA Laboratories (Les Mureaux, France). Ovalbumin (OVA, grade V) was purchased from Sigma-Aldrich (St Louis, MO, USA). Anti-mouse CD4-coated magnetic beads were from Miltenyi Biotech (Gladbach, Germany). FITC-conjugated antibodies to mouse CD154, CD40 and P-Selectin, an APC-conjugated antibody to mouse CD41, PerCP-Cy5.5-conjugated antibodies to mouse CD4, and anti-IFN-γ, anti-IL-4 and anti-mouse Foxp3 staining sets were from eBioscience (San Diego, CA). The rabbit anti-mouse thrombocyte antibody (AIA31440) was from Accurate Chemical & Scientific Corporation (NY, USA). Recombinant human TGF-β was from PeproTech. Mouse IL-4, mouse IL-13 and mouse TGF-β ELISA kits were from R&D (MN, USA).
Regulatory T-cell induction
Regulatory T cell (Treg) induction was carried out according to methods previously described25 with minor modifications. CD4+ T cells were enriched from C57BL/6 splenocytes via positive selection with magnetic beads, and the purity of enriched cells was confirmed to be over 90% by FACS. CD4+ T cells were stimulated with plate-bound anti-CD3 (5 µg/ml), soluble anti-CD28 (2 µg/ml), rhTGF-β (10 ng/ml), anti-IFN-γ (10 µg/ml) and anti-IL-4 (10 µg/ml) in the absence or presence of platelets from wild-type or Cd154−/− mice for 72 h. Intracellular staining of Foxp3 was then performed.
Flow cytometry
For cell surface staining, the single-cell suspensions were incubated with the antibody cocktails for 20 min at 4 °C. Intracellular Foxp3 staining was then performed with an anti-mouse Foxp3 staining set (eBioscience) according to the manufacturer's instructions. Data were obtained on an LSR II and analyzed with FACSDiva software (both from BD Biosciences, CA, USA).
Cytokine assays
Levels of IL-4, IL-13 and TGF-β were detected using ELISA kits according to the manufacturer's protocols (R&D).The serum concentration of OVA-specific IgE was determined by ELISA. Briefly, 96-well plates (Nunc, Rochester, NY, USA) were coated with 10 µg/ml OVA overnight, incubated with diluted serum samples (1∶200) for 2 h and then incubated with anti-mouse IgE-HRP (eBioscience) for 1 h. Plates were washed five times with wash buffer between each step. The results were analyzed spectrophotometrically at 450/590 nm. All incubation steps occurred at room temperature.
Isolation and activation of murine platelets
Murine platelets were prepared as previously described.6 In brief, peripheral blood from C57BL/6 mice was collected via cardiac puncture into syringes containing 0.5 ml acid-citrate dextrose buffer (10.0 g/l D-glucose, 12.5 g/l Na-Citrate, and 6.85 g/l citric acid), and pipetted into tubes containing 5 ml PIPES buffer (150 mM NaCl and 20 mM PIPES, pH 6.5). Blood samples were centrifuged at 100g for 10 min, and the supernatant was collected and mixed with 1 U/ml apyrase and 1 M prostaglandin E1. The supernatant was centrifuged at 1000g for 10 min. The platelet pellet was resuspended in Tyrodes buffer (134 mM NaCl, 20 mM HEPES, 12 mM NaHCO3, 2.9 mM KCL, 0.34 mM Na2PO4, 1 mM MgCl2, 5 mM glucose and 0.5 mg/ml BSA, pH 6.5). To activate platelets, 0.5 U/ml thrombin was added to platelets in Tyrodes buffer. All steps were performed at room temperature.
Induction of allergic asthma
BALB/c mice were immunized intraperitoneally with 100 µg OVA emulsified in 100 µg aluminum hydroxide on days 1, 8 and 15. On days 22, 24 and 26, mice were aerosol-challenged for 30 min with 1% OVA diluted in 5 ml PBS delivered by a PARI-Boy nebulizer (PARI GmbH, Starnberg, Germany). Platelets (from either wild-type or Cd154−/− mice) or anti-platelet antibodies were given intratracheally 30 min before each OVA aerosol challenge. Control mice were not immunized with OVA, but received the normal OVA aerosol challenge. After the last aerosol challenge, mice were sacrificed and analyzed for bronchoalveolar lavage (BAL) fluid platelet activation, leukocyte infiltration and Th2 cytokine expression, serum OVA-specific IgE levels and lung histology.
Histological analysis
Histology analysis was carried out according to previously described protocols.26 In brief, lungs were inflated with 4% paraformaldehyde, embedded in paraffin, sectioned and stained with H&E.
Statistical analysis
The statistical significance of differences for paired samples was analyzed by two-tailed Student's t-tests. Statistical significance was determined as P<0.05.
Results
Ovalbumin aerosols induce platelet activation in the airway
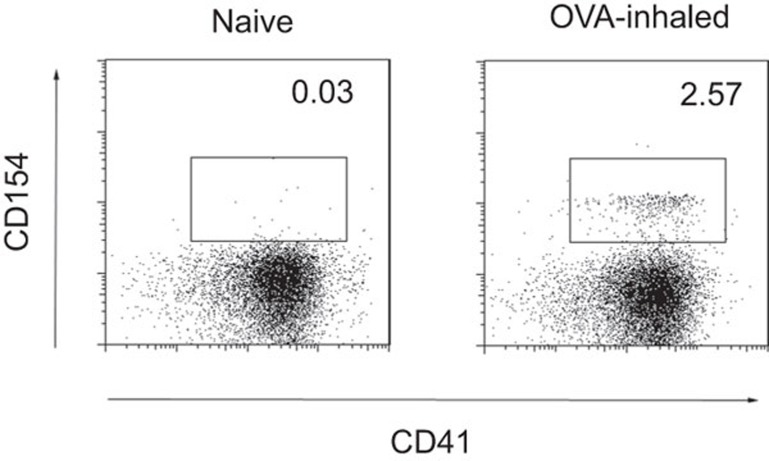
Platelets can be activated by various physiological and pharmacological stimuli such as thrombin, adenosine diphosphate and arachidonic acid, among others. To address whether airway platelets can be activated by allergens during antigen sensitization, we induced allergic asthma in BALB/c mice with OVA aerosol challenge and analyzed platelet activation in BAL fluid. CD41 is a platelet-specific marker, whereas CD154 is inducibly expressed by activated platelets. We found that platelets harvested from OVA-exposed mice had significantly increased expression of the activation marker CD154, whereas those from control mice did not express CD154 (Figure 1). Thus, OVA aerosol is sufficient to induce platelet activation in the airway.
Figure 1.
OVA induces platelet activation in the airway. BAL fluids collected from BALB/c mice, either untreated (naive) or challenged with OVA aerosol (OVA-inhaled), were analyzed for CD41 and CD154 expression by FACS. Data are representative of three independent experiments with similar results. BAL, bronchoalveolar lavage; OVA, ovalbumin.
Platelet transfer exaggerates allergic asthma, whereas platelet depletion ameliorates allergic asthma
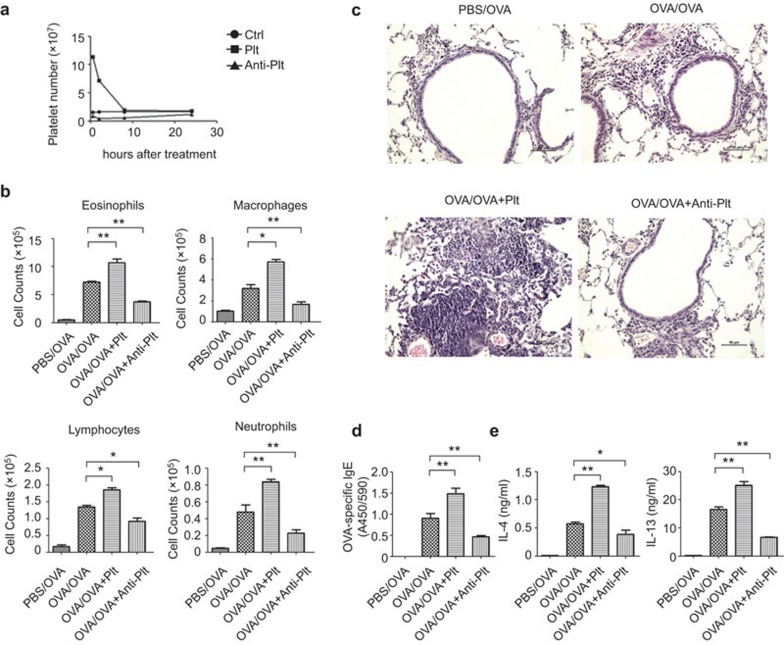
We next investigated whether platelets could affect the development of allergic asthma through the adoptive transfer of platelets or depletion of platelets (administration of an anti-platelet antibody) before each OVA inhalation. The number of CD41+ platelets in BAL fluid significantly increased after platelet transfer and decreased after platelet depletion over an 8-h period (Figure 2a). Giemsa staining of BAL fluid showed increased numbers of eosinophils, macrophages, lymphocytes and neutrophils after platelet transfer and decreased numbers after platelet depletion in asthma-induced mice (Figure 2b). Consistently, lung histology showed increased mononuclear infiltration and tissue inflammation after platelet transfer but decreased infiltration after platelet depletion (Figure 2c). Serum IgE is closely associated with the severity of asthma. We found that platelet transfer increased the expression of serum OVA-specific IgE, whereas platelet depletion decreased the expression (Figure 2d). Asthma is characterized by enhanced Th2-type responses. We also found that platelet transfer increased IL-4 and IL-13 levels in BAL fluid, whereas platelet depletion decreased those levels (Figure 2e). Together, we found more severe disease progression of allergic asthma in asthma-induced mice after platelet transfer and, accordingly, reduced disease severity of allergic asthma after platelet depletion. Thus, platelets promote the progression and severity of allergic asthma.
Figure 2.
Platelet transfusion enhances the progression of allergic asthma, whereas platelet depletion alleviates disease progression. (a) Changes of platelet numbers in BAL fluid after platelet transfer or depletion. Mice were left untreated (Ctrl) or were administered platelets (Plt) or anti-platelet antibody (Anti-Plt). (b–e) BALB/c mice were administered platelets (OVA/OVA+Plt), anti-platelet antibody (OVA/OVA+Anti-Plt) or PBS (OVA/OVA) before exposure to OVA-aerosol. Control BALB/c mice were not immunized but received OVA aerosol challenge (PBS/OVA). After the last OVA inhalation, the mice were killed for disease progression analyses. BAL fluid was collected and stained with Giemsa for leukocyte counts (b). Lung tissues were prepared for paraffin-embedded sectioning and H&E staining. Scale bars=50 µm (c). Sera were collected, and OVA-specific IgE was measured by ELISA (d). BAL fluid was collected, and the concentrations of IL-4 and IL-13 were assessed by ELISA (e). *P<0.05 and **P<0.01 (two-tailed Student's t-test). Data are from three independent experiments (a, b, d, e; the mean and s.d. of five determinants) or are representative of three independent experiments with similar results (c). BAL, bronchoalveolar lavage; OVA, ovalbumin.
CD154 is required for platelets to promote asthma development
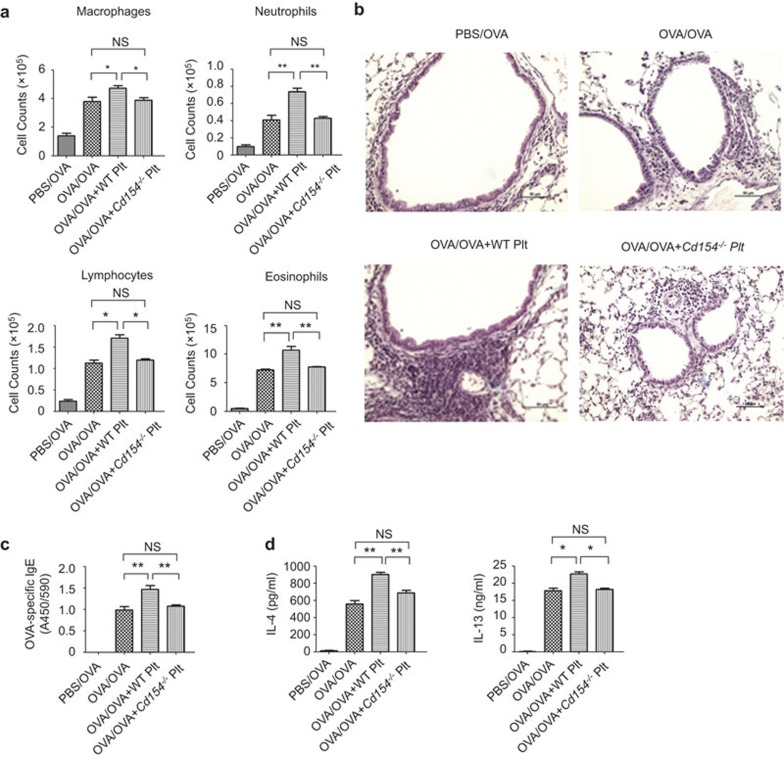
Activated platelets release various immune modulatory factors and upregulate a series of membrane bound ligands, which contribute to the regulation of immune responses. Platelet-derived CD154 has been shown to affect adaptive immune responses. However, the exact role of CD154 in allergic asthma remains unknown. Unlike wild-type platelets, which enhanced lung leukocyte infiltration (Figure 3a and b), serum OVA-specific IgE levels (Figure 3c), and lung IL-4 and IL-13 expression (Figure 3d), platelets from Cd154−/− mice failed to enhance asthma development (Figure 3). Therefore, Cd154 deficiency blocks the function of platelets to promote asthma progression. Thus, CD154 is required for platelets to promote asthma development.
Figure 3.
CD154 is required for platelets to promote asthma. BALB/c mice were administered platelets derived from wild-type mice (OVA/OVA+WT Plt) or Cd154−/− mice (OVA/OVA+Cd154−/− Plt) or were administered PBS (OVA+PBS) before exposure to OVA-aerosol. Control BALB/c mice were not immunized but received OVA aerosol challenge (PBS/OVA). After the last OVA inhalation, the mice were sacrificed for disease progression analyses. (a) BAL fluid was collected and stained with Giemsa for leukocyte counts. (b) Lung tissues were prepared for paraffin-embedded sectioning and H&E staining. Scale bars=50 µm. (c) Sera were collected, and OVA-specific IgE was measured by ELISA. (d) BAL fluid was collected, and the concentrations of IL-4 and IL-13 were assessed by ELISA. NS, not significant, *P<0.05 and **P<0.01 (two-tailed Student's t-test). Data are from three independent experiments (a, c, d; the mean and s.d. of five determinants) or are representative of three independent experiments with similar results (b). BAL, bronchoalveolar lavage; OVA, ovalbumin.
Platelets inhibit Treg generation via CD154
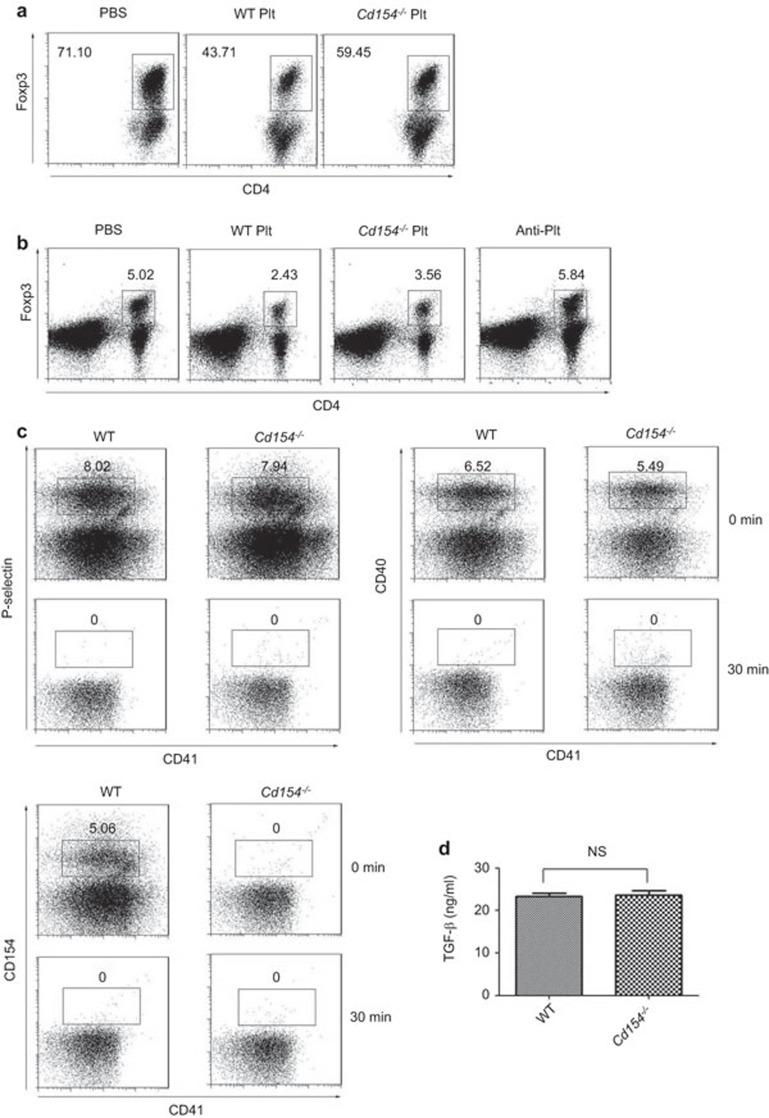
We next wondered about the mechanism by which platelet CD154 promotes asthma progression. It was shown that the inhibition of Tregs was associated with enhanced Th2-type inflammation in asthma, so we studied the impact of platelet activation on Treg generation. Under Treg polarization conditions in vitro, Foxp3 expression was considerably reduced in the presence of platelets, suggesting that platelets inhibit Treg induction in vitro (Figure 4a). Moreover, Cd154−/− platelets had a reduced ability to inhibit Treg induction compared with wild-type platelets, suggesting that platelets inhibit Treg generation at least partially via CD154 expression (Figure 4a). We next examined the effect of platelets in Treg generation in vivo. We found a reduced frequency of Tregs in the mediastinal lymph nodes of asthma-induced mice after platelet transfer. Consistently, Cd154−/− platelets had a reduced ability to inhibit Treg generation in vivo compared with wild-type platelets (Figure 4b). Together, platelets inhibit Treg induction both in vitro and in vivo at least partially via CD154. Because we did not include APC in our in vitro culture system, the above data suggest a direct inhibitory role of platelets on Treg differentiation. In addition to the abundant CD154 expression, platelets also have high expression of other immune regulatory molecules such as P-Selectin, CD40 and TGF-β. However, there was no significant difference in the expression of P-Selectin, CD40 and TGF-β between these two strains (Figure 4c and d), indicating that the reduced inhibitory capacity of Cd154−/− platelets on Treg differentiation is not due to the altered expression of these molecules.
Figure 4.
Platelets inhibit the induction of Treg generation in vitro and in vivo via CD154. (a) CD4+ T cells were cultured under Treg polarization conditions in the presence of platelets from wild-type (WT Plt) or Cd154−/− mice (Cd154−/− Plt) or PBS for 72 h and analyzed for Foxp3 expression. Numbers indicate percentages of Foxp3+ cells in the CD4+ gate. (b) BALB/c mice were administered platelets from wild-type mice (WT Plt) or Cd154−/− mice (Cd154−/− Plt), PBS (PBS) or anti-platelet antibody (Anti-Plt) before exposure to OVA-aerosol. Mediastinal lymph nodes were collected after the last OVA inhalation and were analyzed for Foxp3 expression. Numbers indicate percentages of Foxp3+ cells of the total cells. Data are representative of three independent experiments with similar results. (c) Expression of P-Selectin, CD40, CD154 on BAL platelets from OVA-sensitized WT or Cd154−/− mice at 0 (0 min) or 30 min (30 min) after OVA sensitization. Data are representative of three independent experiments with similar results. (d) Platelets harvested from WT or Cd154−/− mice via cardiac puncture. A total of 2.5×108 platelets were resuspended in 1 ml Tyrodes buffer and activated with 0.5 U thrombin. Supernatants were collected and assayed for TGF-β. NS, not significant (two-tailed Student's t-test). Data are from three independent experiments (d; the mean and s.d. of three determinants) or are representative of three independent experiments with similar results (a–c). BAL, bronchoalveolar lavage; OVA, ovalbumin; Treg, regulatory T cell.
Discussion
Platelets are classically viewed as crucial mediators of thrombosis and coagulation. However, increasing evidence has demonstrated the regulatory role of platelets in various immune processes and in the development of various autoimmune diseases. It had been noticed that platelet activation was related to the progression of asthma, yet the exact role and underlying mechanisms of platelets in asthma progression remained elusive. In our study, we demonstrated that platelets promote the progression of allergic asthma through their expression of CD154, thus providing a new mechanistic explanation for the immunological mechanism of allergic asthma and outlining attractive intervention strategies for allergic diseases.
Asthma is a chronic airway inflammatory disorder associated with Th2 overactivation, elevated IgE production and strengthened mast cell and eosinophil recruitment. Upon exposure to airway allergens, APCs trigger the initiation of innate immune signaling, undergo maturation and migration, and subsequently activate and regulate T cell-dependent adaptive immune responses. The pulmonary microenvironment can also induce the generation of regulatory DCs, which contribute to the maintenance of immune homoeostasis and the control of lung inflammation.27 In particular, Th2-type cytokines are the major driver of asthma through the induction of Th2 cell survival (IL-4), IgE production (IL-4 and IL-13), and mast cell and eosinophil recruitment, activation and maturation (IL-3, IL-5, IL-9, IL-13 and GM-CSF).28 Considerable efforts have been made to develop strategies to treat allergic inflammation through the modulation of innate and adaptive immunity. However, clinical trials with inhibitors of IL-4, IL-13 and GM-CSF have been successful in some studies focusing on asthma therapy but not in others.29 Therefore, it would be more effective to treat asthma by simultaneously suppressing multiple cytokines and disconnected pathological processes. In this study, we show that platelet depletion and Cd154 depletion could significantly attenuate asthma progression by inhibiting IL-4, IL-13 and IgE production and leukocyte infiltration, suggesting a promising strategy for the treatment of allergic asthma.
The CD40–CD154 interaction was shown to promote the production of IgE and pro-inflammatory mediators in asthma. For example, engaging CD40 on epithelial cells promoted asthma progression,30 while silencing Cd40 gene expression in epithelial cells resulted in reduced disease progression.31 CD40–CD154 crosstalk between B cells and mast cells was shown to induce airway inflammation and remodeling in allergic asthma.32 Moreover, Cd40 gene polymorphisms were identified in patients with asthma and were related to elevated serum IgE levels in asthma patients.33 Consistent with these previous studies, our study provides the first evidence, through the use of Cd154−/− mice, that CD154 on platelets promotes IgE production, leukocyte infiltration and Th2-type responses in a mouse model of allergic asthma.
However, another study focusing on the CD40–CD154 interaction in asthma progression yielded paradoxical results. In Cd40 deficient mice, asthma progression was more severe, as demonstrated by elevated airway hyperactivity, eosinophilia and predominant Th2 cell accumulation in airway, indicating a suppressive role of CD40 in both asthma and Th2 differentiation.34 The detailed mechanism and biological significance for this controversial function of the CD40–CD154 interaction in asthma requires further investigation. It might be caused by different functions of CD40–CD154 in different cell types or at different phases of the immune response. In this respect, studies with cell type-specific overexpression or silencing of Cd40 or Cd154 could provide valuable evidence to discern the cellular mechanisms involved in this discrepancy.
Both naturally occurring thymus-derived CD4+CD25+Foxp3+ Tregs and inducible Tregs suppress the development of allergies through multiple mechanisms, including the inhibition of other effector Th1, Th2, and Th17 cells, eosinophils, mast cells, basophils, inflammatory DCs and inflammatory cell migration to tissues.35 The effect of CD154 on Treg induction and activation has been elusive. It was shown that soluble CD154 induced the activation of the immune-regulatory enzyme IDO, IL-10 production and programmed death-1 expression by T cells, and the expansion and maintenance of Tregs and therefore, played an immunosuppressive role in HIV infection and cancer.36,37 Furthermore, B cells activated by CD40 are more potent in inducing and expanding Tregs than immature DCs.38 On the contrary, activated platelets expressing CD154 were found to promote atherogenesis and inflammation by inhibiting the recruitment of Tregs.39 Therefore, it is important to clarify the cell intrinsic role of CD154 in Treg induction. In our study, we showed that CD154 on platelets directly inhibits the induction of Tregs both in vitro and in vivo, providing a mechanistic explanation for the promotion of inflammatory responses and disease progression by CD154. However, further study is required to determine how platelet CD154 regulates Treg induction. It remains unknown whether CD154 is involved in the transcriptional or translational control of Foxp3 expression, whether CD154 exerts an inhibitory function in Treg differentiation through CD40/CD154 signaling or through other unknown mechanisms, and whether antigen-presenting cells such as DCs or monocytes are involved in this process. These issues should be addressed in the future.
Further investigation is also required to determine the reason for the enhanced Th2-type responses after platelet transfer in asthma-induced mice. This might be due to the inhibition of Tregs by CD154 expression in platelets, as we have demonstrated in this study. This possibility could be assessed by Treg transfer experiments in the future. However, it may also be related to the elevated function of antigen-presenting cells or expression of chemotactic factors or to disordered Th1/Th17 cell activation. The direct effect of platelet CD154 in the differentiation of Th1, Th2 or Th17 cells is another intriguing issue. One study found that activated platelets promoted production of Th1 cytokines including IFN-γ and TNF-α but not Th2 cytokines such as IL-4 and IL-5.40 CD40 was also shown to be involved in TCR expression in the periphery through interactions with RAG1 and RAG2.41 Therefore, it remains of interest to clarify more detailed mechanisms behind the regulatory role of platelets and their activation marker CD154 in the context of the complex immune network of antigen-presenting cells and different types of T cells.
Author contributions
XC designed and supervised the research; JT and TZ conducted the experiments; ZG contributed reagents and analytical tools; JT, JL and XC analyzed the data and wrote the paper.
Acknowledgments
We thank R Zhang and N Dong for their technical assistance. This work was supported by the National Key Basic Research Program of China (2013CB530502) and the National Natural Science Foundation of China (31270966, 81123006).
The authors declare no competing financial interests.
References
- 1Semple JW, Italiano JEJr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 2011; 11: 264–274. [DOI] [PubMed] [Google Scholar]
- 2Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol 2014; 12: 426–437. [DOI] [PubMed] [Google Scholar]
- 3Morrell CN1, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood 2014; 123: 2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Mantovani A1, Garlanda C. Platelet-macrophage partnership in innate immunity and inflammation. Nat Immunol 2013; 14: 768–770. [DOI] [PubMed] [Google Scholar]
- 5Grewal IS1, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 1998; 16: 111–135. [DOI] [PubMed] [Google Scholar]
- 6Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998; 391: 591–594. [DOI] [PubMed] [Google Scholar]
- 7Elzey BD, Grant JF, Sinn HW, Nieswandt B, Waldschmidt TJ, Ratliff TL. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol 2005; 78: 80–84. [DOI] [PubMed] [Google Scholar]
- 8Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood 2008; 111: 5028–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Elzey BD, Schmidt NW, Crist SA, Kresowik TP, Harty JT, Nieswandt B et al. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood 2008; 111: 3684–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med 2005; 11: 1167–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity 2003; 19: 9–19. [DOI] [PubMed] [Google Scholar]
- 12Gawaz M, Dickfeld T, Bogner C, Fateh-Moghadam S, Neumann FJ. Platelet function in septic multiple organ dysfunction syndrome. Intensive Care Med 1997; 23: 379–385. [DOI] [PubMed] [Google Scholar]
- 13Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 2007; 357: 2482–2494. [DOI] [PubMed] [Google Scholar]
- 14Russ M, Seliger B, Hauptmann S, Marty R, Bukur J, Eriksson U et al. Platelet-depletion ameliorates cardiac function and disease severity in experimental autoimmune myocarditis. Circulation 2008; 118: S_516. [Google Scholar]
- 15Duffau P, Seneschal J, Nicco C, Richez C, Lazaro E, Douchet I et al. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med 2010; 2: 47ra63. [DOI] [PubMed] [Google Scholar]
- 16Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 2010; 327: 580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Wang F, Wang NS, Yan CG, Li JH, Tang LQ. The significance of platelet activation in rheumatoid arthritis. Clin Rheumatol 2007; 26: 768–771. [DOI] [PubMed] [Google Scholar]
- 18Gasparyan AY, Stavropoulos-Kalinoglou A, Mikhailidis DP, Douglas KM, Kitas GD. Platelet function in rheumatoid arthritis: arthritic and cardiovascular implications. Rheumatol Int 2011; 31: 153–164. [DOI] [PubMed] [Google Scholar]
- 19Pamuk GE, Vural O, Turgut B, Demir M, Pamuk ON, Cakir N. Increased platelet activation markers in rheumatoid arthritis: are they related with subclinical atherosclerosis? Platelets 2008; 19: 146–154. [DOI] [PubMed] [Google Scholar]
- 20Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012; 18: 673–683. [DOI] [PubMed] [Google Scholar]
- 21Yamamoto H, Nagata M, Tabe K, Kimura I, Kiuchi H, Sakamoto Y et al. The evidence of platelet activation in bronchial asthma. J Allergy Clin Immunol 1993; 91: 79–87. [DOI] [PubMed] [Google Scholar]
- 22Pitchford SC, Page CP. Platelet activation in asthma: integral to the inflammatory response. Clin Exp Allergy 2006; 36: 399–401. [DOI] [PubMed] [Google Scholar]
- 23Benton AS, Kumar N, Lerner J, Wiles AA, Foerster M, Teach SJ et al. Airway platelet activation is associated with airway eosinophilic inflammation in asthma. J Investig Med 2010; 58: 987–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Kowal K, Pampuch A, Kowal-Bielecka O, Iacoviello L, Bodzenta-Lukaszyk A. Soluble CD40 ligand in asthma patients during allergen challenge. J Thromb Haemost 2006; 4: 2718–2720. [DOI] [PubMed] [Google Scholar]
- 25Liu J, Han C, Xie B, Wu Y, Liu S, Chen K et al. Rhbdd3 controls autoimmunity by suppressing the production of IL-6 by dendritic cells via K27-linked ubiquitination of the regulator NEMO. Nat Immunol 2014; 15: 612–622. [DOI] [PubMed] [Google Scholar]
- 26Chen W, Han C, Xie B, Hu X, Yu Q, Shi L et al. Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell 2013; 31: 467–478. [DOI] [PubMed] [Google Scholar]
- 27Li Q, Guo Z, Xu X, Xia S, Cao X. Pulmonary stromal cells induce the generation of regulatory DC attenuating T-cell-mediated lung inflammation. Eur J Immunol 2008; 38: 2751–2761 [DOI] [PubMed] [Google Scholar]
- 28Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity 2009; 31: 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Hansbro PM, Kaiko GE, Foster PS. Cytokine/anti-cytokine therapy—novel treatments for asthma? Br J Pharmacol 2011; 163: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Propst SM, Denson R, Rothstein E, Estell K, Schwiebert LM. Proinflammatory and Th2-derived cytokines modulate CD40-mediated expression of inflammatory mediators in airway epithelia: implications for the role of epithelial CD40 in airway inflammation. J Immunol 2000; 165: 2214–2221. [DOI] [PubMed] [Google Scholar]
- 31Suzuki M, Zheng X, Zhang X, Ichim TE, Sun H, Kubo N et al. Inhibition of allergic responses by CD40 gene silencing. Allergy 2009; 64: 387–397. [DOI] [PubMed] [Google Scholar]
- 32Hong GU, Park BS, Park JW, Kim SY, Ro JY. IgE production in CD40/CD40L cross-talk of B and mast cells and mediator release via TGase 2 in mouse allergic asthma. Cell Signal 2013; 25: 1514–1525. [DOI] [PubMed] [Google Scholar]
- 33Park JH, Chang HS, Park CS, Jang AS, Park BL, Rhim TY et al. Association analysis of CD40 polymorphisms with asthma and the level of serum total IgE. Am J Respir Crit Care Med 2007; 175: 775–782. [DOI] [PubMed] [Google Scholar]
- 34Takahashi H, Ebihara S, Kanda A, Kamanaka M, Sato T, Habu S et al. Increased susceptibility to airway responses in CD40-deficient mice. Clin Exp Immunol 2003; 133: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur J Immunol 2010; 40: 1232–1240. [DOI] [PubMed] [Google Scholar]
- 36Jenabian MA, Patel M, Kema I, Vyboh K, Kanagaratham C, Radzioch D et al. Soluble CD40-ligand (sCD40L, sCD154) plays an immunosuppressive role via regulatory T-cell expansion in HIV infection. Clin Exp Immunol 2014; doi: 10.1111/cei.12396. [DOI] [PMC free article] [PubMed]
- 37Huang J, Jochems C, Talaie T, Anderson A, Jales A, Tsang KY et al. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood 2012; 120: 3030–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Zheng J, Liu Y, Lau YL, Tu W. CD40-activated B cells are more potent than immature dendritic cells to induce and expand CD4+ regulatory T cells. Cell Mol Immunol 2010; 7: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix IC et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 2010; 116: 4317–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Gerdes N, Zhu L, Ersoy M, Hermansson A, Hjemdahl P, Hu H et al. Platelets regulate CD4+ T-cell differentiation via multiple chemokines in humans. Thromb Haemost 2011;106: 353–362. [DOI] [PubMed] [Google Scholar]
- 41Vaitaitis GM, Wagner DHJr. CD40 interacts directly with RAG1 and RAG2 in autoaggressive T cells and Fas prevents CD40-induced RAG expression. Cell Mol Immunol 2013; 10: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]