Abstract
CD4+CD25+FoxP3+ regulatory T cells (Tregs) are increased in patients with chronic hepatitis C, which may contribute to the sustained suppression of hepatitis C virus (HCV)-specific T-cell responses and viral persistence in HCV-infected individuals. We postulated that HCV core protein (HCVc) directly contributes to the expansion of Tregs in HCV-infected patients, and we provide evidence to support this hypothesis in the report. Peripheral blood mononuclear cells (PBMCs) and sera were collected from 87 treatment-naïve chronic HCV-infected patients, CD4+CD25+ Tregs were measured by flow cytometry, and HCV RNA and HCVc levels were detected using qPCR and enzyme-linked immunosorbent assay (ELISA), respectively. CD4+, CD8+, CD4+CD25+ and CD4+CD25− T cells were purified from healthy donors and cultured with recombinant HCVc and Toll-like receptor (TLR) ligands. Flow cytometry was used to analyze cell proliferation, and ELISA was performed to measure cytokine production. In the 87 chronic HCV-infected patients, HCVc showed a significant correlation with HCV RNA and CD4+CD25+ Tregs. Mechanistic studies showed that HCVc, together with anti-CD3 antibody, augmented CD4+CD25+ Treg proliferation, but inhibited CD4+CD25− T-cell proliferation and IFN-γ production, in a dose-dependent and Treg-dependent manner. Moreover, unlike the TLR3 ligand (poly I:C) and the TLR4 ligand (lipopolysaccharide, LPS), the TLR2 ligand (lipoteichoic acid, LTA) and HCVc both inhibited TCR-induced CD4+ T-cell proliferation and IFN-γ secretion in a Treg-dependent manner. These data indicate that HCVc, like other TLR2 ligands, triggers CD4+CD25+ Treg activation and expansion to inhibit host immune responses, which may play a critical role in viral persistence in HCV-infected patients.
Keywords: CD4+CD25+ regulatory T cells, HCV core, Toll-like receptor
Introduction
Chronic viral hepatitis due to hepatitis C Virus (HCV) is accompanied by immune failure. Features of this failure include the persistence of viremia, the exhaustion of effector T cells and the increased activity of regulatory T cells (Tregs).1,2,3,4,5 However, the relationships between these biomarkers of immune failure are not entirely clear. We sought to clarify these relationships through a study of T cells isolated from a cohort of 87 HCV-infected patients.
We focused on the core protein of hepatitis C virus (HCVc) because it mediates diverse immunosuppressive effects. One of these actions is mediated by binding to the Toll-like receptor 2 (TLR2) pattern recognition receptor of Kupffer cells. In this context, HCVc induces diverse pro-inflammatory and anti-inflammatory cytokines but strongly inhibits the secretion of the anti-viral cytokines IFN-α and IFN-β and inhibits the upregulation of the TNF receptor-associated apoptosis-inducing ligand on the cell surface.6 The HCVc protein also engages TLR2 on human dendritic cells, skewing their differentiation with increased expression of both macrophage markers, and PD-L1.7
Tregs isolated from HCV-infected patients are immunosuppressive and can inactivate both HCV-specific and bystander CD4+ and CD8+ T cells.8,9 Here, we show that the concentration of HCVc in the blood of HCV-infected patients directly correlates with the frequency of Tregs. Furthermore, HCVc directly caused proliferation of Tregs and caused them to secrete immunosuppressive cytokines.
While it was previously documented that HCVc inhibits T-cell proliferation,10 here we reveal the mechanism: HCVc inhibited CD4+ T-cell proliferation and IFN-γ secretion in a Treg-dependent manner. Both of these effects were also seen with the TLR2 ligand lipoteichoic acid (LTA). Therefore, we conclude that these effects of HCVc on T cells are also mediated by TLR2.
These results clarify the multifaceted problem of immunosuppression and exhaustion in the context of chronic HCV infection and may also shed light on the immunology of other chronic infections, such as hepatitis B Virus infection, in which exhausted effector T cells co-exist with Tregs.11 Therapeutic interventions to reverse exhaustion and initiate immunological self-cure in chronic hepatitis patients depend on a full understanding of these mechanisms.
Materials and methods
Samples
Eighty-seven treatment-naive chronic HCV-infected patients were enrolled in this study (Table 1). Venous blood was withdrawn for serum and peripheral blood mononuclear cells (PBMCs) collection. These studies were approved by the IRB of Jilin University, The First Hospital. Buffy coats from five healthy donors were provided by the Changchun Blood Center, and informed consent was provided according to the protocols of the Changchun Blood Center.
Table 1. Characteristics of study population.
| HCVc serum level (fmol/l) | |||
|---|---|---|---|
| <1000 | 1000–5000 | >5000 | |
| Age, years | |||
| (mean±s.e.m.) | 48.9±15.1 | 45.5±10.9 | 48.2±16.44 |
| Sex | |||
| Female/male | 20/24 | 11/13 | 8/11 |
| ALT, IU/ml | |||
| (mean±s.e.m.) | 71.21±9.36 | 68.24±20.73 | 60.34±13.94 |
| HCV-DNA | |||
| (mean±s.e.m.) | 4.86×105 | 3.05×106 | 1.11×107 |
| ±1.98×105 | ±6.01×105 | ±2.53×106 | |
| HCV genotype | |||
| (2a/1b/n.a) | 29/11/4 | 13/10/1 | 14/5/0 |
Abbreviations: n.a.: not applicable; SEM: standard error of mean.
Serum viral load and HCVc level assay
HCV RNA was detected using the Cobas-TaqMan assay or Amplicor-HCV-Monitor (Roche Diagnostics, Mannheim, Germany). HCVc was quantified by automated immunoassay (i System i2000, Architect; Abbott, Wiesbaden, Germany).
HCVc, TLR agonists and reagents
Recombinant HCVc (aa 2–192 of the HCV polyprotein) was purchased from Biodesign (Saco, ME, USA); TLR agonists LTA, poly I:C and lipopolysaccharide (LPS) were purchased from Invivogen (San Diego, CA, USA).
Limulus amebocyte lysate assay for detection of LPS contamination
The QCL-1000 chromogenic LAL endpoint assay (Cam-brex, Cottonwood, AZ, USA) was used to detect endotoxin contamination of HCVc following the manufacturer's protocol as described.6,7 Briefly, protein-free, phenol-water extracted ultrapure Escherichia coli LPS was used to create a linear standard curve from 1 to 100 pg/ml. HCVc was serially diluted to determine a concentration of agonist within the standard curve of the assay. HCVc preparations were determined to have <1 pg/ml LPS contamination.
Isolation of T lymphocyte subsets from peripheral blood
PBMCs were isolated by Ficoll/Paque density gradient centrifugation. For magnetic cell sorting, the CD8 Multisort kit (Miltenyi Biotec, Bergisch Gladbach, Germany) was used for the purification of CD8+ T cells without ligation of any of their surface molecules. The CD4+CD25+ Treg Isolation kit was used for the purification of CD4+ T cells, CD4+CD25− T cells and CD4+CD25+ Tregs. The purity of the T-cell subsets was determined by flow cytometry and was always in the 90%–95% range.
Enzyme-linked immunosorbent assay (ELISA)
Concentrations of IFN-γ, IL-10 and TGF-β in cell culture supernatants were measured by ELISA according to the manufacturer's instructions.6,7
T lymphocyte subsets proliferation assays
CD8+, CD4+, CD4+CD25+ Treg and CD4+CD25− T- cell subsets were stained with 5 µM CFSE (Molecular Probes, Grand Island, NY. USA) according to the manufacturer's recommended protocol. CD3 activation was performed by using 10 µg/ml OKT3 (CD3 mAb; eBioscience, San Diego, CA. USA) bound to the plastic in the wells for subsequent cross-linking of the T-cell receptors on responding T cells. Plates could also be prepared the night before an experiment and kept in the refrigerator overnight. CFSE-labeled T lymphocyte subsets were cultured in wells pre-coated with OKT3 in the presence of HCVc and/or TLR agonists. After 5 days, when clumps were visible, cells were collected and stained with anti-CD25-APC (BD Biosciences, San Jose, CA, USA), and the proliferating T lymphocyte subsets were characterized by flow cytometry.
Flow cytometry
Staining of cells and analysis on a flow cytometer (FACScan; BD Biosciences) were done as described.6,7 CD4+CD25+ Tregs were identified using the following anti-human mAb: FITC Mouse Anti-Human CD4 (Clone: RPA-T4), APC Mouse Anti-Human CD25 (Clone: M-A251) and PE Mouse Anti-Human FoxP3 (Clone: 259D/C7). Intracellular FoxP3 staining and cell surface CD4 and CD25 staining were performed according to the manufacturers recommended protocol. All the antibodies were obtained from BD Biosciences. The data acquired were analyzed with FlowJo (Treestar software, Ashland, OR, USA).
Statistical analysis
All data were analyzed and found to be significant using the D'Agostino and Pearson omnibus normality test. Mean values were compared using either a paired t-test (two groups) or ANOVA (>two groups), followed by a Bonferroni correction for multiple comparisons test. P values <0.05 were considered to be significant. All statistical tests were performed by Prism software (GraphPad, San Diego, CA, USA).
Results
HCVc correlation with HCV RNA and CD4+CD25+ Tregs
A cohort of 87 treatment-naive chronic HCV-infected patients was enrolled in this study and divided into three groups based on serum HCVc levels (44 with HCVc levels <1000 fmol/l, 24 with HCVc levels between1000 and 5000 fmol/l and 19 with HCVc levels >5000 fmol/l) (Table 1). Serum HCV RNA and HCVc levels were detected using qPCR and ELISA, respectively.
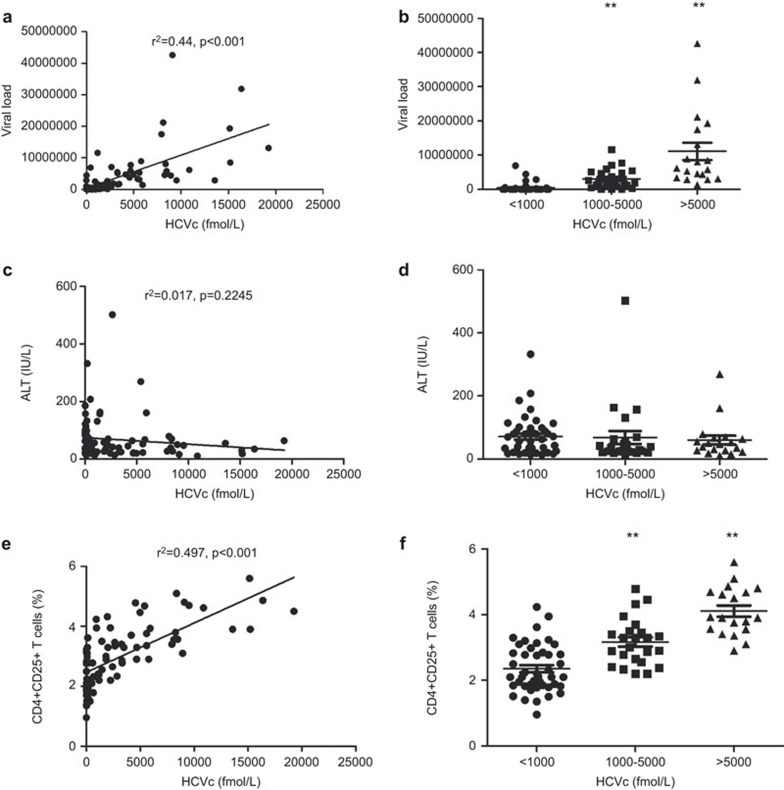
Figure 1a and b show that HCVc levels positively correlate with HCV RNA levels (r2=0.44, P<0.001), but not with ALT levels (r2=0.017, P=0.2245) (Figure 1c and d), indicating that HCVc concentration represents a stable and reliable marker of HCV viral replication. CD4+CD25+ Tregs from PBMCs of chronic HCV-infected patients were measured by flow cytometry. Intracellular FoxP3 staining showed that 87% of the CD4+CD25+ Tregs, 11% of the CD4+CD25dim cells and 0.81% of the CD4+CD25− cells are FoxP3+ (Supplementary Figure 1). The frequency of CD4+CD25+ Tregs was positively correlated with serum HCVc levels (r2=0.497, P<0.001) (Figure 1e). Further analysis shows that the frequency of CD4+CD25+ Tregs was significantly higher among the 19 chronic HCV-infected patients with HCVc levels >5000 fmol/l (4.107±0.1693, N=19) than in the 24 with HCVc levels between 1000 and 5000 fmol/l (3.167±0.1452, N=24) (P<0.001) and the 44 with HCVc levels <1000 fmol/l (2.352±0.1093, N=44) (P<0.001) (Figure 1f and Supplementary Figure 2). These results suggest that the HCV-encoded core protein circulating in the blood may induce the expansion of natural CD4+CD25+ Tregs or stimulate their generation from CD4+ T cells.
Figure 1.
Correlation of HCVc levels with that of HCV RNA and CD4+CD25+ Tregs. Data are shown for 87 treatment-naive chronic HCV-infected patients. Serum HCV RNA and HCVc levels were detected using qPCR and ELISA, respectively. The frequency of CD4+CD25+ Tregs from PBMCs was measured by flow cytometry. A positive correlation was shown between the serum level of HCVc and HCV RNA (r2=0.44, P<0.001) (a). The 87 chronic HCV-infected patients were divided into three groups according to serum HCVc levels (19 with HCVc level >5000 fmol/l, 24 with HCVc level 1000–5000 fmol/l and 44 with HCVc level <1000 fmol/l). The correlation between serum HCVc levels and the serum level of HCV RNA (b), ALT (c) or the frequency of CD4+CD25+ Tregs from PBMCs (d), was also analyzed. HCV, hepatitis C virus; HCVc, HCV core protein; PBMC, peripheral blood mononuclear cell; Treg, regulatory T cell.
HCVc induces proliferation of CD4+CD25+ Tregs
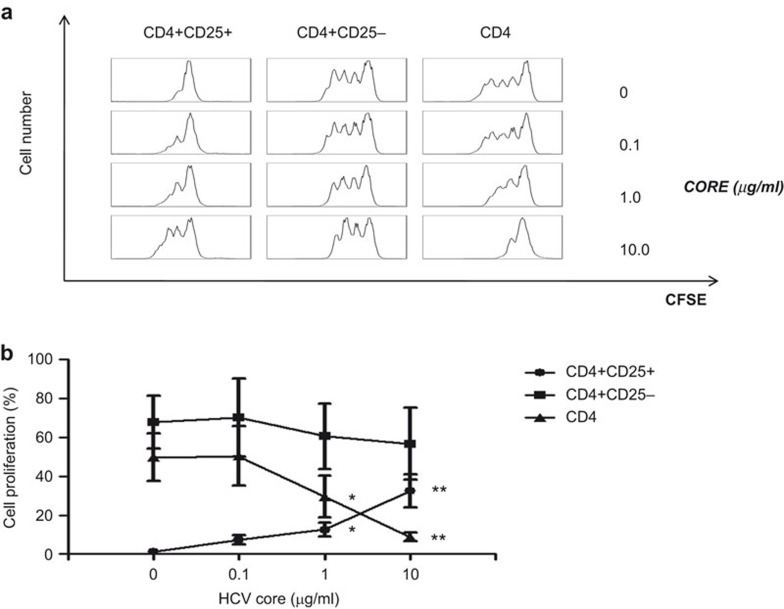
In order to analyze the effects of HCVc on the proliferation of CD4+CD25+ Tregs, freshly isolated CD4+, CD4+CD25+ Tregs and CD4+CD25− T cells from healthy HCV-negative blood donors were activated by anti-CD3 mAb for 5 days in the presence of increasing concentrations of HCVc protein. Our results show that HCVc induced CD4+CD25+ Treg proliferation in a dose-dependent manner but inhibited total CD4+ T-cell proliferation (Figure 2b). However, depletion of CD4+CD25+ Tregs from the total CD4+ T-cell population abrogated the inhibitory effect of HCVc on anti-CD3-induced CD4+ T-cell proliferation (Figure 2a and b) supplementary Figure 3. Additionally, HCVc alone did not induce T-cell proliferation (data not shown). These data suggest that the HCVc protein may stimulate Tregs to actively suppress other CD4+ T cells.
Figure 2.
HCV core protein induces CD4+CD25+ T-cell proliferation. Primary CD4+ T cells were isolated from peripheral blood of healthy donors by using a CD4 isolation kit first, and further purification of CD4+CD25+ and CD4+CD25− T cells was performed by using a Treg isolation kit according to the manufacturer's instructions. The purified total CD4+, CD4+CD25+ and CD4+CD25− T cells were labeled with CFSE and then cultured for 5 days in plates pre-coated with OKT3 (CD3 mAb) and various concentrations of HCVc. Flow cytometry was used to analyze cell proliferation. Representative experiment results from one donor out of five are shown (a) and the percentage of cell proliferation as mean±s.d. from five separate experiments are also shown (b) (n=5, *P<0.05, **P<0.01). HCV, hepatitis C virus; HCVc, HCV core protein; Treg, regulatory T cell.
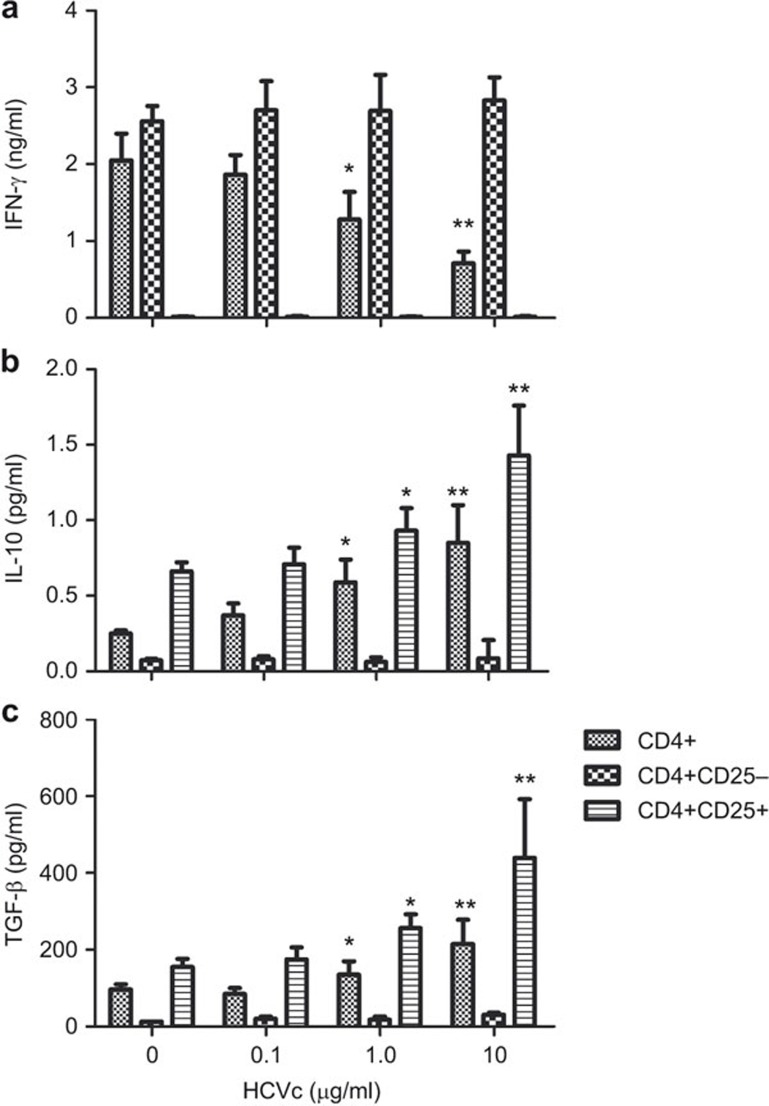
HCVc induced production of IL-10 and TGF-β in CD4+CD25+ T cells and inhibited IFN-γ production in CD4+ T cells through CD4+CD25+ Tregs
To test whether HCVc affected Treg function and to assess its effects on IFN-γ secretion from other T cells, freshly isolated HCV-negative, human CD4+, CD4+CD25+ Tregs and CD4+CD25− T cells were activated by anti-CD3 mAb in the absence or presence of HCVc for 2 days. We found that HCVc inhibited TCR-induced IFN-γ secretion of CD4+ T cells in a dose-dependent manner, and depletion of CD4+CD25+ Tregs from the CD4+ T cells abrogated the inhibitory effect of HCVc on IFN-γ production. HCVc did not induce IFN-γ production in CD4+CD25+ Tregs (Figure 3a). More importantly, we also found that HCVc induced IL-10 and TGF-β secretion by CD4+CD25+ Tregs, but showed no effect on cytokine production by CD4+CD25− T cells (Figure 3b and c).
Figure 3.
HCVc induces IL-10 and TGF-β production in CD4+CD25+ T cells and inhibits IFN-γ production in CD4+ T cells through CD4+CD25+ T cells. Total CD4+, CD4+CD25+ and CD4+CD25− T cells were cultured for 2 days in wells pre-coated with OKT3 (CD3 mAb) and various concentrations of HCVc. The supernatants were then harvested and assayed by ELISA for IFN-γ (a), IL-10 (b) and TGF-β (c) levels (n=5, *P<0.05, **P<0.01). HCVc, HCV core protein.
HCVc and LTA inhibit the proliferation of CD4+ T cells in a Treg-dependent fashion
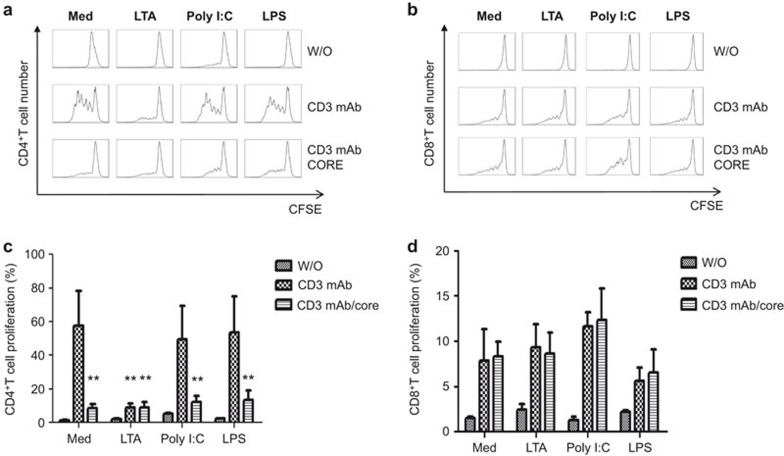
HCVc is a ligand for TLR2, which induces the production of inflammatory cytokines by activating the MyD88-dependent TLR signaling pathway.12 Therefore, we further examined whether HCVc triggers the expansion and activation of CD4+CD25+ regulatory T cells via TLR2 signaling. CD4+ T cells and CD8+ T cells were purified from the peripheral blood of healthy donors by CD4 and CD8 microbeads and then activated by anti-CD3 mAb and ligands of TLR2, TLR3 and TLR4 (LTA, poly I:C and LPS, respectively) in the absence or presence of HCVc. The results showed that the TLR2 ligand LTA, like HCVc, inhibited the proliferation of CD4+ T cells, but poly I:C and LPS did not affect CD4+ T-cell proliferation (Figure 4a and c). In addition, neither HCVc nor any of the ligands of TLR2, TLR3 and TLR4 affected the proliferation of purified CD8+ T cells (Figure 4b and d).
Figure 4.
Both HCVc and LTA inhibit the proliferation of CD4+ T cells but not CD8+ T cells. Primary CD4+ T cells and CD8+ T cells were purified, and CFSE-labeled CD4+ and CD8+ T cells were cultured for 5 days in wells pre-coated with OKT3 (CD3 mAb) and HCVc in the presence of different TLR ligands. Flow cytometry was used to analyze cell proliferation. Representative experiment results from one donor out of five are reported in (a) and (b), and the percentage of proliferating CD4+ and CD8+ T cells and mean±s.d. from five separate experiments are shown as (c) and (d) (n=5, *P<0.05, **P<0.01). HCVc, HCV core protein; LTA, lipoteichoic acid; TLR, Toll-like receptor.
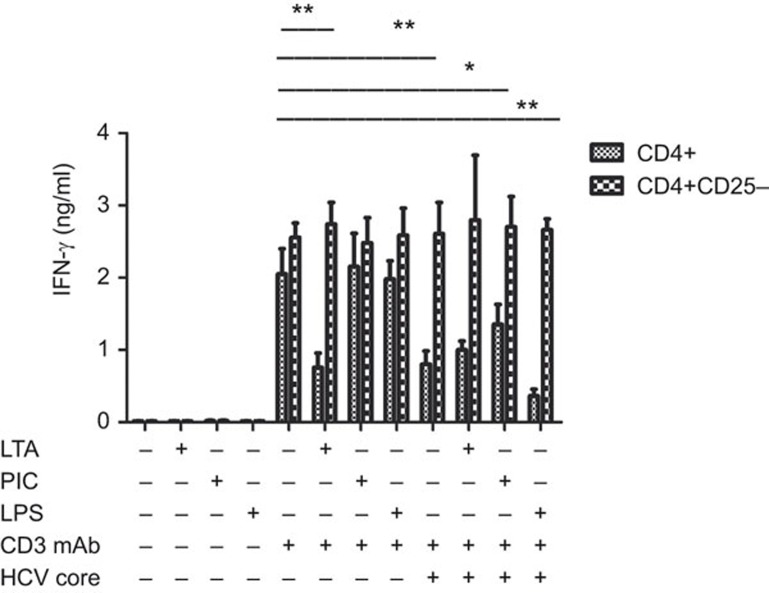
LTA and HCVc inhibit IFN-γ production in TCR-activated CD4+ T cells
To explore whether LTA also inhibits IFN-γ secretion in CD4+ T cells through CD4+CD25+ Tregs, total CD4+ T cells and CD4+CD25− T cells were purified from healthy HCV-negative donors and activated by anti-CD3 mAb and TLR2, TLR3 and TLR4 ligands for 2 days in the presence of HCVc as described above. We found that neither TLR2, TLR3 and TLR4 ligands nor HCVc alone induced CD4+ T-cell IFN-γ production, whereas anti-CD3 mAb induced IFN-γ secretion from both CD4+ and CD4+CD25− T cells (Figure 5). However, LTA and HCVc inhibited anti-CD3 mAb-induced IFN-γ secretion from CD4+ T cells, but not from CD4+CD25− T cells. Poly I:C and LPS did not affect IFN-γ secretion in TCR-activated CD4+ T cells. Therefore, HCVc inhibited CD4+ T-cell activation in a Treg-dependent fashion.
Figure 5.
HCVc and LTA inhibit IFN-γ production by activated CD4+ T cells in a Treg-dependent fashion. Purified CD4+ and CD8+ T cells were cultured with pre-coated OKT3 (CD3 mAb) and HCVc and TLR ligands for 2 days. The supernatants were then harvested and assayed by ELISA for IFN-γ levels (n=5, *P<0.05, **P<0.01). HCVc, HCV core protein; LTA, lipoteichoic acid; Treg, regulatory T cell; TLR, Toll-like receptor.
Discussion
CD4+CD25+FoxP3+ Tregs are increased in patients with chronic hepatitis C, and this may mediate the sustained suppression of HCV-specific T-cell responses and the viral persistence in HCV-infected individuals.13,14,15 The mechanism of Treg proliferation and its activity in HCV infection are not completely understood. In the current study, we confirm that HCVc showed a significant, positive correlation with HCV RNA and the frequency of CD4+CD25+ Tregs in 87 chronic HCV-infected patients. Furthermore, we evaluated the role of HCVc in Treg proliferation and suppressive functions and found that HCVc induced CD4+CD25+ Treg proliferation and IL-10 and TGF-β secretion in a dose-dependent manner. Moreover, HCVc inhibited CD4+ T-cell proliferation and IFN-γ production in a Treg-dependent fashion. In addition, a TLR2 agonist, LTA, inhibited CD4+ T-cell proliferation and IFN-γ production to the same extent as HCVc did, but poly I:C (TLR3 agonist) and LPS (TLR4 agonist) did not. These results suggest that HCVc, as a ligand for TLR2, may play a role in activating Treg proliferation and inducing their suppressive function. This mechanism may contribute to the sustained suppression of HCV-specific T-cell responses and the consequently persistent HCV infection.
HCV has developed different strategies to evade the host's innate and adaptive immune responses. HCV has been shown to interfere with innate antiviral defenses through multiple mechanisms. HCVc protein induces the expression of SOCS-3, which suppresses JAK-STAT signaling. Also, the NS3/4A protease antagonizes IRF-3 activation and interferon-β expression by blocking RIG-I and TLR3 signaling.16,17 In addition, HCV proteins have been shown to upregulate TLR2 and TLR4 expression on Raji cells and peripheral blood mononuclear cells, thereby modulating the cell's pro-inflammatory response.18,19 Moreover, several studies by our group and others have provided evidence that the HCV core can activate TLR2 on human monocytes, macrophages and Kupffer cells, which induces production of inflammatory cytokines by activating the MyD88-dependent TLR signaling pathway.6,12,19 An effect of HCV core expression on CD4+ T cells has been reported, showing that this protein induces a state of unresponsiveness similar to clonal anergy or T-cell exhaustion.20,21,22 HCVc can bind the human complement receptor C1qR, thus inhibiting T-cell responses.23,24 More recently, using a lentiviral vector to express HCVc in CD4+ Jurkat T cells, Dominguez-Villar et al.25 report that FoxP3 and CTLA-4 (cytotoxic T-lymphocyte antigen-4) were upregulated in HCVc-expressing Jurkat cells and that HCVc-transfected Jurkat cells were able to suppress CD4+ and CD8+ T-cell responses to anti-CD3 plus anti-CD28 stimulation.25 In agreement with these findings, our results showed that HCVc inhibited CD4+ T-cell proliferation and IFN-γ production in the presence of anti-CD3 mAb, and further mechanistic studies showed that HCVc triggers CD4+CD25+ Treg expansion and activation to mediate the inhibition.
Our results shed new light on the expansion of Treg cells by HCVc and provide evidence that this HCV protein is responsible for enhanced Treg activity in the periphery during chronic HCV infections. Interestingly, the canonical TLR2 ligand lipoteichoic acid had the same effect on Tregs as HCVc, suggesting that other pathogens that engage TLR2 may also induce the expansion and activation of CD4+CD25+ Tregs.
Acknowledgments
This work was supported by the Natural Science Foundation of China (81373143, to ZT) and by NIH (AI095097, to LS). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the paper.
The authors declare that they have no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- 1Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol 2006; 80: 11398–11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol 2007; 81: 2545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Sekyere SO, Suneetha PV, Kraft A, Zhang S, Dietz J, Sarrazin C et al. A heterogeneous hierarchy of co-regulatory receptors regulates exhaustion of HCV-specific CD8 T cells in patients with chronic hepatitis C. J Hepatol 2014; in press. doi: 10.1016/j.jhep.2014.08.008. [DOI] [PubMed]
- 4Manigold T, Shin EC, Mizukoshi E, Mihalik K, Murthy KK, Rice CM et al. Foxp3+CD4+CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood 2006; 107: 4424–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Rezaei N, Amirzargar AA, Shakiba Y, Mahmoudi M, Moradi B, Aghamohammadi A. Proinflammatory cytokine gene single nucleotide polymorphisms in common variable immunodeficiency. Clin Exp Immunol 2009; 155: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Tu Z, Pierce RH, Kurtis J, Kuroki Y, Crispe IN, Orloff MS. Hepatitis C virus core protein subverts the antiviral activities of human Kupffer cells. Gastroenterology 2010; 138: 305–314. [DOI] [PubMed] [Google Scholar]
- 7Tu Z, Hamalainen-Laanaya HK, Nishitani C, Kuroki Y, Crispe IN, Orloff MS. HCV core and NS3 proteins manipulate human blood-derived dendritic cell development and promote Th 17 differentiation. Int Immunol 2012; 24: 97–106. [DOI] [PubMed] [Google Scholar]
- 8Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P et al. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol 2005; 79: 7852–7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Smyk-Pearson S, Golden-Mason L, Klarquist J, Burton JR Jr, Tester IA, Wang CC et al. Functional suppression by FoxP3+CD4+CD25high regulatory T cells during acute hepatitis C virus infection. J Infect Dis 2008; 197: 46–57. [DOI] [PubMed] [Google Scholar]
- 10Kittlesen DJ, Chianese-Bullock KA, Yao ZQ, Braciale TJ, Hahn YS. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J Clin Invest 2000; 106: 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005; 41: 771–778. [DOI] [PubMed] [Google Scholar]
- 12Dolganiuc A, Oak S, Kodys K. Hepatitis C core and non-structural 3 proteins trigger Toll-like receptor 2-mediated path-ways and inflammatory activation. Gastroenterology 2004; 127: 1513–1524. [DOI] [PubMed] [Google Scholar]
- 13Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C et al. An immunomodulatory role for CD4+ CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology 2004; 40: 1062–1071. [DOI] [PubMed] [Google Scholar]
- 14Boettler T, Spangenberg HC, Neumann-Haefelin C. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8þT cells during chronic hepatitis C virus infection. J Virol 2005; 79: 7860–7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Ebinuma H, Nakamoto N, Li Y, Price DA, Gostick E, Levine BL et al. Identification and in vitro expansion of functional antigen-specific CD25FoxP3 regulatory T cells in hepatitis C virus infection. J Virol 2008; 82: 5043–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Gale M Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature 2005; 436: 939–945. [DOI] [PubMed] [Google Scholar]
- 17Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 2008; 454: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Machida K, Cheng KT, Sung VM, Levine AM, Foung S, Lai MM. Hepatitis c virus induces Toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol 2006; 80: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M et al. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol 2006; 177: 6758–6768. [DOI] [PubMed] [Google Scholar]
- 20Fernandez-Ponce C, Dominguez-Villar M, Aguado E, Garcia-Cozar F. CD4+ primary T cells expressing HCV-core protein upregulate Foxp3 and IL-10, suppressing CD4 and CD8 T cells. PLoS One 2014; 9: e85191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Doumba PP, Serti E, Boutsikou M, Konstadoulakis MM, Georgopoulou U, Koskinas J. Phenotypic and functional alterations of primary human PBMCs induced by HCV non-enveloped capsid-like particles uptake. Cell Mol Life Sci 2013; 70: 3463–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Sundstrom S, Ota S, Dimberg LY, Masucci MG, Bergqvist A. Hepatitis C virus core protein induces an anergic state characterized by decreased interleukin-2 production and perturbation of mitogen-activated protein kinase responses. J Virol 2005; 79: 2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Kittlesen DJ, Chianese-Bullock KA, Yao ZQ, Braciale TJ, Hahn YS. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J Clin Invest 2000; 106: 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Cummings KL, Rosen HR, Hahn YS. Frequency of gC1qR+CD4+ T cells increases during acute hepatitis C virus infection and remains elevated in patients with chronic infection. Clin Immunol 2009; 132: 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Dominguez-Villar M, Fernandez-Ponce C, Munoz-Suano A, Gomez E, Rodríguez-Iglesias M, Garcia-Cozar F. Up-regulation of FOXP3 and induction of suppressive function in CD4-Jurkat T-cells expressing hepatitis C virus core protein. Clin Sci 2012; 123: 15–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.