Abstract
γδ T cells form an important part of adaptive immune responses against infections and malignant transformation. The molecular targets of human γδ T cell receptors (TCRs) remain largely unknown, but recent studies have confirmed the recognition of phosphorylated prenyl metabolites, lipids in complex with CD1 molecules and markers of cellular stress. All of these molecules are upregulated on various cancer types, highlighting the potential importance of the γδ T cell compartment in cancer immunosurveillance and paving the way for the use of γδ TCRs in cancer therapy. Ligand recognition by the γδ TCR often requires accessory/co-stimulatory stress molecules on both T cells and target cells; this cellular stress context therefore provides a failsafe against harmful self-reactivity. Unlike αβ T cells, γδ T cells recognise their targets irrespective of HLA haplotype and therefore offer exciting possibilities for off-the-shelf, pan-population cancer immunotherapies. Here, we present a review of known ligands of human γδ T cells and discuss the promise of harnessing these cells for cancer treatment.
Keywords: cancer, γδ T cells, immunotherapy, phosphoantigens, T cell receptor
INTRODUCTION
The adaptive immune compartment comprises of three distinct cell subsets, namely B cells, T cells expressing an αβ T cell receptor (TCR) and T cells expressing a γδ TCR. These cells generate their defining B cell receptors (BCRs), or TCRs using somatic V(D)J recombination which enables them to recognise a vast spectrum of antigens. Despite the fact that gene segments used in rearrangement leading to αβ and γδ TCRs were discovered almost at the same time,1 the elucidation of αβ T cell biology progressed rapidly, while the complexities of γδ T cells have been slower to emerge. Nevertheless, the tripartite organisation of the lymphocytic immune system appears to be a fundamental requirement for efficient function, as this organisation is present both in jawed and jawless vertebrates, even though the manner of generating the defining receptors completely differs between these lineages.2 The unique functions of γδ T cells, particularly in terms of antigen recognition and kinetics of response, provide further evidence that γδ T cells represent an important and non-redundant lymphocyte subset.
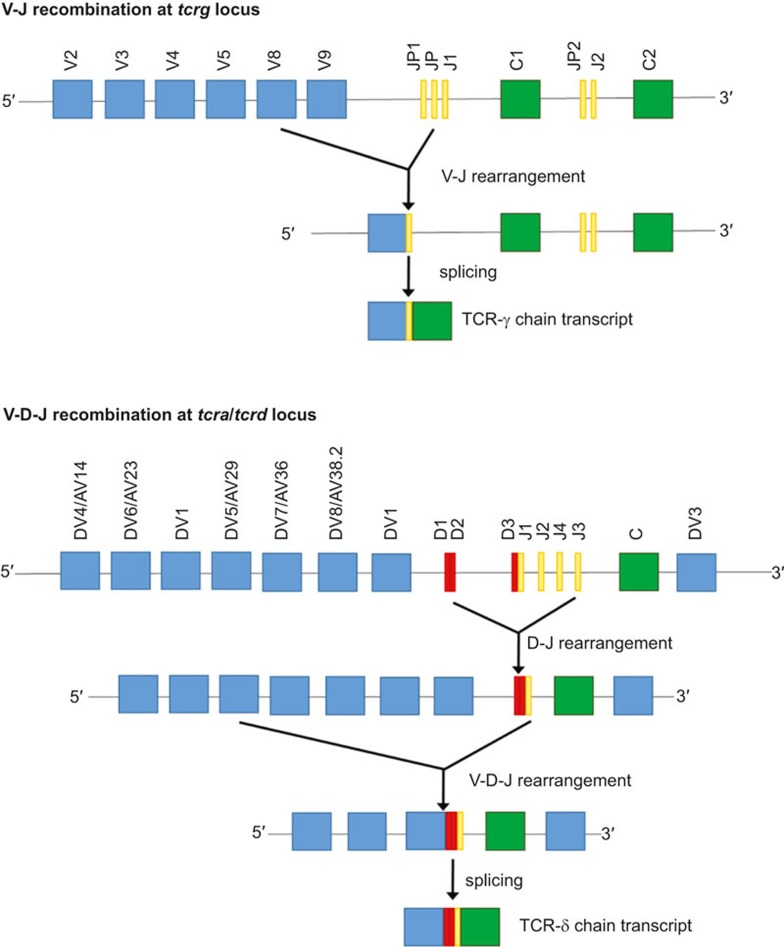
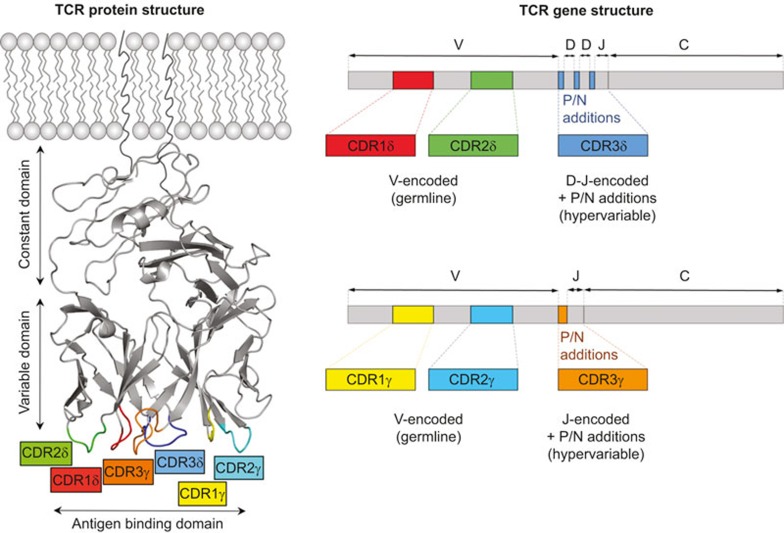
V(D)J recombination of the γ and δ chain genes is shown in Figure 1 (IMGT nomenclature3). There are 4–6 (depending on haplotype) functional variable (V)γ, and 8 Vδ gene segments in humans.4 Some Vδ segments can be used to generate both the α and δ chains of the TCR, as they are located within the tcra locus.5,6 The number of V segments that can be used for γδ T cells is much smaller than that for αβ T cells (46 Vα and 48 Vβ segments). However, the potential diversity of γδ TCR surpasses that of αβ TCR, owing to extensive N-region nucleotide additions and presence of distinct D segments (present only in tcrd but not tcrg locus) which can be used simultaneously and read in all three frames. This junctional variability results in the generation of hyperdiversity focused on the complementarity determining region (CDR)3 loops which are crucial for antigen recognition.7 Furthermore, the length of the CDR3s of both α and β chains is constrained, due to the requirement to make a well-defined contact with peptide-MHC complexes, while CDR3 in the δ chain is usually more variable and longer than its γ counterpart.8 With regard to CDR3 length, the γδ TCR resembles the BCR more than αβ TCR. This greater variability of γδ TCRs may translate into recognition of both proteins and smaller molecules. The CDRs form loops in the γδ TCR structure to provide a highly variable antigen-binding domain at the membrane-distal end of the molecule (Figure 2).
Figure 1.
V(D)J recombination at the tcrg (upper panel) and tcra/tcrd (lower panel) locus. Only the functional gene segments are shown. The TCR-γ chain is produced using only a single V-J recombination, with P/N additions occurring at the V-J junction. The TCR-δ chain is produced using V-D-J recombinations that can involve either 2 or 3 D segments, leading to the creation of up to 4 N diversity regions. For the clarity of the figure, only the gene segments that can be used in TCR-δ chain production are presented (lower panel). The organisation of loci tcrg and tcra/tcrd was adapted from IMGT database.3
Figure 2.
9C2 γδ TCR protein structure (left panel) and γ and δ chain mRNA architecture (right panel). The CDR loops are colour-coded. PDB ID: 4LHU.37
After arising from a common progenitor in the thymus, the maturation pathways of γδ and αβ T cells diverge. Notably, the development of γδ TCR+ thymocytes does not require the expression of Aire,9 a transcriptional regulator crucial for the negative selection of autoreactive αβ T cells. The mechanism by which T cells become committed to the αβ or γδ lineage is not yet fully understood as thymocytes rearrange β, γ and δ genes at the same time which can lead to simultaneous expression of the γδ TCR and pre-TCR (invariant Tα paired with TCR-β).10 However, recent evidence suggests that thymocytes adopt the γδ T cell lineage after receiving a strong signal via γδ TCR, which can be additively enforced by additional signalling via pre-TCR – thus enabling weak ligands to drive γδ T cell lineage commitment as well.11 If cells fail to receive this survival signal they silence the γδ TCR and undergo TCR-α rearrangement.12 This signal strength model implies that γδ T cells need to encounter a cognate ligand in the thymus. However, to date only one molecule, namely Skint-1, has been described as a thymically expressed ligand necessary for development of a subset of mouse γδ T cells.13 The identity of other ligands required for positive selection of γδ remains to be elucidated. Strong γδ TCR-mediated interactions in the thymus have been shown to result in upregulation of CD73, the earliest identified marker of γδ lineage commitment.14 CD73 is expressed by the vast majority of γδ T cells in the periphery, supporting the notion that recognition of the ligand in the thymus is a common occurrence in γδ T cell development. Another striking difference in development between αβ and γδ T cells is the acquisition of effector functions. Conventional αβ T cells acquire their effector phenotype, in terms of produced cytokines, upon interactions with their targets in the periphery, while γδ T cell functions, like their anatomical location, appear to be pre-determined in the thymus by the chain usage of their TCR.15
In humans, γδ T cells constitute 0.5–10% of T cells in peripheral blood but are substantially enriched in epithelial tissues (e.g. in skin, lungs, intestine). The majority of peripheral blood γδ T cells express the Vδ2 chain, while the tissue-resident γδ T cells are mainly Vδ1pos or Vδ3pos. The precise reasons for this tissue specificity and the mechanisms that underlie it are yet to be elucidated. The role of γδ T cells in infection is well established and has been amply reviewed elsewhere.16 More recent discoveries suggest that γδ T cells play a role in anticancer immunity, and it has been known for over a decade that mice lacking the γδ TCR are more susceptible to some cancers through unknown mechanisms.17 Here, we review recent advances in discovering cancer-associated antigens recognised by human γδ T cells and the evidence that there is a broad spectrum of γδ T cell ligands waiting to be discovered. We also provide a brief overview of therapeutic applications of γδ T cells in cancer immunotherapy.
γδ TCR RECOGNITION OF CANCER-ASSOCIATED ANTIGENS
Known ligands of human γδ TCR are scarce when compared with the vast spectrum of antigens recognised by αβ T cells. However, significant progress in this area has been made in the recent years, linking the observed recognition of tumour cells to specific ligands confirmed by biochemical and biophysical data. The main targets encompass phosphorylated prenyl antigens, endo- and exogenous lipids presented by CD1-family proteins, and cell stress molecules that can indicate DNA damage, viral infection or malignant transformation.
Recognition of phosphoantigens
Phosphorylated isoprenoid metabolites, commonly referred to as phosphoantigens (PAgs), can be produced by both bacterial and eukaryotic cells, using non-mevalonate and mevalonate biosynthetic pathways, respectively. The accumulation of PAgs, such as isopentenyl pyrophosphate (IPP), is a result of metabolic dysregulation that commonly occurs in tumour cells18 and therefore the enzymes of the mevalonate pathway are a valid target for anticancer drugs (reviewed in Clendening and Penn19). Importantly, PAgs have long been known to be recognised by γδ T cells expressing Vγ9Vδ2 TCR. The presence of this peripheral blood subset of γδ T cells is restricted to higher primates, with a few exceptions.20 Importantly, mice and other rodents do not possess any corresponding T cell subsets that respond to PAgs.
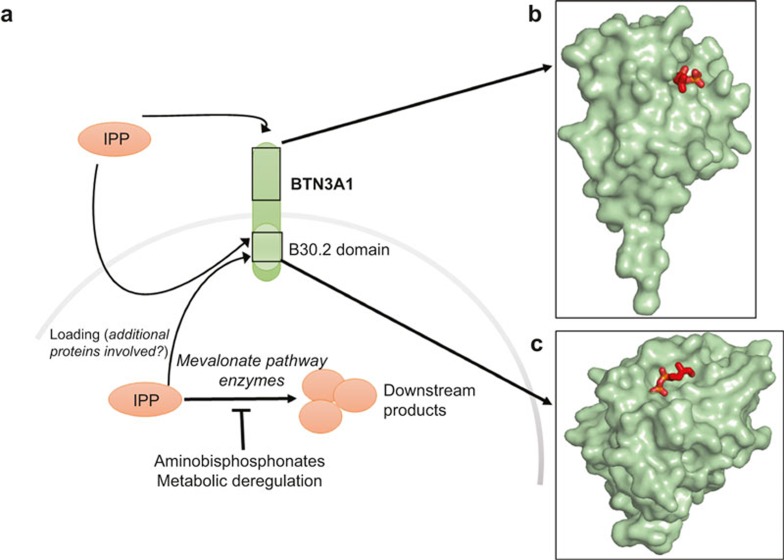
The mode of PAg recognition has only recently been resolved. Early studies indicated that recognition of PAgs by human Vγ9Vδ2 T cells required cell surface presentation by a species-specific molecule.21 One of the proposed molecules presenting the antigens was F1-ATPase which is expressed on the surface of a wide range of tumour cells.22 Further research suggested that PAgs could bind F1-ATPase in the form of a nucleotide derivative, increasing the efficiency of T cell activation. However, as antibody blocking of F1-ATPase did not abrogate T cell recognition, other molecules were thought to be involved in PAg presentation.23 The cell surface-expressed Butyrophilin molecules are encoded within the MHC class I locus and offer attractive potential candidates as PAg-presenting molecules. Butyrophilin-3A (BTN3A/CD277) is present in humans in three isoforms (BTN3A1, BTN3A2 and BTN3A3).24 Recent studies have shown that antibodies specific for BTN3A1 could either mimic PAg-mediated activation of the TCR (antibody 20.1) or abrogate this stimulatory effect (antibody 103.2).25 Biophysical analysis of the underlying interactions suggested that 20.1 antibody induced/stabilised a TCR-activating conformation of BTN3A1.26 Additionally, PAgs and 20.1 antibody activated the same intracellular signalling pathways in the responding γδ T cells, suggesting a common recognition process.27 Interestingly, transduction studies revealed that while the expression of BTN3A1 alone is sufficient for the activation mediated by 20.1, additional genes located on chromosome 6 are required for PAg-mediated recognition.28 These studies suggest the possible existence of molecules responsible for efficient loading of PAgs into butyrophilin-3A1 in a conceptually similar manner to loading peptides into MHC, or that there is a requirement for a co-stimulatory ligand. Two main models explaining the role of BTN3A1 have been proposed, involving intracellular or extracellular binding of pyrophosphate antigens (Figure 3a). Sandstrom et al. demonstrated that BTN3A1 acts as a sensor of intracellular PAgs by binding them in a surface pocket located in an intracellular domain termed B30.2.29 This result was confirmed by the crystal structure of the B30.2-PAg complex (Figure 3c) and by mutational analysis. Introduction of the B30.2 domain of BTN3A1 into the non-activating isoform BTN3A3 was shown to confer PAg binding and T cell stimulation. This result falls in line with observations by Wang et al. who demonstrated a lack of high-affinity binding of PAgs to the extracellular regions of BTN3A1.30 In direct contrast, De Libero and colleagues resolved the crystal structure of two PAgs (IPP and HMBPP) in complex with the extracellular domain of BTN3A1 where the antigen was bound in a shallow surface pocket (Figure 3b).31 The binding affinity of Vγ9Vδ2 TCR to BTN3A1 was relatively weak (KD < 0.1 mM) but increased in the presence of IPP. These two mechanisms need not be mutually exclusive and it remains possible that PAgs bind to both the intracellular and extracellular domains of BTN3A1 to provide a failsafe against aberrant TCR triggering and autoimmunity. The binding affinities of IPP to BTN3A1 and the Vγ9Vδ2 TCR interaction with BTN3A1-IPP are summarised in Table 1. Further investigation will be required to determine the exact mechanism of antigen presentation by BTN3A1.
Figure 3.
(a) Schematic representation of the phosphoantigen presentation pathways by human cells showing phosphoantigen (IPP) binding in the extracellular pocket of BTN3A1 (b) and phosphoantigen (cHDMAPP) binding in the intracellular domain (B30.2) of BTN3A1 (C). PDB IDs: 4JKW (B)31 and 4N7U (C).29
Table 1. The interaction affinities of IPP binding to the intracellular or extracellular domains of BTN3A1, and of TCR binding to BNT3A1 in presence of IPP.
| Interaction | KD (μM) | Ref. |
|---|---|---|
| IPP: extracellular BTN3A1 | 69.9 | 31 |
| IPP:B30.20 | ≈500 | 29 |
| Vγ9Vδ2 TCR:BTN3A1(IPP) | 340 | 31 |
γδ T cells recognise specific ligands in the context of antigen-presenting molecules from the CD1 family
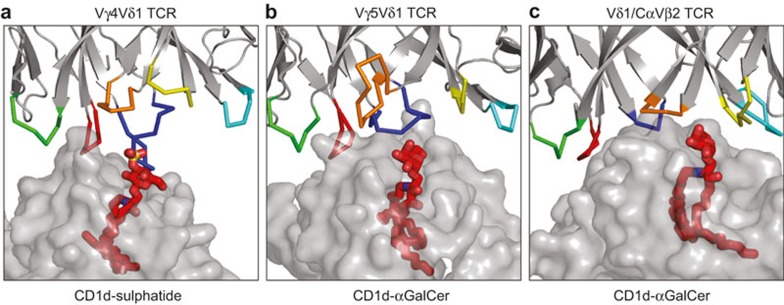
Four members of the human CD1 family of proteins (CD1a–CD1d) present both endogenous and exogenous lipids that can be recognised by both αβ and γδ T cells.32 Early reports demonstrated that mucosal γδ T cell clones showed broad reactivity towards these four CD1 isoforms loaded with exogenous phospholipids.33 Further studies showed that T cells expressing the Vδ3 chain were capable of killing tumour cells expressing CD1d (but not other CD1 isoforms) without addition of exogenous lipids.34 Additionally, the γδ T cell compartment of peripheral blood was shown to recognise CD1d in complex with self-lipids termed sulphatides (sulphated galactosylceramides).35 CD1d-reactive γδ T cells expressed similar Vδ1-Jδ1 chains paired with different Vγ chains. Notably, some of the generated clones could bind CD1d without any loaded lipid. Recent studies provided the structural basis for CD1d-sulphatide recognition, demonstrating that the binding occurred solely via the δ chain of the TCR – the germline-encoded residues made contact with the CD1d molecule whereas the CDR3δ loop interacted with sulphatide (Figure 4a).36 As the CDR3 region is the most variable part of the γδ TCR, it is possible that a subset of γδ T cells can recognise diverse lipid cargoes presented by CD1 molecules, in a similar way to αβ TCR recognition of peptides bound to MHC. A similar mode of interaction was reported for CD1d-α-galactosylceramide (αGalCer) and a Vδ1 TCR (9C2) where CD1d was bound by germline-encoded residues in the δ chain while the lipid was recognised by the hypervariable CDR3γ loop (Figure 4b).37 Interestingly, Pellicci et al. have recently discovered a novel T cell subgroup recognising CD1d-αGalCer complex, expressing a Vδ1 domain linked to permissive Jα-Cα segments, paired with diverse TCR-β chains (termed δ/αβ T cells).38 Again, the binding of the TCR to CD1d was mainly driven by germline-encoded residues in the Vδ1 domain while the specific recognition of the bound lipid was exclusively mediated by the β chain of the TCR (Figure 4c). The affinity of the interaction between TCRδ/αβ and CD1d-αGalCer was, however, much higher than described for 9C2 γδ TCR (the binding affinities of TCRs to CD1d complexed with lipid ligands are summarised in Table 2). Importantly, functional δ/αβ T cells comprise a high proportion of human CD1d-reactive T cells. αGalCer has been so far investigated only in context of boosting NK T cell anticancer activity,39 but it could potentially be used to activate αGalCer-reactive γδ T cells, similarly to using PAgs. Notably, a recent study showed that small quantities of αGalCer and other α-glycosylceramides can be endogenously produced in mammalian species to modulate the immune response.40 Additionally, CD1d may be an important target for cancer immunotherapy as its high expression was reported on chronic lymphocytic leukaemia cells, correlating with disease progression.41 Similarly, CD1c on acute leukaemia cells was shown to present a novel class of immunogenic self-lipids, called methyl-lysophosphatidic acids (mLPAs). Recognition of CD1c-mLPA complex by γδ T cells has yet to be demonstrated,42 but recent studies showing that CD1 family members can present self-lipids make these molecules attractive targets for future exploration.
Figure 4.
Complex formation between TCR and CD1 ligands. CDR loops are colour coded as in Figure 2: CDR1δ-red, CDR2δ-green, CDR3δ-blue, CDR1γ-yellow, CDR2γ-cyan and CDR3γ-orange. The blue loop in panel C is CDR3δ/α. PDB IDs: 4MNG (A),36 4LHU (B),37 4WO4 (C).38
Table 2. The interaction affinities of TCR binding the CD1d-lipid complex.
| TCR | Ligand | KD (μM) | PDB code | Ref. |
|---|---|---|---|---|
| Vγ4 Vδ1 | CD1d-sulphatide | 5.6 | 4MNG | 36 |
| Vγ5 Vδ1 | CD1d-αGalCer | 16 | 4LHU | 37 |
| Vδ1/Cα Vβ2 | CD1d-αGalCer | 0.066 | 4WO4 | 38 |
γδ TCR recognition of general stress ligands
γδ T cells are known to expand in response to some viral infections, with cytomegalovirus (CMV) providing the best-studied example to date. CMV infection results in preferential expansion of γδ T cells expressing Vδ1, Vδ3 and Vδ5 (commonly termed ‘non-Vδ2 T cells').43,44 These cells can also recognise and lyse intestinal tumour cells, in line with the fact that they are enriched in epithelial tissues where they perform stress surveillance.44 Interestingly, infection with CMV, regarded as a treatment-related complication in kidney transplant recipients, serves a protective role against cancer in those patients.45 Taken together with the fact that Vδ2neg compartment is selectively expanded in CMV carriers and does not diminish with age, it is possible that CMV-mediated activation of γδ T cells confers an adaptive-like, lifelong reduction of cancer risk.46
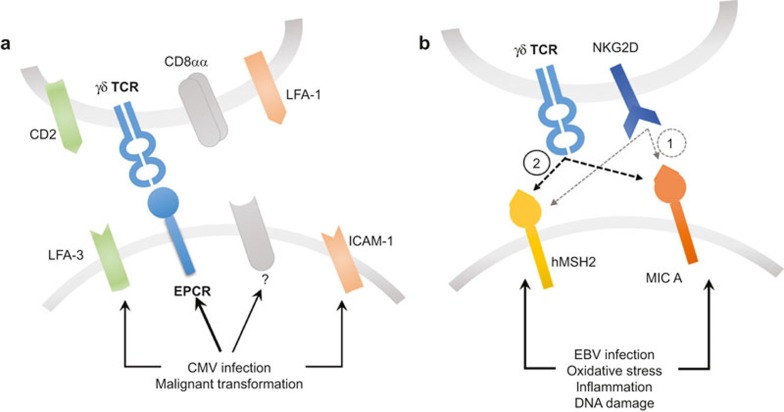
The ligands of dual reactive γδ T cell clones recognising CMV-infected and transformed cells remain unknown, with one notable exception. Recently, Déchanet-Merville and colleagues identified the ligand of one of such clone, named LES. The LES γδ T cell clone expressed Vγ4 and Vδ5 TCR chains and was substantially enriched (25% of peripheral blood T cells) in a CMV-infected transplant recipient. LES was shown to recognise a range of solid tumour lines and CMV-infected cells via its TCR rather than natural killer (NK)-type receptors. The LES TCR was demonstrated to bind endothelial protein C receptor (EPCR).47 EPCR exhibits sequence and structural homology with the MHC-like protein family CD1, and can present phospholipids bound in the antigen-presenting groove.48 EPCR plays a dual role in cancer, as it can both promote and inhibit metastases, presumably depending on whether it is expressed on tumour cells or endothelium (reviewed in Mohan Rao et al.49). Additionally, EPCR signalling can activate antiapoptotic or proapoptotic pathways through unresolved mechanisms. Importantly, direct binding of the LES TCR to EPCR was confirmed by surface plasmon resonance, showing relatively low binding affinity (KD ≈ 90 µM) (Table 3). EPCR, however, does not appear to be a common, innate-like target for dual reactive γδ T cell clones, as the recognition of the tumour lines by such clones, isolated from other patients, was not abrogated by antibody blocking of EPCR. Moreover, the LES TCR recognition was mediated by the hypervariable CDR3 loop of the Vγ4 chain, supporting the hypothesis that EPCR binding was part of an adaptive response. Interestingly, the expression of EPCR was not sufficient to induce T cell activation, suggesting that functional recognition required a cell stress context and additional co-stimulatory molecules. Indeed, CMV infection conferred the recognition of fibroblasts and endothelial cells without altering EPCR expression suggesting that recognition of this receptor occurs in a stress-related co-stimulatory context. The identity of these co-stimulatory ligands remains unknown.
Table 3. γδ TCR and NKG2D binding affinities to stress-related ligands EPCR, hMSH2 and MIC A.
| Receptor | Ligand | KD (μM) | Ref. |
|---|---|---|---|
| Vγ4 Vδ5 TCR (LES) | EPCR | 96 | 47 |
| Vδ2 TCRs | hMSH2 | N/A | 54 |
| NKG2D | hMSH2 | 0.132 | 54 |
| Vγ4 Vδ1 TCR | MIC A | 110–900 | 70 |
| NKG2D | MIC A | 0.3–21 | 70 |
EPCR is not the only cell stress marker that requires an additional co-stimulatory context for efficient recognition by γδ T cells. Other examples described so far encompass the human homologue of the bacterial MutS (hMSH2) protein,50 being a part of the DNA mismatch repair system (MMR), and MHC class I polypeptide-related sequence A (MIC A),51 a broadly recognised stress marker. Both molecules are recognised by both highly variable γδ TCR and the invariant NKG2D receptor (discussed in the next section). hMSH2 is a key protein involved in repairing mutations resulting from DNA recombination or replication. Unsurprisingly, defects in hMSH2 have been reported in various tumour histologies including lung, colon, breast and prostate cancers.52 Although hMSH2 is normally restricted to the nucleus, it can be translocated during cellular stress.53 Recent studies have demonstrated that hMSH2 can be a ligand for some Vδ2 γδ T cells when it is ectopically expressed, inducing cytotoxicity and IFN-γ production.54 Surface expression of hMSH2 can be induced by Epstein-Barr virus (EBV) infection,54 oxidative stress and proinflammatory cytokines such as IL-18 corroborating its role in generalised stress.
Interestingly, oxidative stress promoted the expression of MIC A in a similar manner to hMSH2, involving p38/JNK signalling pathways.55 Another group reported MIC A/B upregulation resulting from ataxia telangiectasia mutated (ATM) protein kinase signalling in response to DNA damage, contributing to γδ T cell-mediated lysis of ovarian cancer cell lines.56 Additionally, the level of MIC A expression on breast cancer cell lines correlated with their susceptibility to γδ T cell-mediated cytotoxicity.57 Induction of surface expression of MIC A/B was also reported in primary glioblastoma after chemotherapy.58 EBV infection induces MIC A expression as well, showing further similarities between expression of MIC and hMSH2.
γδ TCRs RECOGNISE TUMOURS IN A CO-STIMULATORY STRESS CONTEXT
As mentioned above, the recognition of cell stress markers by γδ T cells appears not to be driven solely by the interaction between the TCR and its cognate ligand but rather requires a wider stress context provided by co-stimulatory receptors on the T cell and additional self-antigens. In the case of the LES clone recognising EPCR, part of the co-stimulatory effect was provided by LFA-1 on the T cells binding to ICAM-1 (overexpressed as a result of CMV infection).47 CD2 interaction with CD58 (LFA-3) was also implicated. Blocking of TCR binding to EPCR completely abrogated the recognition while disrupting the ICAM-1-LFA-1 and CD2-CD58 axes merely decreased T cell activation, demonstrating the dominance of TCR signalling and the possible co-existence of complementary co-stimulatory pathways. Furthermore, another group showed that the CD8αα homodimer could act as a coreceptor for recognition of CMV-infected cells by Vδ1 TCR chains (Figure 5a).59 This observation is in line with the findings that T cells expressing CD8αα play an important role in immunosurveillance of the epithelial tissues against viral60 and bacterial61 infections.
Figure 5.
Recognition of γδ TCR ligands requires a cell stress context. (a) Recognition of EPCR requires co-stimulatory ligands on the surface of the target cells, and accessory molecules on the surface of the T cell. (b) Both hMSH2 and MIC A become upregulated and ectopically expressed in response to cell stress stimuli and are dually recognised by NKG2D and TCR. The sequential model of recognition implies that the initial contact provided by a transient high-affinity NKG2D–ligand interaction (1) is followed by formation of a stable low affinity TCR-ligand complex (2).
The requirement for co-stimulation is well established for αβ T cells and it has been known for some time that CD8αβ heterodimer acts to co-receive peptide-MHC class I, stabilising the TCR interaction62 and ensuring full phosphorylation of the CD3ζ chain63 to increase sensitivity to antigen by up to a million-fold64 (reviewed in Cole et al.65). Thus the αβ T cell co-receptors, CD4 and CD8, ensure that this T cell subset is MHC-restricted66,67 and determine the fate of the developing T cell.68 In an analogous fashion, co-stimulation via stress-induced ligands might maintain the correct focus and function of the γδ T cell compartment. In keeping with this concept, both hMSH2 and MIC A/B are dually recognised by cognate TCRs and the NKG2D receptor (Figure 5b). NKG2D is commonly expressed on the surface of NK cells, αβ T cells and γδ T cells, and binds to cell stress molecules from the UL16-binding protein (ULBP1–6) family (reviewed in Ullrich et al.69). The interactions of germline-encoded NKG2D with such diverse ligands are possible because of conserved fragments within the α1α2 superdomain in the ligands. The involvement of the γδ TCR and NKG2D in recognition of the MIC A molecule has recently been studied. Xu et al. showed that both TCR and NKG2D bound overlapping fragments of MIC A, with different affinity and kinetics – for NKG2D, the affinity was much higher than for the TCR (Table 3).70 Nevertheless, the complex between TCR and MIC A was particularly stable, suggesting a sequential model of binding where the initial contact by NKG2D is followed by the more stable TCR:MIC A complex. The role of TCR chains in complex formation remains to be fully resolved as the studies implicated partial contributions of the germline-encoded CDR loops from Vδ1 TCR70 while other studies showed that MIC A-associated recognition of tumour cells could be mediated by both Vδ171 and Vδ256,57,72 TCRs. Notably, TCR engagement has been shown to be indispensable for γδ T cell-mediated cytotoxicity whereas NKG2D played only a co-stimulatory role; thus indicating the importance of TCR:MIC A complex formation.73 ULBP molecules may be recognised in a similar manner as it has been shown that ULBP-4 engages both NKG2D and Vγ9Vδ2 TCRs.74
The sequential recognition of different targets by γδ T cells could therefore play an important role in immunosurveillance as it allows the T cell to rapidly scan the target cells for the signs of stress, indicating a possible infection or transformation. The requirement for a multicomponent stress context for full T cell activation could then provide fail-safe protection against autoimmunity. The apparent co-existence of diverse co-stimulatory axes decreases the chances of immune evasion.
HARNESSING γδ T CELLS FOR THERAPY
Stimulating γδ T cells in vivo with phosphoantigens
As mentioned above, the Vγ9Vδ2 T cell subset that predominates in human peripheral blood is capable of responding to prenyl pyrophosphates. This feature has been employed to redirect Vγ9Vδ2 T cells to tumours by manipulating isoprenoid metabolism in the cancer cells. Such manipulation can be achieved using aminobisphosphonates (e.g. zoledronate, pamidronate, risedronate) which are structural analogues of prenyl pyrophosphates that contain an amino moiety. As a result, aminobisphosphonates act indirectly by inhibiting farnesyl pyrophosphate synthase (FPPS) in the mevalonate pathway leading to the accumulation of prenyl pyrophosphate substrates.75,76 Recently, Idrees et al. screened over 50 tumour cell lines, showing a direct correlation between zoledronate-induced FPPS inhibition and tumour recognition by Vγ9Vδ2 T cells.77 The FPPS-inhibiting concentration of zoledronate was on average two orders of magnitude lower than the concentration required for inhibition of tumour proliferation, thus indicating that T cell activation was not the result of cell death. Another group showed that risedronate caused PAg accumulation in the tumour cells which were then recognised by Vγ9Vδ2 T cells.78 Upon recognition, T cells produced IFNγ which caused upregulation of ICAM-1 on tumour cells, contributing to a positive feedback loop and subsequent cytotoxicity. Additionally, aminobisphosphonates,79 as well as conventional chemotherapy, can sensitise the neoplastic cells to cytototoxic activity of Vγ9Vδ2 T cells. This may be an attractive therapeutic option for eradication of cancer-initiating cells, which are often resistant to conventional therapy and can contribute to the relapse of the disease. Finally, γδ T cells are known to acquire the phenotype of professional antigen-presenting cells upon activation.80 Therefore, they may be used as a potent anticancer vaccine in addition to their broad antineoplastic cytotoxicity.
Redirecting T cells to tumours
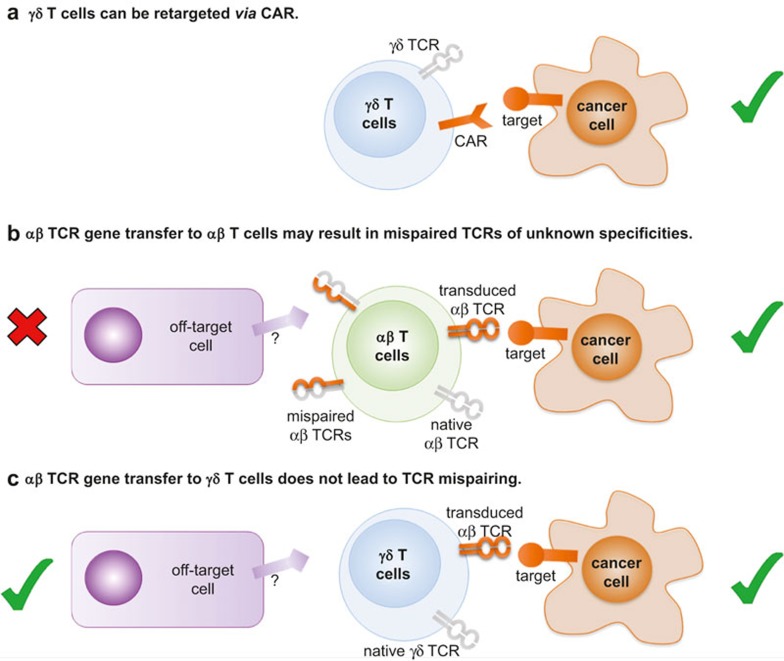
In the absence of known ligands, γδ T cells can be redirected to tumours using antibodies. The efficacy of bispecific antibodies, where one part recognised a tumour surface marker (for example, EpCAM on liver tumours or HER2/neu on pancreatic cancer) while the other binding site targets CD3 or the Vγ9 chain of the TCR, has been demonstrated in pre-clinical models.81,82 An interesting approach was described by Zheng et al. where they cloned out the extracellular domains of a Vγ9Vδ2 TCR from ovarian cancer tumour-infiltrating lymphocytes (TILs) and conjugated them with Fc domain of human IgG1.83 This bispecific construct bound to a range of ovarian cancer cells, recognising an unknown ubiquitous antigen, and mediated the killing of the cells via antibody-dependent cellular cytotoxicity (ADCC). ADCC can be mediated by binding of CD16 (FcγRIII) to the Fc region of IgGs, constituting yet another means of target recognition by γδ T cells, in addition to TCR and NKG2D. CD16 can be upregulated on both Vδ2pos and Vδ2neg T cells, depending on circumstances, while binding of its target may trigger either cytotoxicity or other effector functions (e.g. IFNγ secretion).84,85 A similar approach involves transducing T cells with chimeric antigen receptors (CARs; Figure 6). CARs are usually derived from single chain Fv fragments of antibodies, thus enabling the CAR-transduced T cells to recognise conformational epitopes independently of their TCR (recently reviewed in Maus et al.86). To date, most CAR transduction experiments have been conducted on αβ T cells – nevertheless, γδ T cells are also an appealing target, due to their broad antitumour cytotoxicity and numerous effector functions. Recently, Deniger et al. have transduced polyclonal γδ T cells with a CD19-specific CAR, demonstrating their efficacy in killing CD19+ leukaemia lines.87 CAR-mediated signalling resulted in a similar cytokine secretion profile as TCR activation, and induced unbiased expansion of γδ T cell subsets. Notably, CAR technology has been combined with the generation of induced pluripotent stem cells (iPSC) from human peripheral blood T cells.88 Such cells exhibited a phenotype closest to γδ T cells of all lymphocyte lineages and exerted similar in vivo antitumour activity to CAR-transduced γδ T cells.
Figure 6.
Genetic modification of γδ T cells for adoptive therapy approaches to cancer. (a) γδ T cells can be redirected to kill cancer cells using a chimeric antigen receptor (CAR) made from an antibody that targets a tumour-specific molecule at the cancer cell surface. (b) αβ T cells can be redirected to kill cancer cells by transducing them with a cancer-specific αβ TCR. Such αβ TCR gene transfer could result in the expression of up to four different αβ TCRs at the T cell surface: (i) the endogenous TCR; (ii) the transduced TCR; (iii) a hybrid TCR consisting of the endogenous TCRα chain paired with the transduced TCRβ chain; and (iv) endogenous TCRβ chain paired with the transduced TCRα chain. Neither hybrid will have undergone the rigours of thymic selection and therefore these TCRs have the potential of being autoreactive. (c) Transduction of γδ T cells with an αβ TCR provides a means of circumventing the potential mispairing problem seen in b.
Finally, T cells can be redirected to tumours by introducing an exogenous TCR of known anticancer specificity into patient-derived peripheral lymphocytes prior to adoptive transfer of these cells. Most TCR gene studies have involved transduction of αβ TCRs into αβ T cells.89 However, this strategy comes with the inherent risk of αβ TCR mispairing between the endo- and exogenous TCR chains, resulting in receptors of unknown and potentially autoreactive specificities (Figure 6b). Such autoreactivity has been observed both in ex vivo human samples90 and in mouse models.91 γδ T cells offer an attractive way to circumvent this problem as tumour-reactive αβ TCRs can be introduced into these cells without the risk of mispairing (Figure 6c).92,93 Additionally, γδ T cells transduced with αβ TCRs retain the functionality of their original TCR and respond to stimuli transferred via either TCR with rapid, γδ-like kinetics.94 The main obstacle associated with αβ TCR transfer, however, is that γδ T cells usually do not express CD4 or CD8 co-receptors required for the efficient recognition of peptide-MHC. Thus efficient function might require co-transduction with a coreceptor95 or use of enhanced affinity TCRs.96 It is also possible to transduce peripheral lymphocytes (both of γδ and αβ origin) with a specific γδ TCR. Zhao et al. demonstrated that T cells transduced with a Vγ9Vδ2 TCR, modified with a CDR3δ loop specific for unidentified antigens on ovarian carcinoma, exerted an anticancer activity in vivo.97
Similarly, the transduction of a PAg-reactive Vγ9Vδ2 TCR into αβ T cells successfully redirected them towards cancer cells, as well as led to downregulation of their endogenous αβ TCRs, thus abrogating alloreactive responses.98 It is therefore likely that the detailed characterisation of cancer-specific γδ TCRs will bring about new studies examining the efficacy of such TCRs in the TCR gene transduction setting.
Generating clinically relevant numbers of tumour-reactive γδ T cells
The main hurdle that needs to be overcome in order to use γδ T cells as a therapeutic is obtaining these cell in clinically meaningful numbers. For αβ T cell-based therapies, this is a relatively straightforward task as the requirements for effective αβ T cell expansion have been widely studied, and the cognate ligands can be readily used to stimulate the expanding cells.99 In the case of γδ T cells, the only known common ligands are PAgs which are recognised by the Vγ9Vδ2 subset. As aminobisphosphonates such as zoledronate cause accumulation of PAgs in the cells, they can be used to preferentially expand that T cell subset both ex vivo, prior to re-infusion, and directly in patients. However, PAgs and aminobisphosphonates expand only the Vγ9Vδ2 subset of γδ T cells, and this bias may have a negative impact on clinical efficacy. In an alternative approach, immobilised anti-γδ TCR antibody has been demonstrated to promote the expansion of all γδ T cell subsets, without modulating their antitumour function.100,101 This method, however, may lead to mitogen-induced T cell death and has not yet been used in a published clinical trial. Deniger et al. have recently proved that T cells with polyclonal γδ TCR repertoires can be expanded to large numbers (>109) using IL-2, IL-21 and clinical grade artificial antigen-presenting cells, engineered to express co-stimulatory molecules CD19, CD64, CD86, CD137L and membrane-bound IL-15.102 Notably, the antigen-presenting cells were derived from a tumour line K562, broadly recognised by γδ T cells, and γ-irradiated prior to T cell exposure. The irradiation step could potentially enhance the recognition even further, as it leads to expression of cell stress molecules. Polyclonal γδ T cell lines showed superior toxicity compared to those expressing only Vδ2 chains, thus highlighting the possibility of enhancing γδ T cell-focused immunotherapy. Similarly, Anderson and colleagues used the same antigen-presenting cell line to generate unbiased γδ T cell products from peripheral blood of cancer patients.84
Finally, manipulations of the CD3 co-receptor can be applied to increase therapeutic efficacy of γδ T cells. Dopfer et al. recently showed that two anti-CD3 antibodies affect the functional activity of γδ T cells differentially.103 The reported results indicated that an anti-CD3 antibody, OKT3, induced strong proliferation and cytokine secretion with decreased cytotoxic potential, while another anti-CD3 antibody, UCHT1, enhanced tumour killing via Ras/Erk and PI3K/Akt pathways, without an increase in cytokine secretion or proliferation. These results might pave the way for new therapeutic approaches focused on manipulation for CD3-mediated signal transduction to favour expansion of large numbers of γδ T cells, with desired effector functions such as cytotoxicity.
Lessons learned from clinical trials
Numerous attempts to use γδ T cells in cancer immunotherapy have been made over the past decade, with variable efficacy and a good overall safety profile.
Two recent trials investigated the anticancer effect of zoledronate combined with low dose IL-2. In renal cell carcinoma, the combined treatment resulted only in a minor expansion of Vδ2pos cells which diminished after repeated treatment cycles without achieving the objective response in any of the enrolled patients.104 A similar study by Kunzmann and colleagues showed no objective response in patients with solid tumours (melanoma and renal cell carcinoma), and a partial response in acute myeloid leukaemia patients.105 Surprisingly, the treatment resulted in increased vascular endothelial growth factor levels, an indication of augmented angiogenesis, having a negative impact on the therapeutic outcome. No severe side effects (apart from grade 4 fever) were observed. A recent phase IV clinical trial of zoledronate in cancer-free patients demonstrated that the severity of transient inflammation-related side effects, caused by the treatment, could easily be predicted by ex vivo measurements of IFN-γ production by zoledronate-activated peripheral blood cells.106 Notably, repeated zoledronate treatment led to the depletion of the central memory subset of blood Vγ9Vδ2 T cells, as well as a long-lasting decrease of their absolute count.106,107 This phenomenon may be caused by peripheral blood neutrophils which have been shown to ingest zoledronate and subsequently inhibit the proliferation of the T cells by production of hydrogen peroxide.108 Repeated stimulation with PAgs, either accumulated in response to aminobisphosphonates or as a result of an accelerated mevalonate pathway, can also lead to a differentiation shift of Vγ9Vδ2 T cells and functional exhaustion.109 Zoledronate therefore appears to be insufficient to generate an effective antitumour response per se – nevertheless, two multicentre studies demonstrated that treatment with zoledronate combined with chemotherapy improved the survival of breast cancer and multiple myeloma patients.110,111
The adoptive cell transfer (ACT) of ex vivo expanded γδ T cells appears to be a more effective treatment option than using zoledronate as a single agent. In metastatic renal cell carcinoma, two groups reported re-infusing autologous PAg-expanded Vδ2pos T cells.112,113 When the T cells were infused with IL-2 only, 6 out of 10 patients experienced stabilisation of the disease whereas the infusion of the cells with both IL-2 and zoledronate resulted in 5 out of 10 disease stabilisations and 1 complete response. However, as both studies included only a small number of patients, it is possible that the observed superior efficacy of using both IL-2 and zoledronate was purely coincidental. When cells were expanded using IL-2 and zoledronate but infused without further exogenous stimulation, the partial response was observed in 3 out of 10 patients with various solid tumours.114 As the patients were treated with chemotherapy together with ACT, the response could be contributed to the additive/synergistic effect of the combination therapy. A similar effect was observed by Nicol and colleagues as the complete response (1/17) and partial response (2/17) patients received both cell infusion and chemo/hormone therapy.115 However, the patient cohorts in these studies were small and highly diverse, in terms of cancer type, additional therapy and composition of the T cell infusion. These variables make it difficult to assign the exact clinical contribution of γδ T cells. ACT of γδ T cells and other cytotoxic cell types can also increase the efficacy of radiofrequency ablation.116 The aforementioned trials utilised patients' autologous peripheral blood T cells for ACT – however, in some instances the effector functions of autologous T cells are severely impaired.109 A recent study by Wilhelm et al. demonstrated that it is possible to infuse patients with a γδ T cell product obtained from haploidentical donors, with no evidence of graft versus host disease, showing substantial clinical efficacy (3 out of 4 patients with advanced haematological malignancies experienced complete regression).117 The donor γδ T cells persisted for 28 days and expanded in vivo, in response to exogenous IL-2 and zoledronate. Another group also showed that prolonged persistence of infused γδ T cells can occur without IL-2 supplementation, probably relying on endogenous IL-15.118 Moreover, exogenous IL-18 can be a potent co-stimulator of γδ T cell proliferation.119,120
Overall, while γδ T cell therapy has a good safety profile, clinical performance to date has been underwhelming.121 Anticipated discoveries of the various ligands recognised on tumour cells by the Vδ2neg T cell subset will lead to a better understanding of how these cells operate and an increased capacity to beneficially harness these mechanisms for immunotherapy.
CONCLUSIONS AND FURTHER DIRECTIONS
The evolutionary conservation of γδ T cells in all jawed vertebrates and of an analogous third lymphocyte lineage in their jawless ancestors2 attests to the critical importance of these cells in the maintenance of immune integrity. γδ T cells are known to play an important role in infection16; further recent discoveries have shown that this T cell lineage can respond to stress signals and thereby play an important role in cancer immunosurveillance. Indeed, mice lacking the γδ TCR have increased cancer risk.17 Despite the importance of γδ T cells, very few ligands for the human γδ TCR have been confirmed by biochemical and biophysical data and this field is still in its infancy. Interestingly, certain similarities exist between the few known human γδ TCR ligands that might point to some generalised mechanisms by which these cells patrol the periphery. BTN3A1, MIC A/B and CD1-family proteins belong to the immunoglobulin superfamily (while EPCR is highly homologous to CD1d), and both infections and cellular stress seem to play a role in activating γδ T cells via these immunoglobulin structures. Microbial infections lead to a stimulatory conformational change and/or direct metabolite presentation by BTN3A1 while herpesvirus (CMV, EBV) infections cause upregulation of EPCR, MIC A and hMSH2, respectively. Similarly, cell stress, including neoplasms, can involve deregulation of the mevalonate pathway122 and subsequent PAg activation of BNT3A1 in addition to ectopic expression of cell stress markers such as EPCR, MIC and hMSH2, while CD1-family proteins can present endogenous lipids acting as a marker of malignant transformation (CD1c)42 or viral infection (CD1d).123 Interestingly, both CD1d and EPCR are capable of lipid presentation, and both can be recognised by γδ T cell clones regardless of the lipid cargo – thus indicating that both molecules can act as an immunogenic marker per se.35,47 Requirement for a wider cell stress context for γδ T cell recognition potentially restrains these immune cells and provides a failsafe against autoimmune reactions. Cancer-associated γδ TCR targets are essentially self-antigens. Accordingly, γδ TCR interactions with the known targets are of low affinity (KD = 10–4–10–6 M; Tables 1–3), similar to αβ TCR affinities for self-derived peptides.124,125 To date, only two structures of human γδ TCR in complex with a ligand have been resolved (Figure 4). In both cases (CD1d-sulphatide and CD1d-αGalCer), the germline-encoded CDR loops engaged the antigen-presenting CD1d molecule while hypervariable CDR3 loops made contacts with the lipid ligand, suggesting that discrimination between subtly different lipid cargoes is possible. In contrast, PAg recognition may be mediated mostly by germline-encoded CDR loops from Vδ2 and/or Vγ9 chains as PAg-BTN3A1 can be recognised by a wide range of different Vδ2 Vγ9 T cell clones. However, the exact mode of PAg recognition awaits a BTN3A1: γδ TCR complex structure.
In summary, γδ T cell therapy for cancer has demonstrated a good overall safety profile although results to date have not lived up to their early promise. Nevertheless, we anticipate that many more ligands will be found for these important immune cells in the next 5 years thereby greatly expanding the therapeutic horizon. Therapeutic approaches to cancer treatment based around the αβ TCR in gene transfer99 or as soluble bispecifics126,127 have burgeoned in the last two years. Importantly, γδ TCRs do not appear to suffer the limitation of being HLA-restricted so might offer exciting possibilities for new off-the-shelf, pan-population cancer immunotherapies in the very near future.
Acknowledgments
Mateusz Legut is a Cancer Research UK funded PhD student, David Cole is a Wellcome Trust Career Development Fellow and Andrew Sewell is a Wellcome Trust Senior Investigator.
References
- 1Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature 1988; 334: 395–402. [DOI] [PubMed] [Google Scholar]
- 2Hirano M et al. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature 2013; 501: 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Lefranc MP et al. IMGT®, the international ImMunoGeneTics information system® 25 years on. Nucleic Acids Res 2014. doi:10.1093/nar/gku1056 [DOI] [PMC free article] [PubMed]
- 4Lefranc M.-P. APPENDIX 1O nomenclature of the human T cell receptor genes. In: Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober WW (eds.) Current Protocols in Immunology. John Wiley & Sons, 2001. Available from: http://doi.wiley.com/10.1002/0471142735.ima01os40 [DOI] [PubMed] [Google Scholar]
- 5Clark SP, Arden B, Kabelitz D, Mak TW. Comparison of human and mouse T-cell receptor variable gene segment subfamilies. Immunogenetics 1995; 42: 531–540. [DOI] [PubMed] [Google Scholar]
- 6Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 2000; 18: 975–1026. [DOI] [PubMed] [Google Scholar]
- 7Davis MM. The evolutionary and structural ‘logic' of antigen receptor diversity. Semin Immunol 2004; 16: 239–243. [DOI] [PubMed] [Google Scholar]
- 8Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med 1994; 179: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Tuovinen H et al. gammadelta T cells develop independently of Aire. Cell Immunol 2009; 257: 5–12. [DOI] [PubMed] [Google Scholar]
- 10Burtrum DB, Kim S, Dudley EC, Hayday AC, Petrie HT. TCR gene recombination and alpha beta-gamma delta lineage divergence: productive TCR-beta rearrangement is neither exclusive nor preclusive of gamma delta cell development. J Immunol 1996; 157: 4293–4296. [PubMed] [Google Scholar]
- 11Zarin P, Wong GW, Mohtashami M, Wiest DL, Zuniga-Pflucker JC. Enforcement of -lineage commitment by the pre-T-cell receptor in precursors with weak -TCR signals. Proc Natl Acad Sci USA 2014; 111: 5658–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Hayes SM, Love PE. Strength of signal: a fundamental mechanism for cell fate specification. Immunol Rev 2006; 209: 170–175. [DOI] [PubMed] [Google Scholar]
- 13Barbee SD et al. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci USA 2011; 108: 3330–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Coffey F et al. The TCR ligand-inducible expression of CD73 marks lineage commitment and a metastable intermediate in effector specification. J Exp Med 2014; 211: 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Narayan K et al. Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat Immunol 2012; 13: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Zheng J, Liu Y, Lau YL, Tu W. γδ-T cells: an unpolished sword in human anti-infection immunity. Cell Mol Immunol 2013; 10: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Girardi M et al. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001; 294: 605–609.11567106 [Google Scholar]
- 18Gober HJ et al. Human T cell receptor cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 2003; 197: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Clendening JW, Penn LZ. Targeting tumor cell metabolism with statins. Oncogene 2012; 31: 4967–4978. [DOI] [PubMed] [Google Scholar]
- 20Karunakaran MM, Göbel TW, Starick L, Walter L, Herrmann T. Vγ9 and Vδ2 T cell antigen receptor genes and butyrophilin 3 (BTN3) emerged with placental mammals and are concomitantly preserved in selected species like alpaca (Vicugna pacos). Immunogenetics 2014; 66: 243–254. [DOI] [PubMed] [Google Scholar]
- 21Green AE et al. Recognition of nonpeptide antigens by human V gamma 9V delta 2 T cells requires contact with cells of human origin. Clin Exp Immunol 2004; 136: 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Scotet E et al. Tumor recognition following Vγ9Vδ2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 2005; 22: 71–80. [DOI] [PubMed] [Google Scholar]
- 23Mookerjee-Basu J et al. F1-adenosine triphosphatase displays properties characteristic of an antigen presentation molecule for V 9V 2 T Cells. J Immunol 2010; 184: 6920–6928. [DOI] [PubMed] [Google Scholar]
- 24Afrache H, Gouret P, Ainouche S, Pontarotti P, Olive D. The butyrophilin (BTN) gene family: from milk fat to the regulation of the immune response. Immunogenetics 2012; 64: 781–794. [DOI] [PubMed] [Google Scholar]
- 25Harly C et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human T-cell subset. Blood 2012; 120: 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Palakodeti A et al. The molecular basis for modulation of human V 9V 2 T cell responses by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J Biol Chem 2012; 287: 32780–32790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Decaup E et al. Phosphoantigens and butyrophilin 3A1 induce similar intracellular activation signaling in human TCRVγ9+ γδ T lymphocytes. Immunol Lett 2014; 161: 133–137. [DOI] [PubMed] [Google Scholar]
- 28Riaño F et al. Vγ9Vδ2 TCR-activation by phosphorylated antigens requires butyrophilin 3 A1 (BTN3A1) and additional genes on human chromosome 6: antigen processing. Eur J Immunol 2014; 44: 2571–2576. [DOI] [PubMed] [Google Scholar]
- 29Sandstrom A et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T Cells. Immunity 2014; 40: 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Wang H et al. Butyrophilin 3A1 plays an essential role in Prenyl pyrophosphate stimulation of human V 2V 2 T cells. J Immunol 2013; 191: 1029–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Vavassori S et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol 2013; 14: 908–916. [DOI] [PubMed] [Google Scholar]
- 32Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol 1999; 17: 297–329. [DOI] [PubMed] [Google Scholar]
- 33Russano AM et al. CD1-restricted recognition of exogenous and self-lipid antigens by duodenal gammadelta+ T lymphocytes. J Immunol 2007; 178: 3620–3626. [DOI] [PubMed] [Google Scholar]
- 34Mangan BA et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human V 3 T cells. J Immunol 2013; 191: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Bai L et al. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vδ1 TCR: clinical immunology. Eur J Immunol 2012; 42: 2505–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Luoma AM et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity 2013; 39: 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Uldrich AP et al. CD1d-lipid antigen recognition by the γδ TCR. Nat Immunol 2013; 14: 1137–1145. [DOI] [PubMed] [Google Scholar]
- 38Pellicci DG et al. The molecular bases of δ/αβ T cell-mediated antigen recognition. J Exp Med 2014; 211: 2599–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol 2009; 9: 28–38. [DOI] [PubMed] [Google Scholar]
- 40Kain L et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian α-linked glycosylceramides. Immunity 2014; 41: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Bojarska-Junak A et al. CD1d expression is higher in chronic lymphocytic leukemia patients with unfavorable prognosis. Leuk Res 2014; 38: 435–442. [DOI] [PubMed] [Google Scholar]
- 42Lepore M et al. A novel self-lipid antigen targets human T cells against CD1c+ leukemias. J Exp Med 2014; 211: 1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Déchanet J et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest 1999; 103: 1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Halary F. Shared reactivity of V 2neg T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 2005; 201: 1567–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Couzi L et al. Cytomegalovirus-induced T cells associate with reduced cancer risk after kidney transplantation. J Am Soc Nephrol 2010; 21: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Alejenef A et al. Cytomegalovirus drives Vδ2neg γδ T cell inflation in many healthy virus carriers with increasing age: CMV distorts γδ T cells over time. Clin Exp Immunol 2014; 176: 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Willcox CR et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol 2012; 13: 872–879. [DOI] [PubMed] [Google Scholar]
- 48Oganesyan V. The crystal structure of the endothelial protein C receptor and a bound phospholipid. J Biol Chem 2002; 277: 24851–24854. [DOI] [PubMed] [Google Scholar]
- 49Mohan Rao LV, Esmon CT, Pendurthi UR. Endothelial cell protein C receptor: a multiliganded and multifunctional receptor. Blood 2014; 124: 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Chen H et al. Identification of human T cell receptor gammadelta-recognized epitopes/proteins via CDR3delta peptide-based immunobiochemical strategy. J Biol Chem 2008; 283: 12528–12537. [DOI] [PubMed] [Google Scholar]
- 51Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998; 279: 1737–1740. [DOI] [PubMed] [Google Scholar]
- 52Martin SA, Lord CJ, Ashworth A. Therapeutic targeting of the DNA mismatch repair pathway. Clin Cancer Res 2010; 16: 5107–5113. [DOI] [PubMed] [Google Scholar]
- 53Nadin SB, Cuello-Carrión FD, Sottile ML, Ciocca DR, Vargas-Roig LM. Effects of hyperthermia on Hsp27 (HSPB1), Hsp72 (HSPA1A) and DNA repair proteins hMLH1 and hMSH2 in human colorectal cancer hMLH1-deficient and hMLH1-proficient cell lines. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group 2012; 28: 191–201. [DOI] [PubMed] [Google Scholar]
- 54Dai Y, Chen H, Mo C, Cui L, He W. Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human T cells to induce innate anti-tumor/virus immunity. J Biol Chem 2012; 287: 16812–16819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Mo C, Dai Y, Kang N, Cui L, He W. Ectopic expression of human MutS homologue 2 on renal carcinoma cells is induced by oxidative stress with interleukin-18 promotion via p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) signaling pathways. J Biol Chem 2012; 287: 19242–19254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Lu J et al. Induction of ATM/ATR pathway combined with Vγ2Vδ2 T cells enhances cytotoxicity of ovarian cancer cells. Biochim Biophys Acta BBA – Mol Basis Dis 2014; 1842: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57Aggarwal R et al. Human Vγ2Vδ2 T cells limit breast cancer growth by modulating cell survival-, apoptosis-related molecules and microenvironment in tumors: human Vγ2Vδ2 T cells limit breast cancer growth. Int J Cancer 2013; 133: 2133–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58Lamb LS et al. Engineered drug resistant γδ T cells kill glioblastoma cell lines during a chemotherapy challenge: a strategy for combining chemo- and immunotherapy. PLoS One 2013; 8: e51805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Scheper W et al. γδT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 2013; 27: 1328–1338. [DOI] [PubMed] [Google Scholar]
- 60Zhu J et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 2013; 497: 494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61Van Kaer L et al. CD8αα+ innate-type lymphocytes in the intestinal epithelium mediate mucosal immunity. Immunity 2014; 41: 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62Wooldridge L et al. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor-antigen complexes at the cell surface. J Biol Chem 2005; 280: 27491–27501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63Purbhoo MA et al. The human CD8 coreceptor effects cytotoxic T cell activation and antigen sensitivity primarily by mediating complete phosphorylation of the T cell receptor zeta chain. J Biol Chem 2001; 276: 32786–32792. [DOI] [PubMed] [Google Scholar]
- 64Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity 2003; 18: 255–264. [DOI] [PubMed] [Google Scholar]
- 65Cole DK et al. The molecular determinants of CD8 co-receptor function. Immunology 2012; 137: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66Van Laethem F, Tikhonova AN, Singer A. MHC restriction is imposed on a diverse T cell receptor repertoire by CD4 and CD8 co-receptors during thymic selection. Trends Immunol 2012; 33: 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67Van Laethem F et al. Deletion of CD4 and CD8 coreceptors permits generation of alphabetaT cells that recognize antigens independently of the MHC. Immunity 2007; 27: 735–750. [DOI] [PubMed] [Google Scholar]
- 68Adoro S et al. Coreceptor gene imprinting governs thymocyte lineage fate. EMBO J 2012; 31: 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69Ullrich E, Koch J, Cerwenka A, Steinle A. New prospects on the NKG2D/NKG2DL system for oncology. Oncoimmunology 2013; 2: e26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70Xu B et al. Crystal structure of a T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci USA 2011; 108: 2414–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71Knight A et al. CMV-independent lysis of glioblastoma by ex vivo expanded/activated Vδ1+ γδ T cells. PLoS One 2013; 8: e68729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72Xiang Z et al. Targeted activation of human Vγ9Vδ2-T cells controls Epstein-Barr virus-induced B cell lymphoproliferative disease. Cancer Cell 2014; 26: 565–576. doi:10.1016/j.ccr.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 73Yin S et al. Vav1-phospholipase C- 1 (Vav1-PLC- 1) pathway initiated by T cell antigen receptor (TCR) activation is required to overcome inhibition by ubiquitin ligase Cbl-b during T cell cytotoxicity. J Biol Chem 2013; 288: 26448–26462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74Kong Y et al. The NKG2D ligand ULBP4 binds to TCR 9/2 and induces cytotoxicity to tumor cells through both TCR and NKG2D. Blood 2009; 114: 310–317. [DOI] [PubMed] [Google Scholar]
- 75Hewitt RE et al. The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood gd T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol 2005; 139: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76Wang H et al. Indirect stimulation of human V 2V 2 T cells through alterations in isoprenoid metabolism. J Immunol 2011; 187: 5099–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77Idrees ASM et al. Comparison of γδ T cell responses and farnesyl diphosphate synthase inhibition in tumor cells pretreated with zoledronic acid. Cancer Sci 2013: 104: 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78Benzaid I, Monkkonen H, Bonnelye E, Monkkonen J, Clezardin P. In vivo phosphoantigen levels in bisphosphonate-treated human breast tumors trigger V 9V 2 T-cell antitumor cytotoxicity through ICAM-1 engagement. Clin Cancer Res 2012; 18: 6249–6259 [DOI] [PubMed] [Google Scholar]
- 79Todaro M et al. Chemotherapy sensitizes colon cancer initiating cells to Vγ9Vδ2 T cell-mediated cytotoxicity. PLoS One 2013; 8: e65145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80Brandes M. Professional antigen-presentation function by human gd T cells. Science 2005; 309: 264–268. [DOI] [PubMed] [Google Scholar]
- 81Hoh A et al. The activity of γδ T cells against paediatric liver tumour cells and spheroids in cell culture. Liver Int 2013; 33: 127–136. [DOI] [PubMed] [Google Scholar]
- 82Oberg HH et al. Novel bispecific antibodies increase T-cell cytotoxicity against pancreatic cancer cells. Cancer Res 2014; 74: 1349–1360. [DOI] [PubMed] [Google Scholar]
- 83Zheng J, Guo Y, Ji X, Cui L, He W. A novel antibody-like TCRγδ-Ig fusion protein exhibits antitumor activity against human ovarian carcinoma. Cancer Lett 2013; 341: 150–158. [DOI] [PubMed] [Google Scholar]
- 84Fisher J et al. Neuroblastoma killing properties of V-delta 2 and V-delta2 negative gamma delta T cells following expansion by artificial antigen presenting cells. Clin Cancer Res 2014; 20: 5720–5732. doi:10.1158/1078-0432.CCR-13-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85Couzi L et al. Antibody-dependent anti-cytomegalovirus activity of human T cells expressing CD16 (Fc RIIIa). Blood 2012; 119: 1418–1427. [DOI] [PubMed] [Google Scholar]
- 86Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014; 123: 2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87Deniger DC et al. Bispecific T-cells expressing polyclonal repertoire of endogenous γδ T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther 2013; 21: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88Themeli M et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol 2013; 31: 928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89Morgan RA et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006; 314: 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90Van Loenen MM et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci USA 2010; 107: 10972–10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91Bendle GM et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med 2010; 16: 565–570, 1p following 570. [DOI] [PubMed] [Google Scholar]
- 92Van der Veken LT et al. Alphabeta T-cell receptor engineered gammadelta T cells mediate effective antileukemic reactivity. Cancer Res 2006; 66: 3331–3337. [DOI] [PubMed] [Google Scholar]
- 93Van der Veken LT et al. Alpha beta T cell receptor transfer to gamma delta T cells generates functional effector cells without mixed TCR dimers in vivo. J Immunol 2009; 182: 164–170. [DOI] [PubMed] [Google Scholar]
- 94Hiasa A et al. Rapid alphabeta TCR-mediated responses in gammadelta T cells transduced with cancer-specific TCR genes. Gene Ther 2009; 16: 620–628. [DOI] [PubMed] [Google Scholar]
- 95Xue SA et al. Human MHC class I-restricted high avidity CD4(+) T cells generated by co-transfer of TCR and CD8 mediate efficient tumor rejection in vivo. Oncoimmunology 2013; 2: e22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96Tan MP et al. TCR binding affinity governs the functional profile of cancer-specific CD8(+) T cells. Clin Exp Immunol 2014. doi:10.1111/cei.12570. [DOI] [PMC free article] [PubMed]
- 97Zhao H, Xi X, Cui L, He W. CDR3δ -grafted γ9δ2T cells mediate effective antitumor reactivity. Cell Mol Immunol 2012; 9: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98Marcu-Malina V et al. Redirecting T cells against cancer cells by transfer of a broadly tumor-reactive T-cell receptor. Blood 2011; 118: 50–59. [DOI] [PubMed] [Google Scholar]
- 99Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 2012; 12: 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100Kang N et al. Adoptive immunotherapy of lung cancer with immobilized anti-TCRgammadelta antibody-expanded human gammadelta T-cells in peripheral blood. Cancer Biol Ther 2009; 8: 1540–1549. [DOI] [PubMed] [Google Scholar]
- 101Zhou J, Kang N, Cui L, Ba D, He W. Anti-γδ TCR antibody-expanded γδ T cells: a better choice for the adoptive immunotherapy of lymphoid malignancies. Cell Mol Immunol 2012; 9: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102Deniger DC et al. Activating and propagating polyclonal gamma delta T cells with broad specificity for malignancies. Clin Cancer Res 2014; 20: 5708–5719. doi:10.1158/1078-0432.CCR-13-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103Dopfer EP et al. The CD3 conformational change in the γδ T cell receptor is not triggered by antigens but can be enforced to enhance tumor killing. Cell Rep 2014; 7: 1704–1715. [DOI] [PubMed] [Google Scholar]
- 104Lang JM et al. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother 2011; 60: 1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105Kunzmann V et al. Tumor-promoting versus tumor-antagonizing roles of γδ T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother 2012; 35: 205–213. [DOI] [PubMed] [Google Scholar]
- 106Welton JL et al. Monocytes and γδ T cells control the acute-phase response to intravenous zoledronate: insights from a phase IV safety trial. J Bone Miner Res 2013; 28: 464–471. [DOI] [PubMed] [Google Scholar]
- 107Rossini M et al. Long-term effects of amino-bisphosphonates on circulating γδ T cells. Calcif Tissue Int 2012; 91: 395–399. [DOI] [PubMed] [Google Scholar]
- 108Kalyan S, Chandrasekaran V, Quabius ES, Lindhorst TK, Kabelitz D. Neutrophil uptake of nitrogen-bisphosphonates leads to the suppression of human peripheral blood γδ T cells. Cell Mol Life Sci 2014; 71: 2335–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109Coscia M et al. Dysfunctional V 9V 2 T cells are negative prognosticators and markers of dysregulated mevalonate pathway activity in chronic lymphocytic leukemia cells. Blood 2012; 120: 3271–3279. [DOI] [PubMed] [Google Scholar]
- 110Gnant M et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol 2011; 12: 631–641. [DOI] [PubMed] [Google Scholar]
- 111Morgan GJ et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. The Lancet 2010; 376: 1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112Bennouna J et al. Phase-I study of Innacell γδTM, an autologous cell-therapy product highly enriched in γ9δ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2008; 57: 1599–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother 2011; 60: 1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114Noguchi A et al. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy 2011; 13: 92–97. [DOI] [PubMed] [Google Scholar]
- 115Nicol AJ et al. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer 2011; 105: 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116Cui J et al. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma: radiofrequency ablation with cellular immunotherapy for HCC. Int J Cancer 2014; 134: 342–351. [DOI] [PubMed] [Google Scholar]
- 117Wilhelm M et al. Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. J Transl Med 2014; 12: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118Izumi T et al. Ex vivo characterization of γδ T-cell repertoire in patients after adoptive transfer of Vγ9Vδ2 T cells expressing the interleukin-2 receptor β-chain and the common γ-chain. Cytotherapy 2013; 15: 481–491. [DOI] [PubMed] [Google Scholar]
- 119Nussbaumer O et al. Essential requirements of zoledronate-induced cytokine and T cell proliferative responses. J Immunol 2013; 191: 1346–1355. [DOI] [PubMed] [Google Scholar]
- 120Sugie T et al. Zoledronic acid-induced expansion of γδ T cells from early-stage breast cancer patients: effect of IL-18 on helper NK cells. Cancer Immunol Immunother 2013; 62: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121Wada I et al. Intraperitoneal injection of in vitro expanded V γ 9V δ 2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med 2014; 3: 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122Clendening JW et al. Dysregulation of the mevalonate pathway promotes transformation. Proc Natl Acad Sci USA 2010; 107: 15051–15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123Priatel JJ, Chung BK, Tsai K, Tan R. Natural killer T cell strategies to combat Epstein–Barr virus infection. Oncoimmunology 2014; 3: e28329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124Cole DK et al. Human TCR-binding affinity is governed by MHC class restriction. J Immunol 2007; 178: 5727–5734. [DOI] [PubMed] [Google Scholar]
- 125Aleksic M et al. Different affinity windows for virus and cancer-specific T-cell receptors: implications for therapeutic strategies. Eur J Immunol 2012; 42: 3174–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126Liddy N et al. Monoclonal TCR-redirected tumor cell killing. Nat Med 2012; 18: 980–987. [DOI] [PubMed] [Google Scholar]
- 127Sheridan C. Monoclonal T-cell receptor drugs pique pharma's interest. Nat Biotechnol 2013; 31: 950–951. [DOI] [PubMed] [Google Scholar]