Abstract
Shigella species cause severe bacillary dysentery in humans and are associated with high morbidity and mortality. The Invasion plasmid antigen (IpaB) protein, which is conserved across all Shigella spp., induces macrophage cell death and is required to invade host cells. The present study evaluates the immunogenicity and protective efficacy of the recombinant (r) domain region of IpaB (rIpaB) of S. flexneri. rIpaB was administered either alone or was co-administered with the rGroEL (heat shock protein 60) protein from S. Typhi as an adjuvant in a mouse model of intranasal immunization. The IpaB domain region (37 kDa) of S. flexneri was amplified from an invasion plasmid, cloned, expressed in BL21 Escherichia coli cells and purified. Immunization with the rIpaB domain alone stimulated both humoral and cell-mediated immune responses. Furthermore, robust antibody (IgG, IgA) and T-cell responses were induced when the rIpaB domain was co-administered with rGroEL. Antibody isotyping revealed higher IgG1 and IgG2a antibody titers and increased interferon-gamma (IFN-γ) secretion in the co-administered group. Immunization of mice with the rIpaB domain alone protected 60%–70% of the mice from lethal infection by S. flexneri, S. boydii and S. sonnei, whereas co-administration with rGroEL increased the protective efficacy to 80%–85%. Organ burden and histopathological studies also revealed a significant reduction in lung infection in the co-immunized mice compared with mice immunized with the rIpaB domain alone. This study emphasizes that the co-administration of the rIpaB domain and rGroEL protein improves immune responses in mice and increases protective efficacy against Shigella infection. This is also the first report to evaluate the potential of the GroEL (Hsp 60) protein of S. Typhi as an adjuvant molecule, thereby overcoming the need for commercial adjuvants.
Keywords: GroEL, heat shock protein, IpaB, Shigella, S. Typhi
Introduction
Shigellosis or bacillary dysentery is an acute diarrheal disease caused by the Gram-negative bacillus Shigella. It is a major health problem worldwide, with more than 163 million cases reported annually.1 Shigella spp. invade and destroy the host intestinal epithelium, leading to watery diarrhea that contains blood and mucus.2,3,4 The genus Shigella includes four species, namely, Shigella dysenteriae (13 serotypes), Shigella flexneri (15 serotypes), Shigella boydii (18 serotypes) and Shigella sonnei (1 serotype).1 The emergence of antibiotic resistance and the presence of multiple serotypes emphasize the need for a safe and effective vaccine against Shigella that is protective across all of the serotypes. To the best of our knowledge, there is no available vaccine against Shigella, and only a few vaccines are under development at different phases of clinical trials.5
All Shigella spp. contain a large 220 kb virulence plasmid for invasion. The virulence plasmid encodes a 30 kb Mxi-Spa type III secretion system (T3SS). The T3SS includes a needle tip that acts as a molecular syringe and is comprised of several effector proteins. Of these effector proteins, the invasion plasmid antigens (IpaA, IpaB, IpaC and IpaD) have been reported to be essential for the invasion of epithelial cells and for the release of the bacteria into the host cell cytosol.3,6,7 These Ipa proteins are reported to be the dominant antigens responsible for inducing humoral immune responses in the convalescent sera of infected monkeys and humans.8 The IpaB molecule is conserved in all pathogenic Shigella spp. and plays a major role in the bacterial invasion of epithelial cells.9 In particular, IpaB performs unique functional roles within the T3SS during pathogenesis. For example, IpaB induces apoptosis in host macrophages and dendritic cells, which leads to inflammation and forms a translocon complex that is inserted as a pore into the targeted host cell membranes to direct the entry of other effector proteins into the host cell cytoplasm.3,10,11,12,13 Recent studies in a mouse lung model have also indicated the immunogenicity and protective efficacy of the invasion complex (IpaB, IpaC and lipopolysaccharide) of Shigella against Shigella infections.14 The interaction between IpaB and IpaD at the needle tip mediates host cell sensing and allows translocon insertion into the host cell membrane.15,16,17,18,19,20 Recently, the crystal structure of a protease-stable fragment that was identified within the N-terminal region of IpaB from S. flexneri was presented; this fragment has substantial similarity to the coiled-coil regions of pore-forming proteins from other Gram-negative pathogens.15 Early studies predicted that the domain regions of IpaB were necessary for invasion, phagosome escape, caspase-1 binding and cytotoxicity.21,22,23,24,25,26 In the IpaB domain, amino acids (aa) 44–310 have been reported to contain the regions necessary for the process of invasion. Among aa 44–310 of the IpaB domain, aa 51–72 contain the cognate chaperone invasion plasmid gene C binding site which provides stability to the IpaB protein and prevents its degradation in the cytoplasm; aa 75–310 contain the region responsible for cytotoxicity, invasion, lysing phagosome and macrophage death.15,16,17,18,19 Therefore, in this study, the IpaB domain region (44–310 aa) was explored for its immunogenicity and protective efficacy against Shigella infection.
Heat shock proteins (HSPs) are evolutionarily conserved intracellular molecules that are found in all forms of life. They function as molecular chaperones in numerous processes, such as protein folding and transport, and are induced under stressful conditions. They are potent inducers of the immune system, inducing both innate and adaptive immune responses.27,28 Many microbial HSPs are reported to be dominant antigens that elicit host immune responses to a variety of pathogens29,30,31,32,33,34 and act as effective adjuvants.35 Previously, we developed recombinant (r) Hsp 60 (GroEL) and rHsp70 (DnaK) from Salmonella enterica serovar Typhi, and we reported that these candidate vaccine molecules stimulate both arms of immunity against S. Typhi and S. typhimurium infection in mice.36,37,38,39 HSPs share high sequence homology among various spp., and we have previously reported the in vitro and in vivo cross-protective efficacy of rGroEL of S. Typhi against multiple bacterial pathogens.40
With this background, the present study was designed to evaluate the immunogenicity and protective efficacy of the rIpaB domain of Shigella alone (without any adjuvant) and to investigate the adjuvant effect of rGroEL of S. Typhi when co-administered with the rIpaB domain in mice against infection with S. flexneri, S. boydii and S. sonnei. Because IpaB is conserved in all serotypes of Shigella spp., it would be a potent cross-protective antigen for the development of a safe and effective vaccine against all Shigella serotypes.
Materials and methods
Animals
Four- to six-week-old female BALB/c mice were used in all experiments. All of the animals were reared and housed in the Experimental Animal Facility, Defence Institute for Physiology and Allied Sciences, Delhi, India, under standard laboratory conditions. Food and sterile water were provided ad libitum. All animal protocols were approved by CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Government of India, and were carried out according to the guidelines of the Institutional Ethical Committee.
Growth conditions and maintenance of bacterial strains and vectors
Shigella flexneri 2a, Shigella boydii and Shigella sonnei were clinically isolated pathogens collected from the All India Institute for Medical Sciences, New Delhi, India. All Shigella strains were grown in Tryptic Soy Broth (Difco, Sparks, MD, USA) at 37 °C. Virulent plasmid bearing Shigella colonies were isolated by growing the strains regularly in Tryptic Soy Agar with 0.02% Congo red. Escherichia coli DH5α cells were maintained in LB medium, E. coli BL21 (DE3) pLysS cells were maintained in LB medium with 35 µg/ml chloramphenicol and pRSET A vector was maintained in LB medium with 50 µg/ml ampicillin at 37 °C.
Plasmid DNA was isolated using GenElute Plasmid DNA Isolation kits according to the manufacturer's instructions (Sigma, St. Louis, MO, USA), and isolated plasmid DNA was stored at −20 °C.
Cloning, expression and purification of recombinant the IpaB domain region of S. flexneri
The 220 kb invasion plasmid of S. flexneri was isolated and used as a template to amplify the 801 bp domain region of IpaB using the following IpaB primers:
IpaB forward primer: 5′-agttctcgaggatcttactgctaaccaaaat-3′
IpaB reverse primer: 3′-ggtactgcagaacacaacccattactctgt-5′
The primers were designed such that the amplified gene contained XhoI and PstI restriction sites at the 5′ and 3′ ends, respectively. The PCR reaction was carried out using the following conditions: 95 °C for 5 min (initial denaturation), 95 °C for 1 min (denaturation), 57 °C for 1 min (annealing) and 72 °C for 2 min (extension). This cycle was repeated 37 times, followed by a final extension step of 72 °C for 10 min. The 801-bp amplified PCR product was digested with XhoI and PstI, gel eluted and purified. The digested product was then ligated into the expression vector pRSET A, which was further digested and purified with XhoI and PstI. The 801-bp IpaB domain was in frame with histidine6 tag sequence at the N-terminal end. The ligated, cloned product was transformed into DH5α cells to screen for the recombinants by CaCl2 transformation. The presence of the cloned product was confirmed by colony PCR and double digestion. The recombinant pRSET A IpaB was finally transformed into E. coli BL21 pLysS cells.
Transformed E. coli BL21 pLysS cells were inoculated into LB broth (500 ml) and incubated until the OD600 reached 0.5. Then, the cells were induced with 1 mM isopropylthiogalactoside (IPTG) for 4 h at 37 °C, followed by centrifugation at 5000g. The resulting pellet was suspended in lysis buffer containing 8 M urea, 200 mM NaCl, 2 mM imidazole and 50 mM Tris-Cl, and the pellet was sonicated using a sonicator (Vibra, Sonics and Materials Inc, CT, USA) under cold conditions. The sonicated sample was purified by NiNTA slurry according to the manufacturer's instructions (Qiagen, Hilden, Germany). The recombinant protein was eluted from the NiNTA slurry using elution buffer with 0.5 M imidazole. The purification of recombinant protein through Ni-NTA yielded a highly purified protein without any bacterial contaminants. The purified protein was refolded in vitro using 1 M arginine at 4 °C. Using a dialysis membrane, the recombinant protein was dialyzed in dialysis buffer containing 50 mM Tris and 1 mM EDTA for 48 h. The dialysis buffer was changed every 6 h to remove the urea and imidazole residues. The purified and dialyzed recombinant protein was concentrated using Amicon filtration columns (Millipore, Billerica, MA, USA). The purity of the protein was determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). The expressed rIpaB domain protein was confirmed by western blotting using an IpaB antibody (Santacruz, CA, USA). LPS content in the purified protein was found to be negligible (<1 EU/mg protein) as determined by limulus amoebocyte lysate (Sigma).
Expression and purification of recombinant GroEL protein of S. Typhi
The S. Typhi GroEL gene was cloned, expressed and purified previously in our laboratory. Briefly, transformed E. coli BL21 cells were grown in LB medium (500 ml) and induced with 0.5 mM IPTG. The expressed protein was analyzed by SDS–PAGE. The expressed recombinant GroEL protein was purified by Ni-NTA chromatography under denaturing conditions according to the manufacturer's instructions (Qiagen). The purified protein was refolded in vitro using 1 M arginine and then dialyzed and concentrated using Amicon filtration columns.37
Immunization of mice
Four groups of female BALB/c mice (n=6/group) were taken for immunization studies. Group 1 was immunized intranasally (i.n.) with rIpaB alone without any adjuvant (40 µg/mouse dissolved in 25 µl sterile phosphate-buffered saline (PBS)). Group 2 was immunized with rIpaB (i.n., 40 µg/mouse in 25 µl sterile PBS) along with rGroEL as an adjuvant molecule (i.n., 40 µg/mouse in 25 µl sterile PBS). The third group of mice (Group 3) was immunized with rGroEL adjuvant alone (i.n., 40 µg/mouse in 25 µl sterile PBS). The fourth group of mice (Group 4) was immunized with an equal amount of sterile PBS (25 µl/mice i.n.) and served as the control group. Subsequent booster doses were given on both the seventh and twenty-eighth days.
Determination of antibody titers
Seven days after the last booster dose, blood was drawn from both the control and immunized mice via the retro-orbital sinus. Serum was collected and stored at −20 °C for further immunological studies. Additionally, BAL fluid (BALF) was collected 1 week after the last immunization by inflating the lungs with 1.0 ml of ice cold PBS into the trachea and aspirating through a 25-G needle. The lavage fluid was then separated from the tissue debris by centrifugation at 2700g for 10 min at 4 °C. The clarified BAL samples were stored at −20 °C for further analysis. The levels of IpaB-specific IgG and IgA were measured in serum, and IgA was measured in BALF by enzyme-linked immunosorbent assay. Briefly, 200 µl of the coating buffer (0.1 M bicarbonate, pH 9.3) containing 1 µg of rIpaB domain alone/rGroEL adjuvant alone was added to 96-well microtiter plates (Grenier, Frickenhausen, Germany) and incubated overnight at 4 °C. The plates were washed 3 times with PBS-0.05% Tween-20 (PBST) and blocked with 5% BSA in PBS for 2 h. Again, the plates were washed three times with PBST and then incubated with serially diluted mice sera/BALF at room temperature for 2 h. The plates were then washed three more times with PBST. The bound immunoglobulins were detected by incubating the plates with diluted anti-mouse IgG/IgA HRP conjugate (1∶2000) for 1 h at room temperature. To measure IgG isotypes in the serum, bound antibodies were detected by incubation with anti-mouse IgG1/IgG2a HRP conjugate (1∶3000) for 1 h at room temperature. Then, the plates were washed three times with PBST, and a color change was elicited by adding 200 µl of TMB/H2O2 substrate (BD Biosciences, San Diego, CA, USA). The plates were incubated in the dark for 30 min, and the reaction was stopped by adding 50 µl of 2 N H2SO4. The absorbance was read at 450 nm using a multimode reader (Molecular devices, Sunnyvale, CA, USA).
Lymphocyte proliferation
The control and immunized mice were killed on the seventh day after the last booster dose was administered; splenocytes were then collected from the spleen. Briefly, 200 µl of splenocytes was cultured in RPMI 1640 medium (1×105 cells/well) in 96-well microtiter plates and stimulated with 5 µg of rIpaB domain/rGroEL adjuvant separately. The plates were incubated for 72 h at 37 °C in a 5% CO2 incubator. Lymphocyte proliferation was determined by adding 10 µl of MTT reagent (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) (5 mg/ml) to each well, and the plates were incubated for 3 h in the dark. The plates were centrifuged at 1500 r.p.m. for 10 min. The supernatant was stored for cytokine estimation, and the pellet was incubated for 15 min in 100 µl of dimethyl sulphoxide. The absorbance was measured at 570 nm.
Cytokine estimation
Splenocytes from control and immunized mice were isolated as described above. Cells were stimulated with 5 µg of rIpaB domain alone/rGroEL adjuvant alone as described above, and the supernatant was collected. Interferon-gamma (IFN-γ) was estimated from the culture supernatants collected using enzyme-linked immunosorbent assay kits (BD Biosciences, CA, USA) according to the manufacturer's instructions. The absorbance was read at 450 nm.
Challenge studies
To study the protective efficacy of rIpaB domain against Shigella infection, four groups of mice (n=10/group) were used for the challenge studies. Group 1 was immunized with rIpaB domain alone, group 2 was immunized with rIpaB domain+rGroEL protein, group 3 was immunized with rGroEL alone and group 4 was administered sterile PBS as a control. The lethal dose of Shigella spp. was determined by performing serial dilutions in agar plates and counting colonies after incubation overnight. Protective efficacy was evaluated 15 days after the last booster dose was administered. Each group of mice was challenged i.n. with a lethal dose (1×107 colony forming units (CFU)/mouse) of S. flexneri, S. boydii and S. sonnei. The mice were observed for mortality for 30 days.
Passive immunization
To assess antibody-mediated protection, immune sera collected from all four groups of mice (7 days after the last booster was administered, as described above) was heated at 56 °C for 30 min to inactivate the complement system.41 Another four groups of mice (n=10/group) were passively immunized (i.n.) with 30 µl of this heat-inactivated sera. After 24 h, the mice were challenged with a lethal dose of S. flexneri, S. boydii and S. sonnei (i.n., 1×107 CFU/mouse). The mice were monitored for mortality for 30 days.
Organ burden
To assess the bacterial load in the control and immunized mice, lungs were collected from individual animals aseptically after 30 days. Tissues were homogenized in 5 ml of ice-cold PBS using a tissue homogenizer (Kinematica, AG, Luzern, Switzerland). The resulting homogenates were plated in 10-fold serial dilutions on LB agar plates followed by incubation at 37 °C for 16–18 h. The number of CFU were counted in each plate and expressed as the number of CFU/ml.
Histopathology
Lung tissue from control and immunized groups after challenge was excised, fixed in 10% formalin and embedded in paraffin blocks. Sections were stained with hematoxylin and eosin and analyzed by microscopic examination.
Statistical analysis
All experimental data were statistically analyzed using one-way analysis of variance in SPSS, the Statistical Package for the Social Sciences (SPSS Inc. Version 16.0, Chicago). A P-value of <0.05 was considered significant in all experiments, and the data are presented as the mean±s.d. Protection studies were expressed using Kaplan–Meier survival curves. All experiments were performed in triplicate.
Results
Cloning, expression and purification of the S. flexneri IpaB domain
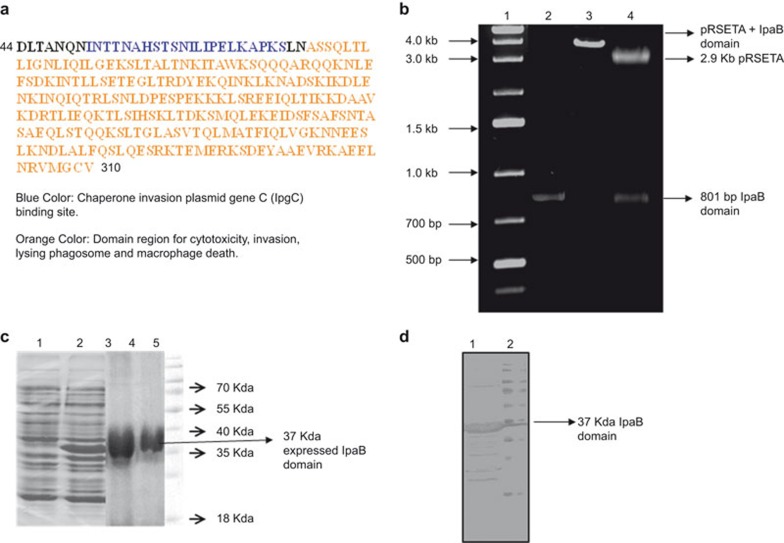
The 801-bp IpaB domain was ligated into a pRSET A vector and transformed into E. coli BL21 cells. The transformants were confirmed by amplification of the IpaB domain by colony PCR and by restriction digestion. The 801-bp IpaB domain gene was identified by PCR amplification. The generation of restriction fragments on a 1% agarose gel of 2.9 kb pRSET A vector and 801-bp IpaB provided confirmation of the cloned products in BL21 cells.
The transformed cells were induced with 1 mM IPTG and purified by Ni-NTA chromatography. The rIpaB domain protein (Figure 1a) was refolded, dialyzed and concentrated using Amicon filtration columns. The expression of the purified rIpaB domain (37 kDa) was confirmed by SDS–PAGE (Figure 1b), followed by western blotting with IpaB antibody (Figure 1c).
Figure 1.
Cloning, expression and purification of the rIpaB domain region. (a) Schematic diagram representing the aa 44–310 domain region of IpaB. Blue indicates aa residues 51–72, the cognate chaperone IpgC binding site, and orange indicates the 75–310 aa region for cytotoxicity, invasion, lysing phagosome and macrophage death. (b) Cloning of the rIpaB domain region in pRSET A, Lane 1: 1 kb DNA ladder. Lane 2: 801 bp IpaB domain region amplified by colony PCR. Lane 3: XhoI digested plasmid showing 3.7 kb product (2.9 kb pRSET A+801 bp IpaB). Lane 4: Xho I and Pst I digested plasmid showing 2.9 kb pRSET A and 801 bp IpaB gene. (c) SDS–PAGE showing the expression and purification of the rIpaB domain region in E. coli cells. The transformed cells were induced with 1 mM IPTG for 4 h at 37 °C, and the expressed recombinant protein was purified by Ni-NTA affinity chromatography. Lane 1: un-induced cells; Lane 2: IPTG-induced cells with over-expressed 37 kDa protein; Lane 3: eluted protein; Lane 4: dialyzed and purified protein; Lane 5: molecular mass marker. (d) Western blot. Lane 1: expression of the 37 kDa rIpaB domain protein when probed with IpaB antibody was confirmed by western blotting; Lane 2: Molecular mass marker. aa, amino acid; IpgC, invasion plasmid gene C; IPTG, isopropylthiogalactoside; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
rGroEL improves humoral immune responses generated by rIpaB
Antibody titers
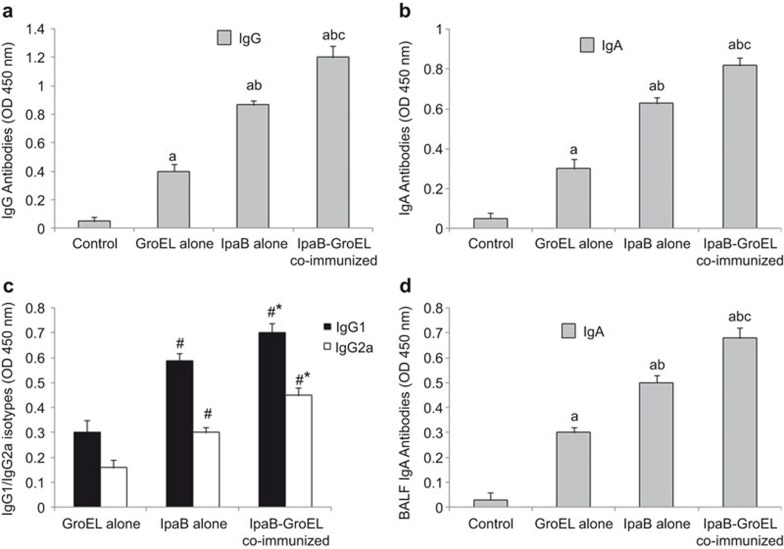
To assess humoral immune responses, sera collected from all immunized and control groups of mice were screened for the presence of the antigen-specific antibodies IgA and IgG. All three immunized groups showed high antibody titers (IgG, IgA) compared with the control groups; however, there was a significant difference in the magnitude of antibody response when rIpaB was administered either alone or with rGroEL. The highest antibody response was generated when rIpaB was co-administered with rGroEL. Maximum titers were observed 1 week after the last immunization in all groups (Figure 2a and b).
Figure 2.
Effects of rIpaB and rIpaB+rGroEL (co-administration) immunization on antibody levels in mice. Groups of female BALB/c mice (n=6) were immunized i.n. with 40 µg/mouse of recombinant GroEL alone (adjuvant control)/rIpaB alone/rIpaB+rGroEL. Subsequent booster doses were given on the seventh and twenty-eighth days. The control group of mice (n=6) was immunized with sterile PBS. Mice were killed 1 week after the last booster, and sera were collected to estimate the antibody titers by ELISA. (a) Serum IgG, (b) Serum IgA, (c) IgG1 and IgG2a and (d) BALF IgA antibody titers. No detectable IgG1/IgG2a isotope antibodies were observed in the control group. The sera samples which gave an o.d. value >0.3 were measured for scoring. Data were represented as the mean±s.d. P<0.001, a vs. Control, b vs. GroEL, c vs. IpaB. P<0.001, # vs. GroEL, * vs. IpaB. BALF, BAL fluid; ELISA, enzyme-linked immunosorbent assay; PBS, phosphate-buffered saline.
IgG isotyping
The isotype profile of antibody responses depends on the cytokines produced by antigen-specific T cells and is an indirect measure of Th1 and Th2 type cytokines. The effect of rIpaB/rIpaB+rGroEL/rGroEL immunization was studied on the Th1 and Th2 profile by determining IgG1 and IgG2a antibody levels. High IgG1 and IgG2a levels in the immunized groups compared with the control groups indicate the induction of both Th1 and Th2 immune responses. However, the increase was more pronounced in the group co-administered rIpaB+rGroEL compared with the groups administered only rIpaB or rGroEL (Figure 2c). High levels of IgA were observed in BALF of the immunized groups compared with the control groups but the maximum level of IgA was observed when rIpaB was administered along with rGroEL, suggesting a robust induction of mucosal immunity (Figure 2d).
Cell-mediated immune responses
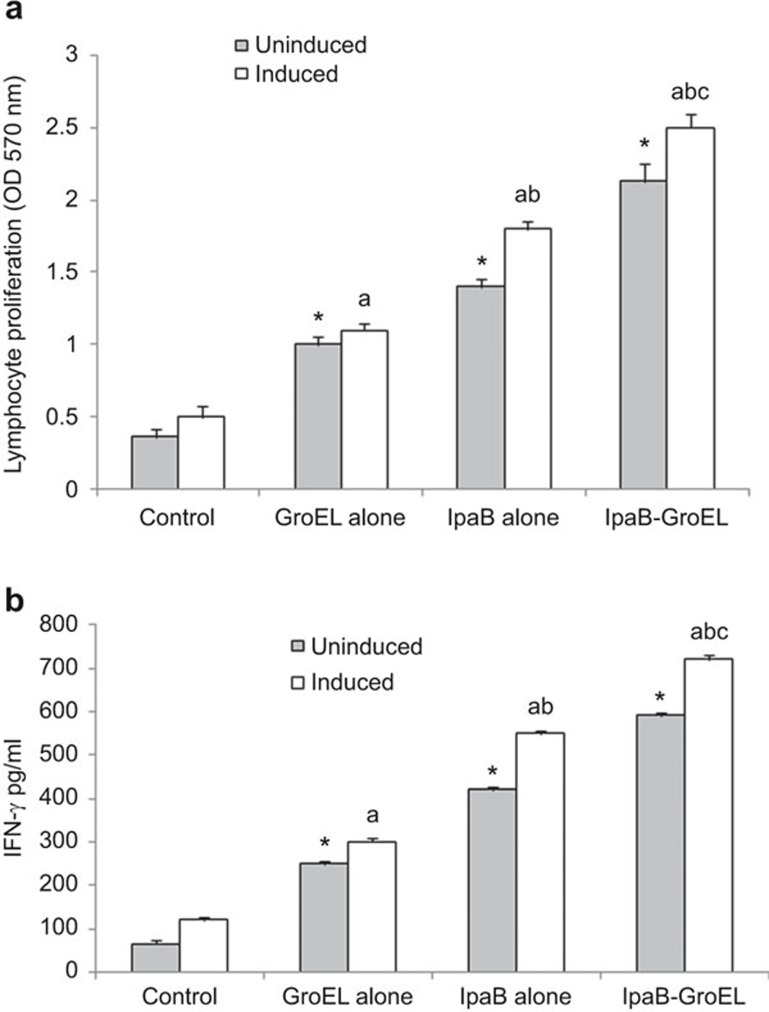
To analyze T-cell responses in immunized mice, lymphocyte proliferation was measured by isolating the splenocytes from individual animals stimulated in vitro with rIpaB alone/rIpaB+rGroEL/rGroEL adjuvant 1 week after the last immunization. Splenocytes of all groups of immunized mice showed significant lymphocyte proliferation, even in the absence of external stimulation, compared with the control groups. A further increase in the proliferation was observed upon in vitro stimulation in the rIpaB immunized group compared with the rGroEL adjuvant/control groups, and significantly higher proliferation was observed in the rIpaB+rGroEL co-administered group than in the rIpaB immunized group (P<0.001) (Figure 3a).
Figure 3.
Effects of rIpaB and rIpaB+rGroEL (co-administration) immunization on lymphocyte proliferation and IFN-γ production. (a) Groups of female BALB/c mice (n=6) were immunized i.n. with 40 µg/mouse of rGroEL/rIpaB alone/rIpaB+rGroEL. Subsequent booster doses were given on the seventh and twenty-eighth days. The control group of mice (n=6) was immunized with sterile PBS. After the last immunization, the splenocytes were isolated and cultured ((1×105 cells/well) either in the absence (unstimulated) or in the presence (stimulated) of rIpaB/rGroEL for 72 h in a CO2 incubator at 37 °C. The absorbance was measured at 570 nm. (b) After the last immunization, the splenocytes were isolated and cultured for 72 h in a CO2 incubator at 37 °C to estimate the IFN-γ production. The absorbance was measured in culture supernatants at 450 nm. P<0.001, * vs. control UI, a vs. control-induced, b vs. IpaB-induced, c vs. IpaB-GroEL-induced. IFN-γ, interferon-gamma; i.n., intranasally.
To further characterize the type of immune response generated, splenocytes from all of the groups were cultured in vitro for 72 h and IFN-γ was measured in the culture supernatants. There was a significant increase in the IFN-γ level in all of the immunized groups compared with the control group. The level of IFN-γ was appreciably higher in the co-administered group stimulated in vitro with the rGroEL+ rIpaB domain compared to the group stimulated with the rIpaB domain alone, confirming the shift toward a Th1 immune response (P<0.001) which was also revealed by IgG isotyping (Figure 3b).
rIpaB domain co-administered with rGroEL improves protective efficacy
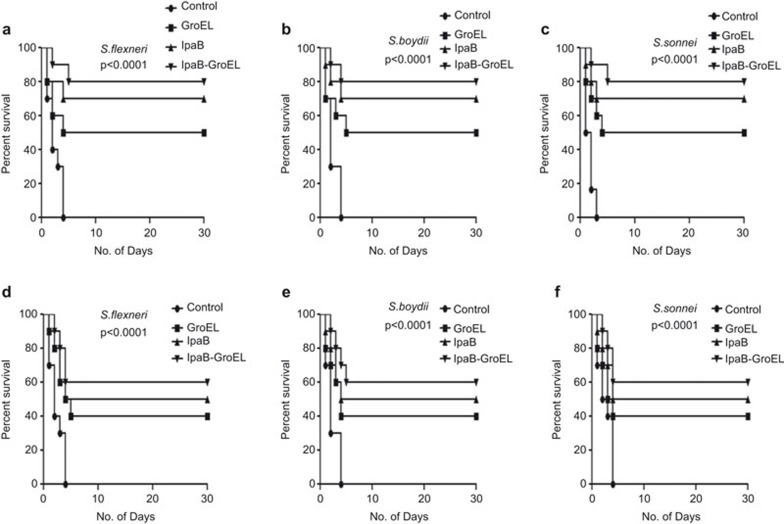
The significant increase in antibody titers and IFN-γ led us to evaluate the protective efficacy of the rIpaB domain against S. flexneri, S. boydii and S. sonnei. Fifteen days after the last immunization, control/rIpaB domain immunized/co-administered/rGroEL adjuvant alone mice (n=10/group) were challenged with the lethal dose of 1×107 CFU/mouse i.n. All of the control mice died within 3–4 days, whereas 50% of the rGroEL immunized mice survived and 60%–70% of the rIpaB domain immunized mice survived when challenged with all three of the Shigella spp. Interestingly, rIpaB domain co-administered with rGroEL protein protected 80%–85% of the mice challenged with Shigella spp. (Figure 4a–c).
Figure 4.
Protective efficacy of rIpaB and rIpaB+rGroEL immunization on survival. Groups of female BALB/c mice (n=10) were immunized i.n. with 40 µg/mouse of rGroEL/rIpaB/rIpaB+rGroEL. Subsequent booster doses were given on the seventh and twenty-eighth days. The control group of mice (n=10) was immunized with sterile PBS. Fifteen days after the last booster dose, mice were challenged with a lethal dose of 1×107 CFU/mouse i.n. of (a) S. flexneri, (b) S. boydii or (c) S. sonnei. Another group of 10 mice was passively immunized i.n. with 30 µl of heat inactivated immune sera raised from the mice immunized with rGroEL/rIpaB/rIpaB+rGroEL and these mice were challenged with a lethal dose of 1×107 CFU/mouse i.n. of (d) S. flexneri, (e) S. boydii or (f) S. sonnei. All groups of mice were observed for mortality for 30 days. CFU, colony forming units; i.n., intranasally.
Passive immunization
Antibody-mediated protection against Shigella infection was assessed by immunizing the mice with heat inactivated anti-IpaB and anti-IpaB+GroEL antiserum. The mice were challenged after 24 h with S. flexneri, S. boydii and S. sonnei i.n. Fifty percent of the animals passively immunized with rIpaB antisera survived against lethal infection by all of the Shigella spp., whereas the survival rate was higher (60%) in the mice passively immunized with the rIpaB domain co-administered with rGroEL antisera (Figure 4d–f).
Organ burden and histopathology
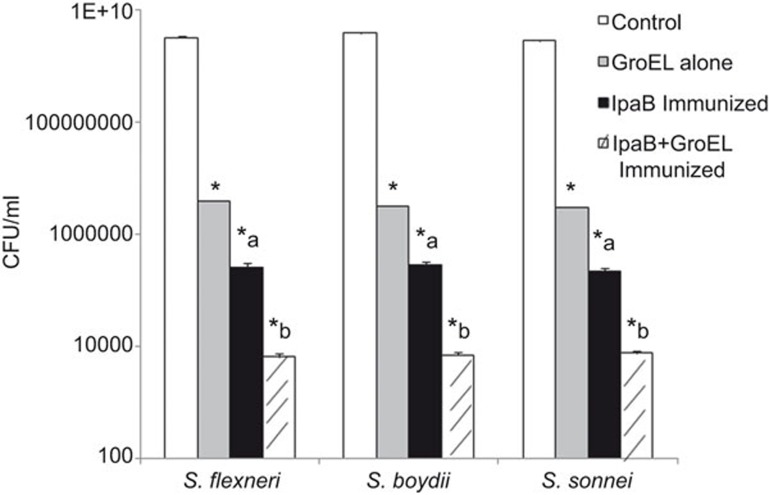
Bacterial load was determined from the lungs of control and immunized mice challenged with Shigella spp. Organ burden studies showed a decrease in CFU in the lungs of rIpaB domain immunized mice compared to the controls (P<0.0001). However, the maximum reduction in CFU was observed in the rIpaB+rGroEL co-administered group compared with the control/rIpaB domain groups (P<0.0001) (Figure 5).
Figure 5.
Organ burden studies after challenge of immunized mice with a lethal dose of 1×107 CFU/mouse i.n. of S. flexneri, S. boydii and S. sonnei. Lung tissues were homogenized in 5 ml of ice-cold PBS. The resulting homogenates were plated in 10-fold serial dilutions on LB agar plates followed by incubation at 37 °C for 16–18 h. CFU were counted and recorded. * vs. control, P<0.0001; a vs. rGroEL, P<0.0001; b vs. a, P<0.0001. CFU, colony forming units; i.n., intranasally; PBS, phosphate-buffered saline.
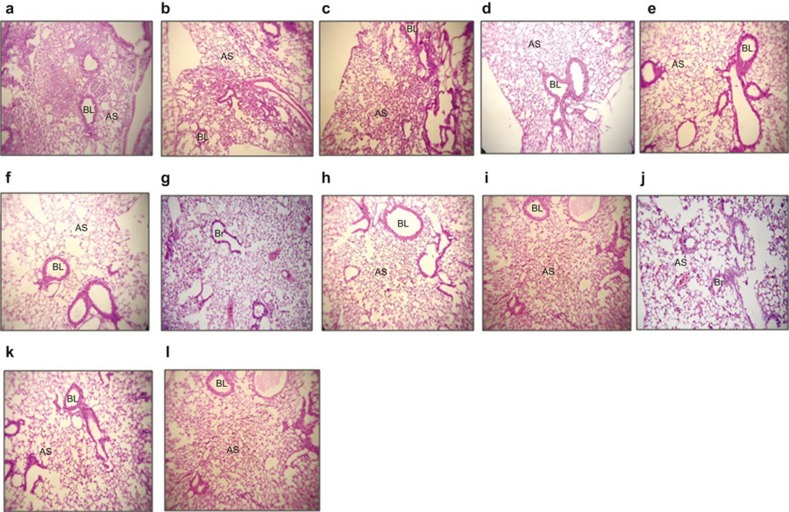
Histopathology of lung tissues immunized with the rIpaB domain protein revealed that these tissues had a more normal morphology with uniform alveoli compared with the controls. The lung sections of the rIpaB+rGroEL co-administered group challenged with Shigella spp. showed normal lung parenchyma with no inflammatory infiltrates or edema in the alveolar spaces or bronchial lumina compared with the rIpaB group (Figure 6a–l).
Figure 6.
Histopathology of control and immunized (rIpaB alone and rIpaB+rGroEL co-administered) mouse lung tissue. Sections of lung tissue were stained with hematoxylin and eosin and analyzed by microscopic examination. (a–c) Lung sections of control mice challenged with S. flexneri, S. boydii or S. sonnei, respectively, showing lung parenchyma with two large areas of consolidation with heavy neutrophilic cell infiltration into the lung parenchyma. (d–f) Lung sections of adjuvant control GroEL immunized mice challenged with Shigella spp. showing improved lung histology. (g–i) IpaB immunized lung section from a mouse challenged with Shigella spp. showing improved lung parenchyma with no edema in the alveolar spaces or bronchial lumina. (j–l) Lung section of a mouse co-immunized with IpaB-GroEL and challenged with Shigella spp. showing normal lung parenchyma with no inflammatory infiltrates or edema in the alveolar spaces or bronchial lumina. Images shown are at ×100 magnification. AS, alveolar space; BL, bronchial lumen.
Discussion
The development of an effective vaccine against Shigella is of global importance; however, such a vaccine has yet to be successfully developed. The emergence of antibiotic resistance among Shigella serotypes limits the number of drugs available for the treatment of shigellosis.42,43,44,45,46 The vaccine strategies currently being developed against this pathogen include live attenuated, conjugate, broad spectrum, LPS-based and proteasome-based vaccines.47 However, broad protection against all four Shigella spp. and their serotypes must be considered in the development of vaccines against shigellosis. Such a vaccine could be developed by exploiting a common molecule that is conserved in all the Shigella spp. Therefore, in our present study, we focused on the IpaB domain protein, which is a conserved molecule across all Shigella spp. that plays a major role in the pathogenesis of the bacteria. Furthermore, we tested the adjuvanticity of rGroEL of S. Typhi that we previously developed.37,38
We evaluated the immunogenicity and protective efficacy of the domain region of the IpaB protein (37 kDa) against Shigella spp. It has been reported that these IpaB domains are recognized by human convalescent sera and that they are found to be immunogenic during natural infection.16 The antibodies produced against these IpaB antigens efficiently prevent the colonization of Shigella in the host cells, making them effective as a candidate molecule for vaccine development.
We cloned and expressed the IpaB domain region in E. coli BL21 cells and purified the recombinant protein. Our study showed that immunization of mice i.n. with the rIpaB domain region resulted in an increase of both IgG and IgA antibody titers in the sera compared with the control. Antibody isotyping revealed elevated levels of both IgG1 and IgG2a antibodies, indicating the induction of both Th1 and Th2 immune responses. It has been reported that intranasal immunization stimulates a higher IgA antibody response in the mucosal surfaces and also primes the systemic immune responses with the development of serum antibodies.48 We also observed an increase in IgA antibodies in BALF among all of the immunized groups compared with the control groups, but the maximum titer was observed in the group co-administered rIpaB+rGroEL. The secretion of IgA is important because it is necessary to prevent mucosal enteric infection in humans. Significantly higher circulating IgA antibody secreting-cells have been reported in patients infected with S. flexneri after the onset of the disease.49 Although the mechanisms of protection against Shigella are not well defined, mucosal immune defenses may play an important role in neutralizing the Shigella T3SS interactions in the epithelial barrier, preventing pathogenic attachment and thereby preventing colonization of the host epithelial cells by the bacteria. The protection provided by the recombinant IpaB domain was 60%–70% against a lethal infection with S. flexneri, S. boydii and S. sonnei. To the best of our knowledge, we are the first to report the induction of protective immunity against Shigella infection by the rIpaB domain region in mice.
Furthermore, to increase the immunogenicity and protective efficacy of the rIpaB domain, the mice were co-administered both rIpaB and the recombinant S. Typhi GroEL. Co-administration of the rIpaB domain with rGroEL in mice resulted in an increase in IgG, IgG1 and IgG2a compared with the administration of IpaB alone, indicating the stimulation of both the Th1 and Th2 immune responses. The increased production of IFN-γ observed in the splenocytes isolated from all of the immunized groups suggests that T cells also contribute to the protection against Shigella infection. Cell-mediated immunity favors the production of IFN-γ, which boosts the production of the IgG2a isotype by murine B cells.50 Interestingly, the titers were higher in the co-administered group than they were in the other groups. Moreover, the ratio of IgG1 and IgG2a decreased significantly in the co-administered group, suggesting a shift toward the Th1 response. This finding is in line with the observation that higher production of IFN-γ was observed in the rIpaB+rGroEL co-administered group compared with the IpaB group. This finding is also supported by the observation of higher lymphocyte proliferation in the splenocytes isolated from the co-administered group compared with the other groups. The requirement of IFN-γ for protection against Shigella infection in mice has been reported previously.51,52 IFN-γ knockout mice lacking this cytokine showed an increased susceptibility to Shigella infection compared with wild-type controls, and the Shigella infection was not cleared in the IFN-γ-deficient mice.53 In shigellosis patients, the expression of gamma interferon was twofold higher during convalescence than during the acute stage.54 It has been reported that both humoral and T-cell immune responses were induced when Shigella IpaB/IpaD was co-administered with double mutant heat labile toxin and that this protected the mice from Shigella challenge.20,55,56,57
Furthermore, we observed that the rIpaB domain region alone conferred 60%–70% protection against Shigella spp. Interestingly, when rGroEL was administered along with the rIpaB domain, the survival percentage of the mice increased to 80%–85% protection against the lethal infection by Shigella spp., indicating an adjuvant effect of rGroEL. Because Shigella is an intracellular pathogen, cell-mediated immunity is also required to fight off infection through the secretion of pro-inflammatory mediators that further promote innate immune cells and T helper cytokines that support antibody production.55 Passive immunization with anti-IpaB or anti-GroEL+anti-IpaB immune sera conferred 50%–60% protection in the mice, demonstrating the importance of the humoral response for protection against shigellosis. This antibody-mediated protection observed in the present study could be due to the surface localization of IpaB, which is an important constituent of T3SS, an essential virulence determinant of many Gram-negative bacterial pathogens.23,24,25,26 The Shigella T3SS consists of a cytoplasmic bulb, a transmembrane region and a hollow ‘needle' protruding from the bacterial surface. The needle is directly involved in sensing the host cells and leads to the secretion and assembly of pore that are formed by IpaB and IpaC in the host membrane, through which the proteins that facilitate host cell invasion are translocated. The localization of IpaB at the surface of needle tips has been demonstrated by many researchers, thus facilitating the recognition of IpaB antigen by antibodies.17,18,19,20
In vaccine development, the choice of the adjuvant is as vital as the selection of the vaccine antigen. HSPs are conserved immunodominant molecules, and we have previously reported that an in vitro serum bactericidal assay with S. Typhi rGroEL antisera exhibited 50%–55% inhibition of cells of Shigella spp. and that in vivo cross-protection studies with rGroEL of S. Typhi demonstrate 60% protection against Shigella spp. i.p.40 In this study, we have shown that immunization with rGroEL i.n. also confers 50% protection by inducing immune responses. An ideal vaccine adjuvant should induce both humoral and cell-mediated immunity and should be non-toxic and without any side effects.58 rGroEL boosted the immune responses and the protection rate of rIpaB. In this regard, recombinant S. Typhi GroEL can be considered an effective adjuvant because it induces both arms of immunity, as we reported previously.35 In the present study, co-administration of the rIpaB domain with rGroEL augmented the immune responses and the protective efficacy against Shigella infection in mice. Recent studies have reported that the double mutant heat labile toxin increases the efficacy of Shigella IpaB/IpaD.21,55,56,57 This is the first report evaluating the use of recombinant GroEL of S. Typhi as an adjuvant, surmounting the side effects of conventional adjuvants.
The organ burden studies presented here revealed a significant reduction in the number of colonies of S. flexneri, S. boydii and S. sonnei in the lung tissues of mice that were co-administered both the rIpaB domain and rGroEL compared with mice administered the rIpaB domain alone. Histopathological studies of lung tissue also showed improved tissue morphology in the co-administered mice challenged with Shigella spp. compared with the rIpaB domain-immunized group.
In conclusion, we report the recombinant IpaB domain of S. flexneri to be immunogenic and protective against Shigella species and the recombinant GroEL of S. Typhi to be an effective adjuvant. When co-administered, rIpaB+rGroEL showed robust humoral and cellular immune responses and provided protection against Shigella infections. Therefore, this study highlights the potential of the recombinant IpaB domain along with rGroEL for the development of broadly protective subunit vaccines against Shigellosis.
Acknowledgments
We would like to thank Mr Bhagwat Singh of the Experimental Animal Facility for his valuable support and technical assistance with animal handling. Ms S T S Chitradevi thankfully acknowledges the financial assistance provided by the Defence Research and Development Organization. This work was supported by the Defence Research and Development Organization, Ministry of Defence, Government of India. There are no conflicts of interest for the authors to report.
References
- 1Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 1999; 77: 651–666. [PMC free article] [PubMed] [Google Scholar]
- 2Philpott DJ, Edgeworth JD, Sansonetti PJ. The pathogenesis of Shigella flexneri infection: lessons from in vitro and in vivo studies. Philos Trans R Soc Lond 2000; 355: 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Sansonetti PJ. Microbes and microbial toxins: paradigms for microbial–mucosal interactions III. Shigellosis: from symptoms to molecular pathogenesis. Am J Physiol Gastrointest Liver Physiol 2001; 280: G319–G323. [DOI] [PubMed] [Google Scholar]
- 4Torres AG. Current aspects of Shigella pathogenesis. Rev Latinoam Microbiol 2004; 46: 89–97. [PubMed] [Google Scholar]
- 5WHO. WHO Initiative for Vaccine Research (IVR). Geneva: WHO, 2006. [Google Scholar]
- 6Menard R, Sansonetti PJ, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol 1993; 175: 5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Jennison AV, Verma NK. Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol Rev 2004; 28: 43–58. [DOI] [PubMed] [Google Scholar]
- 8Oaks EV, Hale TL, Formal SB. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun 1986; 53: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9High N, Mounier J, Prevost MC, Sansonetti PJ. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J 1992; 11: 1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Blocker A, Gounon P, Larquet E, Niebuhr K. The Tripartite Type III Secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J Cell Biol 1999; 147: 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Menard R, Prevost M, Gounon P, Sansonetti P, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci USA 1996; 93: 1254–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Schroeder GN, Hilbi H. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev 2008; 21: 134–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Senerovic L, Tsunoda SP, Goosmann C, Brinkmann V, Zychlinsky A, Meissner F et al. Spontaneous formation of IpaB ion channels in host cell membranes reveals how Shigella induces pyroptosis in macrophages. Cell Death Dis 2012; 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Turbyfill KS, Kaminski RW, Oaks EV. Immunogenicity and efficacy of highly purified invasin complex vaccine from Shigella flexneri 2a. Vaccine 2008; 26: 1353–1364. [DOI] [PubMed] [Google Scholar]
- 15Mills JA, Buysse JM, Oaks EV. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect Immun 1988; 56: 2933–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Barzu S, Nato F, Rouyre S, Mazie J, Sansonetti P, Phalipon A. Characterization of B-cell epitopes on IpaB, an invasion-associated antigen of Shigella flexneri: identification of an immunodominant domain recognized during natural Infection. Infect Immun 1993; 61: 3825–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Shen D, Saurya S, Wagner C, Nishioka H, Blocker AJ. Domains of the Shigella flexneri Type III Secretion System IpaB protein involved in secretion regulation. Infect Immun 2010; 78: 4999–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Menard R, Sansonetti P, Parsot C, Vasselon T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasions of S. flexneri. Cell 1994; 79: 515–525. [DOI] [PubMed] [Google Scholar]
- 19Guichon A, Hersh D, Smith MR, Zychlinsky A. Structure–function analysis of the Shigella virulence factor IpaB. J Bacteriol 2001; 183: 1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Martinez-Becerra FJ, Scobey M, Harrison K, Choudhari SP, Quick AM, Joshi SB et al. Parenteral immunization with IpaB/IpaD protects mice against lethal pulmonary infection by Shigella. Vaccine 2013; 31: 2667–2672. [DOI] [PubMed] [Google Scholar]
- 21Heine SJ, Diaz-McNair J, Martinez-Becerra FJ, Choudhari SP, Clements JD, Picking WL et al. Evaluation of immunogenicity and protective efficacy of orally delivered Shigella type III secretion system proteins IpaB and IpaD. Vaccine 2013; 31: 2919–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Barta ML1, Dickenson NE, Patil M, Keightley A, Wyckoff GJ, Picking WD et al. The structures of coiled-coil domains from type III secretion system translocators reveal homology to pore-forming toxins. J Mol Biol 2012; 417: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Veenendaal AK, Hodgkinson JL, Schwarzer L, Stabat D, Zenk SF, Blocker AJ. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol Microbiol 2007; 63: 1719–1730. [DOI] [PubMed] [Google Scholar]
- 24Johnson S, Roversi P, Espina M, Olive A, Deane JE, Birket S et al. Self-chaperoning of the type III secretion system needle tip proteins IpaD and BipD. J Biol Chem 2007; 282: 4035–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Stensrud KF, Adam PR, La Mar CD, Olive AJ, Lushington GH, Sudharsan R et al. Deoxycholate interacts with IpaD of Shigella flexneri in inducing the recruitment of IpaB to the type III secretion apparatus needle tip. J Biol Chem 2008; 283: 18646–18654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Olive AJ, Kenjale R, Espina M, Moore DS, Picking WL, Picking WD. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect Immun 2007; 75: 2626–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Kaufmann SH. Heat shock proteins and the immune response. Immunol Today 1990; 11: 129–136. [DOI] [PubMed] [Google Scholar]
- 28Zugel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev 1999; 12: 19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Lee JY, Yi NN, Kim US, Choi JS, Kim SJ, Choi JI. Porphyromonas gingivalis heat shock protein vaccine reduces vaccine reduces the alveolar bone loss induced by multiple periodontopathogenic bacteria. J Periodont Res 2006; 41: 10–14. [DOI] [PubMed] [Google Scholar]
- 30Gomez FJ, Allendoerfer R, Deepe GS. Vaccination with recombinant heat shock protein 60 from Histoplasma capsulatum protects mice against pulmonary histoplasmosis. Infect Immun 1995; 63: 2587–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Wilhelm V, Zoza C, Martinez R, Rosemblatt M, Bursio LO, Valenzuela PD. Production and immune response of recombinant Hsp60 and Hsp70 from the salmon pathogen Piscirickettsia salmonis. Biol Res 2005; 38: 69–82. [DOI] [PubMed] [Google Scholar]
- 32Noll A, Autenreith IB. Immunity against Yersinia enterocolitica by vaccination with Yersinia hsp60 immunostimulating complexes or Yersinia hsp60 plus interleukin-12. Infect Immun 1996; 64: 2955–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Lowrie DB, Silva CL, Colston MJ, Ragno S, Tascon RE. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine 1997; 15: 834–838. [DOI] [PubMed] [Google Scholar]
- 34Ferrero RL, Thilberge JM, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA 1995; 92: 6499–6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Segal BH, Wang XY, Dennis CG, Youn R, Repasky EA, Manjili MH et al. Heat shock proteins as vaccine adjuvants in infections and cancer. Drug Discov Today 2006; 11: 534–540 [DOI] [PubMed] [Google Scholar]
- 36Sagi SSK, Paliwal P, Bansal A, Mishra C, Khan N, Mustoori SR et al. Studies on immunogenicity and protective efficacy of DnaJ of Salmonella Typhi against lethal infection by Salmonella Typhimurium in mice. Vaccine 2006; 24: 7135–7141. [DOI] [PubMed] [Google Scholar]
- 37Paliwal PK, Bansal A, Sagi SSK, Sairam M, Govindaswamy I. Cloning, expression and characterization of heat shock protein 60 (GroEL) of Salmonella enteric serovar Typhi and its role in protective immunity against lethal Salmonella infection in mice. Clin Immunol 2008; 126: 89–96. [DOI] [PubMed] [Google Scholar]
- 38Bansal A, Paliwal PK, Sagi SS, Sairam M. Effect of adjuvants on immune response and protective immunity elicited by recombinant Hsp60 (GroEL) of Salmonella Typhi against S. Typhi infection. Mol Cell Biochem 2010; 337: 213–221. [DOI] [PubMed] [Google Scholar]
- 39Paliwal PK, Bansal A, Sagi SS, Sairam M. Intraperitonial immunization of recombinant Hsp 70 (DnaK) of Salmonella Typhi induces a predominant Th2 response and protective immunity in mice against lethal Salmonella infection. Vaccine 2011; 29: 6532–6539. [DOI] [PubMed] [Google Scholar]
- 40Chitradevi ST, Kaur G, Singh K, Sugadev R, Bansal A. Recombinant Heat shock protein 60 (Hsp60/GroEL) of Salmonella enterica serovar Typhi elicits cross-protection against multiple bacterial pathogens in mice. Vaccine 2013; 31: 2035–2041. [DOI] [PubMed] [Google Scholar]
- 41Triglia RP, Linscott WD. Titers of nine complement components, conglutinin and C3b-inactivator in adult and fetal bovine sera. Mol Immunol 1980; 17:741–748. [DOI] [PubMed] [Google Scholar]
- 42Taylor DN. The growing problem of antimicrobial resistance among enteric pathogens. Clin Updates Infect Dis 2003; 6: 1–4. [Google Scholar]
- 43Dutta D, Bhattacharya MK, Dutta S, Datta A, Sarkar D, Bhandari B et al. Emergence of multidrug-resistant Shigella dysenteriae Type 1 causing sporadic outbreak in and around Kolkata, India. J Health Popul Nutr 2003; 21: 79–80. [PubMed] [Google Scholar]
- 44Seidlein LV, Kim DR, Ali M, Lee H, Wang X, Thiem VD et al. A multicentre study of Shigella diarrhoea in six asian countries: Disease burden, clinical manifestations, and microbiology. PLoS Med 2006; 3: 1556–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Pazhani GP, Niyogi SK, Singh AK, Sen B, Taneja N, Kundu M et al. Molecular characterization of multidrug-resistant Shigella species isolated from epidemic and endemic cases of shigellosis in India. J Med Microbiol 2008; 57: 856–863. [DOI] [PubMed] [Google Scholar]
- 46Antoine B, Adjehi D, Nathalie G, Valerie G. Virulence factors and resistance profile of Shigella isolated during infectious diarrhea in Abidjan, Cote D'Ivoire. J Appl Sci Res 2010; 5: 594–599. [Google Scholar]
- 47Kweon M. Shigellosis: the current status of vaccine development. Curr Opin Infect Dis 2008; 21:313–318. [DOI] [PubMed] [Google Scholar]
- 48Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev 2001; 14: 430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Rasolofo-Razanamparany V, Cassel-Beraud AM, Roux J, Sansonetti PJ, Phalipon A. Predominance of serotype-specific mucosal antibody response in Shigella flexneri-infected humans living in an area of endemicity. Infect Immun 2001; 69: 5230–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Finkelmen FD, Katona IM, Mosmann TR, Coffman RL. Interferon γ regulates the isotypes of immunoglobulin secreted during in vivo humoral responses. J Immunol 1988; 140: 1022–1027. [PubMed] [Google Scholar]
- 51Le- Barillec K, Magalhaes JG, Corcuff E, Thuizat A, Sansonetti PJ, Phalipon A. Roles for T and NK cells in the innate immune response to Shigella flexneri. J Immunol 2005; 175: 1735–1740. [DOI] [PubMed] [Google Scholar]
- 52Jehl SP, Nogueira CV, Zhang X, Starnbach MN. IFN γ inhibits the cytosolic replication of Shigella flexneri via the cytoplasmic RNA sensor RIG-I. PLoS Pathog 2012; 8: e1002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53Way SS, Borczuk AC, Dominitz R, Goldberg MB. An essential role for Gamma interferon in innate resistance to Shigella flexneri infection. Infect Immun 1998; 66: 1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Raqib R, Ljungdahl A, Lindberg AA, Andersson U, Andersson J. Local entrapment of interferon-gamma in the recovery from Shigella dysenteriae type 1 infection. Gut 1996; 38: 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G et al. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect Immun 2012; 80: 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Martinez-Becerra FJ, Chen X, Dickenson NE, Choudhari SP, Harrison K, Clements JD et al. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine. Infect Immun 2013; 81: 4470–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57Heine SJ, Diaz-McNair J, Andar AU, Drachenberg CB, van de Verg L, Walker R et al. Intradermal delivery of Shigella IpaB and IpaD type III secretion proteins: kinetics of cell recruitment and antigen uptake, mucosal and systemic immunity, and protection across serotypes. J Immunol 2014; 192: 1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58Kurella S, Monacha M, Sabhnani L, Thomas B, Rao DN. New age adjuvants and delivery systems for subunit vaccines. Indian J Clin Biochem 2000; 15: 83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]