Abstract
Vascular development and maintenance of proper vascular function through various regulatory mechanisms are critical to our wellbeing. Delineating the regulatory processes involved in development of vascular system and function is one of the most important topics in human physiology and pathophysiology. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), a cell adhesion molecule with proangiogenic and proinflammatory activity, has been subject of numerous studies. Here we will review the important roles PECAM-1 and its isoforms play during angiogenesis, and its molecular mechanisms of action in the endothelium. In the endothelium, PECAM-1 not only plays a role as an adhesion molecule but also participates in intracellular signaling pathways which impact various cell adhesive mechanisms and endothelial nitric oxide (eNOS) expression and activity. In addition, recent studies from our laboratory have revealed an important relationship between PECAM-1 and endoglin expression. Endoglin is an essential molecule during angiogenesis, vascular development and integrity whose expression and activity are compromised in the absence of PECAM-1. Here we will discuss the roles PECAM-1 isoforms may play in modulation of endothelial cell adhesive mechanisms, eNOS and endoglin expression and activity, and angiogenesis.
Keywords: PECAM-1, eNOS, endoglin, angiogenesis, vasculogenesis
INTRODUCTION
Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), a 130 kDa transmembrane glycoprotein, is a member of immunoglobulin (Ig) gene superfamily. In the 1980s, PECAM-1 was characterized by a number of groups independently as a 130 kDa cell surface protein in the vascular compartment, including endothelial cells (EC), platelets, and leukocytes, and identified as an endothelial cell junction molecule [1–5]. PECAM-1 is highly expressed on the surface of EC and at lower levels on hematopoietic and immune cells, including macrophages, monocytes, neutrophils, natural killer cells, naive T cells, and B cells [6]. In cultured EC, a large amount of PECAM-1 is expressed on the cell surface (~106 molecules per cell [7]) and mainly localizes to sites of cell-cell contacts [1]. However, the exact role of PECAM-1, and more specifically its isoforms, in these activities is not fully understood.
The PECAM-1 gene, 65 kbp in length, is localized to the long arm of human chromosome 17 [8]. Cloning of the human PECAM-1 gene demonstrated that the full length human cDNA encodes an open reading frame of 738 amino acids (aa) consisting of a signal peptide (27 aa), an Ig-like extracellular domain (574 aa), a hydrophobic transmembrane domain (19 aa), and a relatively long cytoplasmic domain (118 aa) bearing multiple potential sites for phosphorylation and posttranslational modifications such as carbohydrate and lipid modifications [9]. The murine PECAM-1 cDNA was also cloned and it revealed 70–80% sequence homology with human PECAM-1 [10]. While the cytoplasmic domain of other cell adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1), are encoded by a single exon, the PECAM-1 cytoplasmic domain is encoded by multiple exons. The PECAM-1 gene contains 16 exons with introns ranging in size from 86 to 12,000 bp; exons1–2 encode 5’-untranslated region and the signal peptide, exons 3–8 encode the six Ig-like extracellular domain, exon 9 encodes the hydrophobic transmembrane domain, and exons 10–16 encode the long cytoplasmic domain [8].
The PECAM-1 gene produces a number of isoforms which only differ in the length of their cytoplasmic domain. These are generated by the alternative splicing of exons 10–16. Although the PECAM-1 cytoplasmic domain lacks kinase activity, it participates in signaling cascades via regulated phosphorylation of specific tyrosine residues in exon 13 and 14. The expression of PECAM-1 isoforms in the vascular bed of various tissues including brain, heart, kidney, liver, and lung from human and mouse revealed that the alternative splicing of human PECAM-1 gene is less frequent than mouse PECAM-1 [11, 12]. The alternative splicing of human PECAM-1 generates 6 isoforms including full-length, and those lacking exon 12 (Δ12), Δ13, Δ14, Δ15, and Δ14&15, and the full length isoform is the major form of PECAM-1 in human tissues and EC [12, 13]. However, 8 different PECAM-1 isoforms including full-length, Δ12, Δ14, Δ15, Δ12&14, Δ12&15, Δ14&15, Δ12, 14&15 are detected in most mouse tissues. In contrast to human, Δ14&15 is the predominant isoform detected in various mouse tissues and EC [11, 13, 14]. In addition, alternative splicing of PECAM-1 generates a number of these isoforms during differentiation and/or activation of hematopoietic cells and platelets [15, 16].
Investigation of PECAM-1 isoform expression in mice demonstrated a regulated expression pattern during development [13, 14, 17]. For example, in kidney, exon 14 expression was detected early in development, but not in later stages [11]. Since each isoform has distinct cytoplasmic domain bearing different signaling potential, PECAM-1 appears to impact angiogenesis and/or vasculogenesis processes through alternative splicing of its cytoplasmic domain. However, delineating the physiological significance of these isoforms in regulation of EC function remains very challenging and unexplored.
Endothelial cells line the inside of blood vessels, and play critical roles in vascular function. Endothelial cell precursors which arise from hematopoietic progenitors differentiate to EC during vasculogenesis [18]. Although EC are sufficient for the formation and function of small blood vessels, larger blood vessels require EC interactions with pericytes or smooth muscle cells (SMC) for vessel stabilization and integrity. In blood vessels, EC exert their function through direct interactions with bloodstream and sensing variety of stimuli such as hypoxia, growth factors, cytokines, and shear stress. These activities are mediated through production of a variety of mediators and modulation of intracellular signaling pathways, including those by PECAM-1 (Figure 1).
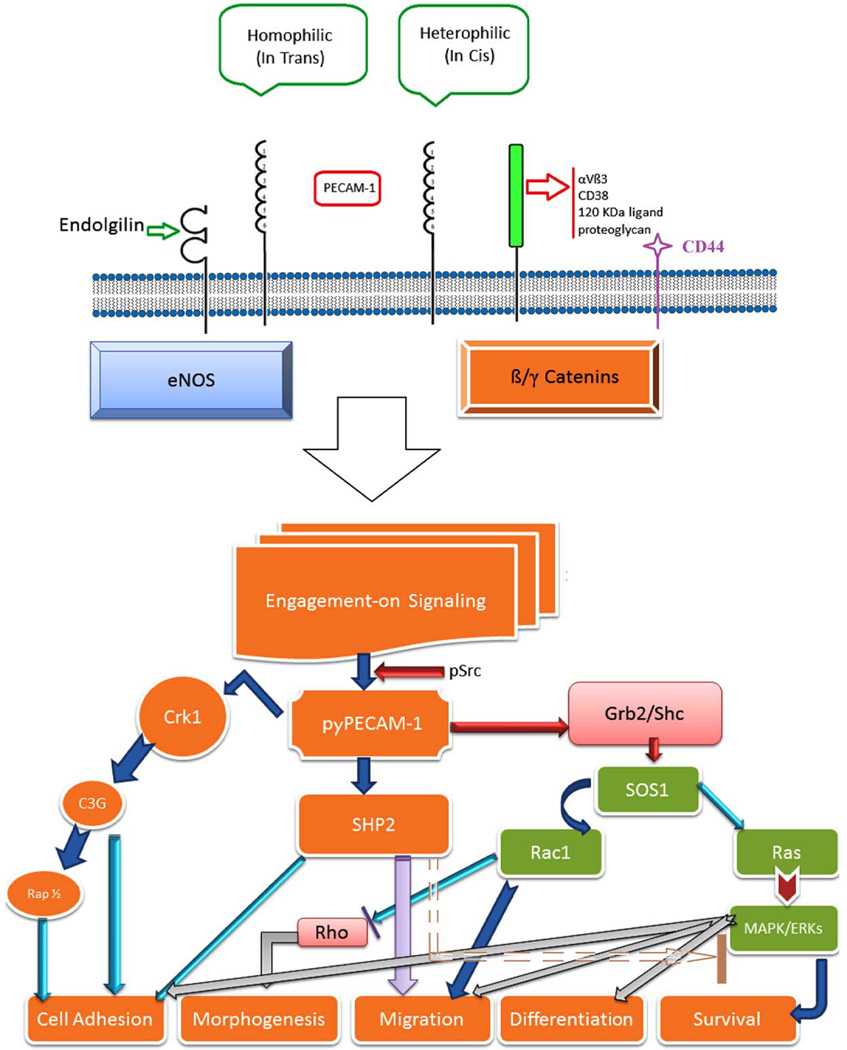
Figure 1. PECAM-1-mediated signal transduction.
PECAM-1 extracellular domain is involved in the interactions between various membrane molecules including PECAM-1 itself. Homophillic interaction between two PECAM-1 isoforms without exon14 regulates cell-cell adhesion and adherens junction formation. While, exon14 containing isoforms mediate heterophillic interaction with other molecules on the cell surface imapcting various cellular processes, including aggregation and transendothelial migration. These activities occur through engagement of various signaling molecules. In addition, PECAM-1 cytoplasmic domaine participates in modulation of cell adhesion and migration through interaction with various intracellular proteins (e.g. β/γ-catenin and eNOS). For additional details please see [44, 187]
PECAM-1 SIGNAL TRANSDUSCTION
The human PECAM-1 cytoplasmic domain contains 12 serine, 5 threonine, and 5 tyrosine residues, and serine/threonine residues in the cytoplasmic domain of PECAM-1 are constitutively phosphorylated [19, 20]. However, the phosphorylation of tyrosine residues in exon13 (Y663) and exon14 (Y686) are actively regulated by protein-tyrosine kinases (PTKs; e.g. Src and Csk family of kinases) and SHP-2 phosphatase [21, 22]. These tyrosine phosphorylations are modulated in response to physiological stimuli, including platelet aggregation [23], engagement of the high affinity IgE receptor on basophils and mast cells [24], triggering of the antigen receptor on T-cells [25] and mechanical stimulation of EC [26, 27]. Distinct cellular status of EC exhibits different levels of PECAM-1 phosphorylation; decreased phosphorylation in migrating cells and maximum phosphorylation in attached confluent EC [28]. Examination of the regulation of PECAM-1 cytoplasmic domain in its junctional localization has revealed that deletion of PECAM-1 cytoplasmic domain results in lack of cell-cell junctional localization in mice fibroblast L-cell [29, 30]. Thus, it is now becoming apparent that intracellular signaling for cell migration and cell-cell junction formation is regulated by phosphorylation of PECAM-1 cytoplasmic domain [22, 31].
PECAM-1 cytoplasmic sequence expanding Y663 and Y686 shares common features of immunoreceptor tyrosine-based inhibitory motifs (ITIM)[32–34], that upon phosphorylation exerts inhibitory function by recruiting and activating protein-tyrosine phosphatases (PTPs), including SHP-1 and SHP-2 [35–38]. Transient overexpression of mouse PECAM-1 in COS cells demonstrated that phosphorylation of PECAM-1 by Src family kinases was sufficient to promote binding of SHP-1 and SHP-2 [22]. Thus, phosphorylated tyrosine residues in PECAM-1 cytoplasmic domain provide docking sites for SH2 domain containing signaling molecules, predominantly SHP-2, and bound SHP-2 phosphatase inhibits PECAM-1-mediated signaling cascade [39]. Lack of phosphorylation of PECAM-1 results in failure to recruit SHP-2 phosphatase and enhanced MAPK/ERKs signaling in EC, which results in enhanced EC migration and abrogated cell-cell adherens junction formation [40] (Figure 2). Thus, the regulation of SHP-2 phosphatase activity is essential in PECAM-1 mediated cellular signaling cascades, especially MAPK/ERKs which impact EC proliferation, survival, differentiation, and migration (Figure 1) [32, 40–43].
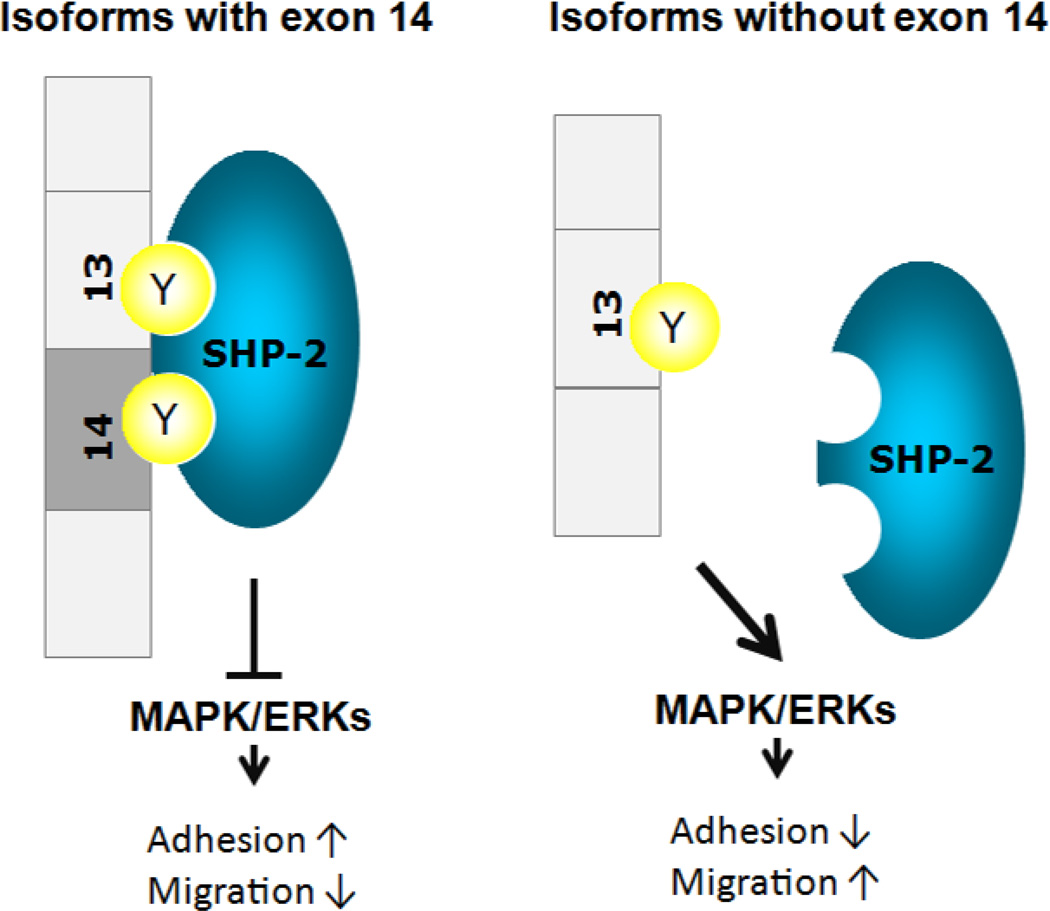
Figure 2. PECAM-1-mediated signaling pathways through interactions between phosphorylated tyrosine residues in the cytoplasmic domain and SHP-2.
Phosphorylated tyrosine residues in exon 13 (Y663) and exon 14 (Y686) serve as binding site for SHP-2 phosphatase [187], and bound SHP-2 attenuates EC migration by inhibition of MAPK/ERKs signaling pathway [44].
PECAM-1 cytoplasmic domain contains tyrosine residues in exon 13 (Y663) and exon 14 (Y686) and alternative splicing of PECAM-1 cytoplasmic domain could produce isoforms with or without exon 14 (Y686) [11–14]. As a result, each isoform has a different binding capacity for SH2 domain containing signaling molecules. PECAM-1 isoform-expressing Madin-Darby canine kidney (MDCK) cells revealed that interaction between PECAM-1 and Src kinase is exon 14-independent, whereas SHP-2 association is exon14-dependent [44]. Investigation of PECAM-1 isoform-specific functions in signaling pathways was conducted by re-expressing Δ15 or Δ14&15 PECAM-1 isoform in MDCK epithelial cells lacking PECAM-1 expression or PECAM-1 −/− bEND cells; a Polyoma middle T-transformed line of brain EC prepared from PECAM-1−/− mice) [39, 44, 45]. In PECAM-1 −/− bEND cells, phosphorylation level of Δ15 PECAM-1 isoform was higher than that of Δ14&15 isoform, and consequently activation level of MAPK/ERKs in Δ15 isoform expressing cell was comparable to parental PECAM-1 −/− cells cells [45]. In contrast, Δ14&15 PECAM-1 isoform expression enhanced MAPK/ERKs activation and resulted in abrogated cell-cell adherens junction formation and enhanced migration of PECAM-1 −/− bEND cells [39, 44, 45]. Furthermore, re-expression of isoforms lacking exon 14 (Δ14 or Δ14&15) in PECAM-1−/− kidney and retinal EC restored proangiogenic properties, including migration, capillary morphogenesis, and cell adhesion to extracellular matrix (ECM) proteins [45, 46]. Taken together, exon14 dependent SHP-2 association with PECAM-1 cytoplasmic domain makes a significant contribution to signaling pathways impacting angiogenesis. This may occur through competition between SHP-2 and Src for Y663 (exon 13).
VASCULAR FUNCTION OF PECAM-1
Vascular development
Nutrients and oxygen are provided to organs through blood circulation. Accordingly the development of the vascular network is one of the most critical events in embryogenesis. The development of vasculature fundamentally occurs via three processes including vasculogenesis, angiogenesis, and vascular remodeling. Vasculogenesis refers to the de novo vascular network formation by differentiated EC from vascular endothelial precursor cells termed angioblasts [47]. During early embryogenesis, the primary vasculature is formed by vasculogenesis. Angiogenesis is the process by which blood vessels are formed from pre-existing capillaries, and mediates vascular development until the vasculature is formed [48]. After completion of development, angiogenesis is restricted only to the ovarian cycle and placenta during pregnancy [49]. However, some physiological stimuli reactivate angiogenesis in adulthood such as wound healing and hypoxia [50].
Angiogenesis is tightly regulated by a balanced production of inhibitory (e.g. pigment epithelium-derived factor (PEDF), thrombospondin-1(TSP1), TSP2, angiostatin, endostatin) and stimulatory (e.g. vascular endothelial growth factor (VEGF) family, fibroblast growth factor (FGF) family, epidermal growth factor (EGF), PECAM-1) factors [51]. Unregulated angiogenesis is involved in over 70 disorders including cancer, inflammatory disorders, obesity, asthma, diabetes, autoimmune diseases, and various eye diseases [18, 50]. These are generally associated with increased production of proangiogenic factors and decreased production of antiangiogenic factors, which tip the angiogenic balance towards angiogenesis.
Vascular remodeling is an adaptive structural alteration process occurring in response to long-term changes in hemodynamic conditions. The process is modulated by locally generated growth factors, vasoactive substances, and hemodynamic stimuli, and is accomplished by changes in cellular processes including cell growth, cell death, cell migration, and production or degradation of extracellular matrix (ECM) [52]. Our studies of retinal postnatal vascular development have demonstrated an important role for TSP1, a matricellular protein with antiangiogenic activity, in retinal vascular maturation [53].
Endothelial Cells and Angiogenesis
Vessel formation is initiated by the production of angiogenic growth factors, including VEGF, placental growth factor (PlGF), angiopoietin-1, inhibitors of differentiation (Id) proteins and cytokines [54–57]. Following binding to their respective receptors on EC, these factors promote EC proliferation, migration, and capillary morphogenesis, which are stabilized by recruitment and interaction with pericytes or SMC. In the process of vessel formation, EC’s distinctive features including cell migration and capillary morphogenesis play essential roles. Capillary morphogenesis refers to the process of forming tube-like networks between EC, which is a unique and pivotal feature of these cells. Migration is regulated by the interaction between integrins on EC surface and the ECM proteins which are produced by EC, pericyte, and SMC, and fills the extracellular space. Integrins, receptors for ECM proteins, and immunoglobulin superfamily of cell adhesion molecules mediate cell migration through activation of intracellular signaling pathways including focal adhesion kinase (FAK), Src, and many other kinases [58]. Through forming functional actin-filament and focal-adhesions, EC migrate with directivity toward the source of promigratory signals. The role of PECAM-1 in these activities and how these activities are impacted by various isoforms of PECAM-1 need further investigation.
PECAM-1 in Vascular Development and Remodeling
To further investigate the role of PECAM-1 in vasculogenesis and angiogenesis, Duncan and colleagues disrupted PECAM-1 gene in embryonic stem cells to generate PECAM-1-deficient (PECAM-1−/−) mice. PECAM-1−/− mice reported to be viable and born without critical vascular defects [59]. However, they were later shown to exhibit attenuated alveolarization, an angiogenesis dependent process during lung development [60], postnatal retinal vascular development, and brain angiogenesis abnormalities [61–63]. In addition, PECAM-1 −/− mice showed decreased retinal vascular density, abnormal secondary branch formations, and increased vessel diameter, and also failed to undergo neovascularization during oxygen-induced ischemic retinopathy [61]. These observations suggest an important role for PECAM-1 in normal vascular development and angiogenesis, and potential compensatory mechanisms which may minimize embryonic defects in vivo.
The formation of a functional vessel requires proper remodeling in response to a chronic stimulus on vessel wall. During vascular remodeling, EC sense the stimulus on vessel wall and accordingly vessels enlarge or reduce lumen size. The blood flow is one of the important stimuli for vascular remodeling [52]. Important role of PECAM-1 in sensing fluid shear stress (FSS) has been addressed by defining PECAM-1 as a major component of the mechanosensory complex sensing shear-stress [27, 64] through changes in its phosphorylation [26, 65, 66]. Actually previous studies demonstrated that PECAM-1 −/− mice are defective in responding to shear stress-mediated vascular remodeling [67], and collateral vessel formation, which were more dilated and shorter than those in wild type mice [68].
PECAM-1 and Inflammation
The vascular endothelium is not only important in angiogenesis but also in immune responses. During immunosurveillance and inflammation, leukocytes undergo migration from bloodstream to targeted tissues. Transmigration of leukocytes through the endothelium is a critical step that is accomplished through the interaction of cell adhesion molecules between leukocytes and EC and/or between EC. The multistep leukocyte trafficking process named transendothelial migration (TEM) is conducted through well-established sequential steps of leukocyte rolling, activation, adhesion, and migration through the endothelium. Since leukocyte migration is mediated by the interaction between active receptor on the leukocytes and ligands on the EC, the expression and activation of adhesion molecules are critical determinant of this process [69]. During leukocyte rolling step, interactions with selectins and glycosylated ligands slow their migration [70]. Activated leukocytes exposed to chemokines then enhance integrin activity and increase their adhesions to cell adhesion molecules, the member of immunoglobulin superfamily including PECAM-1, ICAM-1, ICAM-2, and VCAM-1 [71]. Loosening of cell-cell adhesions between EC by the disruption of the homophilic interactions of adhesion molecules (e.g. junctional adhesion molecule (JAM)-JAM and PECAM-1-PECAM-1), results in diapedesis of leukocytes and completion of TEM. Recent studies also demonstrate an active role for perivascular supporting cells in these activities [72]. However, the contribution of PECAM-1 to prevascular supporting cell’s activities during inflammation remains unknown.
Muller and colleagues suggested a role for PECAM-1 in leukocyte transmigration through the observation that PECAM-1 specific antibodies inhibited leukocyte transmigration through the endothelium [73]. Additional studies have now demonstrated a critical role for PECAM-1 in leukocyte transmigration in vivo [74–76] and in vitro [75, 77, 78]. In PECAM-1 −/− mice, the absence of PECAM-1 resulted in attenuation of leukocyte TEM, neutrophil recruitment, and inflammatory responses [59, 79]. However, there are contrasting studies indicating minimal involvement of PECAM-1 in transmigration of leukocytes [59] and mice strain-specific defects with PECAM-1 deficiency may be a contributing factor [80]. Furthermore, transmigration of leukocytes is not necessarly undesirable; for example perkowsky et al [81] showed that inhibtiton of this reaction by blocking PECAM-1 function does not alleviate pulmonary edema.
PECAM-1 expression on EC and leukocytes mediate homophilic interactions between EC and leukocytes and between EC themselves. During transmigration, PECAM-1 expressed on leukocytes contributes to chemokine-mediated directional migration of leukocytes to the sites of inflammation [82]. Following leukocyte adhesion to the endothelium, PECAM-1 homophilic interactions between leukocytes and EC promote leukocyte migration through cell-cell junctions [83, 84] and enhance the activation of integrins on leukocytes which promotes migration across the perivascular basement membrane [6, 59, 74, 84]. A number of studies have suggested that the function of PECAM-1in this process is independent of its SHP-2 recruitment [85, 86]. However, the detailed signaling pathways involved and the potential contribution of perivascular supporting cells to these activities require further delineation.
HOMOPHILIC AND HETEROPHILIC INTERACTIONS OF PECAM-1
The PECAM-1 six Ig-like homology domain of the extracellular region exhibits similar characteristics to other Ig superfamily members, and play important roles in cell adhesion through homophilic binding between PECAM-1 [3, 87, 88] and heterophilic binding [89, 90] between PECAM-1 and other cell surface molecules, including αvβ3 [91–93], CD38 [94], 120 kDa ligand on T cells [95], and heparin-dependent proteoglycans [89]. Previous studies have demonstrated that the PECAM-1 cytoplasmic domain modulates its heterophilic and homophilic binding characteristics [96, 97]. Deletion of the entire PECAM-1cytoplasmic domain impairs PECAM-1-mediated cell-cell interactions, while its partial deletion differentially impacts these activities [30]. Furthermore, PECAM-1 isoforms showed different cell aggregation capacity depending on the presence or absence of exon14 in their cytoplasmic domain. While isoforms containing exon14 showed heterophilic, calcium-dependent, and heparin-sensitive aggregation, isoforms lacking exon14 exhibited homophilic, calcium-independent and heparin-insensitive aggregation [29, 98]. Thus, PECAM-1-mediated cell-cell interactions through heterophilic and homophilic interactions are regulated by the presence or absence of exon14. However, the majority of these studies were conducted in non-EC, which lacked PECAM-1 expression and perhaps other EC specific molecules that may impact PECAM-1 function. Therefore, the adhesion properties of these isoforms in EC, which lack PECAM-1 is of great interest and should provide important insight into activities of these isoforms.
PROANGIOGGENIC ROLES OF PECAM-1
Cell-Cell Junction Formation
In human a number of pathological conditions, including ischemic stroke and inflammation, are due to defects in endothelial permeability. Endothelial monolayers form cell-cell junctions through the interactions of adhesion molecules on EC, and disruption of cell-cell junction causes altered vascular permeability and fragility. Numerous genes involved in vascular remodeling and vessel integrity have been identified, and their deletion or down regulation results in embryonic lethality due to defective vascular development [50].
In the endothelium, adhesion and communication between EC is mediated by intercellular junctions including adherens junctions (AJ), tight junctions (TJ), and gap junctions (GJ). As a communication structure, GJ provide the passage for small molecular weights solutes between neighboring cells. In EC, AJ and TJ are major types of junctions [99–101] and the function of AJ and TJ are distinctive. Adherens junctions are involved in the initiation of cell-cell contacts and their maturation, and TJ participate in the passage of the ions and solutes. Through the formation of intercellular junctions EC sense their position, regulate cell growth and apoptosis, and develop tubular structures [102–104]. Junction formation in EC is mediated by the homophilic bindings of transmembrane proteins, including VE-cadherin, catenin, ZO-1 and PECAM-1 for AJ and occludins, claudins, and ZO-1 for TJ [102].
The observation of increased vascular permeability in PECAM-1 −/− mice supports a role for PECAM-1in AJ formation and function [62]. In cultured EC, PECAM-1 localizes at the edge of cells together with other proteins, and cell confluency enhance its junctional localization [1, 46, 105]. Incubation of bovine EC with anti-PECAM-1 antibody revealed that PECAM-1 functions to establish and maintain cell-cell junction. In this study, junction formation was affected when EC was incubated with PECAM-1 antibody before forming a confluent EC monolayer, however after complete junction formation, PECAM-1 antibody did not influence cell-cell junctions [1]. Additional insight into the role of PECAM-1 isoforms in cell-cell junction formation was investigated by expressing a specific PECAM-1 isoform in MDCK cells, which like EC form various types of junctions but lack PECAM-1 expression, and PECAM-1−/− bEND cells prepared from PECAM-1 deficient mice [39, 40]. These studies indicated, for the first time, that the junction formation is actively modulated by PECAM-1 in an isoform specific manner through modulation of intracellular signaling pathways and junctional localization of PECAM-1 is dependent on formation of AJ as discussed above.
Endothelial Cell Migration and Adhesion
Endothelial cell migration during angiogenesis is conducted by three major mechanisms: (i) chemotaxis, migration toward gradient of chemoattractants, (ii) haptotaxis, migration toward a gradient of immobilized ligands, and (iii) mechanotaxis, the migration mediated by mechanical forces [106]. While growth factors such as VEGF and basic fibroblast growth factor (bFGF) induce chemotactic migration of EC, binding of integrins to ECM is involved in haptotaxis of EC [41, 107]. In order to migrate, the cytoskeleton needs to undergo constant remodeling into filopodia, lamellipodia and formation of actin stress fibers.
The first indication that PECAM-1regulates cell migration was demonstrated with the expression of full-length PECAM-1 in NIH3T3 cells, a line of mouse fibroblast which normally lacks PECAM-1 expression [108]. NIH3T3 cells expressing full-length PECAM-1 showed enhanced cell adhesion, and reduced cell migration. Since then numerous studies utilized PECAM-1 antibodies to demonstrate the role of PECAM-1 in EC migration, and incubation with PECAM-1 antibodies inhibited EC migration [60, 63, 109] and capillary morphogenesis [109–114]. More specific understanding of the role of PECAM-1 in EC migration was provided by studying isolated EC from PECAM-1 −/− mice. Lack of PECAM-1in EC resulted in abrogated cell migration [46, 63, 115] and capillary morphogenesis [46, 116]. In contrast, enhanced PECAM-1 expression increased EC motility [46, 109, 111]. Reduced PECAM-1 −/− EC migration was characterized with defective remodeling of cytoskeleton into filopodia and lamellipodia, and enhanced adhesion to ECM proteins [46, 63].
PECAM-1 modulates EC migration by impacting intracellular signaling cascades through phosphorylation of its cytoplasmic tyrosine residues. The expression of tyrosine residue-mutated or cytoplasmic domain truncated PECAM-1in EC increased the migration and phosphorylation of other migration regulators, including β-catenin and focal adhesion kinase [97, 105, 117]. Recent studies demonstrated that loss of exon 14 enhances migration by activation of MAPK/ERKs signaling and decreasing cell adhesion to ECM protein [41, 44, 45, 117]. This isoform-specific effect on cell migration provides meaningful insight into the understanding of the differential role PECAM-1 isoforms may play during tissue development [13, 14, 17].
Endothelial Cell Proliferation
In the endothelium, the confluent monolayer of EC remains in a contact-inhibition state, which limits their proliferation and migration, and PECAM-1 may exert a regulatory function in this process [118]. Proliferation of EC is a critical process during angiogenesis, which is promoted by proangiogenic growth factors including VEGF and bFGF. The role of PECAM-1 in angiogenesis has been approached by applying blocking antibodies against PECAM-1 in vivo, and the investigations revealed abrogated cytokine-induced rat corneal angiogenesis, bFGF-induced angiogenesis in Matrigel implant assays [110], and tumor angiogenesis [112, 119]. In addition, cell proliferation in the retinal vasculature was enhanced in PECAM-1 −/− mice [61]. However, the effect of PECAM-1 on EC proliferation in vitro varies depending on the source of EC, and the methods used to down-regulate PECAM-1 function or expression (e.g. PECAM-1 specific antibodies and siRNAs). PECAM-1 +/+ and PECAM-1 −/− lung EC showed comparable level of proliferation [63], whereas PECAM-1 −/− bEND cells and retinal EC (both from the central nervous system) were more proliferative compared with PECAM-1 +/+ cells [46, 115]. Thus, the role of PECAM-1 in EC proliferation is tissue specific and PECAM-1 deficiency level effects on proliferating process, which may be linked to expression and/or activity of CD44 through Hippo pathway [120, 121].
PECAM-1 and Endothelial Targeting
The specific expression of PECAM-1 in the endothelium at high levels has been utilized as a potential target for specific delivery of various materials with therapeutic potential [122, 123]. Muzykantov and collegues initally showed that clostering of biotinylated anti-PECAM-1 antibody with streptavidin significantly enhanced uptake and intenaliztion of anti-PECAM-1 antibody by cells expressing PECMA-1. Furthermore, conjugation of catalase with biotinylated-streptavidin anti-PECAM-1 antibody was taken up by endothelial cells and protected them from H2O2-mediated injury, both in culure and in perfused lungs [122]. Using a similar stratagies they later showed the utility of this imunotargeting in clinical lung transplantation and perhaps other disorders with an endothelial component [123–131]. These studies also led to identification of a novel endocytic pathway for anti-PECAM-1 antibody conjugates independent of clathrin-mediated or caveolar endocytosis [132]. However, agents that inhibit macropinocytosis resulted in reduced internalization of clustered PECAM-1. Additional studies demonstrated an important role for PECAM-1 cytoplasmic domain including Y686 for Rho activation and actin polymerization, and endocytosis of anti-PECAM-1 antibodies [133]. Thus, PECAM-1 signaling and interaction with the cytoskeleton provides a safe intra-endothelial drug delivery for anti-PECAM-1 carriers, and may be influenced by distinct targeted PECAM-1 epitope [134].
PECAM-1, ENOS, AND REGULATION OF NO PRODUCTION
Biology of eNOS
Nitric oxide (NO) is a short-lived signaling molecule participating in the regulation of vascular tone, vascular remodeling, endothelial permeability, and angiogenesis [135–137]. Nitric oxide synthase (NOS) catalyzes NO production from L-arginine. There are three main isoforms of NOS including nNOS (neuronal NOS; NOS1), iNOS (inducible NOS; NOS2), and eNOS (endothelial NOS; NOS3). The endothelial NOS (eNOS/NOS3) is the predominant NOS isoform in EC. During angiogenesis, NO plays a role through regulating physiological processes, including cell survival, proliferation, and migration. The activity of eNOS is modulated by the phosphorylation of Ser1177 (activation), Thr495 (inhibitory) [138], and dephosphorylation of Ser113 site, which is known to be involved in VEGF-mediated NO production [139, 140]. Thus, eNOS activity is highly impacted by post-translational modifications of specific serine and threonine residues.
Endothelial cells line the inside of blood vessels and consequently are exposed to fluid shear stress (FSS) of blood stream, which impacts EC morphology, gene expression , and physiology [137]. Fluid shear stress is the most essential physiological stimulus for eNOS activation and NO production in the endothelium [141–143]. While eNOS localizes to the perinuclear region, specifically Golgi complexes [144] under subconfluent conditions, main localization of eNOS in confluent monolayers is at sites of cell-cell contacts [135, 145, 146]. Since NO functions to regulate permeability of the endothelium by responding to stimuli, the presence of eNOS at cell-cell contacts site is critical for its activation [135].
Caveolae, a plasma membrane structure shaped like cavity, is a site enriched with various signaling molecules, ion channels, eNOS and protein kinases [143, 147–149], and is pointed as a place where eNOS is activated after FSS [143]. Noel et al recently demonstrated an important role for PECAM-1 and caveolae in enhanced angiogenic signaling in pulmonary endothelium following a stopped flow challenge [150]. These studies demonstrated that PECAM-1 and caveolae are parts of the mechanosensing complex which modulates NOX2 activation and production of superoxides, which promote neutrophil influx and proangiogenic signaling with loss of shear. These activities were attenuated in the absence of PECAM-1, perhaps as a result of failure to sense loss of shear and the need for PECAM-1 in neutrophil infiltration. However, the detailed mechanisms involved in PECAM-1 caveolae localization, and the potential role PECAM-1 isoforms and eNOS interactions may make to the formation of the mechanosensing complex and these activities remain to be determined.
As indicated above antibody to PECAM-1 has provided a suitable carrier for delivery and uptake of various agents to the endothelium. Shear stress impact on endothelial cells may also impact their interactions with and uptake of multivalent nanocarriers coated with PECAM-1 antibodies. These carriers are taken up via a noncanonical endocytic pathway that is related to macropinocytosis [132]. Han et al [151] recently showed that endothelial cell chronic adaptation to flow inhibited the endocytosis of nanocarrier coated with PECAM-1 antibody in arterial compared with capillary vessels. However, acute flows without stress fiber formation stimulated the endocytosis of nanocarries incubated with PECAM-1. Furthermore, deletion of PECAM-1 cytoplamic domain and disruption cholesterol-rich plasmalemma domains attenuated the stimulation of endocytosis of nanocarrier incubated with PECAM-1 by acute flow. Thus, the local microenvironment has a significant impact on uptake of nanocarriers by the endothelium, and cell culture models of nanoparticle uptake should reflect the microenvironment and phenotype of target cells. In addition, co-adminsteration of a paired monoclonal antibody directed to an adjacent, yet distinct PECAM-1 epitope, increased the binding of PECAM-1 directed antibodies with significant enhancement of functional activity of the nanocarrier [126].
PECAM-1 Deficiency and eNOS
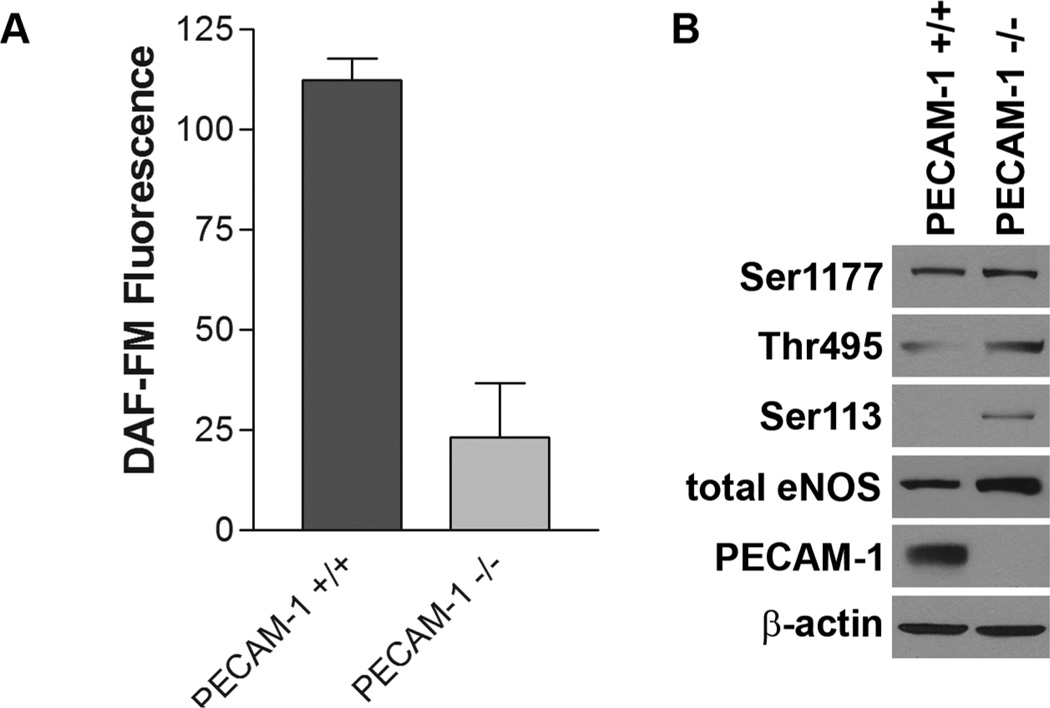
Bagi and colleagues showed that PECAM-1−/− mice exhibit defective vasodilatory activities in their artery, which suggested critical role for PECAM-1 in regulation of eNOS activity and NO production [152]. Dilated retinal vessels in PECAM-1 deficient mice provide further insight into the role of PECAM-1 in NO production [61]. Retina of PECAM-1 deficient mice expressed lower level of eNOS [61] and retinal EC from PECAM-1 −/− mice expressed significantly reduced level of eNOS and its phosphorylated active form, and as a result there is a dramatic decrease in NO production [46]. However, the effect of PECAM-1 deficiency on eNOS expression is tissue-dependent. Aorta from PECAM-1−/− mice exhibited comparable level of eNOS to PECAM-1+/+ mice [152] and PECAM-1−/− bEND cells expressed higher level of eNOS than PECAM-1+/+ bEND cells (Figure 3). Although eNOS expression level was not down regulated by the lack of PECAM-1, PECAM-1 −/− bEND cells were defective in NO production (Figure 3). Thus, PECAM-1 expression may contribute to appropriate activation of eNOS.
Figure 3. Activation of eNOS in PECAM-1 +/+ and PECAM-1 −/− bEND cells.
(A) NO production level in PECAM-1 +/+ and PECAM-1 −/− bEND cells were measured by DAF-FM assay as previously described [185]. (B) Phosphorylation level of Ser1177, Thr495, and Ser113 sites of eNOS were analyzed by Western blot analysis as previously described [185]. All antibodies were from Cell Signaling (Boston, MA). Anti-PECAM-1 was from R&D Systems (Minneapolis, MN) and anti-eNOS was from Santa Cruz (Santa Cruz, CA). Please note diminished production of NO in PECAM-1−/− bEND cells perhaps as a result of increased Ser113 and Thr495 phosphorylation despite similar expression of eNOS and Ser1177 phosphorylation as PECAM-1+/+ bEND cells. (S.Park, CM Sorenson and N. Sheibani, unpublished work)
As explained above, eNOS activity is regulated by phosphorylation and dephosphorylation of several sites on eNOS (e.g. Ser1177, Thr495, and Ser113), and PECAM-1 appears to be involved in the regulation of these phosphorylation and dephosphorylation processes. Lack of PECAM-1 did not affect the phosphorylation of Ser1177 whose phosphorylation activate NO production. However, increased phosphorylation level of Thr495 (inhibitory site), and Ser113 whose dephosphorylation is critical in VEGF-mediated NO production [139, 140] were observed in PECAM-1 −/− bEND cells. These altered phosphorylation status seems to contribute to defective NO production in PECAM-1 −/− bEND (Figure 3), and desrve further investigation.
PECAM-1 deficient retinal EC exhibited abrogated NO production from down-regulated eNOS expression level [46]. Interestingly, decreased NO production was observed in PECAM-1 −/− bEND cells which expressed even higher level of eNOS and comparable level of Ser1177 phosphorylation (Figure 3). These results indicated that PECAM-1 is involved in NO production of EC not just by regulating eNOS expression but also its phosphorylation for the activation through undetermined processes.
PECAM-1-Mediated Regulation of eNOS Activity
Considerable number of studies have demonstrated various FSS sensors, including ion channels, tyrosine kinase receptors, G-protein-coupled receptors, caveolae, adhesion molecules including integrins, PECAM-1, glycocalyx, and primary cilia [147]. In confluent EC monolayer PECAM-1 localizes at sites of cell-cell contacts, and application of FSS in these cells induces phosphorylation of tyrosine residues in PECAM-1 cytoplasmic domain [26, 66, 153, 154], which mediate “mechanosignal transduction” through activation of MAPK/ERKs and recruitment of SHP-2 [23, 155].
Phosphorylation of tyrosine residues in PECAM-1upon FSS induces phosphorylation and activation of eNOS. The down-regulation of PECAM-1 expression in EC induces defective phosphorylation of eNOS [156]. Moreover recent studies have demonstrated that PECAM-1 is important in the activation of eNOS through direct protein-protein interactions at cell-cell junctions [135, 152, 156, 157] (Figure 4). These studies indicated that PECAM-1 may play a role in restricting eNOS localization to cell-cell junctions, and PECAM-1 association with eNOS results in its inactivation. Following the phosphorylation of PECAM-1 upon FSS, the association of PECAM-1 and eNOS is disrupted, and the dissociation enhances eNOS activity [156, 158]. Thus, PECAM-1 is implicated in the mechanotransduction and regulation of eNOS activity through its direct association at cell-cell contacts. However, the detailed mechanisms involved, and whether these activities are impacted in a PECAM-1 isoform specific manner, remain unclear and are active area of investigation.
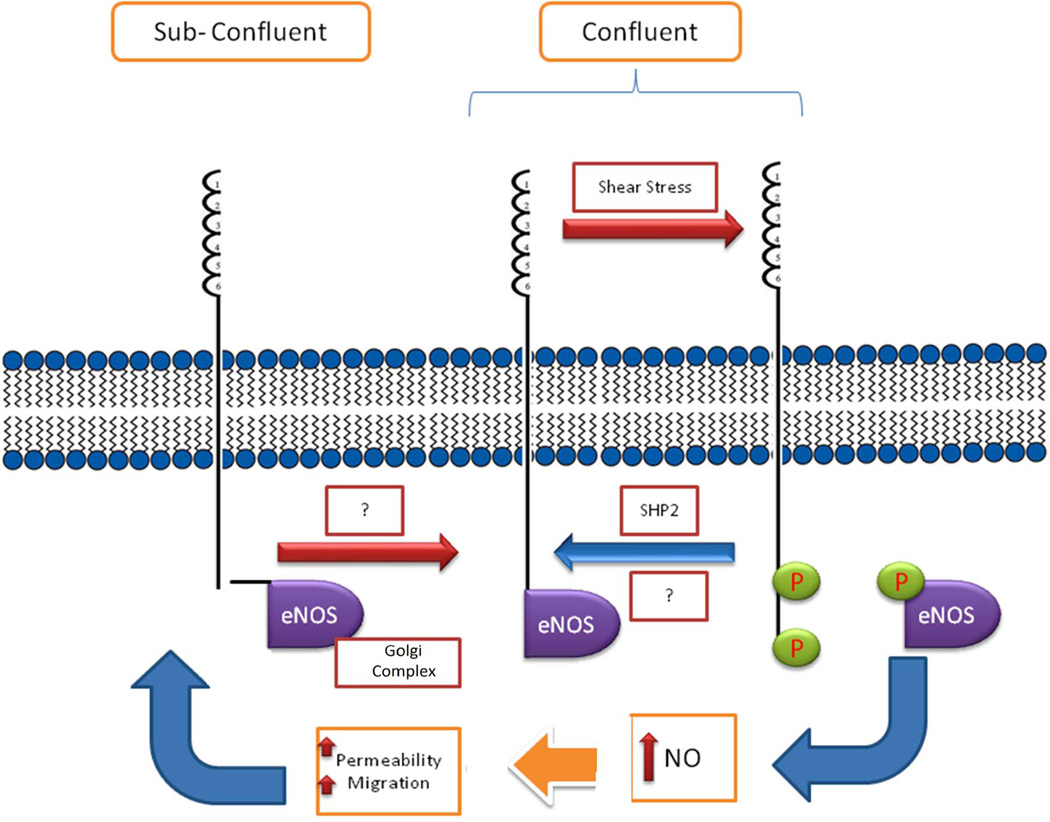
Figure 4. Proposed regulation of eNOS activity through interactions with PECAM-1.
Under subconfluent conditions eNOS localizes to perinuclear region, especially Golgi complex. Under confluent conditions PECAM-1 modulates cell-cell border localization of eNOS. Although eNOS is shown to associate with PECAM-1, how this association occurs and results in inactivation of eNOS remains to be determined. Phosphorylation of PECAM-1 in response to shear stress disrupts eNOS interaction with PECAM-1 and results in eNOS activation [185]. Interaction of phosphorylated PECAM-1 and its association with SHP2 may play a role in inactivation of eNOS and its reassociation with PECAM-1 upon removal of shear stress. This pathway may provide a mechanism for inactivation of eNOS and its junctional localization following its activation. However, the details of this mechanism and the exact role PECAM-1 plays remain elucive and deserve further confirmation.
PECAM-1 Isoforms and Caveolae Localization of eNOS
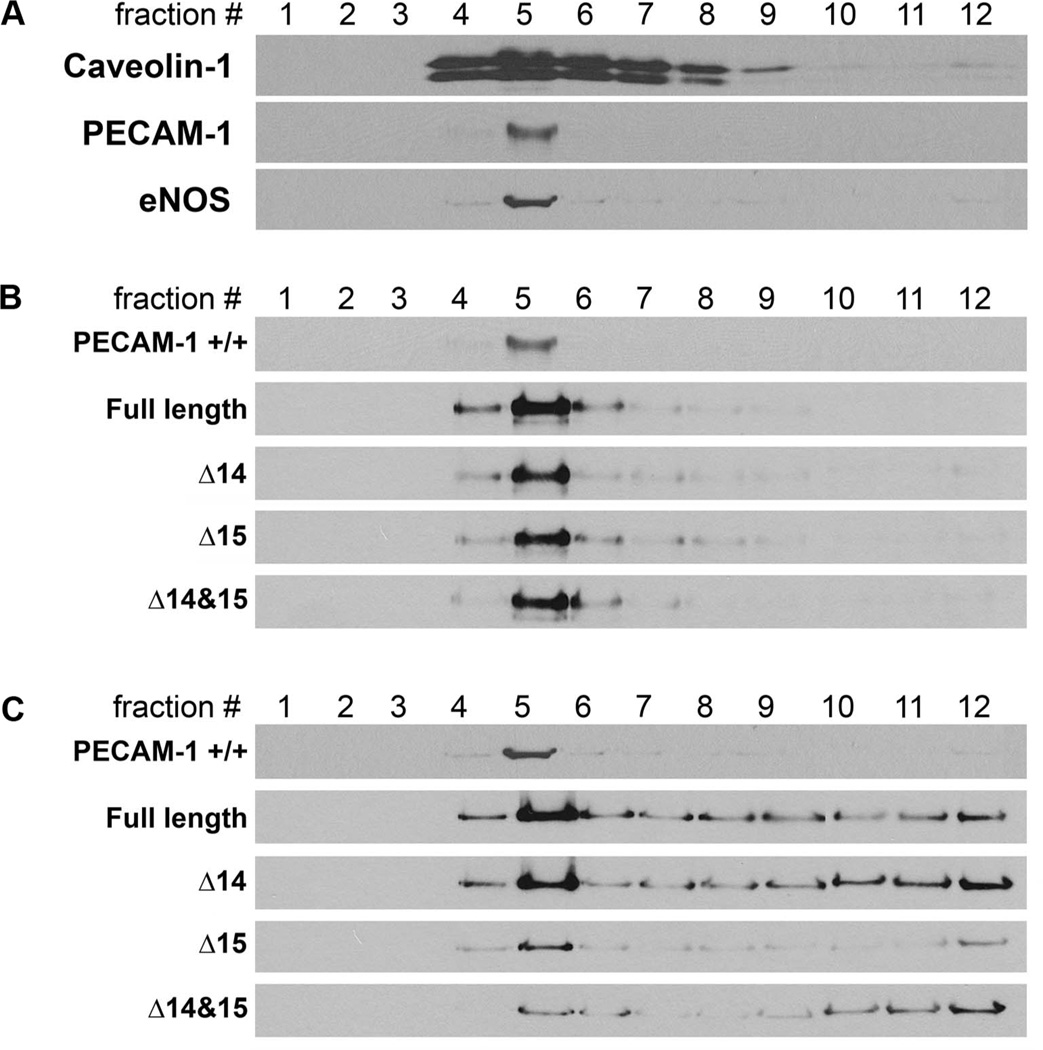
In cell membrane, a number of molecules including PECAM-1 and eNOS are enriched in caveolae structures for efficient interaction and signaling. It has been demonstrated that endothelial junctional localization of eNOS is modulated by its interaction with PECAM-1 [135, 156, 158]. However, our understanding of the impact of PECAM-1 isoforms on eNOS activity is quite limited. To determine the effect of PECAM-1 isoforms on caveolae localization of eNOS, each PECAM-1 isoform expressing EC were analyzed after caveolae fractionation upon ultracentrifugation. Since PECAM-1 −/− retinal EC express significantly decreased level of eNOS compared to PECAM-1 +/+ EC [46], PECAM-1 −/− retinal EC were transfected with a specific isoform of PECAM-1 and eNOS for these assays. Following fractionation upon ultracentrifugation, caveolae fractions were examined for caveolin-1, a protein component of the caveolae structure which was mainly present in fraction 5 (Figure 5A). In PECAM-1 +/+ retinal EC, both PECAM-1 and eNOS were predominantly localized in fraction 5 (Figure 5). Expression of PECAM-1 isoforms with and without exon 14 did not affect PECAM-1 and eNOS caveolae localization in EC (Figure 5B and C). The eNOS, in PECAM-1 +/+ EC was mainly localized to caveolae (fraction 5). However, eNOS in PECAM-1 −/− EC expressing a specific isoform of PECAM-1 exhibited a more diffused localization such as Golgi (fractions 6–8), endoplasmic reticulum, microsomes (fractions 9–11), and nuclear pellet (fraction 12) (Figure 5). Analyzing PECAM-1 isoform expressing PECAM-1 −/− bEND cells, which do express significant amount of eNOS, exhibited a similar result (not shown). Thus, caveolae localization of PECAM-1 appear to be isoform-independent, and altered localization of eNOS in PECAM-1 −/− deficient EC expressing a specific isoform of PECAM-1 requires further investigation and may be influenced by FSS.
Figure 5. PECAM-1 is not sufficient for caveolae localization of eNOS.
Caveolae localization was analyzed by caveolae fractionation using sucrose gradient ultracentrifugation. Briefly, PECAM-1 +/+ (A) and PECAM-1 −/− (B, C) retinal EC expressing a specific PECAM-1 isoform and eNOS were cultured in 100 mm dishes. Confluent EC were washed and scraped off the plates with ice-cold PBS containing protease inhibitor cocktail, 3 mM Na3VO4, and 5 mM NaF and then sonicated. Protein concentration was determined using BCA protein assay kit (Pierce) and 2 ml of cell lysate (1 mg of protein) were mixed with 2 ml of 90% sucrose prepared in MES buffer (25 mM MES, 150 mM NaCl, pH 6.5) and vortexed vigorously. The sample was then added to the bottom of the centrifuge tube (Beckman, Palo Alto, CA), and 4 ml of 35% sucrose and 4 ml of 5 % sucrose in MBS (25 mM MES, 150 mM NaCl, 250 mM NaHCO3) were sequentially added. The tubes were centrifuged in a L-70 ultracentrifuge (Beckman Instrument, Palo Alto, CA) equipped with a swing rotor (SW41 Ti) at 39,000 rpm for 16 h. The gradient was separated into 1 ml fractions from the top to obtain fraction #1 (the lowest density fraction in the sucrose gradient) to 12 (the highest density fraction). A sample of each fraction was separated on SDS-PAGE and Western blotted with antibodies to caveolin-1 (BD Biosciences), anti-PECAM-1 (R&D Systems) and anti-eNOS (Santa Cruz). Retinal PECAM-1 −/− EC (B, C; express no PECAM-1 and little or no eNOS) were infected with adenovirus encoding full length, Δ14, Δ15, or Δ14&15 PECAM-1 and with eNOS, and the fractions were separated by SDS-PAGE and Western blotted with antibodies to PECAM-1 (B) or eNOS (C). Please note the caveolae localization of PECAM-1 isoforms (#5) and diffused localization of eNOS in PECAM-1 −/− EC expressing a specific PECAM-1 isoform. Although PECAM-1 caveolae localization was isoform indepepdnet, the localization of eNOS was more defuse. Full length and Δ14 isoform showed most caveolae localization. (S.Park, CM Sorenson and N. Sheibani, unpublished work)
Isoform Specific Interaction of PECAM-1 and eNOS
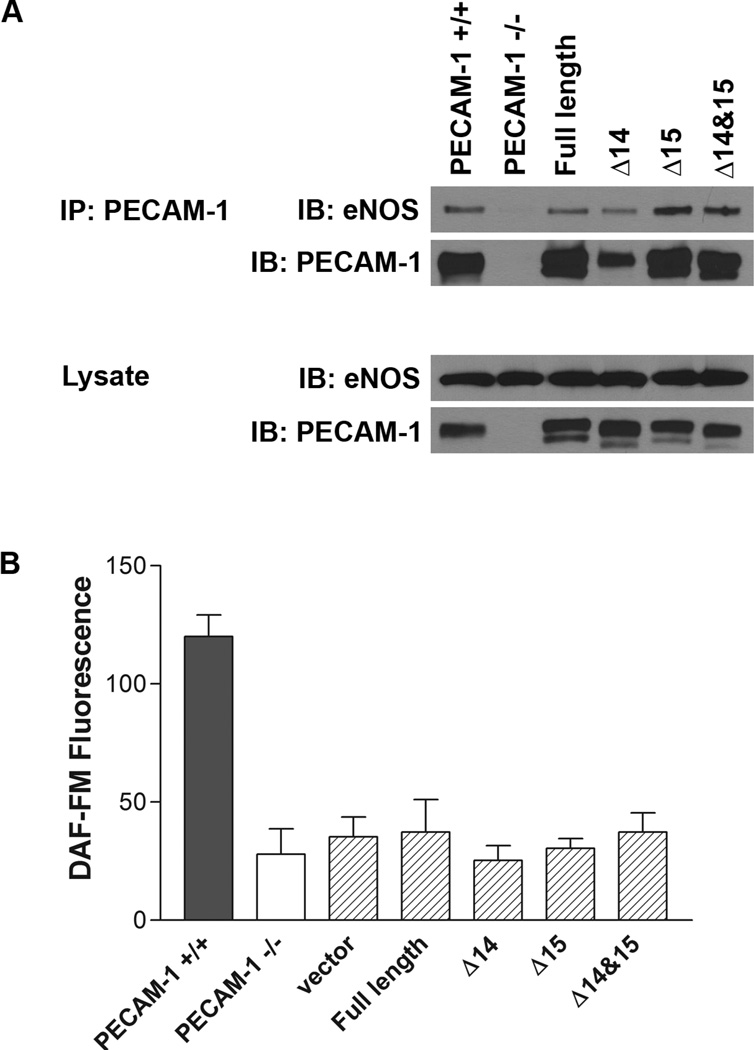
PECAM-1 is known to modulate eNOS activity through protein-protein interactions at site of cell-cell contact [135, 156, 158]. However, the role of PECAM-1 isoforms in this process is still unclear. To enhance our understanding of this process, PECAM-1 isoform-specific interaction with eNOS in PECAM-1 −/− bEND cells expressing a specific isoform of PECAM-1 was analyzed by immunoprecipitation (IP) assays. Among various isoforms, Δ15 and Δ14&15 isoforms showed intense interaction with eNOS (Figure 6A). However, the relative amount of PECAM-1-bound to eNOS was significantly lower than free eNOS (Figure 6A; ~10%: IP vs. lysate). Also, PECAM-1 isoform expression in PECAM-1 −/− cells did not affect the abrogated NO production (Figure 6B) and phosphorylation of eNOS (not shown). Here the experiments were performed under normal culture conditions with EC isolated from retina and brain microvessels. These results suggested that interaction between PECAM-1 and eNOS in microvessels may not be the major step in control of NO production, and fluid shear stress might be the most potent stimulator of the PECAM-1 and eNOS interaction. Therefore, investigation of applied shear stress to PECAM-1-deficient EC expressing a specific isoform of PECAM-1 may provide a better understanding of the PECAM-1 isoform specific interaction with eNOS and its implication in EC function.
Figure 6. PECAM-1 isoform specific interaction with eNOS and the effect of PECAM-1 expression on NO production.
(A) The interaction between PECAM-1 isoforms and eNOS was assessed by immunoprecipitation (IP) analysis of lysates prepared from PECAM-1 −/− bEND cells expressing a specific isoform of PECAM-1under normal cell culture condition. The amount of total protein for lysates was 1/20 of that applied for IP analysis. (B) Intracellular NO levels in PECAM-1 −/− bEND cells expressing a specific isoform of PECAM-1 was measured using the DAF-FM assay as previously described [185]. Please note that all the PECAM-1 isoforms interacted with PECAM-1. The isoforms lacking exona 14, 15 or both showed higher levels of eNOS compared to full length PECAM-1 or wild type cells. However, expression of none of these isoforms resulted in activation of eNOS and NO production as seen in wild type cells. Thus, simple expression of PECAM-1 and its interaction with eNOS is not sufficient to restore NO levels in null cells. (S.Park, CM Sorenson and N. Sheibani, unpublished work)
PECAM-1 AND ENDOGLIN
Biology of Endoglin
Endoglin (CD105) is a 180 kDa homodimeric transmembrane glycoprotein that functions as an auxiliary receptor in Transforming Growth Factor-β (TGFβ) signaling. After its initial identification in pre-B lymphoblastic HOON cell line [159], expression of endoglin on vascular EC, acute lymphoblastic and myelocytic leukemia cells, and bone marrow cells was reported [159–161]. Loss of endoglin is embryonic lethal due to defects in cardiovasculature development [162, 163]. Heterozygous mutations in the external domain of endoglin results in haploinsufficiency, and is implicated in hemorrhagic telangiectasia type 1 (HHT1) and arteriovenous malformations (AVM) in humans [164]. In mice endoglin haploinsufficiency also results in vascular defects similar to HHT1 [163].
Endoglin participates in angiogenesis through regulation of EC proliferation, migration, and capillary morphogenesis [165, 166]. Based on the observations of highly increased endoglin expression in active EC upon tumor angiogenesis [167, 168], the development of cancer therapy targeting endoglin is under intense investigation [169]. These activities of endoglin are mediated through both canonical and non-canonical TGFβ signaling pathways. However, the detailed implication of endoglin in these mechanisms still remains poorly understood.
Endoglin and TGFβ Signaling Pathways
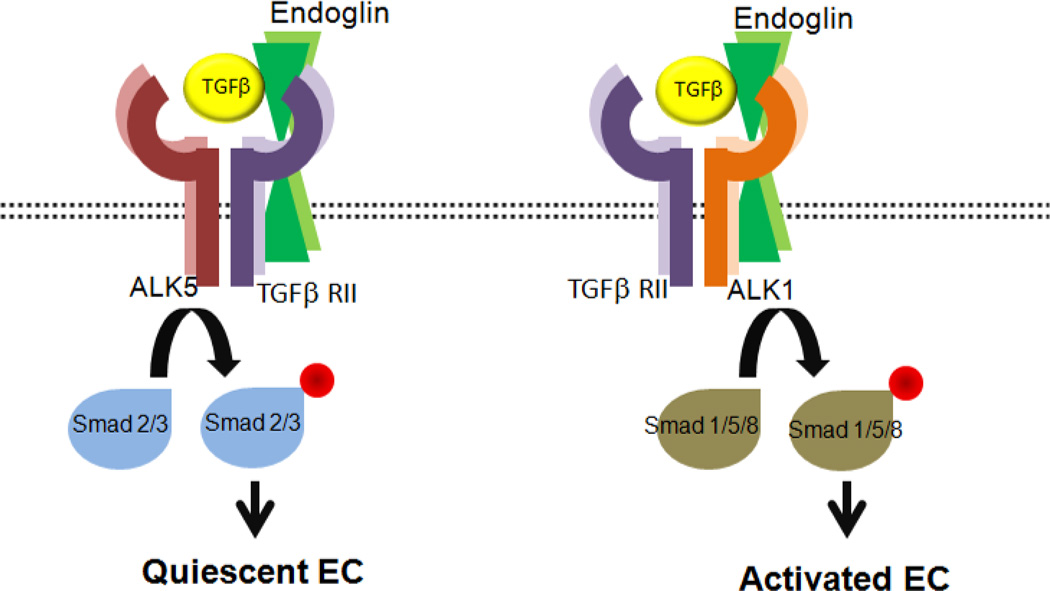
TGFβ is a cytokine with important roles in EC proliferation, differentiation, migration, and survival [170–172], and includes three isoforms TGFβ1, TGFβ2, and TGFβ3. TGFβ is shown to both inhibit and stimulate angiogenesis depending on experimental conditions [173, 174]. The main function of TGFβ is mediated through tyrosine/threonine kinase receptors on the cell surface including TGFβ type II receptor (TGFβ RII), TGFβ type I receptor (TGFβ RI), and endoglin [175]. There are two distinct types of TGFβ RI including activin receptor-like kinase 1 (ALK1), which is exclusively expressed on EC, and ALK5 with a more universal expression. Following TGFβ ligand binding to TGFβ RII, TGFβ RI is recruited and heteromeric complex of two TGFβRII and two TGFβRI is formed. The TGFβRII then phosphorylates TGFβRI, and the phosphorylated TGFβ RI mediates the signaling cascade by phosphorylating intracellular effector proteins, Smads [176, 177]. TGFβ is known to participate in angiogenesis by stimulating or inhibiting the activation of EC through a balance in ALK5 and ALK1 signaling. While activated ALK5 phosphorylates Smad2/3 and induces quiescence of EC, phosphorylation of Smad1/5/8 following activation of ALK1 activates EC to migrate and proliferate (Figure 7) [178].
Figure 7. The proposed role of endoglin in TGFβ signaling pathways.
TGFβ bound TGFβRII recruits TGFβRI (ALK5 or ALK1), forms a heteromeric complex, and phosphorylates TGFβRI. The phosphorylated ALK5 and ALK1 phosphorylate Smad 2/3 and Smad 1/5/8, respectively. Activated Smad2/3 promotes the quiescence of EC, while activated Smad 1/5/8 promote EC proliferation and migration. Endoglin is phosphorylated by TGFβRII and ALK5/ALK1 complex and phosphorylated endoglin by ALK1 modulates ALK1 dependent EC growth and adhesion [185]. In addition, endoglin participate in none-cananical signaling pathways such as MAPK pathways. Our recent studies showed that appropriate expression of endoglin in EC is essential for attenuation of MAPK/ERKs pathway and regulation of their angiogenic properties [185].
Endoglin, as an auxiliary TGFβ receptor, is a component of the TGFβ receptor complex system and binds TGFβ1 and TGFβ3 [179, 180]. Activated ALK5, ALK1, and TGF β RII interact with endoglin and phosphorylate serine and threonine residues in the endoglin cytoplasmic domain, and the phosphorylation of endoglin by endothelial-specific ALK1 regulates ALK1-dependent EC growth and adhesion [181, 182].TGFβ also regulates angiogenesis through non-Smad-dependent signaling pathways, including MAPK, Rho GTPase, and PI3K/AKT pathways [183, 184]. How these signaling pathways contribute to the promotion and/or inhibition of angiogenesis needs further elucidation.
Endoglin and PECAM-1
Endoglin expression is highly up-regulated during angiogenesis. PECAM-1 deficient mice exhibited defects in angiogenesis perhaps due to reduced endoglin expression and failure to up-regulate endoglin expression during acute neovascularization [61]. Very limited knowledge exists regarding the relationship between PECAM-1 and endoglin and the effect of a PECAM-1 deficiency on endoglin expression and function. In recent studies altered angiogenic properties and TGFβ signaling cascade of retinal EC prepared from endoglin +/− mice was observed and these studies were first in delineating the cell autonomous impact of endoglin haploinsufficiency on EC functions [185]. This study revealed interesting correlation between PECAM-1 and endoglin expression that reduced endoglin expression causing down regulation of PECAM-1. Through undetermined mechanisms, PECAM-1 and endoglin influence the expression of each other, and their altered expression generate defective angiogenic properties of EC through intracellular signaling pathways impacted by TGFβ. However, the detailed regulatory mechanisms engaged during angiogenesis, which result in proper expression of endoglin need further elucidation.
CONCLUSIONS AND FUTURE DIRECTIONS
PECAM-1 not only regulates angiogenesis but also affects inflammation. In spite of intensive research, the function of PECAM-1 still awaits further delineation. Although PECAM-1 undergoes alternative splicing the full length isoform is the predominant isoform in human endothelium. In contrast, PECAM-1 isoform lacking exons 14 and 15 is the predominant isoform in mouse endothelium. Thus, PECAM-1 activity may be differentially regulated in humans and mice, phosphorylation in humans and alternative splicing in mice. However, the significance of these different mechanisms for regulation of PECAM-1 activity remains unclear.
The role of PECAM-1 isoform specific function in various signaling pathways and regulation of eNOS activity remains unexplored. Lack of PECAM-1 is also associated with altered expression of other proangiogenic molecules, including eNOS and endoglin in a tissue specific manner. However, the detailed mechanisms involved are unknown. Recently CD44 expression also proposed to be a component of the regulatory axis discussed here, and its deficiency results in decreased level of PECAM-1. This is reported to be mediated through Hippo pathway. However, the impact of CD44 deficiency on expression and activity of eNOS and endoglin needs further investigation.
The direct phosphorylation of endoglin by Src, more specifically in the 612YIY614 membrane proximal motif, induces its internalization and degradation with a significant impact on EC proliferation, migration, and capillary morphogenesis [186]. We have shown PECAM-1 interacts with Src through its exon 13, which is present in all murine PECAM-1 isoforms [44]. How interaction of PECAM-1 with Src may impact endoglin phosphorylation and EC function remains to be explored. Furthermore, determining whether expression of PECAM-1 isoforms, which differentially modulate EC migration, impact the phosphorylation state of endoglin, will provide additional insight into the mechanisms of PECAM-1 and endoglin interactions during angiogenesis.
Collectively the studies presented here establish an important role for PECAM-1and its potential partners in regulation of angiogenesis and vascular function. These activities are specifically impacted by phosphorylation and/or alternative splicing of PECAM-1. Thus, understanding the critical role of PECAM-1 in regulation of angiogenesis and delineation of its isoform specific functions will likely deepen our understanding of angiogenesis process and provide novel targets for its modulation and potential therapeutic applications.
Acknowledgments
This work was supported by grants R01 EY016995, R24 EY022883, P30 EY016665, and P30 CA014520 UW Paul P. Carbone Cancer Center Support Grant from the National Institutes of Health and an unrestricted departmental award from Research to Prevent Blindness. NS is a recipient of a Research Award from American Diabetes Association, 1-10-BS-160 and Retina Research Foundation. SYP was supported by a Predoctoral Award from AstraZeneca. CMS was supported by grant from the National Institutes of Health R21EY023024 and the Retina Research Foundation/Daniel M. Albert Chair. We greatly appreciate the help of Dr. M. Ali Saghiri with preparation of figures.
REFERENCES
- 1.Albelda SM, Oliver PD, Romer LH, Buck CA. EndoCAM: a novel endothelial cell-cell adhesion molecule. J. Cell Biol. 1990;110:1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyert SM, Ferrero EM, Seremetis SV, Winchester RJ, Silver J, Mattison AC. Biochemistry and expression of myelomonocytic antigens. J. Immunol. 1986;137:3909–3914. [PubMed] [Google Scholar]
- 3.Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J. Exp. Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohto H, Maeda H, Shibata Y, Chen RF, Ozaki Y, Higashihara M, Takeuchi A, Tohyama H. A novel leukocyte differentiation antigen: two monoclonal antibodies TM2 and TM3 define a 120-kd molecule present on neutrophils, monocytes, platelets, and activated lymphoblasts. Blood. 1985;66:873–881. [PubMed] [Google Scholar]
- 5.van Mourik JA, Leeksma OC, Reinders JH, de Groot PG, Zandbergen-Spaargaren J. Vascular endothelial cells synthesize a plasma membrane protein indistinguishable from the platelet membrane glycoprotein IIa. J. Biol. Chem. 1985;260:11300–11306. [PubMed] [Google Scholar]
- 6.Woodfin A, Voisin M-B, Nourshargh S. PECAM-1: A Multi-Functional Molecule in Inflammation and Vascular Biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 7.Newman PJ. The role of PECAM-1 in vascular cell biology. Ann. N. Y. Acad. Sci. 1994;714:165–174. doi: 10.1111/j.1749-6632.1994.tb12041.x. [DOI] [PubMed] [Google Scholar]
- 8.Kirschbaum NE, Gumina RJ, Newman PJ. Organization of the gene for human platelet/endothelial cell adhesion molecule-1 shows alternatively spliced isoforms and a functionally complex cytoplasmic domain. Blood. 1994;84:4028–4037. [PubMed] [Google Scholar]
- 9.Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, Muller WA., 2nd PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 10.Xie Y, Muller WA. Molecular Cloning and Adhesive Properties of Murine Platelet/Endothelial Cell Adhesion Molecule 1. PNAS. 1993;90:5569–5573. doi: 10.1073/pnas.90.12.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheibani N, Sorenson CM, Frazier WA. Tissue specific expression of alternatively spliced murine PECAM-1 isoforms. Dev. Dyn. 1999;214:44–54. doi: 10.1002/(SICI)1097-0177(199901)214:1<44::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Su X, Sorenson CM, Sheibani N. Tissue-specific distributions of alternatively spliced human PECAM-1 isoforms. Am J Physiol Heart Circ Physiol. 2003;284:H1008–H1017. doi: 10.1152/ajpheart.00600.2002. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Repyak K, Sheibani N. Expression pattern of alternatively spliced PECAM-1 isoforms in retinal vasculature. Mol. Vis. 2004;10:103–111. [PubMed] [Google Scholar]
- 14.Wang Y, Su X, Wu Z, Sheibani N. Thrombospondin-1 deficient mice exhibit an altered expression pattern of alternatively spliced PECAM-1 isoforms in retinal vasculature and endothelial cells. J. Cell. Physiol. 2005;204:352–361. doi: 10.1002/jcp.20290. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Sheibani N. Expression pattern of alternatively spliced PECAM-1 isoforms in hematopoietic cells and platelets. J. Cell. Biochem. 2002;87:424–438. doi: 10.1002/jcb.10321. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Su X, Sorenson CM, Sheibani N. Modulation of PECAM-1 expression and alternative splicing during differentiation and activation of hematopoietic cells. J. Cell. Biochem. 2003;88:1012–1024. doi: 10.1002/jcb.10451. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 19.Newman PJ, Hillery CA, Albrecht R, Parise LV, Berndt MC, Mazurov AV, Dunlop LC, Zhang J, Rittenhouse SE. Activation-dependent changes in human platelet PECAM-1: phosphorylation, cytoskeletal association, and surface membrane redistribution. J. Cell Biol. 1992;119:239–246. doi: 10.1083/jcb.119.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zehnder JL, Hirai K, Shatsky M, McGregor JL, Levitt LJ, Leung LL. The cell adhesion molecule CD31 is phosphorylated after cell activation. Down-regulation of CD31 in activated T lymphocytes. J. Biol. Chem. 1992;267:5243–5249. [PubMed] [Google Scholar]
- 21.Lu TT, Barreuther M, Davis S, Madri JA. Platelet endothelial cell adhesion molecule-1 is phosphorylatable by c-Src, binds Src-Src homology 2 domain, and exhibits immunoreceptor tyrosine-based activation motif-like properties. J. Biol. Chem. 1997;272:14442–14446. doi: 10.1074/jbc.272.22.14442. [DOI] [PubMed] [Google Scholar]
- 22.Cao MY, Huber M, Beauchemin N, Famiglietti J, Albelda SM, Veillette A. Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J. Biol. Chem. 1998;273:15765–15772. doi: 10.1074/jbc.273.25.15765. [DOI] [PubMed] [Google Scholar]
- 23.Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J. Biol. Chem. 1997;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- 24.Sagawa K, Swaim W, Zhang J, Unsworth E, Siraganian RP. Aggregation of the high affinity IgE receptor results in the tyrosine phosphorylation of the surface adhesion protein PECAM-1 (CD31) J. Biol. Chem. 1997;272:13412–13418. doi: 10.1074/jbc.272.20.13412. [DOI] [PubMed] [Google Scholar]
- 25.Sagawa K, Kimura T, Swieter M, Siraganian RP. The protein-tyrosine phosphatase SHP-2 associates with tyrosine-phosphorylated adhesion molecule PECAM-1 (CD31) J. Biol. Chem. 1997;272:31086–31091. doi: 10.1074/jbc.272.49.31086. [DOI] [PubMed] [Google Scholar]
- 26.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J. Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins C, Guilluy C, Welch C, O’Brien ET, Hahn K, Superfine R, Burridge K, Tzima E. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr. Biol. 2012;22:2087–2094. doi: 10.1016/j.cub.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bird IN, Taylor V, Newton JP, Spragg JH, Simmons DL, Salmon M, Buckley CD. Homophilic PECAM-1(CD31) interactions prevent endothelial cell apoptosis but do not support cell spreading or migration. J Cell Sci. 1999;112:1989–1997. doi: 10.1242/jcs.112.12.1989. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, Williams J, Yan HC, Amin KM, Albelda SM, DeLisser HM. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J. Biol. Chem. 1996;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- 30.DeLisser HM, Chilkotowsky J, Yan HC, Daise ML, Buck CA, Albelda SM. Deletions in the cytoplasmic domain of platelet-endothelial cell adhesion molecule-1 (PECAM-1, CD31) result in changes in ligand binding properties. J. Cell Biol. 1994;124:195–203. doi: 10.1083/jcb.124.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kogata N, Masuda M, Kamioka Y, Yamagishi A, Endo A, Okada M, Mochizuki N. Identification of Fer tyrosine kinase localized on microtubules as a platelet endothelial cell adhesion molecule-1 phosphorylating kinase in vascular endothelial cells. Mol. Biol. Cell. 2003;14:3553–3564. doi: 10.1091/mbc.E03-02-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;369:340. doi: 10.1038/369340a0. [DOI] [PubMed] [Google Scholar]
- 33.D’Ambrosio D, Fong DC, Cambier JC. The SHIP phosphatase becomes associated with Fc gammaRIIB1 and is tyrosine phosphorylated during ‘negative’ signaling. Immunol. Lett. 1996;54:77–82. doi: 10.1016/s0165-2478(96)02653-3. [DOI] [PubMed] [Google Scholar]
- 34.Scharenberg AM, Kinet JP. The emerging field of receptor-mediated inhibitory signaling: SHP or SHIP? Cell. 1996;87:961–964. doi: 10.1016/s0092-8674(00)81790-0. [DOI] [PubMed] [Google Scholar]
- 35.Jackson DE, Kupcho KR, Newman PJ. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J. Biol. Chem. 1997;272:24868–24875. doi: 10.1074/jbc.272.40.24868. [DOI] [PubMed] [Google Scholar]
- 36.Henshall TL, Jones KL, Wilkinson R, Jackson DE. Src homology 2 domain-containing protein-tyrosine phosphatases, SHP-1 and SHP-2, are required for platelet endothelial cell adhesion molecule-1/CD31-mediated inhibitory signaling. J. Immunol. 2001;166:3098–3106. doi: 10.4049/jimmunol.166.5.3098. [DOI] [PubMed] [Google Scholar]
- 37.Hua CT, Gamble JR, Vadas MA, Jackson DE. Recruitment and activation of SHP-1 protein-tyrosine phosphatase by human platelet endothelial cell adhesion molecule-1 (PECAM-1). Identification of immunoreceptor tyrosine-based inhibitory motif-like binding motifs and substrates. J. Biol. Chem. 1998;273:28332–28340. doi: 10.1074/jbc.273.43.28332. [DOI] [PubMed] [Google Scholar]
- 38.Newman DK, Hamilton C, Newman PJ. Inhibition of antigen-receptor signaling by Platelet Endothelial Cell Adhesion Molecule-1 (CD31) requires functional ITIMs, SHP-2, and p56(lck) Blood. 2001;97:2351–2357. doi: 10.1182/blood.v97.8.2351. [DOI] [PubMed] [Google Scholar]
- 39.Sheibani N, Sorenson CM, Frazier WA. Differential modulation of cadherin-mediated cell-cell adhesion by platelet endothelial cell adhesion molecule-1 isoforms through activation of extracellular regulated kinases. Mol. Biol. Cell. 2000;11:2793–2802. doi: 10.1091/mbc.11.8.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J, Sheibani N. Modulation of VE-cadherin and PECAM-1 mediated cell-cell adhesions by mitogen-activated protein kinases. J. Cell. Biochem. 2003;90:121–137. doi: 10.1002/jcb.10600. [DOI] [PubMed] [Google Scholar]
- 41.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan G, Kalaitzidis D, Neel BG. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev. 2008;27:179–192. doi: 10.1007/s10555-008-9126-y. [DOI] [PubMed] [Google Scholar]
- 43.Dance M, Montagner A, Salles JP, Yart A, Raynal P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell. Signal. 2008;20:453–459. doi: 10.1016/j.cellsig.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Sheibani N. PECAM-1 isoform-specific activation of MAPK/ERKs and small GTPases: Implications in inflammation and angiogenesis. J. Cell. Biochem. 2006;98:451–468. doi: 10.1002/jcb.20827. [DOI] [PubMed] [Google Scholar]
- 45.DiMaio TA, Sheibani N. PECAM-1 isoform-specific functions in PECAM-1-deficient brain microvascular endothelial cells. Microvasc. Res. 2008;75:188–201. doi: 10.1016/j.mvr.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park S, DiMaio TA, Scheef EA, Sorenson CM, Sheibani N. PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions. Am J Physiol Cell Physiol. 2010;299:C1468–C1484. doi: 10.1152/ajpcell.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Risau W, Flamme I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 48.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 49.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 50.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 51.Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q. J. Nucl. Med. 2003;47:149–161. [PubMed] [Google Scholar]
- 52.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N. Engl. J. Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev. Dyn. 2003;228:630–642. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 54.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 55.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 56.Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat. Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 58.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, et al. Genetic Evidence for Functional Redundancy of Platelet/Endothelial Cell Adhesion Molecule-1 (PECAM-1): CD31-Deficient Mice Reveal PECAM-1-Dependent and PECAM-1-Independent Functions. J. Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- 60.DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, et al. Loss of PECAM-1 Function Impairs Alveolarization. J. Biol. Chem. 2006;281:8724–8731. doi: 10.1074/jbc.M511798200. [DOI] [PubMed] [Google Scholar]
- 61.Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. Dev. Biol. 2008;315:72–88. doi: 10.1016/j.ydbio.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J. Clin. Invest. 2002;109:383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao G, Fehrenbach ML, Williams JT, Finklestein JM, Zhu JX, Delisser HM. Angiogenesis in platelet endothelial cell adhesion molecule-1-null mice. Am J Pathol. 2009;175:903–915. doi: 10.2353/ajpath.2009.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 65.Chiu Y-J, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J. Cell Biol. 2008 doi: 10.1083/jcb.200801062. jcb.200801062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujiwara K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J. Intern. Med. 2006;259:373–380. doi: 10.1111/j.1365-2796.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1067–1073. doi: 10.1161/ATVBAHA.109.186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Z, Rubin J, Tzima E. Role of PECAM-1 in arteriogenesis and specification of preexisting collaterals. Circ Res. 2010;107:1355–1363. doi: 10.1161/CIRCRESAHA.110.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salmi M, Jalkanen S. How do lymphocytes know where to go: current concepts and enigmas of lymphocyte homing. Adv. Immunol. 1997;64:139–218. doi: 10.1016/s0065-2776(08)60889-5. [DOI] [PubMed] [Google Scholar]
- 70.Shimizu Y, Rose DM, Ginsberg MH. Integrins in the immune system. Adv. Immunol. 1999;72:325–380. doi: 10.1016/s0065-2776(08)60024-3. [DOI] [PubMed] [Google Scholar]
- 71.Johnson-Leger C, Aurrand-Lions M, Imhof BA. The parting of the endothelium: miracle, or simply a junctional affair? J Cell Sci. 2000;113(Pt 6):921–933. doi: 10.1242/jcs.113.6.921. [DOI] [PubMed] [Google Scholar]
- 72.Wang S, Cao C, Chen Z, Bankaitis V, Tzima E, Sheibani N, Burridge K. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS ONE. 2012;7:e45499. doi: 10.1371/journal.pone.0045499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thompson RD, Noble KE, Larbi KY, Dewar A, Duncan GS, Mak TW, Nourshargh S. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 2001;97:1854–1860. doi: 10.1182/blood.v97.6.1854. [DOI] [PubMed] [Google Scholar]
- 75.Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J. Exp. Med. 1994;179:1059–1064. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wakelin MW, Sanz MJ, Dewar A, Albelda SM, Larkin SW, Boughton-Smith N, Williams TJ, Nourshargh S. An anti-platelet-endothelial cell adhesion molecule-1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through the basement membrane. J. Exp. Med. 1996;184:229–239. doi: 10.1084/jem.184.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Christofidou-Solomidou M, Nakada MT, Williams J, Muller WA, DeLisser HM. Neutrophil platelet endothelial cell adhesion molecule-1 participates in neutrophil recruitment at inflammatory sites and is down-regulated after leukocyte extravasation. J. Immunol. 1997;158:4872–4878. [PubMed] [Google Scholar]
- 78.Nakada MT, Amin K, Christofidou-Solomidou M, O’Brien CD, Sun J, Gurubhagavatula I, Heavner GA, Taylor AH, Paddock C, Sun QH, et al. Antibodies against the first Ig-like domain of human platelet endothelial cell adhesion molecule-1 (PECAM-1) that inhibit PECAM-1-dependent homophilic adhesion block in vivo neutrophil recruitment. J. Immunol. 2000;164:452–462. doi: 10.4049/jimmunol.164.1.452. [DOI] [PubMed] [Google Scholar]
- 79.Solowiej A, Biswas P, Graesser D, Madri JA. Lack of Platelet Endothelial Cell Adhesion Molecule-1 Attenuates Foreign Body Inflammation because of Decreased Angiogenesis. Am J Pathol. 2003;162:953–962. doi: 10.1016/S0002-9440(10)63890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schenkel AR, Chew TW, Muller WA. Platelet Endothelial Cell Adhesion Molecule Deficiency or Blockade Significantly Reduces Leukocyte Emigration in a Majority of Mouse Strains. J. Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- 81.Perkowski S, Scherpereel A, Murciano JC, Arguiri E, Solomides CC, Albelda SM, Muzykantov V, Christofidou-Solomidou M. Dissociation between alveolar transmigration of neutrophils and lung injury in hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1050–L1058. doi: 10.1152/ajplung.00067.2006. [DOI] [PubMed] [Google Scholar]
- 82.Wu Y, Stabach P, Michaud M, Madri JA. Neutrophils lacking platelet-endothelial cell adhesion molecule-1 exhibit loss of directionality and motility in CXCR2-mediated chemotaxis. J. Immunol. 2005;175:3484–3491. doi: 10.4049/jimmunol.175.6.3484. [DOI] [PubMed] [Google Scholar]
- 83.Liao F, Ali J, Greene T, Muller WA. Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J. Exp. Med. 1997;185:1349–1357. doi: 10.1084/jem.185.7.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J. Exp. Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dasgupta B, Dufour E, Mamdouh Z, Muller WA. A novel and critical role for tyrosine 663 in platelet endothelial cell adhesion molecule-1 trafficking and transendothelial migration. J. Immunol. 2009;182:5041–5051. doi: 10.4049/jimmunol.0803192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Brien CD, Lim P, Sun J, Albelda SM. PECAM-1-dependent neutrophil transmigration is independent of monolayer PECAM-1 signaling or localization. Blood. 2003;101:2816–2825. doi: 10.1182/blood-2002-08-2396. [DOI] [PubMed] [Google Scholar]
- 87.Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J. Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun QH, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J. Biol. Chem. 1996;271:11090–11098. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- 89.DeLisser HM, Yan HC, Newman PJ, Muller WA, Buck CA, Albelda SM. Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J. Biol. Chem. 1993;268:16037–16046. [PubMed] [Google Scholar]
- 90.Muller WA, Berman ME, Newman PJ, DeLisser HM, Albelda SM. A heterophilic adhesion mechanism for platelet/endothelial cell adhesion molecule 1 (CD31) J. Exp. Med. 1992;175:1401–1404. doi: 10.1084/jem.175.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buckley CD, Doyonnas R, Newton JP, Blystone SD, Brown EJ, Watt SM, Simmons DL. Identification of alpha v beta 3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- 92.Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J. Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong CW, Wiedle G, Ballestrem C, Wehrle-Haller B, Etteldorf S, Bruckner M, Engelhardt B, Gisler RH, Imhof BA. PECAM-1/CD31 trans-homophilic binding at the intercellular junctions is independent of its cytoplasmic domain; evidence for heterophilic interaction with integrin alphavbeta3 in Cis. Mol. Biol. Cell. 2000;11:3109–3121. doi: 10.1091/mbc.11.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J. Immunol. 1998;160:395–402. [PubMed] [Google Scholar]
- 95.Prager E, Sunder-Plassmann R, Hansmann C, Koch C, Holter W, Knapp W, Stockinger H. Interaction of CD31 with a heterophilic counterreceptor involved in downregulation of human T cell responses. J. Exp. Med. 1996;184:41–50. doi: 10.1084/jem.184.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Famiglietti J, Sun J, DeLisser HM, Albelda SM. Tyrosine residue in exon 14 of the cytoplasmic domain of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) regulates ligand binding specificity. J. Cell Biol. 1997;138:1425–1435. doi: 10.1083/jcb.138.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu TT, Yan LG, Madri JA. Integrin engagement mediates tyrosine dephosphorylation on platelet-endothelial cell adhesion molecule 1. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11808–11813. doi: 10.1073/pnas.93.21.11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan H-C, Baldwin HS, Sun J, Buck CA, Albelda SM, DeLisser HM. Alternative Splicing of a Specific Cytoplasmic Exon Alters the Binding Characteristics of Murine Platelet/Endothelial Cell Adhesion Molecule-1 (PECAM-1) J. Biol. Chem. 1995;270:23672–23680. doi: 10.1074/jbc.270.40.23672. [DOI] [PubMed] [Google Scholar]
- 99.Anderson JM, Balda MS, Fanning AS. The structure and regulation of tight junctions. Curr. Opin. Cell Biol. 1993;5:772–778. doi: 10.1016/0955-0674(93)90024-k. [DOI] [PubMed] [Google Scholar]
- 100.Beyer EC. Gap junctions. Int. Rev. Cytol. 1993;137c:1–37. [PubMed] [Google Scholar]
- 101.Gumbiner BM. Breaking through the tight junction barrier. J. Cell Biol. 1993;123:1631–1633. doi: 10.1083/jcb.123.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 103.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 104.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 105.Gratzinger D, Canosa S, Engelhardt B, Madri JA. Platelet endothelial cell adhesion molecule-1 modulates endothelial cell motility through the small G-protein Rho. FASEB J. 2003;17:1458–1469. doi: 10.1096/fj.02-1040com. [DOI] [PubMed] [Google Scholar]
- 106.Li S, Huang NF, Hsu S. Mechanotransduction in endothelial cell migration. J. Cell. Biochem. 2005;96:1110–1126. doi: 10.1002/jcb.20614. [DOI] [PubMed] [Google Scholar]
- 107.Giroux S, Tremblay M, Bernard D, Cardin-Girard JF, Aubry S, Larouche L, Rousseau S, Huot J, Landry J, Jeannotte L, et al. Embryonic death of Mek1- deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 1999;9:369–372. doi: 10.1016/s0960-9822(99)80164-x. [DOI] [PubMed] [Google Scholar]
- 108.Schimmenti LA, Yan HC, Madri JA, Albelda SM. Platelet endothelial cell adhesion molecule, PECAM-1, modulates cell migration. J. Cell Physiol. 1992;153:417–428. doi: 10.1002/jcp.1041530222. [DOI] [PubMed] [Google Scholar]
- 109.Cao G, O’Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A, DeLisser HM. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol Cell Physiol. 2002;282:C1181–C1190. doi: 10.1152/ajpcell.00524.2001. [DOI] [PubMed] [Google Scholar]
- 110.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- 111.O’Brien CD, Cao G, Makrigiannakis A, DeLisser HM. Role of immunoreceptor tyrosine-based inhibitory motifs of PECAM-1 in PECAM-1-dependent cell migration. Am J Physiol Cell Physiol. 2004;287:C1103–C1113. doi: 10.1152/ajpcell.00573.2003. [DOI] [PubMed] [Google Scholar]
- 112.Zhou Z, Christofidou-Solomidou M, Garlanda C, DeLisser HM. Antibody against murine PECAM-1 inhibits tumor angiogenesis in mice. Angiogenesis. 1999;3:181–188. doi: 10.1023/a:1009092107382. [DOI] [PubMed] [Google Scholar]
- 113.Sheibani N, Frazier WA. Down-regulation of platelet endothelial cell adhesion molecule-1 results in thrombospondin-1 expression and concerted regulation of endothelial cell phenotype. Mol. Biol Cell. 1998;9:701–713. doi: 10.1091/mbc.9.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sheibani N, Newman PJ, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates platelet-endothelial cell adhesion molecule-1 expression and endothelial cell morphogenesis. Mol. Biol Cell. 1997;8:1329–1341. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rothermel TA, Engelhardt B, Sheibani N. Polyoma virus middle-T-transformed PECAM-1 deficient mouse brain endothelial cells proliferate rapidly in culture and form hemangiomas in mice. J. Cell. Physiol. 2005;202:230–239. doi: 10.1002/jcp.20114. [DOI] [PubMed] [Google Scholar]
- 116.Kondo S, Scheef EA, Sheibani N, Sorenson CM. PECAM-1 isoform-specific regulation of kidney endothelial cell migration and capillary morphogenesis. Am J Physiol Cell Physiol. 2007;292:C2070–C2083. doi: 10.1152/ajpcell.00489.2006. [DOI] [PubMed] [Google Scholar]
- 117.Kim CS, Wang T, Madri JA. Platelet endothelial cell adhesion molecule-1 expression modulates endothelial cell migration in vitro. Lab. Invest. 1998;78:583–590. [PubMed] [Google Scholar]
- 118.RayChaudhury A, Elkins M, Kozien D, Nakada MT. Regulation of PECAM-1 in endothelial cells during cell growth and migration. Exp Biol Med (Maywood) 2001;226:686–691. doi: 10.1177/153537020222600715. [DOI] [PubMed] [Google Scholar]
- 119.DeLisser H, Liu Y, Desprez PY, Thor A, Briasouli P, Handumrongkul C, Wilfong J, Yount G, Nosrati M, Fong S, et al. Vascular endothelial platelet endothelial cell adhesion molecule 1 (PECAM-1) regulates advanced metastatic progression. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18616–18621. doi: 10.1073/pnas.1004654107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Flynn KM, Michaud M, Madri JA. CD44 deficiency contributes to enhanced experimental autoimmune encephalomyelitis: a role in immune cells and vascular cells of the blood-brain barrier. Am J Pathol. 2013;182:1322–1336. doi: 10.1016/j.ajpath.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsuneki M, Madri JA. CD44 regulation of endothelial cell proliferation and apoptosis via modulation of CD31 and VE-cadherin expression. J. Biol. Chem. 2014;289:5357–5370. doi: 10.1074/jbc.M113.529313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 2003;21:392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 124.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. Journal of controlled release : official journal of the Controlled Release Society. 2001;71:1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 125.Hood ED, Greineder CF, Dodia C, Han J, Mesaros C, Shuvaev VV, Blair IA, Fisher AB, Muzykantov VR. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. Journal of controlled release : official journal of the Controlled Release Society. 2012;163:161–169. doi: 10.1016/j.jconrel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chacko AM, Nayak M, Greineder CF, Delisser HM, Muzykantov VR. Collaborative enhancement of antibody binding to distinct PECAM-1 epitopes modulates endothelial targeting. PLoS One. 2012;7:e34958. doi: 10.1371/journal.pone.0034958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Han J, Shuvaev VV, Muzykantov VR. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J. Pharmacol. Exp. Ther. 2011;338:82–91. doi: 10.1124/jpet.111.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, Madesh M, Nakada M, Muzykantov VR. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J. 2011;25:348–357. doi: 10.1096/fj.10-169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, Arguiri E, Solomides CC, Albelda SM, Harrison DG, et al. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J. Pharmacol. Exp. Ther. 2009;331:404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Danielyan K, Ding BS, Gottstein C, Cines DB, Muzykantov VR. Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema. J. Pharmacol. Exp. Ther. 2007;321:947–952. doi: 10.1124/jpet.107.120535. [DOI] [PubMed] [Google Scholar]
- 131.Ding BS, Gottstein C, Grunow A, Kuo A, Ganguly K, Albelda SM, Cines DB, Muzykantov VR. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood. 2005;106:4191–4198. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116:1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 133.Garnacho C, Shuvaev V, Thomas A, McKenna L, Sun J, Koval M, Albelda S, Muzykantov V, Muro S. RhoA activation and actin reorganization involved in endothelial CAM-mediated endocytosis of anti-PECAM carriers: critical role for tyrosine 686 in the cytoplasmic tail of PECAM-1. Blood. 2008;111:3024–3033. doi: 10.1182/blood-2007-06-098657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garnacho C, Albelda SM, Muzykantov VR, Muro S. Differential intra-endothelial delivery of polymer nanocarriers targeted to distinct PECAM-1 epitopes. Journal of controlled release : official journal of the Controlled Release Society. 2008;130:226–233. doi: 10.1016/j.jconrel.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Govers R, Bevers L, de Bree P, Rabelink TJ. Endothelial nitric oxide synthase activity is linked to its presence at cell-cell contacts. Biochem. J. 2002;361:193–201. doi: 10.1042/0264-6021:3610193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J. Cardiovasc. Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 137.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 139.Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, Figeys D, Harrison DG, Berk BC, Aebersold R, et al. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J. Biol. Chem. 1999;274:30101–30108. doi: 10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 140.Kou R, Greif D, Michel T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. J. Biol. Chem. 2002;277:29669–29673. doi: 10.1074/jbc.M204519200. [DOI] [PubMed] [Google Scholar]
- 141.Cheng C, van Haperen R, de Waard M, van Damme LCA, Tempel D, Hanemaaijer L, van Cappellen GWA, Bos J, Slager CJ, Duncker DJ, et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005;106:3691–3698. doi: 10.1182/blood-2005-06-2326. [DOI] [PubMed] [Google Scholar]
- 142.Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am. J. Physiol. 1994;266:C628–C636. doi: 10.1152/ajpcell.1994.266.3.C628. [DOI] [PubMed] [Google Scholar]
- 143.Rizzo V, McIntosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J. Biol. Chem. 1998;273:34724–34729. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- 144.Sessa WC, Garcia-Cardena G, Liu J, Keh A, Pollock JS, Bradley J, Thiru S, Braverman IM, Desai KM. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J. Biol. Chem. 1995;270:17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- 145.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RGW, Michel T. Acylation Targets Endothelial Nitric-oxide Synthase to Plasmalemmal Caveolae. J. Biol. Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 147.Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circulation journal : official journal of the Japanese Circulation Society. 2009;73:1983–1992. doi: 10.1253/circj.cj-09-0583. [DOI] [PubMed] [Google Scholar]
- 148.Boyd NL, Park H, Yi H, Boo YC, Sorescu GP, Sykes M, Jo H. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H1113–H1122. doi: 10.1152/ajpheart.00302.2003. [DOI] [PubMed] [Google Scholar]
- 149.Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am. J. Physiol. 1998;275:L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 150.Noel J, Wang H, Hong N, Tao JQ, Yu K, Sorokina EM, Debolt K, Heayn M, Rizzo V, Delisser H, et al. PECAM-1 and caveolae form the mechanosensing complex necessary for NOX2 activation and angiogenic signaling with stopped flow in pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2013;305:L805–L818. doi: 10.1152/ajplung.00123.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Han J, Zern BJ, Shuvaev VV, Davies PF, Muro S, Muzykantov V. Acute and Chronic Shear Stress Differently Regulate Endothelial Internalization of Nanocarriers Targeted to Platelet-Endothelial Cell Adhesion Molecule-1. ACS Nano. 2012;6:8824–8836. doi: 10.1021/nn302687n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bagi Z, Frangos JA, Yeh J-C, White CR, Kaley G, Koller A. PECAM-1 Mediates NO-Dependent Dilation of Arterioles to High Temporal Gradients of Shear Stress. Arterioscler Thromb Vasc Biol. 2005;25:1590–1595. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- 153.Osawa M, Masuda M, Harada N, Lopes RB, Fujiwara K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur. J. Cell Biol. 1997;72:229–237. [PubMed] [Google Scholar]
- 154.Harada N, Masuda M, Fujiwara K. Fluid flow and osmotic stress induce tyrosine phosphorylation of an endothelial cell 128 kDa surface glycoprotein. Biochem. Biophys. Res. Commun. 1995;214:69–74. doi: 10.1006/bbrc.1995.2257. [DOI] [PubMed] [Google Scholar]
- 155.Masuda M, Osawa M, Shigematsu H, Harada N, Fujiwara K. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett. 1997;408:331–336. doi: 10.1016/s0014-5793(97)00457-2. [DOI] [PubMed] [Google Scholar]
- 156.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 157.McCormick ME, Goel R, Fulton D, Oess S, Newman D, Tzima E. Platelet-endothelial cell adhesion molecule-1 regulates endothelial NO synthase activity and localization through signal transducers and activators of transcription 3-dependent NOSTRIN expression. Arterioscler Thromb Vasc Biol. 2011;31:643–649. doi: 10.1161/ATVBAHA.110.216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dusserre N, L’Heureux N, Bell KS, Stevens HY, Yeh J, Otte LA, Loufrani L, Frangos JA. PECAM-1 Interacts With Nitric Oxide Synthase in Human Endothelial Cells: Implication for Flow-Induced Nitric Oxide Synthase Activation. Arterioscler Thromb Vasc Biol. 2004;24:1796–1802. doi: 10.1161/01.ATV.0000141133.32496.41. [DOI] [PubMed] [Google Scholar]
- 159.Quackenbush EJ, Gougos A, Baumal R, Letarte M. Differential localization within human kidney of five membrane proteins expressed on acute lymphoblastic leukemia cells. J. Immunol. 1986;136:118–124. [PubMed] [Google Scholar]
- 160.Quackenbush EJ, Letarte M. Identification of several cell surface proteins of non-T, non-B acute lymphoblastic leukemia by using monoclonal antibodies. J. Immunol. 1985;134:1276–1285. [PubMed] [Google Scholar]
- 161.Gougos A, Letarte M. Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J. Immunol. 1988;141:1925–1933. [PubMed] [Google Scholar]
- 162.Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Developmental Biology. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 163.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 164.Matsubara S, Bourdeau A, terBrugge KG, Wallace C, Letarte M. Analysis of endoglin expression in normal brain tissue and in cerebral arteriovenous malformations. Stroke. 2000;31:2653–2660. doi: 10.1161/01.str.31.11.2653. [DOI] [PubMed] [Google Scholar]
- 165.Bernabeu C, Conley BA, Vary CP. Novel biochemical pathways of endoglin in vascular cell physiology. J. Cell. Biochem. 2007;102:1375–1388. doi: 10.1002/jcb.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fonsatti E, Nicolay HJ, Altomonte M, Covre A, Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc. Res. 2010;86:12–19. doi: 10.1093/cvr/cvp332. [DOI] [PubMed] [Google Scholar]
- 167.Miller DW, Graulich W, Karges B, Stahl S, Ernst M, Ramaswamy A, Sedlacek HH, Muller R, Adamkiewicz J. Elevated expression of endoglin, a component of the TGF-beta-receptor complex, correlates with proliferation of tumor endothelial cells. Int. J. Cancer. 1999;81:568–572. doi: 10.1002/(sici)1097-0215(19990517)81:4<568::aid-ijc11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 168.Perez-Gomez E, Del Castillo G, Juan Francisco S, Lopez-Novoa JM, Bernabeu C, Quintanilla M. The role of the TGF-beta coreceptor endoglin in cancer. The Scientific World Journal. 2010;10:2367–2384. doi: 10.1100/tsw.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin. Cancer Res. 2008;14:1931–1937. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 170.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.LI C, HAMPSON IN, HAMPSON L, KUMAR P, BERNABEU C, KUMAR S. CD105 antagonizes the inhibitory signaling of transforming growth factor β1 on human vascular endothelial cells. The FASEB Journal. 2000;14:55–64. doi: 10.1096/fasebj.14.1.55. [DOI] [PubMed] [Google Scholar]
- 172.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 173.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 174.Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 175.Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc. Res. 2005;65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 176.Wieser R, Wrana JL, Massague J. GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 178.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J. Biol. Chem. 1999;274:584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- 180.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J. Biol. Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 181.Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II. J. Biol. Chem. 2002;277:29197–29209. doi: 10.1074/jbc.M111991200. [DOI] [PubMed] [Google Scholar]
- 182.Koleva RI, Conley BA, Romero D, Riley KS, Marto JA, Lux A, Vary CP. Endoglin structure and function: Determinants of endoglin phosphorylation by transforming growth factor-beta receptors. J. Biol. Chem. 2006;281:25110–25123. doi: 10.1074/jbc.M601288200. [DOI] [PubMed] [Google Scholar]
- 183.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 184.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Park S, Dimaio TA, Liu W, Wang S, Sorenson CM, Sheibani N. Endoglin regulates the activation and quiescence of endothelium by participating in canonical and non-canonical TGF-beta signaling pathways. J Cell Sci. 2013;126:1392–1405. doi: 10.1242/jcs.117275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pan CC, Kumar S, Shah N, Hoyt DG, Hawinkels LJ, Mythreye K, Lee NY. Src-mediated post-translational regulation of endoglin stability and function is critical for angiogenesis. J. Biol. Chem. 2014;289:25486–25496. doi: 10.1074/jbc.M114.578609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Newman PJ, Newman DK. Signal Transduction Pathways Mediated by PECAM-1: New Roles for an Old Molecule in Platelet and Vascular Cell Biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]