Abstract
FTY720, an agonist for four of the five known sphingosine-1-phosphate (S1P) receptors, has been reported to inhibit acute graft-versus-host disease (aGVHD). Because FTY720 functions through multiple S1P receptors, the mechanism of action through one or more of these receptors may account for its side effects. Thus, more selective S1P receptor modulators are needed to evaluate the roles of different S1P receptors and their therapeutic efficacies. In this study, we investigated the effect of an S1P1-selective agonist, CYM-5442, on the progression of aGVHD. We showed that CYM-5442 significantly inhibited but did not prevent aGVHD. CYM-5442 did not affect the infiltration of the donor T cells into the target organs, while the number of macrophages in GVHD organs was significantly reduced by CYM-5442 treatment. In vivo proliferation assays showed that the proliferation of macrophages was not suppressed by CYM-5442. Further studies using human endothelial cells demonstrated that CYM-5442 treatment downregulated CCL2 and CCL7 expression in endothelial cells, therefore reducing the migration of monocytes, from which tissue macrophages originate. Our data demonstrate the therapeutic efficacy of an S1P1-selective agonist in aGVHD and its possible mechanism of action. The results suggest that further investigations are needed regarding CYM-5442 as a potential therapeutic regimen for aGVHD.
Keywords: S1P, S1P receptor agonist, Monocyte/MacrophageI, Chemokine
Introduction
Acute graft-versus-host disease (aGVHD) is one of the major complications of allogeneic hematopoietic stem cell transplantation. It is induced by donor T cells and inflammatory cytokines causing damage to host epithelial tissues, predominantly the skin, liver and gastrointestinal tract.1,2,3 Despite pharmacological prophylaxis, about half of the patients receiving allogeneic hematopoietic stem cell transplantation experience severe aGVHD, which requires high-dose steroid treatment and often has a highly unsatisfactory outcome. Therefore, an immunomodulatory regimen is urgently needed for aGVHD prevention and treatment.
The bioactive lipid sphingosine-1-phosphate (S1P) is involved in various cellular responses such as cell proliferation, platelet aggregation, endothelial cell chemotaxis, and lymphocyte trafficking.4,5,6,7 S1P is mainly produced by erythrocytes and endothelial cells. S1P binds to five known G protein-coupled receptors, S1P1–5, and acts as a second messenger during cell signaling.8,9 Most S1P-dependent immune modulations were discovered using the receptor agonist FTY720.10,11 The binding of FTY720 to S1P1, 3, 4, 5 induces receptor internalization and results in functional antagonism.9,12,13 FTY720 has been shown to be a promising immunosuppressive agent for the treatment of autoimmune disease, promotion of solid organ engraftment and inhibition of aGVHD.8,14,15,16,17,18,19,20,21 However, the mechanism underlying the action of FTY720 is not completely known. Clinically, FTY720 mediates both lymphopenia and transient dose-dependent bradycardia on initial dosing in humans.22 Because FTY720 acts through multiple S1P receptors, the mechanism of action through one or more of these receptors may account for its side effects. Thus, more selective S1P receptor modulators are needed to evaluate the roles of the different S1P receptors and their therapeutic efficacy.
It has been suggested that the immunosuppression and lymphocyte sequestration caused by FTY720 is primarily mediated by S1P1.4,5,9,23,24,25 CYM-5442 (CYM) is an S1P1-selective agonist, specifically a sphingosine-like fungal metabolite.24 CYM is a full agonist in vitro for S1P1 internalization and phosphorylation. In vivo, it induces S1P1-dependent lymphopenia in a manner dependent on both dose and time. CYM is modestly orally bioavailable, with a half-life of 3 h. Although S1P1 is expressed on both endothelial cells and lymphocytes, S1P1 signaling in endothelial cells has been shown to be essential for cytokine storms that induce mortality during influenza infection.
Macrophages are recruited from the blood to sites of inflammation by chemokine gradients. CCL2 (MCP-1) and CCL7 (MCP-3) are the primary chemokines that regulate monocyte recruitment in response to inflammation. S1P might also be involved in macrophage trafficking.3,26,27,28,29 FTY720 reduces macrophage infiltration into sites of inflammation in many inflammatory disease models.30,31,32,33 The mechanism of action of FTY720 may involve its effects on endothelial cells to reduce monocyte migration.34
This study shows that the S1P1-selective agonist CYM inhibited aGVHD by reducing macrophage infiltration at GVHD sites. CYM may act directly on endothelial cells to affect CCL2 and CCL7 production. These results provide evidence for the therapeutic potential of using S1P1-selective agonists in treating aGVHD and elucidate the importance of endothelial cells in mediating monocyte migration during CYM treatment.
Materials and methods
Mice
BALB/c (H2d) and C57BL/6 (H2b) mice were purchased from SLAC Laboratory Animal Center (Shanghai, China). Mice were housed in a specific pathogen-free facility and were used at the age of 8–12 weeks. All animal protocols were approved by the Ethical Committee of Soochow University. All invasive procedures were performed under isofurane anesthesia, and efforts were made to minimize suffering at all times. Animals were sacrificed in their home cage by CO2 inhalation, followed by exsanguination by cardiac puncture to obtain blood. All other tissues were harvested in a sterile hood following cardiac puncture.
Reagents and cell lines
CYM (2-(4-(5-(3,4-diethoxyphenyl)-1,2,4-oxadiazol-3-yl)-2,3-dihydro-1H-inden-1-yl amino) ethanol) and RS102895 were purchased from Sigma-Aldrich (St Louis, MO, USA). FTY720 (2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol, hydrochloride) was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Human umbilical vein endothelial cells (HUVECs) were provided by Dr Quansheng Zhou (Cyrus Tang Hematology Center, Soochow University). HUVECs were cultured with Delbecoo's modified eagle media (DMEM) containing 10% fetal bovine serum (FBS).
Establishment of an aGVHD model
On day 0, BALB/c (H2d) recipients were lethally irradiated with 750 cGy total body irradiation from a 60Co source and were infused with 1×107 C57BL/6 (H2b) bone marrow cells and 5×106 whole splenocytes 4 h later. CYM (3 mg/kg),5,27,28 control water, or FTY720 was intraperitoneally injected daily from day −1 to day 3 post allogeneic bone marrow transplantation (BMT). Ten mice per group were monitored daily for survival, were weighed every 3 days, and were thereafter examined for the clinical characteristics of aGVHD. The degree of systemic GVHD was assessed by a scoring system that sums changes in five clinical parameters: weight loss, posture (hunching), activity, fur texture and skin integrity.35
Histology
Livers, lungs and small intestines were harvested from mice 4 days post allo-BMT and were fixed in 10% paraformaldehyde. Livers were perfused before harvesting. Paraffin-embedded fixed tissues were sectioned at 5 µm thickness and were stained with hematoxylin and eosin. Pictures of hematoxylin and eosin sections were acquired in an Olympus upright fluorescence microscope (Tokyo, Japan). Magnification was ×200 or ×400.
Flow cytometry
Mice were killed 4 days after allo-BMT, and spleens, livers, lungs and small intestines of each mouse treated with CYM or the control reagent (six mice per group) were harvested. Livers were perfused before harvesting. Single-cell suspensions were prepared, and red blood cells were lysed. All samples were incubated with Mouse Fc block (anti-mouse CD16/32; BD Biosciences, San Diego, CA, USA) for 10 min and were then stained with the following mouse-specific monoclonal antibodies (BD Biosciences): FTTC-conjugated CD69, PE-conjugated H2Kb, PE-TexasRed-conjugated CD3, PerCPCy5.5-conjugated NK1.1, APC-conjugated CD8, APC-H7-conjugated CD4, FTTC-conjugated H2Kb, PE-conjugated CD11b, PE-TexasRed-conjugated CD3, PerCPCy5.5-conjugated Gr-1, PE-Cy7-conjugated CD11c, PerCPCy5.5-conjugated F4/80.
Intracellular Staining: Single cell suspensions of spleens, livers, lungs and small intestines were prepared and incubated with 1-Methoxy-2-propylacetate (PMA) (50 ng/ml), Iomomycin (500 ng/ml) and brefeldin (BFA) (10 µg/ml) at 37°C and 5% CO2 for 4 h. Suspensions were then stained with PE-conjugated anti-CD4 antibody (BD Biosciences) for 30 min at 4 °C. After the wash with phosphate buffered saline (PBS), the cells were fixed with 4% paraformaldehyde and permeabilized with 1% saponin (Sigma-Aldrich) and were then stained with the following mouse-specific monoclonal antibodies: FTTC-conjugated IL-17A, APC-conjugated IL-4, APC-conjugated IFN-γ or control isotype antibody. Samples were acquired in a FACS Canto II (BD Biosciences) equipped with a 488 nm argon laser and a 635 nm red diode laser. Data were analyzed with FlowJo Software (FlowJo, Ashland, OR, USA).
In vivo cell proliferation assay
Mice were intraperitoneally injected with EdU 24 h before the analysis. Single-cell suspensions of lymphocytes isolated from spleens, livers, lungs and small intestines were incubated with Mouse Fc block (anti-mouse CD16/32; BD Biosciences) for 10 min and then stained with the following mouse-specific monoclonal antibodies (BD Biosciences): FTTC-conjugated H2Kb, PerCPCy5.5-conjugated Gr-1 and APC-H7-conjugated CD4. Then, the suspensions were incubated with Click-iT EdU buffer according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). The cells were then stained with the following mouse-specific monoclonal antibodies (BD Biosciences): PE-conjugated CD11b, PE-TexasRed-conjugated CD3 and PE-Cy7-conjugated CD11c. The cells were washed twice and resuspended with PBS containing 1% FBS. Samples were acquired in a FACS Canto II (BD Biosciences) equipped with a 488 nm argon laser and a 635 nm red diode laser. Data were analyzed with FlowJo Software (FlowJo).
Adoptive transfer of macrophages
Macrophages from bone marrow of donor C57BL/6 mice were sorted and labeled with carboxy fluorescein succinimidyl amino ester (CFSE) (Invitrogen) according to the manufacturer's instructions. Macrophages labeled with CFSE were adoptively transferred into mice treated with CYM or control mice. After 30 h, single-cell suspensions of lymphocytes were isolated from livers, lungs and small intestines, and the samples were acquired in a FACS Canto II (BD Biosciences). Data were analyzed with FlowJo Software (FlowJo).
Real-time PCR
HUVECs were plated at 5×106 per well in six-well plates and were then cultured overnight. The cells were treated with CYM (0.1 µM or 1 µM final concentration) or an equal volume of dimethyl sulfoxide (DMSO) for 24 h. Then, the cells were washed twice with PBS, and total RNA was extracted using TRizol (Invitrogen) according to the manufacturer's instructions. The total RNA was reverse transcribed to prepare cDNA. The mRNA levels of CCL2, CCL7, CXCL1 and CXCL5 were detected by real-time PCR using Power SYBR Green Master Mix (Applied Biosystems, Warrington, UK). β-actin was simultaneously amplified as an internal reference. The primer sequences are as follows: CCL2 forward: agtgtcccaaagaagctgtg; CCL2 reverse: gattcttgggttgtggagtg; CCL7 forward: ttttggtgggttttgaacat, CCL7 reverse: tgcttccatagggacatcat; CXCL1 forward: ttgaaatgtcaaccccaagt, CXCL1 reverse: cctgccttcacaatgatctc; CXCL5 forward: atccagaagccccttttcta and CXCL5 reverse: gaggaatccaggaagaaagc.
Transwell assay
Peripheral blood mononuclear cells (PBMCs) was isolated from the peripheral blood by Ficoll, washed twice with 1× PBS and stained with anti-CD14 monoclonal antibody at 4 °C for 30 min. After washing, CD14+ cells were sorted by FACS Aria (BD Bioscience) . Sorted monocytes were incubated with 10 ng/ml or 100 ng/ml RS102895, or with DMEM at 37 °C and 5% CO2 for 30 min; then, the monocytes were washed with DMEM three times. The monocytes (5×105/200 µl) were added to the upper wells. Five hundred microliters of supernatant of HUVECs treated for 24 h with CYM (1 µM final concentration) or with an equal volume of DMSO was plated in the lower wells. The plate was incubated at 37 °C for 6–8 h, and the cells that migrated to the lower wells were then counted and analyzed.
Serum cytokine measurement
Serum was collected from mice on day 4 after allo-BMT. Serum cytokine levels, including IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ and TNF, were measured using Mouse Th1/Th2/Th17 CBA Kits (BD Bioscience), according to the manufacturer's instructions. Samples were acquired with a FACS Canto II (BD Biosciences), and the data were analyzed with FlowJo Software (FlowJo). Serum levels of CCL2 and CCL7 were measured using the mouse MCP-1/CCL2 ELISA kit (Biolegend, San Diego, CA, USA) and the mouse MCP-3/CCL7 ELISA kit (R&D, Piscataway, NJ, USA) according to the manufacturer's instructions.
Statistical analysis
Survival data were analyzed by a log-rank test. Group comparisons were made with a two-tailed Student's t-test. Data were analyzed using GraphPad Prism 5 software for Windows (GraphPad Software, San Diego, CA, USA), and the differences were considered statistically significant when P<0.05.
Results
CYM-5442 inhibits but does not prevent aGVHD
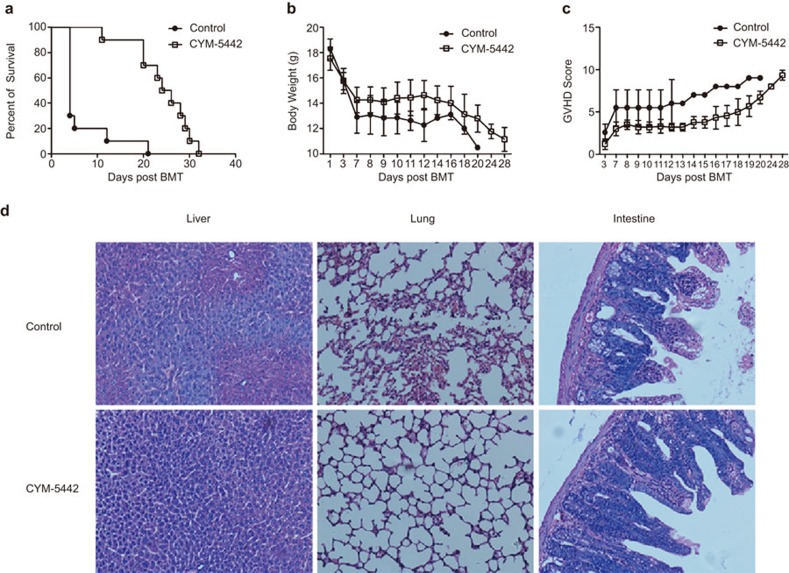
To assess the effect of the S1P1 selective agonist CYM on aGVHD progression, we used a major MHC-mismatched allogeneic BMT model (B6→BALB/c) and transplanted lethally irradiated BALB/c mice with 1×107 C57BL/6 TCD-BM cells and whole spleen cells (5×106). CYM (3 mg/kg) or control reagent was intraperitoneally injected once daily from day −1 to day 3 post bone marrow transplantation. CYM treatment dramatically prolonged the survival of GVHD mice compared with control-treated mice (Figure 1a; P<0.0001). An improved therapeutic effect was observed with CYM treatment compared with FTY720 treatment at the dose of 3 mg/kg in our model (Supplementary Figure 1). Body weight loss of GVHD mice and GVHD scores were also reduced by administration of CYM (Figure 1b and c). However, all the CYM-treated mice eventually died from aGVHD. On day 4 post allogeneic BMT, the target organs in control mice showed typical characteristics of aGVHD, such as incomplete intestinal villus epithelial structure and epithelial cell shedding (Figure 1d). In contrast, the target organs of CYM-treated mice showed reduced GVHD characteristics. Therefore, CYM treatment could significantly prolong the survival of aGVHD mice, but could not completely prevent the incidence of aGVHD.
Figure 1.
CYM inhibits but does not prevent aGVHD. (a) CYM treatment could dramatically prolong the survival of GVHD compared with control treatment in mice (n=10 per group; ***P<0.0001). (b, c) Body weight loss of GVHD mice and GVHD scores (P<0.01) were reduced by administration of CYM. (d) CYM administration dramatically reduced GVHD characteristics of target organs (×400, liver; ×200, lung; ×200, intestine). The data are representative of three independent experiments, each using 10 mice per group. Data are shown as the mean±s.e.m. aGVHD, acute graft-versus-host disease.
CYM does not affect the infiltration of donor T cells to the GVHD target organs, but reduces their activation
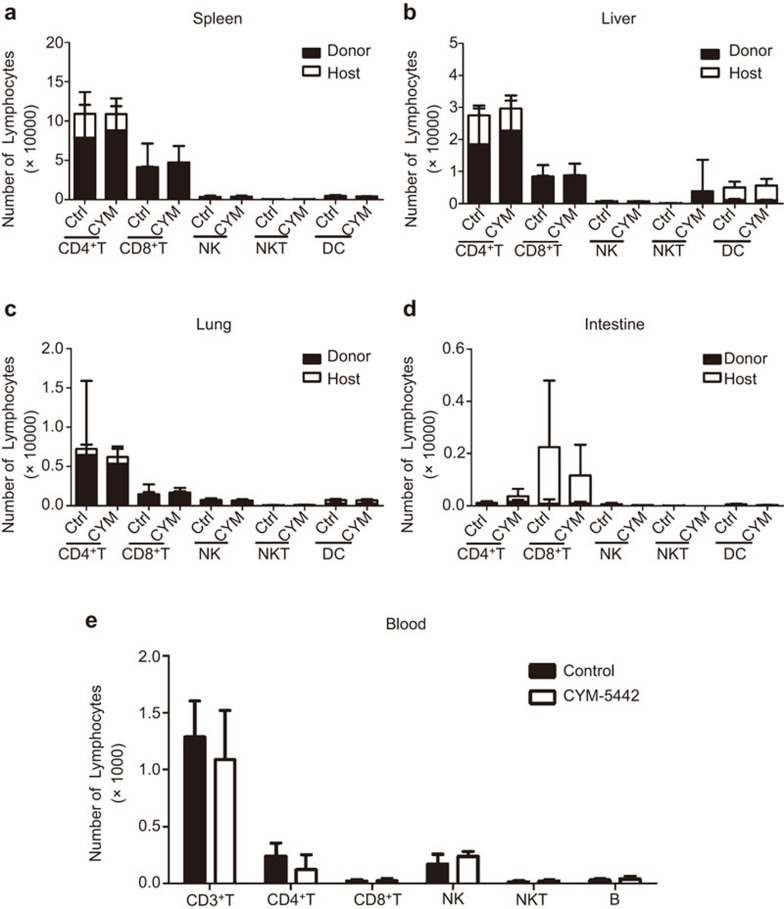
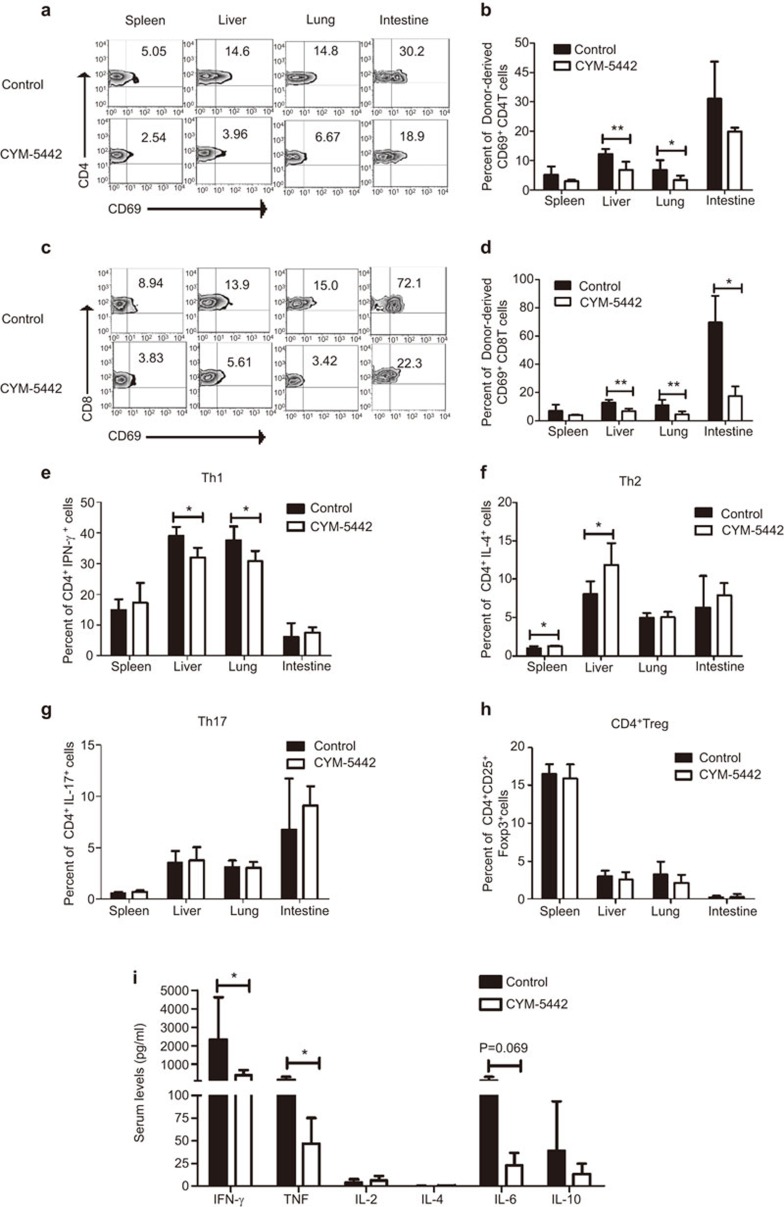
To determine whether CYM treatment could affect donor lymphocyte infiltration to the GVHD target organs, we examined the number of donor- and host-derived CD4+ T cells, CD8+ T cells, natural killer (NK) cells, natural killer K (NKT) cells and dendritic cells (DCs) in the spleen, liver, lung and intestine on day 4 post BMT (Figure 2a–d, Supplemental Figure 2). In all the GVHD target organs, there was no difference in the number of donor- or host-derived cells with or without CYM treatment. Therefore, CYM did not affect the infiltration of donor T cells, NK cells, NKT cells or DCs. The immunohistochemistry staining for infiltrating T cells in the target organs showed similar results (data not shown). The blood counts of CD4+ T cells, CD8+ T cells, B cells, NK cell and NKT cells were not significantly changed by CYM treatment (Figure 2e). However, the expression of the early activation marker CD69 was significantly decreased by CYM treatment on both CD4+ (Figure 3a and b) and CD8+ (Figure 3c and d) T cells. Under CYM treatment, the Th1 response was significantly decreased in the liver and lung (Figure 3e), and the percent of Th2 cells was increased in the spleen and liver (Figure 3f). The percent of Th17 (Figure 3g) and regulatory T (Figure 3h) cells was not affected by CYM treatment. Moreover, the serum levels of IFN-γ, TNF and IL-6 were also reduced (Figure 3i). Our results demonstrated that the infiltration of the donor-derived T cells to the GVHD target organs was not affected, while the activation and Th1 responses of the donor-derived T cells may be hampered by CYM treatment.
Figure 2.
CYM does not affect the infiltration of the donor T cells to the GVHD target organs. (a–d) The number of donor- and host-derived CD4+ T cells, CD8+ T cells, NK cells, NKT cells and DCs in the spleen, liver, lung and intestine day 4 post BMT. Donor-derived cells were identified as H2Kb+ cells, and host-derived cells were identified as H2Kd+ cells; the cell subsets were analyzed by CD3+CD4+ for CD4+ T cells, CD3+CD8+ for CD8+ T cells, CD3−NK1.1+ for NK cells, CD3+NK1.1+ for NKT cells and CD11c+ for DCs. (e) The number of CD4+ T cells, CD8+ T cells, NK cells, NKT cells and B cells in blood day 4 post BMT. The data are representative of three independent experiments, each using 4–5 mice per group. Data are shown as the mean±s.e.m. BMT, bone marrow transplantation; DC, dendritic cell; GVHD, graft-versus-host disease; NK, natural killer; NKT, natural killer T.
Figure 3.
CYM reduces the activation of donor T cells at the GVHD target organs. (a–d) CD69 expression was significantly decreased by CYM treatment on both CD4+ (a, b) and CD8+ T cells (c, d). (e–h)The percentages of Th1, Th2, Th17 and Treg cells in the spleen, liver, lung and intestine were determined using intracellular staining after CYM treatment. (i) The serum levels of IFN-γ, TNF, IL-2, IL-4, IL-6 and IL-10 were measured. The data are representative of three independent experiments, each using 4–5 mice per group. Data are shown as the mean±s.e.m. (*P<0.05, **P<0.01). GVHD, acute graft-versus-host disease; Treg, regulatory T.
CYM significantly reduces the number of macrophages in the aGVHD target organs without directly affecting their proliferation
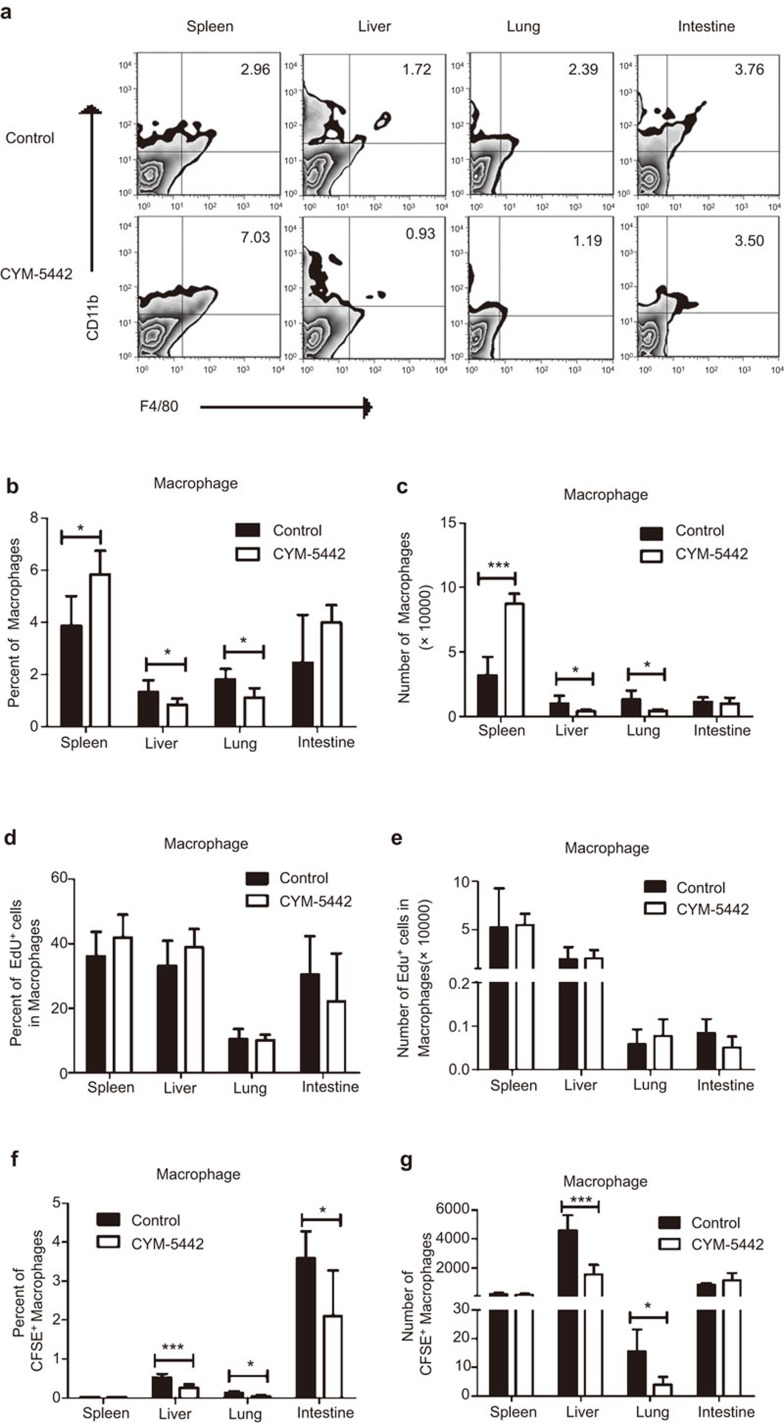
Myeloid cells are reported to play an important role in the early immune response of GVHD mice.29 We also monitored the changes of myeloid cells in the GVHD target organs with and without CYM treatment. As with the DCs shown in Figure 2, neutrophil numbers in the GVHD target organs were not affected by CYM treatment (data not shown). Interestingly, both the percent and number of macrophages were significantly reduced by CYM treatment in the liver and lung, but increased in the spleen on day 4 post BMT (Figure 4a–c). The percent and number of macrophages in the intestine were not affected by CYM treatment. These results suggest that the migration of macrophages from the lymphoid organ to the GVHD target organs, including the liver and lung, was inhibited by CYM. To further determine whether the decreased number of macrophages was caused by a direct effect of CYM on macrophage proliferation, the in vivo EdU assay was performed. CYM showed no effect on both the percentage and number of EdU+ macrophages, suggesting the proliferation of macrophages was not inhibited by CYM treatment (Figure 4d and e). To further determine whether the migration of macrophages to the GVHD target organs were inhibited by CYM treatment, CFSE-labeled bone marrow macrophages were adoptively transferred into CYM-treated and control mice (Figure 4f and g). After 30 h, the transferred macrophages were analyzed in the spleen, liver, lung and intestine. The percent and number of transferred macrophages in the spleen were similar for CYM-treated and control mice. However, the percent of transferred macrophages was significantly lower in all of the GVHD target organs examined for CYM-treated mice compared with the control mice. The numbers of transferred macrophages were also significantly lower in the liver and lung of CYM-treated mice compared with the control mice. Therefore, the results indicate that treatment with the S1P1-selective agonist CYM reduced macrophage migration to GVHD target organs.
Figure 4.
CYM reduces the number of macrophages in the aGVHD target organs without directly affecting their proliferation. (a–c) Both percent and number of macrophages were analyzed in the spleen, liver, lung and intestine after CYM treatment day 4 post BMT. CD11b+F4/80+ cells were identified as macrophages. (d, e) The proliferation of macrophages was analyzed by an in vivo EdU assay, and both the percentage and number of EdU+ macrophages were not affected by CYM treatment. (f, g) The percent and number of transferred CFSE-labeled macrophages were analyzed in the spleen, liver, lung and intestine after CYM treatment day 4 post BMT. The data are representative of three independent experiments, each using 4–5 mice per group. Data are shown as the mean±s.e.m. (*P<0.05, ***P<0.005). BMT, bone marrow transplantation; aGVHD, acute graft-versus-host disease.
CYM inhibits monocyte migration by reducing the production of CCL2 and CCL7 by endothelial cells
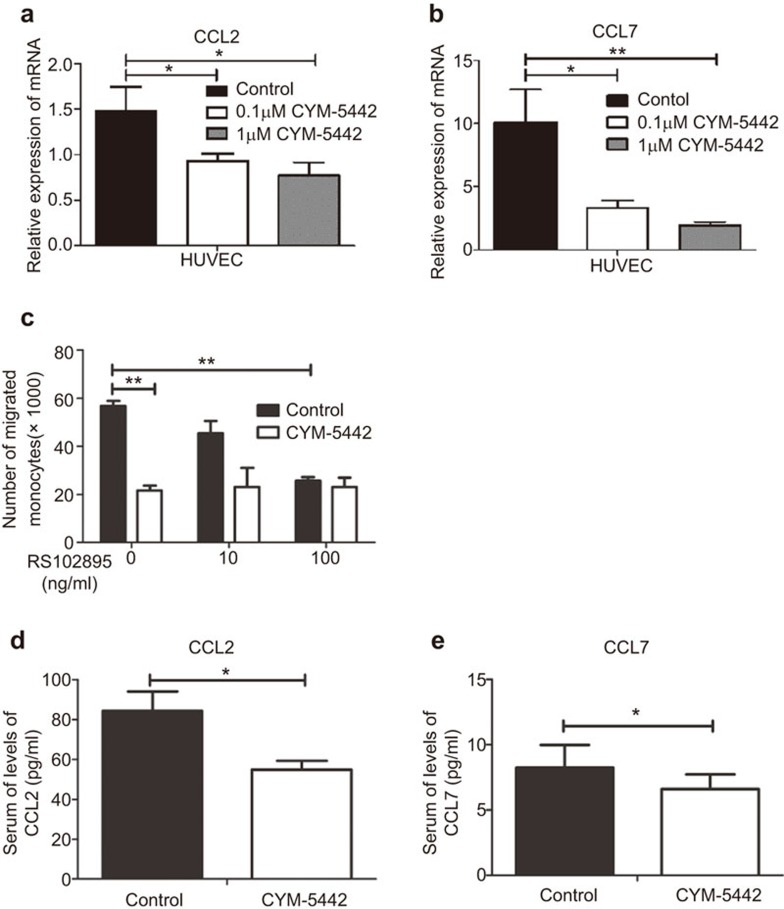
It has been shown that endothelial cells are the key regulators of cytokine storm and innate cell migration to inflammatory sites.5 A potential mechanism by which CYM inhibits macrophage migration to the GVHD target organs is by reducing chemokine production by endothelial cells. To test this hypothesis, we examined the chemokine production of the human endothelial cell line HUVEC, which expressed S1P1 after CYM treatment. Both 0.1 µM and 1 µM of CYM significantly downregulated the mRNA expression of CCL2 and CCL7 in HUVECs (Figure 5a and b). However, two other chemokines that could affect monocyte migration, CXCL1 and CXCL5, were not affected by CYM treatment (Supplementary Figure 3a and b). Another human endothelial cell line that also expresses S1P1, HLMVEC, showed similar results (data not shown).
Figure 5.
CYM inhibits monocyte migration by reducing the production of CCL2 and CCL7 by endothelial cells. (a, b) HUVECs were treated by 0.1 µM or 1 µM CYM or an equal volume of DMSO for 24 h, and mRNA expressions of CCL2 and CCL7 were analyzed. (c) Monocytes from the peripheral blood were sorted and treated with the CCR2 antagonist RS102895 or control media. They were placed in the upper wells in the transwell assay with CYM-treated or control-treated HUVEC supernatant placed in the lower wells. (d, e) The serum levels of CCL2 and CCL7 were analyzed day 4 post BMT. The data are representative of five independent experiments. Data are shown as the mean±s.e.m. (*P<0.05, **P<0.01). BMT, bone marrow transplantation; HUVEC, human umbilical vein endothelial cell.
To determine whether CCL2 and CCL7 could be the key chemokines affected by CYM to reduce monocyte migration through interaction with their receptor CCR2, we sorted monocytes from the peripheral blood and incubated them with or without the CCR2 antagonist RS102895 for 30 min at 37 °C before the transwell assay. Without RS102895 treatment, the number of monocytes that migrated into the lower wells was significantly decreased when the CYM-treated HUVEC supernatant was placed in the lower wells compared with the control-treated HUVEC supernatant (Figure 5c). This result demonstrates that CYM significantly suppressed the chemoattractant ability of endothelial cells for monocyte migration. With RS102895 treatment, monocyte migration to the lower wells with control-treated HUVEC supernatant was significantly reduced in a dose-dependent manner, eventually to a level similar to that when CYM-treated HUVEC supernatant was used. RS102895 treatment did not affect monocyte migration with the CYM-treated HUVEC supernatant, suggesting that the interaction between CCR2 and its ligands was already blocked with CYM treatment. To confirm this finding in vivo, we measured the serum levels of CCL2 and CCL7 in CYM-treated and control mice (Figure 5d and e). Both CCL2 and CCL7 levels were decreased by CYM treatment. These results indicate that the S1P1-selective agonist CYM inhibited CCL2 and CCL7 production by the endothelial cells and therefore reduced both the migration of monocytes through the blood vessels and the infiltration of macrophages to the GVHD target organs.
Discussion
The recruitment of innate immune cells to the GVHD organs and the excessive production of proinflammatory cytokines and chemokines are hallmarks of the initial stage of aGVHD.36 This study demonstrates that the S1P1-selective agonist CYM could inhibit aGVHD by preventing macrophage recruitment to the GVHD target organs. Our results also indicate that CYM could modulate chemokine production by endothelial cells, thereby affecting monocyte migration. This is the first report on the role of an S1P1-selective agonist in aGVHD, and our data indicate that the S1P1 receptor on endothelial cells might be critical for monocyte recruitment during the initial stage of aGVHD.
The S1P1-selective agonist CYM has been shown to induce reversible lymphopenia and ameliorate the murine model of multiple sclerosis.37 However, in the model of atherosclerosis in Low-density lipoprotein (LDL) receptor-deficient mice, FTY720 lowered blood lymphocytes while CYM did not, suggesting that these two S1P receptor agonists may have different mechanisms.38 In our model of aGVHD, CYM did not induce obvious lymphopenia. We did not observe any effect of CYM on the infiltration of donor T cells to the aGVHD organs at an early stage of the disease. Although it has been suggested that FTY720 may modulate lymphocyte trafficking, especially donor-derived T-cell infiltration into target organs, to inhibit aGVHD,20,39,40 another recent study demonstrated that FTY720 did not inhibit donor effector T-cell trafficking into the aGVHD target organs.41 The same report also showed FTY720 reduced the number of DCs in the target organs, which has been suggested to be the mechanism underlying aGVHD inhibition. We did not observe any changes in DCs in the GVHD target organs with CYM treatment. However, CYM treatment was associated with significantly fewer macrophages in GVHD target organs. The difference in the number of DCs induced by FTY720 treatment may not be due to a blockade of the S1P1 receptor, and the numbers of macrophages were not noted specifically in that study. Both FTY720 and CYM were found to reduce the activity of splenic and peritoneal macrophages in the murine atherosclerosis model,38 suggesting that S1P1 signaling could be critical to macrophage activity. In this study, there were more macrophages in the spleen, but their migration to the GVHD target organs was inhibited by CYM treatment. Because the hematopoietic reconstitution is far from complete at the early time points after hematopoietic stem cell transplantation, the numbers of macrophages are very low at the GVHD target organs. Although the percent and number of macrophages showed only slight reductions in the liver and lung after CYM treatment, the differences were statistically significant. Bone marrow macrophages from CYM-treated mice showed even higher levels of tumor necrosis factor (TNF) and IL-6 production upon Lipopolysaccharides (LPS) stimulation than those from the control mice (our unpublished data). This result suggests that the function of macrophages was not suppressed systemically by CYM treatment. The increased cytokine release from the bone marrow macrophages after stimulation may even suggest reduced migration of the activated macrophages into the GVHD organs. Because the number of infiltrating macrophages was very low, it was difficult to sort out enough cells to perform functional assays. Future studies are needed to determine the function of the infiltrating macrophages in the GVHD target organs.
Macrophage infiltration into target organs has been shown to be an important event during aGVHD.42,43 One retrospective clinical study investigated the relationship between the types of infiltrating cells and the clinical outcomes of GVHD.44 The number of macrophages that infiltrated skin lesions was shown to be a significant predictive factor for refractory GVHD and a poor prognosis, but the number of T cells was not. Macrophages may function as antigen-presenting cells during the initial stage of aGVHD, but also to provide pro-inflammatory cytokines to initiate the alloreactive adaptive immune response. Human monocytes express S1P receptors 1, 2 and 4, and human macrophages express S1P receptors 1, 2, 3 and 4.45 An S1P1-selective agonist may act directly on macrophage proliferation and trafficking.26,27,28,29,46 An in vivo proliferation assay demonstrated that CYM did not affect macrophage proliferation. Increasing amounts of data have indicated that the lower number of macrophages in the inflamed tissues of FTY720-treated mice could be an indirect effect of vascular permeability and monocyte recruitment.
CYM inhibited but did not prevent aGVHD when given on day −1 to day 3 post transplantation. Because of the short half-life of CYM, continuous administration could result in better efficacy. Because early events of cell trafficking are critical for aGVHD pathogenesis, we performed all of the analysis on day 4, one day after the last CYM treatment. FTY720 has also been shown to only inhibit aGVHD,41 similar to our results. Additionally, the same study proposes that the primary function of FTY720 may be targeted to host cells, as indicated by the fact that FTY720 treatment prior to transplantation inhibited GVHD, but continuous administration for up to 28 days after transplantation did not significantly prolong survival. This could also be the case for CYM. However, further investigation must be performed before a more effective therapeutic schedule can be developed.
Some S1P receptor agonists, including the S1P1-selective agonist KRP203, are currently in clinical trials for the treatment of autoimmune disease.47 SEW2871, a selective S1P1 agonist, has been shown to be effective in protecting kidneys against ischemia–reperfusion injury by reducing CD4+ T-cell infiltration in mice.48 AUY954, which selectively targets S1P1, is known to sequester lymphocytes into secondary lymphoid tissues. In experimental autoimmune neuritis rats, AUY954 greatly prevented paraparesis if administered from the day of immunization.49 Therefore, a study of the role of other selective S1P1 agonists in acute GVHD is warranted in the future. Moreover, silencing S1P1 expression using other methods including siRNA/shRNA may further confirm the critical role of S1P1 during the pathogenesis of acute GVHD. Binding of S1P receptor agonists, including FTY720 and CYM, to S1P receptors causes internalization and may cause functional antagonism, persistent signaling or both.12,50,51 The ways in which internalization, degradation, and sustained signaling collectively regulate S1P receptor function are not yet understood. Further studies are needed to fully elucidate the molecular mechanism by which the S1P agonists act.
In summary, the current work demonstrates that the S1P1-selective agonist CYM can inhibit aGVHD and provides insight into the mechanism of action. CYM may provide a more specific clinical strategy than FTY720 for controlling aGVHD.
Acknowledgments
This work has been supported by the grants from the National Natural Science Foundation of China (91029703, 81072436 and 81273268), with project funding from Suzhou City (SWG0904, SZS201109), Priority Academic Program Development of Jiangsu Higher Education Institutions, Qing Lan Project of Jiangsu Province, Jiangsu Provincial Innovative Research Team and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1075).
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- 1Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol 2006; 43: 3–10. [DOI] [PubMed] [Google Scholar]
- 2Lee PY, Li Y, Kumagai Y, Xu Y, Weinstein JS, Kellner ES et al. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am J Pathol 2009; 175: 2023–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol 2007; 7: 340–352. [DOI] [PubMed] [Google Scholar]
- 4Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 2004; 279: 15396–5401. [DOI] [PubMed] [Google Scholar]
- 5Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004; 427: 355–360. [DOI] [PubMed] [Google Scholar]
- 6Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol 2008; 8: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 2011; 146: 980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002; 277: 21453–21457. [DOI] [PubMed] [Google Scholar]
- 9Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002; 296: 346–349. [DOI] [PubMed] [Google Scholar]
- 10Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett 2003; 554: 189–193. [DOI] [PubMed] [Google Scholar]
- 11Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, Urtz N et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood 2006; 107: 1454–1458. [DOI] [PubMed] [Google Scholar]
- 12Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA 2011; 108: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Rosen H, Liao J. Sphingosine 1-phosphate pathway therapeutics: a lipid ligand-receptor paradigm. Curr Opin Chem Biol 2003; 7: 461–468. [DOI] [PubMed] [Google Scholar]
- 14Brinkmann V. FTY720: mechanism of action and potential benefit in organ transplantation. Yonsei Med J 2004; 45: 991–997. [DOI] [PubMed] [Google Scholar]
- 15Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 2010; 9: 883–897. [DOI] [PubMed] [Google Scholar]
- 16Brinkmann V, Wilt C, Kristofic C, Nikolova Z, Hof RP, Chen S et al. FTY720: dissection of membrane receptor-operated, stereospecific effects on cell migration from receptor-independent antiproliferative and apoptotic effects. Transplant Proc 2001; 33: 3078–3080. [DOI] [PubMed] [Google Scholar]
- 17Chiba T, Yokosuka O, Goto S, Fukai K, Imazeki F, Kohno Y et al. Clinicopathological features in patients with hepatic graft-versus-host disease. Hepatogastroenterology 2005; 52: 1849–1853. [PubMed] [Google Scholar]
- 18Hashimoto D, Asakura S, Matsuoka K, Sakoda Y, Koyama M, Aoyama K et al. FTY720 enhances the activation-induced apoptosis of donor T cells and modulates graft-versus-host disease. Eur J Immunol 2007; 37: 271–281. [DOI] [PubMed] [Google Scholar]
- 19Kataoka H, Sugahara K, Shimano K, Teshima K, Koyama M, Fukunari A et al. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol 2005; 2: 439–448. [PubMed] [Google Scholar]
- 20Kim YM, Sachs T, Asavaroengchai W, Bronson R, Sykes M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with FTY720. J Clin Invest 2003; 111: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Yopp AC, Ledgerwood LG, Ochando JC, Bromberg JS. Sphingosine 1-phosphate receptor modulators: a new class of immunosuppressants. Clin Transplant 2006; 20: 788–795. [DOI] [PubMed] [Google Scholar]
- 22Budde K, Schmouder RL, Nashan B, Brunkhorst R, Lücker PW, Mayer T et al. Pharmacodynamics of single doses of the novel immunosuppressant FTY720 in stable renal transplant patients. Am J Transplant 2003; 3: 846–854. [DOI] [PubMed] [Google Scholar]
- 23Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G et al. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther 2004; 309: 758–768. [DOI] [PubMed] [Google Scholar]
- 24Gonzalez-Cabrera PJ, Jo E, Sanna MG, Brown S, Leaf N, Marsolais D et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol 2008; 74: 1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med 2005; 201: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G et al. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res 2011; 108: 314–323. [DOI] [PubMed] [Google Scholar]
- 27Massberg S, von Andrian UH. Fingolimod and sphingosine-1-phosphate—modifiers of lymphocyte migration. N Engl J Med 2006; 355: 1088–1091. [DOI] [PubMed] [Google Scholar]
- 28Tolle M, Levkau B, Keul P, Brinkmann V, Giebing G, Schonfelder G et al. Immunomodulator FTY720 Induces eNOS-dependent arterial vasodilatation via the lysophospholipid receptor S1P3. Circ Res 2005; 96: 913–920. [DOI] [PubMed] [Google Scholar]
- 29Tolle M, Pawlak A, Schuchardt M, Kawamura A, Tietge UJ, Lorkowski S et al. HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arterioscler Thromb Vasc Biol 2008; 28: 1542–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther 2003; 305: 70–77. [DOI] [PubMed] [Google Scholar]
- 31Martini S, Kramer S, Loof T, Wang-Rosenke Y, Daig U, Budde K et al. S1P modulator FTY720 limits matrix expansion in acute anti-thy1 mesangioproliferative glomerulonephritis. Am J Physiol Renal Physiol 2007; 292: F1761–F1770. [DOI] [PubMed] [Google Scholar]
- 32Rausch M, Hiestand P, Foster CA, Baumann DR, Cannet C, Rudin M. Predictability of FTY720 efficacy in experimental autoimmune encephalomyelitis by in vivo macrophage tracking: clinical implications for ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. J Magn Reson Imaging 2004; 20: 16–24. [DOI] [PubMed] [Google Scholar]
- 33Zhang Z, Zhang ZY, Fauser U, Schluesener HJ. FTY720 ameliorates experimental autoimmune neuritis by inhibition of lymphocyte and monocyte infiltration into peripheral nerves. Exp Neurol 2008; 210: 681–690. [DOI] [PubMed] [Google Scholar]
- 34Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta 2008; 1781: 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Hill GR, Cooke KR, Teshima T, Crawford JM, Keith JC Jr, Brinson YS et al. Interleukin-11 promotes T cell polarization and prevents acute graft-versus-host disease after allogeneic bone marrow transplantation. J Clin Invest 1998; 102: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Robb RJ, Hill GR. The interferon-dependent orchestration of innate and adaptive immunity after transplantation. Blood 2012; 119: 5351–5358. [DOI] [PubMed] [Google Scholar]
- 37Gonzalez-Cabrera PJ, Cahalan SM, Nguyen N, Sarkisyan G, Leaf NB, Cameron MD et al. S1P1 receptor modulation with cyclical recovery from lymphopenia ameliorates mouse model of multiple sclerosis. Mol Pharmacol 2012; 81: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Poti F, Costa S, Bergonzini V, Galletti M, Pignatti E, Weber C et al. Effect of sphingosine 1-phosphate (S1P) receptor agonists FTY720 and CYM5442 on atherosclerosis development in LDL receptor deficient (LDL-R−/−) mice. Vascul Pharmacol 2012; 57: 56–64. [DOI] [PubMed] [Google Scholar]
- 39Huu DL, Matsushita T, Jin G, Hamaguchi Y, Hasegawa M, Takehara K et al. FTY720 ameliorates murine sclerodermatous chronic graft-versus-host disease by promoting expansion of splenic regulatory cells and inhibiting immune cell infiltration into skin. Arthritis Rheum 2013; 65: 1624–1635. [DOI] [PubMed] [Google Scholar]
- 40Song J, Ito T, Matsuda C, Miao G, Tanemura M, Nishida T et al. Inhibition of donor-derived T cells trafficking into target organs by FTY720 during acute graft-versus-host disease in small bowel transplantation. Clin Exp immunology 2006; 146: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Taylor PA, Ehrhardt MJ, Lees CJ, Tolar J, Weigel BJ, Panoskaltsis-Mortari A et al. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD). Blood 2007; 110: 3480–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Toubai T, Shono Y, Nishihira J, Ibata M, Suigita J, Kato N et al. Serum macrophage migration inhibitory factor (MIF) levels after allogeneic hematopoietic stem cell transplantation. Int J Lab Hematol 2009; 31: 161–168. [DOI] [PubMed] [Google Scholar]
- 43Toubai T, Tanaka J, Nishihira J, Ohkawara T, Hirate D, Kondo N et al. Effect of macrophage migration inhibitory factor (MIF) on acute graft-versus-host disease in a murine model of allogeneic stem cell transplantation. Transpl Immunol 2006; 16: 117–124. [DOI] [PubMed] [Google Scholar]
- 44Nishiwaki S, Terakura S, Ito M, Goto T, Seto A, Watanabe K et al. Impact of macrophage infiltration of skin lesions on survival after allogeneic stem cell transplantation: a clue to refractory graft-versus-host disease. Blood 2009; 114: 3113–3116. [DOI] [PubMed] [Google Scholar]
- 45Duong CQ, Bared SM, Abu-Khader A, Buechler C, Schmitz A, Schmitz G. Expression of the lysophospholipid receptor family and investigation of lysophospholipid-mediated responses in human macrophages. Biochim Biophys Acta 2004; 1682: 112–119. [DOI] [PubMed] [Google Scholar]
- 46Weigert A, Weis N, Brune B. Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology 2009; 214: 748–760. [DOI] [PubMed] [Google Scholar]
- 47O'Sullivan C, Dev KK. The structure and function of the S1P1 receptor. Trends Pharmacol Sci 2013; 34: 401–412. [DOI] [PubMed] [Google Scholar]
- 48Dong J, Wang H, Wu G, Zhao J, Zhang L, Zuo L et al. Oral treatment with SEW2871, a sphingosine-1-phosphate type 1 receptor agonist, ameliorates experimental colitis in interleukin-10 gene deficient mice. Clin Exp Immunol 2014; 177: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Zhang ZY, Zhang Z, Zug C, Nuesslein-Hildesheim B, Leppert D, Schluesener HJ. AUY954, a selective S1P1 modulator, prevents experimental autoimmune neuritis. J Neuroimmunol 2009; 216: 59–65. [DOI] [PubMed] [Google Scholar]
- 50Healy LM, Sheridan GK, Pritchard AJ, Rutkowska A, Mullershausen F, Dev KK. Pathway specific modulation of S1P1 receptor signalling in rat and human astrocytes. Br J Pharmacol 2013; 169: 1114–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol 2009; 5: 428–434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.