Abstract
A major cause of mortality after spinal cord injury is respiratory failure. In normal rats, acute intermittent hypoxia (AIH) induces respiratory motor plasticity, expressed as diaphragm (Dia) and second external intercostal (T2 EIC) long-term facilitation (LTF). Dia (not T2 EIC) LTF is enhanced by systemic adenosine 2A (A2a) receptor inhibition in normal rats. We investigated the respective contributions of Dia and T2 EIC to daily AIH-induced functional recovery of breathing capacity with/without A2a receptor antagonist (KW6002, i.p.) following C2 hemisection (C2HS). Rats received daily AIH (dAIH: 10, 5-min episodes, 10.5% O2; 5-min normoxic intervals; 7 successive days beginning 7 days post-C2HS) or daily normoxia (dNx) with/without KW6002, followed by weekly (reminder) presentations for 8 weeks. Ventilation and EMGs from bilateral diaphragm and T2 EIC muscles were measured with room air breathing (21% O2) and maximum chemoreceptor stimulation (MCS: 7% CO2, 10.5% O2). dAIH increased tidal volume (Vt) in C2HS rats breathing room air (dAIH + vehicle: 0.47 ± 0.02, dNx + vehicle: 0.40 ± 0.01ml/100 g; p<0.05) and MCS (dAIH + vehicle: 0.83 ± 0.01, dNx + vehicle: 0.73 ± 0.01ml/100g; p<0.001); KW6002 had no significant effect. dAIH enhanced contralateral (uninjured) diaphragm EMG activity, an effect attenuated by KW6002, during room air breathing and MCS (p<0.05). Although dAIH enhanced contralateral T2 EIC EMG activity during room air breathing, KW6002 had no effect. dAIH had no statistically significant effects on diaphragm or T2 EIC EMG activity ipsilateral to injury. Thus, two weeks post-C2HS: 1) dAIH enhances breathing capacity by effects on contralateral diaphragm and T2 EIC activity; and 2) dAIH-induced recovery is A2a dependent in diaphragm, but not T2 EIC. Daily AIH may be a useful in promoting functional recovery of breathing capacity after cervical spinal injury, but A2a receptor antagonists (eg. caffeine) may undermine its effectiveness shortly after injury.
Keywords: intermittent hypoxia, spinal plasticity, motor neuron, adenosine receptor, spinal cord injury, breathing
Introduction
A major life-threatening consequence of cervical spinal cord injury (SCI) is the disruption of brainstem-spinal cord projections that give rise to spinal respiratory motor neuron activity and breathing (Winslow and Rozovsky, 2003). One approach to enhance respiratory function in SCI patients is to induce plasticity in spared synaptic pathways to respiratory motor neurons (Dale et al., 2014; Mitchell, 2007; Ramer et al., 2000). Moderate acute intermittent hypoxia (AIH) induces respiratory motor plasticity (Devinney et al., 2013; Feldman et al., 2003; Mahamed and Mitchell, 2007; Mitchell et al., 2001), strengthening synaptic inputs to phrenic motor neurons (Fuller et al., 2003; Golder and Mitchell, 2005; Lovett-Barr et al., 2012). The functional consequences of this plasticity are: 1) persistent increases in phrenic nerve activity in anesthetized rats (phrenic long-term facilitation, pLTF) (Bach and Mitchell, 1996); 2) diaphragm (diaLTF) and second thoracic external intercostal (T2 EIC LTF) muscle long-term facilitation in unanesthetized rats (Navarrete-Opazo and Mitchell, 2014); and 3) ventilatory LTF (largely tidal volume; vLTF) in unanesthetized rats (Nakamura et al., 2010; Olson et al., 2001) and humans (Pierchala et al., 2008; Tester et al., 2014). Thus, repetitive AIH may be a viable strategy to restore function in respiratory muscle activity and breathing capacity (Dale et al., 2014).
Multiple, distinct cellular mechanisms elicit AIH-induced respiratory motor plasticity, including serotonin- and adenosine-dependent pathways (Dale-Nagle et al., 2010; Devinney et al., 2013; Nichols et al., 2012). These serotonin and adenosine-dependent pathways interact via cross-talk inhibition (Dale-Nagle et al., 2010; Hoffman et al., 2010). With moderate AIH, pLTF is serotonin-dependent, and is constrained by concurrent, sub-threshold activation of spinal A2a receptors; thus, spinal and systemic A2a receptor inhibition enhance pLTF in anesthetized rats (Hoffman et al., 2010) and diaLTF in unanesthetized rats (Navarrete-Opazo et al., 2014).
Although it may seem logical to hypothesize that A2a receptor inhibition would enhance the therapeutic benefits of repetitive AIH, different (serotonin- and adenosine-dependent) cellular mechanisms may underlie repetitive AIH-induced functional recovery in rats with acute versus chronic spinal injuries. For example, shortly after C2 spinal hemisections (C2HS), serotonergic innervation of the phrenic motor nucleus is diminished (Saruhashi et al., 1996; Zhou and Goshgarian, 1999), abolishing serotonin-dependent pLTF two weeks post-C2HS (Golder and Mitchell, 2005). However, with time post-injury, serotonergic innervation and the capacity for serotonin-dependent pLTF recover (>8 weeks post-C2HS). Similarly, neither AIH-induced diaphragm nor T2 EIC LTF are expressed 7 days post-C2HS in unanesthetized rats, yet both can be elicited 8 weeks post-injury (Navarrete-Opazo et al., 2014). Although serotonin-dependent respiratory plasticity is compromised two weeks post-C2HS, the capacity for A2a receptor induced phrenic motor facilitation remains (Golder et al., 2008).
Moderate daily AIH (dAIH) partially restores breathing capacity 2 weeks post-C2HS (Lovett-Barr et al., 2012). With this acute SCI, functional recovery may nevertheless have arisen from serotonin-dependent mechanisms since more hypoxic episodes were used in this study (10 episodes/day for 7 days versus 3 episodes in a single day) (Golder and Mitchell, 2005; Lovett-Barr et al., 2012). An alternative hypothesis is that more hypoxic episodes and/or more severe hypoxemia within episodes triggered distinct, A2a receptor-dependent recovery of respiratory function (Nichols et al., 2012). These alternatives can be discriminated by the response to systemic A2a receptor antagonist pre-treatment. In the former case, we predict enhanced dAIH-induced functional recovery with A2a receptor inhibition; in the latter case, the opposite result will be observed (attenuated dAIH-induced functional recovery). The respiratory effects of combined A2a receptor inhibition and dAIH 2 weeks post-C2HS have not been explored. Our working hypothesis is that dAIH elicits plasticity and functional recovery via adenosine-dependent mechanisms with acute SCI, but reverts to serotonin-dependent (adenosine-constrained) mechanisms with chronic injury. Thus, we predicted that A2a receptor antagonist pretreatment impairs dAIH-induced recovery of breathing capacity in acute (2 week) C2HS rats.
Repetitive AIH also improves non-respiratory motor functions after SCI (Hayes et al., 2013; Lovett-Barr et al., 2012; Trumbower et al., 2012), and this functional improvement is greatest when AIH is paired with task-specific training (Hayes et al., 2013). The grooming test (Bertelli and Mira, 1993) utilizes stereotypical, brainstem regulated grooming to evaluate somatic motor recovery following injury. This innate behavior (grooming) does not require pre-training and results are unaffected by repeated testing (Berntson et al., 1988; Bertelli and Mira, 1993).
The duration of functional improvement following repetitive AIH has not been determined. Daily AIH-induced respiratory functional recovery lasts at least 1 day following dAIH (Lovett-Barr et al., 2012), and preliminary data suggest that effects last at least one week post-dAIH (Terada, Vinit, MacFarlane and Mitchell, unpublished). We wondered if weekly AIH “reminders” extend dAIH-induced functional recovery. Such reminders have been effective at prolonging behavioral memories (Martin et al., 2010; Rovee-Collier et al., 1980; Wiltgen and Tanaka, 2013).
Here we studied the impact of dAIH and systemic A2a receptor inhibition on respiratory and non-respiratory motor function in unanesthetized rats following acute C2HS. We measured ventilation with whole-body plethysmography, bilateral diaphragm and T2 external intercostal muscle activity via electromyography (EMG) radiotelemetry, and grooming behavior to evaluate a non-respiratory motor function. We tested the hypotheses that, two weeks post-C2HS: 1) dAIH improves breathing capacity (i.e. tidal volume) and grooming behavior; 2) spontaneous T2 EIC motor recovery ipsilateral to injury is greater than in diaphragm; 3) dAIH enhances the ability to increase diaphragm and T2 EIC muscle activity during maximal chemoreceptor stimulation; 4) systemic A2a receptor antagonist administration before daily AIH impairs functional recovery of breathing capacity and diaphragm muscle activity; and 5) weekly AIH presentations (single day) prolong dAIH-induced functional benefits.
Methods
Animals
All experiments began with 3-4 months old, male Sprague-Dawley rats (310-440 g, colony 211, Harlan, Indianapolis, IN). Rats were individually housed in a controlled environment (12-h light/dark cycle). The Animal Care and Use Committee at the School of Veterinary Medicine, University of Wisconsin approved all experimental procedures in this study.
Experimental preparation
Surgical preparation
For telemetry implantation and C2 cervical hemisection, sterile surgery was performed under isoflurane anesthesia (in 100% O2). The rats were injected with buprenorphine (0.03 mg/kg), carprofen (Rimadyl, 5 mg/kg) and enrofloxacin (Baytril, 4 mg/kg) subcutaneously to minimize potential post-operative pain and infection. Body temperature was maintained at 36.5-37.5°C using a rectal probe and external heating pad. A cannula was inserted into the trachea and the rats were artificially ventilated (tidal volume, 2.0-2.5 ml; Rodent Ventilator, model 683; Harvard Apparatus, South Natick, MA) with 1.5-2.5% isoflurane in 100% O2 during surgery. Effective anesthesia was confirmed by abolition of pedal withdrawal and corneal blink reflexes. Oxygen saturation was monitored by pulse oximetry (model 8600; Nonin Medical Inc. Plymouth, MN) during surgery. At the end of surgery buprenorphine, carprofen and enrofloxacin (see above for dosage) were administered at 12 h intervals for 48 h post-surgery. Rats were visually monitored and weighed daily. In spinally injured rats, post-surgical care included trimming nails after surgery, and cleaning fur, eyes and snout with warm water daily for 7 days to avoid accumulation of porphyrin. Rats had free access to pellets and high caloric nutritional gels inside their cages. In telemetry implantation and spinal injury surgeries, stainless steel staples were used to close the wound, and were removed 7 days post-surgery.
Telemetry transmitter implantation
After anesthetic induction, rats were placed in a supine position and the ventral surface of the abdominal muscle exposed. A sterilized transmitter (model 4ET-S1/2; Data Sciences International [DSI], St. Paul, MN) was inserted into the peritoneal cavity. The transmitter allowed simultaneous and continuous monitoring of electrical bio-potentials, body temperature and general locomotor activity. In the present study, the four bio-potential channels were used to record electromyographic (EMG) activity from bilateral diaphragm and second external intercostal (T2 EIC) muscles. Implantations were performed as follows. First, both right and left hemi-diaphragms were exposed through a midline incision. On both hemidiaphragms, two leads were implanted on the mid-costal area using a 23-G syringe needle guide and tissue adhesive (Vetbond 1469SB; 3M Animal care product, St. Paul, MN) as reported in previous studies (Terada and Mitchell, 2011). Next, right and left T2 EIC muscles were exposed through a 2.5 cm mid-sternum incision, starting in the upper edge of sternum, followed by retraction of pectoralis major and minor on the right and left side. The right and left T2 EIC muscles were implanted 1.0 cm right and left from the sternum respectively and the second interspace was identified by counting from the first interspace. The bi-potential lead pairs targeting T2 EIC muscles were tunneled subcutaneously from the body of the transmitter placed in the peritoneal cavity. As used in the diaphragm, all T2 EIC leads were implanted using a 23-G syringe needle guide and tissue adhesive to keep the leads on place. Finally, abdominal muscles and pectoralis major were sutured in the midline with polysorb 3.0 and the skin was closed with wound staples in both ventral thorax and abdomen.
Cervical C2 hemisection
One week after telemetry implantation, spinal hemisections at the second cervical level (C2HS) were performed. The surgical technique was consistent with previous reports (Dougherty et al., 2012b; Fuller et al., 2009; Vinit et al., 2009). After appropriate anesthesia and pre-operative care, the spinal cord was exposed at C2 via a dorsal laminectomy. The dura matter was cut and a left C2 hemisection (C2HS) performed using a micro-scalpel followed by aspiration. The overlying muscles were sutured with polysorb 3.0 and the skin closed with stainless steel wound clips. Sham rats underwent cervical laminectomy without spinal injury.
Whole-body plethysmography
Rats were placed individually in a 4 L DSI Plethysmography chamber (model 600-1211-001). Pressurized air flowed through the chamber at 4L/min, allowing control of inspired gas composition. The chamber was positioned onto a receiver (see below for telemetry signal acquisition) to measure EMG and plethysmography simultaneously. Compensated whole body plethysmography was used to assess tidal volume (Vt); compensation was based on chamber temperature (model P/N 60-1210-001) and humidity sensors (model P/N 600-1211-001). The system used a transducer (Buxco, model TRD5700) and a gas analyzer (CWE, Gemini). Plethysmography data were analyzed in 1 min bins during baseline (i.e. normoxia, 20 minutes) and during maximum chemoreceptor stimulation (hypoxia: 10.5% O2 and hypercapnia: 7% CO2; 20 min). To obtain more accurate Vt assessments, we used intraperitoneal temperature from the telemetry system to compensate for body temperature changes.
Telemetry signal acquisition
For the AIH protocol (see below), rats were placed in custom-made Plexiglas chambers positioned on receivers (model RPC-2; DSI, St. Paul, MN). Signals from the implanted radiotelemetry transmitter were detected by the receivers and sent to a data exchange matrix (model ACQ-7700; DSI, St Paul, MN). Four channels of EMG, body temperature and general locomotor activity in unanesthetized freely moving rats were monitored during experiments on a laboratory computer (data acquisition system: PONEMAH Physiology Platform; DSI, St. Paul, MN). EMG signals were sampled at 1200 Hz and analyzed with Neuroscore software (DSI, St. Paul, MN).
Grooming Test
Forelimb grooming function was assessed using a scoring system adapted from Bertelli and Mira (Bertelli and Mira, 1993), originally developed to examine recovery in a rat brachial plexus reconstruction model. Cool tap water was applied to the rat’s head and back with soft gauze, and the rat was returned to the chamber. Grooming activity was recorded with a video camera from the onset of grooming through at least two stereotypical grooming sequences (~2 min), which include: 1) licking of the forepaws and face washing, 2) forelimb grooming of the face, 3) repetitive licking of the body, and 4) hindpaw scratching (Berntson et al., 1988). Scoring was done as illustrated in Fig.8. Slow motion video playback was used to score each forelimb independently by the maximal contact made while initiating any part of the grooming sequence. The animals were tested after telemetry implantation, after spinal cord injuries, and weekly after dAIH exposure until the end of the study.
Fig 8.
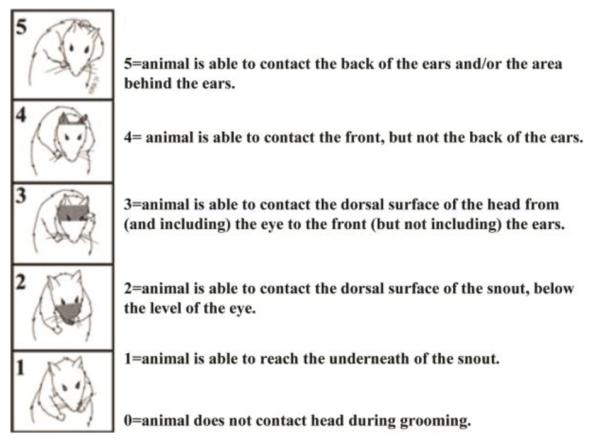
Grooming Test scoring.
Drug preparation
KW6002 (Istradefyline, Sigma-Aldrich) is a selective adenosine A2a receptor antagonist with a molecular weight of 384 and a Ki of 29.6 nM in rats. It’s half-life of 110 min, 97% bio-availability after intraperitoneal injection, and ability to cross the blood brain barrier (Yang et al., 2007), make it a suitable drug for our in vivo experiments. The drug was dissolved in DMSO at 9.3 mg/ml, sonicated and stored at 4°C in a dark vial protected from light. The day of the experiment, the drug was administrated via intraperitoneal injection at a dose of 0.5 mg/kg.
Experimental design
Five days after telemetry implantation, grooming tests were performed, and then simultaneous plethysmography and EMG recordings were made during baseline air breathing (Nx) and maximum chemoreceptor stimulation (MCS) to establish pre-C2HS values. One day after C2HS, the same protocols were done to demonstrate a complete hemisection.
Seven days post-C2HS, 32 rats were randomly allocated into the following groups: 1) dAIH + KW6002 (n=8), 2) dAIH + vehicle (n=8); 3) dNx + KW6002 (n=6); 4) dNx + vehicle (n=6); and 5) Sham (n=4). Group 1 rats received an intraperitoneal injection of KW6002 (0.5 mg/kg) 5 minutes before AIH on 7 consecutive days starting day 7 post-C2HS, and then weekly presentations of AIH with KW6002 (3-7 weeks post-C2HS). Group 2 rats received the same protocol, but KW6002 was replaced with vehicle (DMSO). During the same time window, rats in groups 3 and 4 (time control rats) were exposed to normoxia and KW6002 or vehicle, respectively. Sham rats were exposed to normoxia and did not receive intraperitoneal injections. All groups were compared over time through assessment of weekly video recording of grooming test performance, and simultaneous plethysmography and EMG recordings during normoxia and MCS until the end of the study (see fig.1, experimental design).
Fig. 1.
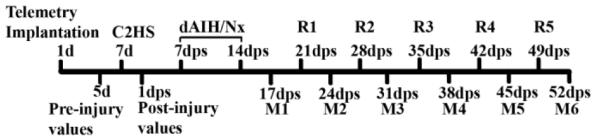
Timeline describing the experimental design from day 1 (1d) until 52 days post-spinal cord injury (dps). C2HS: cervical hemisection in second segment, dAIH/dNx: daily acute intermittent hypoxia or normoxia for seven days depending on groups (see methods), R1-R5: weekly presentations or “reminders” of acute intermittent hypoxia or normoxia, M1-M6: weekly measurements including grooming test and simultaneous plethysmography and electromyography of bilateral diaphragm and second external intercostal muscles.
Acute intermittent hypoxia
Normoxic (21% O2) and hypoxic (10.5% O2) conditions were established in custom-made chambers (Plexiglas cylinder, 12 × 4 inches id; 1 rat per chamber) by mixing O2 and N2 gas with a custom-made computer-controlled mass-flow controller system to obtain the desired inspired oxygen concentrations. Within the chambers, CO2 and O2 levels were continuously monitored during the entire protocol (O2 Analyzer, model 17518; CO2 Analyzer, model 17515; VacuMed Inc, Ventura, CA). Gas flowed through the chamber at a rate of 4 L/min, keeping chamber CO2 concentration less than 0.5% at all times. 95% of the change in O2 levels within hypoxic episodes was achieved in 25 ± 5 sec. At 8:00 am, on the experimental day, rats were placed in the chamber for 2-hour acclimation. Next, intraperitoneal injections of either KW6002 or vehicle were administrated accordingly (see experimental design). Once all rats were in the chambers, the experimental groups were administered the AIH protocol (10, 5-min 10.5% O2 interspersed with 5-min 21% O2, for a total of 95 min). Control and sham rats were administered continuous normoxia (i.e. time controls; TC). Chamber temperature was kept at 22.5-24.5°C during the entire protocol.
Maximum chemoreceptor stimulation
Normoxic (21% O2), hypoxic (10.5% O2) and hypercapnic conditions (7% CO2) were established in plethysmography chambers (see above) by mixing O2, N2 and CO2 gas via a custom-made, computer-controlled mass flow controller system to obtain the desired inspired gas concentrations. After 30 min acclimation, baseline breathing during normoxia was recorded for 20 min, followed by 20 min of maximum chemoreceptor stimulation (MCS) (10.5% O2 and 7% CO2, 20 min). This protocol enables assessment of a standardized, high breathing level elicited by maximal chemoreflex activation (Navarrete-Opazo and Mitchell, 2014). EMGs and whole-body plethysmography were used to measure tidal volume (Vt) and breathing frequency, along with respiratory muscle EMG signals.
Tissue Processing
To verify the extent of hemisections, each spinal cord was removed after completion of the experiments, immersed in paraformaldehyde (4%, overnight at 4°C) and cryoprotected in increasing concentrations of sucrose (20–30%). Tissues were then frozen in isopentane (−45°C) and stored at −80°C. Longitudinal sections of the spinal cord (C1 to C6, 30 μm thick) were stained with cresyl violet and examined histologically with light microscopy to reconstruct the injury in a transverse plane (Vinit et al., 2006), according to Paxinos and Watson (Paxinos and Watson, 1998 ). We used ImageJ software (National Institute of Health; http://rsb.info.nih.gov/jj) to measure and compare the extent of the hemisection among groups.
Data analyses
EMG signals were analyzed with Neuroscore software. The raw signal was filtered (100-624 Hz), rectified, integrated (100 msec) and averaged for each muscle. EMG values during active locomotor activity and grooming behavior were excluded in the analysis. Absolute values of tidal volume and breathing frequency were averaged. EMG amplitudes in each muscle were expressed as a percent change from pre-injury values. Grooming test score (0-5) was assessed separately in right and left forelimb.
All variables were compared among groups, and statistical inferences were made using two-way, repeated measures ANOVA for time (baseline and MCS) and treatment (see experimental design); individual comparisons were made using Fisher’s LSD post hoc tests (Sigma-Stat version 2.03, Systat Software Inc, San Jose, CA, USA). Differences indicated as statistically significant were P < 0.05. All values are expressed as means ± SEM.
Results
Daily acute intermittent hypoxia enhances capacity to increase tidal volume
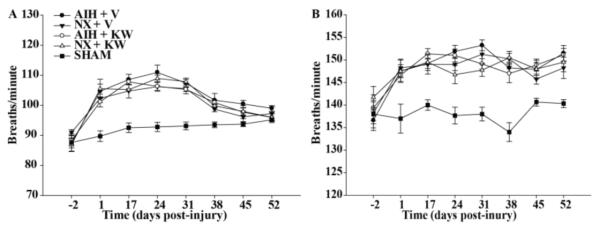
Pre-injury values were established 5 days post-telemeter implantation (Fig.1) in uninjured rats exposed to 20 min of normoxia followed by 20 min of MCS for both plethysmography and EMG recordings. One day post-C2HS, all experimental groups exhibit significant reductions in tidal volume versus pre-injury and sham values when breathing air (Sham: 0.55 ± 0.02 vs. dAIH + vehicle: 0.38 ± 0.01, dAIH + KW6002: 0.39 ± 0.02, dNx + vehicle: 0.40 ± 0.02, dNx + KW6002: 0.40 ± 0.03 ml/100gr rat, p<0.05; Fig.2A) and MCS (Sham: 1.07 ± 0.02 vs. dAIH + vehicle: 0.74 ± 0.02, dAIH + KW6002: 0.71 ± 0.02, dNx + vehicle: 0.72 ± 0.03, dNx + KW6002: 0.72 ± 0.02 ml/100gr rat, p<0.05; Fig.2B), but there were no significant differences among C2HS groups (p>0.05).
Fig. 2.
Absolute values of tidal volume (Vt) per 100 gr rat during normoxia (Nx, A) and maximum chemoreceptor stimulation (MCS, B) in all groups 2 days before spinal injury and then, 1 day up to 52 days post-injury. Note: (1) all groups show reduced Vt 1 dps in A and B compared to shams; (2) AIH elicits an increase in Vt during Nx and MCS, which was not significantly affected by adenosine A2a antagonist. AIH: acute intermittent hypoxia, V: vehicle (DMSO), KW: KW6002, Nx: normoxia. Values are means ± SEM. # significantly different from controls (Nx + V and Nx + KW6002); p < 0.05.
Three days post-dAIH, (i.e. 17 days post-C2HS), dAIH + vehicle and dAIH + KW6002-treated rats had significantly increased Vt versus time control (TC) rats (dNx + vehicle and dNx + KW6002) when breathing room air (dAIH + vehicle: 0.47 ± 0.01, dAIH + KW6002: 0.46 ± 0.02 vs. dNx + vehicle: 0.40 ± 0.01, dNx + KW6002: 0.38 ± 0.03 ml/100 g, p<0.05; Fig.2A). Although this effect is maintained 24 days post-injury, it was no longer significantly different from time controls. TC rats showed considerable spontaneous recovery, which was apparent at 24 days and continued up to the 38 days post-injury; at this time, Vt in control, untreated rats was no longer significantly different from sham rats without C2HS (Fig. 2A, p>0.05). During MCS, dAIH-treated rats showed significantly increased Vt at 17 and 24 days post-C2HS versus TC rats (dAIH + vehicle: 0.83 ± 0.01 vs. dNx + vehicle: 0.73 ± 0.01, dNx + KW6002: 0.72 ± 0.01 ml/100 gr, all p<0.001; Fig 2B), but this was not affected significantly by A2a receptor inhibition (dAIH + vehicle: 0.83 ± 0.01 vs. dAIH + KW6002: 0.80 ± 0.02 ml/100gr, p>0.05; Fig. 2B). At later time-points, there were no significant differences among groups, either when breathing room air or during MCS (p>0.05).
Daily acute intermittent hypoxia does not affect respiratory frequency
Respiratory frequency (breaths per minute, bpm) significantly increased one day post-C2HS versus pre-injury or sham values in all groups breathing air (dAIH + vehicle: 105 ± 2, dAIH + KW6002: 101 ± 2, dNx + vehicle: 102 ± 2, dNx + KW6002: 105 ± 3 bpm versus Sham: 90 ± 2 bpm, all p<0.05; Fig.3A) and during MCS (dAIH + vehicle: 148 ± 2, dAIH + KW6002: 146 ± 2, dNx + vehicle: 148 ± 2, dNx + KW6002: 147 ± 3 bpm versus Sham: 138 ± 3 bpm, all p<0.05; Fig.3B); there were no significant differences among treatment groups with C2HS (p>0.05).
Fig. 3.
Absolute values of respiratory frequency during normoxia (Nx, A) and maximum chemoreceptor stimulation (MCS, B) in all groups studied 2 days before spinal cord injury, and one day up to 52 days post-injury. Respiratory frequency is significantly increased in all groups, compared to sham during normoxia and MCS. AIH: acute intermittent hypoxia, V: vehicle (DMSO), KW: KW6002.
Respiratory frequency remained greater than pre-injury values and sham rats at 17 days post-C2HS during room air breathing (dAIH + vehicle: 109 ± 2, dAIH + KW6002: 107 ± 2, dNx + vehicle: 104 ± 2, dNx + KW6002: 105 ± 2 bpm vs. Sham: 92 ± 2 bpm, all p<0.05; Fig.3A), and then progressively decreased so that, at 45 days post-C2HS, no groups remained significantly different from sham values (dAIH + vehicle: 100 ± 2, dAIH + KW6002: 97 ± 2, dNx + vehicle: 96 ± 2, dNx + KW6002: 98 ± 2 vs. Sham: 93 ± 2 bpm, all p>0.05; Fig.3A). Similarly, during MCS, respiratory frequency remained greater than pre-injury values and sham rats 17 days post-C2HS (dAIH + vehicle: 149 ± 2, dAIH + KW6002: 151 ± 2, dNx + vehicle: 149 ± 2%, dNx + KW6002: 150 ± 3 bpm vs. Sham: 140 ± 2 bpm, all p<0.05; Fig.3B), and then remained greater than sham through the study.
Daily acute intermittent hypoxia does not induce significant ipsilateral motor recovery
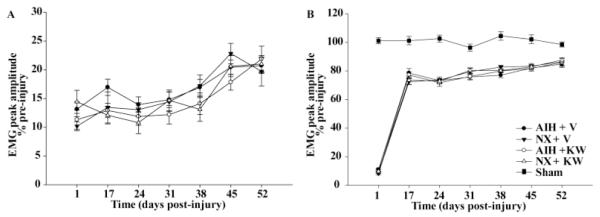
Ipsilateral (injured) diaphragm and second external intercostal (T2 EIC) EMG activity were measured one-day post-C2HS breathing air (Fig. 4,5). Inspiratory amplitude decreased significantly versus pre-injury values and sham rats in diaphragm (Sham: 103 ± 4.1% of pre-injury values; dAIH + vehicle: 16.9 ± 1.4%, dAIH + KW6002: 12.9 ± 1.3%, dNx + vehicle: 13.5 ± 2.8%, dNx + KW6002: 12.1 ± 1.6%, all p<0.05 when comparing sham vs C2HS groups; Fig. 4A, 5D) and T2 EIC (Sham: 101.2 ± 2.1% of pre-injury values; dAIH + vehicle: 11.0 ± 1.2%, dAIH + KW6002: 9.2 ± 1.6%, dNx + vehicle: 8.2 ± 2.0%, dNx + KW6002: 9.7 ± 3.0%, all p<0.05 comparing sham vs C2HS groups; Fig.4B, 5D); there were no significant differences among C2HS groups.
Fig. 4.
Changes in ipsilateral (injured) diaphragm (A) and second external intercostal muscle (T2 EIC, B) muscle amplitude during normoxia (Nx) expressed as percent change of pre-injury values one day up to 52 days post-injury. The spontaneous recovery is remarkable in ipsilateral T2 EIC in all groups (B) and not significant in ipsilateral diaphragm (A). Daily acute intermittent hypoxia (dAIH) alone or combined with A2a inhibition (KW6002) does not have an effect in neither diaphragm nor T2 EIC ipsilateral motor recovery. AIH: acute intermittent hypoxia, V: vehicle (DMSO), KW: KW6002.
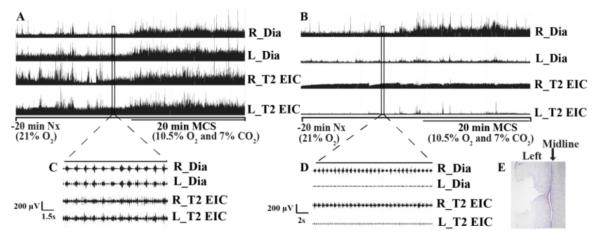
Fig. 5.
Representative integrated (A and B) and raw (C and D) EMG activity of right/left diaphragm (R/L_Dia) and second external intercostal muscle (R/L_T2 EIC) before (A, C) and one day after (B, D) spinal hemisection (C2HS) during maximum chemoreceptor stimulation (MCS). Note: (1) significantly reduced EMG activity in ipsilateral diaphragm and T2 external intercostal in (D), confirming the cervical hemisection; (2) absence of MCS response in injured (left) diaphragm and bilateral T2 EIC muscle in (B). (E) Representative longitudinal spinal cord slide (C1-C6) stained with Cresyl violet showing C2HS.
Cervical hemisection was consistent among groups. Reconstruction of the hemisection (C2HS) demonstrated a similar cross-sectional injury area in all groups (expressed as a percentage of total spinal cross sectional area; dAIH + vehicle: 48.2 ± 1.3 %, dAIH + KW6002: 46.3 ± 1.0%, dNx + vehicle: 51.0 ± 1.7%, dNx + KW6002: 45.4 ± 1.5%, p=0.053; Fig. 5E).
Ipsilateral diaphragm shows greatly reduced EMG amplitude (~13% of pre-injury values) 17 days post-C2HS, with a small (~20% pre-injury values), but not significant spontaneous increase at the end of the study (52 days post-C2HS, p>0.05; Fig 4A). This result is consistent with previous studies showing modest ipsilateral phrenic activity after C2HS (Dougherty et al., 2012b). In contrast, ipsilateral T2 EIC is less affected in any group (dAIH + vehicle: 78.5 ± 3.1%, dAIH + KW6002: 77.1 ± 2.1%, dNx + vehicle: 72.4 ± 2.0%, dNx + KW6002: 73.5 ± 2.9% of pre-injury values, p<0.001) 17 days post-C2HS, with a non-significant trend to increase (~86% of pre-injury values) by the end of experiments (52 days post-C2HS, p>0.05; Fig.4B). Overall, dAIH alone or dAIH combined with A2a receptor inhibition had no significant effect on ipsilateral diaphragm or T2 EIC EMG amplitude at any time-point (1-7 weeks, p>0.05).
Daily acute intermittent hypoxia induce contralateral diaphragm and T2 intercostal recovery
Contralateral (uninjured) diaphragm and T2 EIC muscles had increased EMG amplitude (above pre-injury values) one-day post-C2HS, demonstrating that mechanisms compensating for loss of ipsilateral function begin early after spinal injury. EMG amplitudes were significantly greater than pre-injury values and sham rats in right diaphragm (dAIH + vehicle: 125.6 ± 2.8 %, dAIH + KW6002: 128.0 ± 3.8%, dNx + vehicle: 123.5 ± 3.5%, dNx + KW6002: 128.2 ± 2.5% vs. Sham: 103.3 ± 2.3% of pre-injury values, p<0.05; Fig 6A) and right T2 EIC (dAIH + vehicle: 112.3 ± 1.5 %, dAIH + KW6002: 113.7 ± 2.7%, dNx + vehicle: 111.8 ± 2.5%, dNx + KW6002: 113.4 ± 2.2% vs. Sham: 99.0 ± 1.7% of pre-injury values, p<0.05; Fig 6B); there were no significant differences among C2HS groups. Contralateral (uninjured) diaphragm maintained a 20-30% increase in EMG amplitude above pre-injury values in all groups at all times post-C2HS (Fig.6A, p<0.05). In contrast, contralateral T2 EIC EMG amplitude returned to pre-injury values 17 days post-injury in TC rats (Fig. 6B, p>0.05).
Fig. 6.
Changes in contralateral (uninjured) diaphragm (A) and second external intercostal muscle (T2 EIC, B) muscle amplitude during normoxia (Nx) expressed as percent change of pre-injury values one day up to 52 days post-injury. Note: (1) all groups show significant increase in EMG amplitude compared to sham and pre-injury values during Nx in the diaphragm (A), demonstrating compensatory plasticity; (2) contralateral T2 EIC exhibits a transitory compensatory plasticity in all groups at 17 days post-injury; (3) daily acute intermittent hypoxia (dAIH) significantly increases EMG amplitude at 17 days post-injury in diaphragm and T2 EIC and this effect is impaired by A2a inhibition in diaphragm but not in T2 EIC muscle. AIH: acute intermittent hypoxia, V: vehicle (DMSO), KW: KW6002. Values are mean ± SEM.** significantly different from AIH + KW6002, * significantly different from time controls (TC: Nx + V and Nx + KW6002), # significantly different from sham; p < 0, 05.
Daily AIH significantly increased EMG amplitude in contralateral diaphragm 17 days post-C2HS versus control rats (dAIH + vehicle: 136.3 ± 1.6% vs dNx + vehicle: 124.5 ± 1.2% of pre-injury values, p<0.001; Fig 6A). At the same time-point, dAIH also significantly increased T2 EIC EMG amplitude versus control rats (dAIH + vehicle: 110.5 ± 1.7% vs. dNx + vehicle: 102.8 ± 1.7% of pre-injury values, p<0.001; Fig. 6). At later time-points there were no significant differences between dAIH and control-treated rats in contralateral diaphragm or T2 EIC muscle.
Adenosine 2A receptor inhibition impairs dAIH-enhancement of contralateral diaphragm but not intercostal activity
In contralateral (uninjured) diaphragm, dAIH plus KW6002-treated rats showed significantly decreased EMG activity versus dAIH plus vehicle-treated rats at 17 days post-C2HS (dAIH + vehicle: 136.3 ± 1.6% vs. dAIH + KW6002: 131.0 ± 0.8% of pre-injury values, p=0.015; Fig.6A). Thus, KW6002 impaired the functional benefits of dAIH at this early time post-injury, suggesting that respiratory motor recovery in diaphragm is A2a receptor dependent. In contrast, in contralateral T2 EIC muscle, there were no statistically significant differences between dAIH plus vehicle and dAIH plus KW6002-treated rats at 17 days post-C2HS (dAIH + vehicle: 110.5 ± 1.7%, vs. dAIH + KW6002: 109.5 ± 1.9% of pre-injury values, p=0.601; Fig. 6B).
Taken together, dAIH increases activity in both contralateral diaphragm and T2 EIC muscles. Although the dAIH effect was reduced by A2a receptor inhibition in diaphragm, this treatment had no effect on dAIH-enhanced T2 EIC motor activity. Thus, dAIH-enhanced motor activity is A2a modulated in diaphragm, but not in T2 EIC activity. Furthermore, weekly AIH (reminder) was unable to maintain dAIH-increased motor activity at later time-points in either right diaphragm or T2 EIC muscle.
Variable response to maximum chemoreceptor stimulation
Pre-injury EMG recordings and simultaneous plethysmography showed a robust MCS response (Fig.5A) in both diaphragm and T2 EIC, as demonstrated previously (Navarrete-Opazo and Mitchell, 2014). During MCS one-day post-C2HS, ipsilateral (injured) diaphragm and T2 EIC muscle activity were abolished (Fig.5B). The MCS response was also abolished in contralateral (uninjured) T2 EIC (Fig. 5B), and was significantly reduced in contralateral diaphragm, compared to pre-injury values and sham rats at one-day post-injury (dAIH + vehicle: 91.63 ± 1.1%, dAIH + KW6002: 94.68 ± 1.93%, dNx + vehicle: 91.02 ± 1.58%, dNx + KW6002: 93.18 ± 2.34% vs. Sham: 101.2 ± 2.8% of pre-injury values, all p<0.05; Fig.7A); there were no significant differences among C2HS groups (p>0.05).
Fig. 7.

Changes in contralateral (uninjured) diaphragm during maximum chemoreceptor stimulation (MCS, A) and representative integrated EMG activity of right/left diaphragm (R/L_Dia) and second external intercostal muscle (R/L_T2 EIC) at 52 days post-injury during MCS (B). Note: (1) reduced but consistent MCS response in all groups in uninjured diaphragm (A); (2) daily acute intermittent hypoxia (dAIH) significantly increases EMG amplitude at 17 days post-injury and this effect is impaired by KW6002; (3) small MCS response in uninjured T2 EIC (B); (4) the MCS response in injured T2 EIC remains abolished at all this time-points (B).
Despite the extensive spontaneous recovery of ipsilateral T2 EIC muscle activity during room air breathing, the response with MCS was absent throughout the study; this effect may be explained by a “ceiling effect” where the ipsilateral muscle has fully utilized compensatory mechanisms and is unable to increase motor activity further. Similarly, the ipsilateral diaphragm response to MCS was abolished, except during the last measurement (52 days post-C2HS), with a variable response ranging from −13.4% to 23.5% of pre-injury values (Fig. 7B, p>0.05).
An MCS response in contralateral T2 EIC muscle is observed 38 days post-injury, with variable amplitude ranging from 13 to 71% of pre-injury values, and no significant differences among C2HS groups. Contralateral diaphragm exhibited an attenuated MCS response at all time-points versus pre-injury values and sham rats (Fig. 7A, p<0.001).
Daily AIH-treated rats significantly improved the MCS response in contralateral diaphragm 17 days post-injury versus time control rats (dAIH + vehicle: 97.1 ± 1.2% vs. dNx + vehicle: 90.1 ± 1.1%, dNx + KW6002: 87.85 ± 1.3% of pre-injury values, p<0.001; Fig.7A). This effect was significantly impaired by A2a receptor inhibition (dAIH + vehicle: 97.1 ± 1.2% vs. dAIH + KW6002: 93.2 ± 1.0% of pre-injury values, p=0.015; Fig. 7A), suggesting that dAIH-induced motor recovery in contralateral diaphragm is adenosine versus serotonin-dependent with acute C2HS. At later time-points, contralateral MCS responses were not different among C2HS groups.
Daily acute intermittent hypoxia does not affect forelimb grooming behavior
All rats exhibited normal grooming behavior (score=5, Fig.8) after telemetry implantation but before spinal injury. One day post-spinal injury, injured rats show complete paralysis of the left (injured) limbs; these rats were able to groom only with the right (uninjured) forelimb, but not the left (injured) forelimb (i.e. score = 0). This effect is consistent with complete cervical hemisection. All rats showed normal right forelimb grooming at all times post-C2HS. At 17 days post-injury, half of the rats in all groups (dAIH + vehicle: n=4 rats, dAIH + KW6002: n=5, dNx + vehicle: n=2, dNx + KW6002: n=3) were able to touch the bottom of the snout with left forelimb (score of 1); this effect may reflect limited spontaneous improvement in trunk function (and balance) versus somatic recovery. The remaining rats in each group showed no improvement in grooming behavior with left forelimbs (i.e. score 0). Thus, dAIH had no demonstrable benefit in grooming behavior using the affected limb in rats with acute C2HS.
Discussion
In this study, there were six major findings: 1) spontaneous motor recovery was modest in ipsilateral diaphragm, but substantial in ipsilateral T2 EIC when breathing room air, suggesting a greater contribution of inspiratory intercostals muscles (vs. diaphragm) to spontaneous recovery of respiratory function after SCI; 2) contralateral diaphragm and T2 EIC made more substantial contributions to spontaneous functional recovery, and these contributions were rapid (beginning one day post-C2HS); 3) the contralateral diaphragm shows a consistent but reduced response to MCS, possibly due to a “ceiling effect” for contributions to spontaneous functional recovery from the uninjured diaphragm; 4) dAIH improves Vt when breathing room air and during MCS, but this effect could not be maintained by weekly AIH “reminders;” 5) dAIH primarily improves contralateral (uninjured), but not ipsilateral (injured) diaphragm and T2 EIC activity; and 6) A2a receptor inhibition impairs dAIH-induced functional recovery of contralateral diaphragm, but not T2 EIC muscle activity. Thus, dAIH-induced functional recovery of diaphragm activity is adenosine- and not serotonin-dependent after acute C2HS, and is accounted for by effects on respiratory motor output contralateral to injury.
Altered breathing pattern after C2 hemisection
After C2HS, ipsilateral diaphragm paralysis reduces Vt and increases breathing frequency. This breathing pattern shift is consistent with previous reports in unanesthetized (Fuller et al., 2005; Fuller et al., 2006) and anesthetized rats (Golder et al., 2001b). Changes in breathing pattern after C2HS initially result from diminished pre-motor drive to respiratory motor neurons, thus reducing Vt. Frequency changes may reflect decreased feedback from lung stretch receptors (Golder et al., 2001a), and/or altered afferent inputs to the CNS that were disrupted by the injury (Golder et al., 2011). A prominent role for the latter mechanism is revealed in rats with cervical contusion injuries (which show similar pattern shifts). When these rats undergo vagotomy, they continue to exhibit elevated breathing frequencies (Golder et al., 2011). After cervical SCI, time-dependent increases in Vt occur, reflecting spontaneous spinal plasticity (Dougherty et al., 2012b; Lovett-Barr et al., 2012). Similarly, Vt was substantially reduced one-day post-C2HS, yet exhibited remarkable spontaneous recovery by 24 days post-C2HS (Fig.2A). Although breathing frequency was significantly higher than in sham rats, it slowly declined after 24 days post-C2HS (Fig.3A). Thus, changes in breathing frequency are at least partially reversible, and vary inversely with Vt.
As reported previously (Fuller et al., 2005; Fuller et al., 2006; Fuller et al., 2009), rats with SCI exhibit deficits in ventilatory capacity when challenged with combined hypercapnia/hypoxia (i.e. MCS); thus, C2HS causes respiratory deficits characterized by an inability to respond appropriately to respiratory challenges. This deficit is characterized by diminished ability to increase Vt during MCS (Fig. 2B), with minimal effect on breathing frequency (Fig. 3B).
Spontaneous recovery of ipsilateral motor activity
C2HS disrupts bulbospinal inputs to spinal respiratory motor neurons distal to the lesion. However, partial return of ipsilateral diaphragm/intercostal activity occurs over a period of weeks to months after C2HS (Dougherty et al., 2012a; Dougherty et al., 2012b; Fuller et al., 2006; Mantilla et al., 2013; Nantwi et al., 1999; Vinit et al., 2006). The extent that crossed phrenic phenomenon translates into a functional increases in respiratory capacity is questionable since at least three studies suggest that this spontaneous plasticity contributes minimally to tidal volume generating capacity up to 8 weeks post injury (Dougherty et al., 2012b; Fuller et al., 2006; Golder et al., 2003). After C2HS, the ipsilateral (injured) phrenic nerve and/or hemidiaphragm show little inspiratory activity during normoxia (Dow et al., 2009; Golder and Mitchell, 2005) and we confirm this finding in diaphragm activity in unanesthetized rats. In contrast, the ipsilateral T2 EIC muscle showed remarkable recovery, reaching near normal levels in agreement with previous studies (Dougherty et al., 2012a; Sherrey and Megirian, 1990). Thus, accessory respiratory muscles may make a relatively greater contribution to preserving/restoring breathing capacity after cervical spinal injury. Further studies are warranted to determine mechanisms underlying enhancement of intercostal muscle EMG activity after C2 spinal injury.
Removal of inhibitory reflexes may dis-inhibit intercostal motor neurons after spinal injury. In dogs, diaphragm paralysis rapidly increases activity of the inspiratory intercostal muscles in compensation (De Troyer, 1998). The increase may be due to diminished sensory afferent inputs arising coursing through the phrenic nerve that normally inhibit inspiratory intercostal activity (De Troyer, 1998). Indeed, bilateral stimulation of C5 phrenic afferent neurons decreased intercostal inspiratory activity by 50% (De Troyer, 1998). In our C2 hemisection model, one side of the diaphragm is paralyzed, most likely decreasing afferent feedback and disinhibiting of inspiratory output in the intercostal muscles.
Plasticity in contralateral (uninjured) respiratory motor output has been observed previously after unilateral spinal injury (Katagiri et al., 1994; Mantilla et al., 2013; Rowley et al., 2005; Sherrey and Megirian, 1990; Teitelbaum et al., 1993). With C2HS, right diaphragm and T2 EIC muscle activity increased, reflecting a form of compensatory respiratory plasticity. Presumably muscles on the uninjured side now make a greater proportional contribution to breathing, at least shortly after injury. Contralateral diaphragm EMG amplitude increased ~25% above pre-injury values, and remained elevated throughout the study (Fig. 6A). Similar compensatory (contralateral) plasticity was observed in right T2 EIC muscle activity (Fig. 6B), but this compensation had diminished 24 days post-injury, coinciding with recovery of the left (injured) T2 EIC. Although this reciprocal relationship suggests a causal relationship between these events, its mechanistic basis is not yet clear.
Daily acute intermittent hypoxia induces respiratory functional recovery
Daily AIH improves Vt 17 days post-injury when breathing room air, and up to 24 days post-injury during MCS (Fig.2B). This effect was not maintained by weekly AIH“reminders,” suggesting that more frequent repetitive AIH may be necessary to preserve long-lasting functional benefits. To some extent, the initial dAIH-induced recovery was masked by spontaneous recovery during normoxia, leading to a convergence between treated and untreated animals; thus, dAIH accelerated recovery, but did not lead to a sustained advantage. Since, respiratory frequency did not show significant differences across time, (room air breathing or MCS), spontaneous recovery of Vt and frequency arise from distinct mechanisms.
Increased ventilatory capacity induced by dAIH is likely attributable to increased contralateral motor activity in diaphragm and T2 EIC. A previous study reported that AIH augmented crossed spinal synaptic pathways (phrenic long-term facilitation; pLTF) at 8 weeks, but not 2 weeks, post-injury (Golder and Mitchell, 2005). The timing of recovery in the ability to elicit pLTF below the injury coincides with recovery of serotonin terminal density in the phrenic motor nucleus below the injury. Consistent with this study, we now show that dAIH did not increase motor activity in ipsilateral diaphragm or T2 EIC. In contrast, dAIH contributed to further compensatory plasticity on the uninjured side (both diaphragm and T2 EIC muscles), where serotonergic innervation of phrenic and thoracic motor nuclei remained intact.
A2a receptor inhibition impairs dAIH-enhanced diaphragm activity
One week of dAIH, beginning 1-week post-C2HS, is reported to restore ipsilateral phrenic motor output and breathing capacity in unanesthetized rats (Lovett-Barr et al., 2012), a time when serotonergic innervation of the phrenic motor nucleus remains disrupted (Golder and Mitchell, 2005). Because of this, the observed effects of dAIH may arise from mechanisms that are serotonin-independent. Indeed, KW6002, an A2a receptor antagonist, impairs dAIH-induced recovery of diaphragm activity contralateral to injury at 17 days post-C2HS. In contrast, the lack of any effect of KW6002 on dAIH-induced motor recovery in uninjured T2 EIC suggests that adenosine is not initiating dAIH induced plasticity in this muscle/motor neuron group. Similarly, T2 EIC LTF in uninjured rats is unaffected by A2a receptor inhibition (Navarrete-Opazo et al., 2014). Differential mechanisms (with respect to adenosine) giving rise to dAIH induced recovery of diaphragm versus intercostal motor activity remain to be explored. It is possible that spinal interneurons play a greater role in functional recovery of intercostal versus phrenic motor neurons, or that there is less disruption of serotonergic innervation in the thoracic spinal cord.
This is the first evidence that dAIH induces functional recovery of breathing capacity via adenosine-dependent mechanisms with acute SCI, since dAIH-induced recovery of diaphragm EMG activity was impaired by daily KW6002 pre-treatment. In recent unpublished studies, we also found that dAIH-induced recovery of breathing capacity after acute C2HS does not require serotonin receptor activation (Terada, Vinit, MacFarlane and Mitchell, unpublished observations). Thus, dAIH induced functional recovery results from adenosine-dependent, serotonin-independent mechanisms with acute SCI (2 weeks), a time when serotonergic innervation below the site of injury is minimal (Golder and Mitchell, 2005).
Despite the effects of KW6002 on dAIH-induced functional recovery of diaphragm activity, we did not see similar impairment of dAIH effects on tidal volume, This difference may be explained by shifts to other inspiratory muscles that are less or unaffected by A2a receptor inhibition. Since scalenus medius and intercostal muscles of the cephalic spaces T1, T2 T3 are inspiratory in rats (Megirian et al., 1987), dAIH may increase their motor output (as in T2 EIC); alternately, dAIH may induce respiratory activity in intercostal muscles that are not normally active. The differential effects of KW6002 on tidal volume versus diaphragm function after dAIH strongly suggests that other inspiratory muscles make greater contributors to ventilatory capacity after dAIH and SCI.
Variable maximum chemoreceptor response after acute spinal injury
Numerous studies confirm that ipsilateral phrenic nerve activity (below chronic C2HS) increases during chemoreceptor challenge (Dougherty et al., 2012b; Fuller et al., 2006; Fuller et al., 2009). These observations are consistent with the hypothesis that the CPP primarily enables respiratory behaviors requiring large tidal volumes, such as a sigh or augmented breath (Golder et al., 2003). However, we found minimal (and variable) increase in ipsilateral diaphragm activity 52 days post-injury. Surprisingly, the MCS response was abolished in contralateral T2 EIC muscle for up to 38 days post-C2HS, and was significantly reduced in contralateral diaphragm versus pre-injury values. We speculate that contralateral respiratory motorneurons are recruited after hemisection to compensate for loss of ipsilateral phrenic and thoracic activity and, therefore, are subject to a “ceiling effect” where they cannot increase motor output further. Injured T2 EIC motor recovery may have a functional significance during quiet breathing, but not during respiratory challenge after acute C2HS. In contrast, uninjured diaphragm showed a reduced but consistent MCS response at all time-points, demonstrating that contralateral diaphragm has great functional significance during respiratory challenge. More importanly, dAIH improved breathing (i.e. tidal volume) during MCS 17 days post-injury.
Daily acute intermittent hypoxia does not improve automatic grooming behavior
The failure of dAIH to improve automatic grooming behavior differs in some respects from other studies demonstrating improved limb function in both rats and humans (Lovett-Barr et al., 2012; Trumbower et al., 2012). Even a single AIH presentation increases ankle strength (plantar flexion torque) in humans with motor incomplete chronic spinal injuries (Trumbower et al., 2012). Furthermore, dAIH (beginning 4 week post-C2HS) improves forelimb function in injured rats (Lovett-Barr et al., 2012). In this latter study the authors mention that it was somewhat difficult to discriminate the effects of AIH per se versus paired training. Thus, our study may differ from that of Lovett-Barr and colleagues (2012) in that the dAIH was applied shortly after C2HS (beginning 1 versus 4 weeks post-injury), the latter study used combined treatment (dAIH plus daily ladder walking) and the specific tasks differ in their demands on the animal (automatic grooming versus ladder walking). Considering that dAIH mainly increases contralateral versus ipsilateral diaphragm and T2 EIC function, the lack of ipsilateral forelimb recovery in acute C2HS rats is not unexpected. However, we cannot rule out that the grooming test may not be sensitive enough to detect subtle somatic improvements elicited by dAIH.
Conclusion
Collectively, we found that dAIH improves respiratory function in unanesthetized rats with acute cervical spinal injuries; however, the differential effects (dAIH versus normoxia treated rats) are transient, reflecting slow spontaneous recovery without treatment. Weekly AIH reminders are insufficient preserve relative functional recovery (versus time controls), suggesting that more robust repetitive AIH protocols are necessary to preserve functional advantages of dAIH. Thus, the main benefit of dAIH was to accelerate functional recovery of breathing capacity when applied shortly after spinal injury.
At this time post-injury, the main functional benefits of dAIH are attributable to increased EMG activity in contralateral (uninjured) diaphragm and ipsilateral T2 EIC muscle versus ipsilateral diaphragm—a more frequently studied outcome. Further, A2a receptor inhibition actually impairs the extent of functional recovery induced by dAIH of contralateral diaphragm, suggesting that adenosinergic mechanisms play a key role in the therapeutic effects of repetitive AIH with acute SCI. Systemic administration of selective A2a receptor agonists (CGS21680) minimize tissue damage, locomotor dysfunction and inflammatory profiles when administered within 24 hours post-SCI (Genovese et al., 2009; Paterniti et al., 2011). Although systemic A2a receptor inhibition undermined functional benefits of dAIH with acute injury, we hypothesize that it will enhance the benefits of dAIH with chronic SCI, after spinal serotonergic innervation has recovered. This possibility remains to be explored.
Acknowledgement
This work was supported by NIH grants HL69064 and HL080209. A. Navarrete-Opazo was supported by Fulbright scholarship. S. Vinit and B.J. Dougherty were supported by Craig H. Neilsen foundation post-doctoral fellowships.
Footnotes
Disclosure
Authors declare no conflict of interest, financial or otherwise.
References
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Jang JF, Ronca AE. Brainstem systems and grooming behaviors. Annals of the New York Academy of Sciences. 1988;525:350–362. doi: 10.1111/j.1749-6632.1988.tb38619.x. [DOI] [PubMed] [Google Scholar]
- Bertelli JA, Mira JC. Behavioral evaluating methods in the objective clinical assessment of motor function after experimental brachial plexus reconstruction in the rat. Journal of neuroscience methods. 1993;46:203–208. doi: 10.1016/0165-0270(93)90068-3. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Advances in experimental medicine and biology. 2010;669:225–230. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda, Md.) 2014;29:39–48. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer AD. The canine phrenic-to-intercostal reflex. The Journal of physiology. 1998;508(Pt 3):919–927. doi: 10.1111/j.1469-7793.1998.919bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Annals of the New York Academy of Sciences. 2013;1279:143–153. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Lee KZ, Gonzalez-Rothi EJ, Lane MA, Reier PJ, Fuller DD. Recovery of inspiratory intercostal muscle activity following high cervical hemisection. Respiratory physiology & neurobiology. 2012a;183:186–192. doi: 10.1016/j.resp.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Lee KZ, Lane MA, Reier PJ, Fuller DD. Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J Appl Physiol. 2012b;112:96–105. doi: 10.1152/japplphysiol.00690.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow DE, Zhan WZ, Sieck GC, Mantilla CB. Correlation of respiratory activity of contralateral diaphragm muscles for evaluation of recovery following hemiparesis. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference; 2009; 2009. pp. 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annual review of neuroscience. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. Journal of neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr., Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr., Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respiratory physiology & neurobiology. 2009;165:245–253. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Melani A, Esposito E, Mazzon E, Di Paola R, Bramanti P, Pedata F, Cuzzocrea S. The selective adenosine A2a receptor agonist CGS 21680 reduces JNK MAPK activation in oligodendrocytes in injured spinal cord. Shock (Augusta, Ga.) 2009;32:578–585. doi: 10.1097/SHK.0b013e3181a20792. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Lovett-Barr MR, Vinit S, Resnick DK, Mitchell GS. Breathing patterns after mid-cervical spinal contusion in rats. Exp Neurol. 2011;231:97–103. doi: 10.1016/j.expneurol.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001a;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Davenport PW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol. 2001b;91:2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: A randomized trial. Neurology. 2013 doi: 10.1212/01.WNL.0000437416.34298.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. The Journal of physiology. 2010;588:255–266. doi: 10.1113/jphysiol.2009.180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri M, Young RN, Platt RS, Kieser TM, Easton PA. Respiratory muscle compensation for unilateral or bilateral hemidiaphragm paralysis in awake canines. J Appl Physiol. 1994;77:1972–1982. doi: 10.1152/jappl.1994.77.4.1972. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3591–3600. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Experimental physiology. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 2013;114:380–386. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Pourie G, Bossenmeyer-Pourie C, Jazi R, Gueant JL, Vert P, Daval JL. Conditioning-like brief neonatal hypoxia improves cognitive function and brain tissue properties with marked gender dimorphism in adult rats. Seminars in perinatology. 2010;34:193–200. doi: 10.1053/j.semperi.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Megirian D, Pollard MJ, Sherrey JH. The labile respiratory activity of ribcage muscles of the rat during sleep. The Journal of physiology. 1987;389:99–110. doi: 10.1113/jphysiol.1987.sp016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Gaultier C, editor. Genetic Basis for Respiratory Control Disorders. Springer Publishing Company; New York: 2007. pp. 291–311. [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr. Invited review: Intermittent hypoxia and respiratory plasticity. Journal of applied physiology (Bethesda, Md. : 1985) 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Olson EB, Jr., Terada J, Wenninger JM, Bisgard GE, Mitchell GS. Sleep state dependence of ventilatory long-term facilitation following acute intermittent hypoxia in Lewis rats. Journal of applied physiology (Bethesda, Md. : 1985) 2010;109:323–331. doi: 10.1152/japplphysiol.90778.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehabilitation and Neural Repair. 1999;13:225–234. [Google Scholar]
- Navarrete-Opazo A, Mitchell GS. Recruitment and plasticity in diaphragm, intercostal, and abdominal muscles in unanesthetized rats. Journal of applied physiology (Bethesda, Md. : 1985) 2014;117:180–188. doi: 10.1152/japplphysiol.00130.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo AA, Vinit S, Mitchell GS. Adenosine 2A receptor inhibition enhances intermittent hypoxia-induced diaphragm but not intercostal long-term facilitation. Journal of neurotrauma. 2014 doi: 10.1089/neu.2014.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. Journal of applied physiology (Bethesda, Md. : 1985) 2012;112:1678–1688. doi: 10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EB, Jr., Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. Ventilatory long-term facilitation in unanesthetized rats. Journal of applied physiology (Bethesda, Md. : 1985) 2001;91:709–716. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- Paterniti I, Melani A, Cipriani S, Corti F, Mello T, Mazzon E, Esposito E, Bramanti P, Cuzzocrea S, Pedata F. Selective adenosine A2A receptor agonists and antagonists protect against spinal cord injury through peripheral and central effects. Journal of neuroinflammation. 2011;8:31. doi: 10.1186/1742-2094-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. 1998. [DOI] [PubMed] [Google Scholar]
- Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respiratory physiology & neurobiology. 2008;160:259–266. doi: 10.1016/j.resp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Harper GP, Bradbury EJ. Progress in spinal cord research - a refined strategy for the International Spinal Research Trust. Spinal cord. 2000;38:449–472. doi: 10.1038/sj.sc.3101055. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier CK, Sullivan MW, Enright M, Lucas D, Fagen JW. Reactivation of infant memory. Science (New York, N.Y.) 1980;208:1159–1161. doi: 10.1126/science.7375924. [DOI] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Sieck GC. Respiratory muscle plasticity. Respiratory physiology & neurobiology. 2005;147:235–251. doi: 10.1016/j.resp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Saruhashi Y, Young W, Perkins R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp Neurol. 1996;139:203–213. doi: 10.1006/exnr.1996.0094. [DOI] [PubMed] [Google Scholar]
- Sherrey JH, Megirian D. After phrenicotomy the rat alters the output of the remaining respiratory muscles without changing its sleep-waking pattern. Respir Physiol. 1990;81:213–225. doi: 10.1016/0034-5687(90)90047-3. [DOI] [PubMed] [Google Scholar]
- Teitelbaum J, Borel CO, Magder S, Traystman RJ, Hussain SN. Effect of selective diaphragmatic paralysis on the inspiratory motor drive. J Appl Physiol. 1993;74:2261–2268. doi: 10.1152/jappl.1993.74.5.2261. [DOI] [PubMed] [Google Scholar]
- Terada J, Mitchell GS. Diaphragm long-term facilitation following acute intermittent hypoxia during wakefulness and sleep. J Appl Physiol. 2011;110:1299–1310. doi: 10.1152/japplphysiol.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term facilitation of ventilation in humans with chronic spinal cord injury. American journal of respiratory and critical care medicine. 2014;189:57–65. doi: 10.1164/rccm.201305-0848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26:163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. Journal of neurotrauma. 2006;23:1137–1146. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respiratory physiology & neurobiology. 2009;169:210–217. doi: 10.1016/j.resp.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Tanaka KZ. Systems consolidation and the content of memory. Neurobiology of learning and memory. 2013;106:365–371. doi: 10.1016/j.nlm.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Yang M, Soohoo D, Soelaiman S, Kalla R, Zablocki J, Chu N, Leung K, Yao L, Diamond I, Belardinelli L, Shryock JC. Characterization of the potency, selectivity, and pharmacokinetic profile for six adenosine A2A receptor antagonists. Naunyn-Schmiedeberg's archives of pharmacology. 2007;375:133–144. doi: 10.1007/s00210-007-0135-0. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. Effects of serotonin on crossed phrenic nerve activity in cervical spinal cord hemisected rats. Exp Neurol. 1999;160:446–453. doi: 10.1006/exnr.1999.7213. [DOI] [PubMed] [Google Scholar]