Abstract
B cells use unusual strategies to enable the production of a seemingly unlimited number of antibodies from a very limited amount of DNA; however this approach also dramatically increases the likelihood that proteins will be produced that are unable to fold or assemble properly. Thus these cells are particularly dependent on quality control mechanisms to oversee the production of antibodies. Recent in vitro experiments demonstrate that Ig domains have evolved diverse folding strategies ranging from robust spontaneous folding to intrinsically disordered domains that require assembly with their partner domains in order to fold, and in vivo experiments reveal that these different folding characteristics form the basis for cellular checkpoints in Ig transport. Together these reports provide a detailed understanding of how B cells monitor and ensure the functional fidelity of Ig proteins.
A short overview of antibody biology
Immunoglobulin (Ig) proteins serve as cell surface antigen receptors on B cells, and upon antigen stimulation and plasma cell differentiation they are secreted as soluble effector molecules (antibodies) that provide protection against infections and foreign antigens. In their simplest form, the IgG antibodies, each molecule is composed of two identical heavy chains (HCs) and two identical light chains (LCs) that are linked by disulfide bonds. Both chains are composed of multiple domains of ~100 amino acids each (Figure 1A). The N-terminal domains of both chains vary between antibodies, giving rise to their designation as variable domains (VH and VL), and contain particularly diverse stretches of amino acids (hypervariable regions) that provide the exquisite binding specificity of the antibody molecule. Together these two domains form the antigen binding site (Figure 1A). The remainder of the antibody sequence is conserved within antibody classes (constant domains) and is important for effector functions such as complement activation or recruitment of macrophages and natural killer cells. Five different classes of antibodies are made in most higher vertebrates, IgM, IgG, IgA, IgE, and IgD, that differ in the HC constant regions used. Only two types of LC (κ or λ) exist, which can assemble with all HC classes. However, in a given cell only one HC and one LC allele are expressed, so that antibodies with a single specificity are produced1.
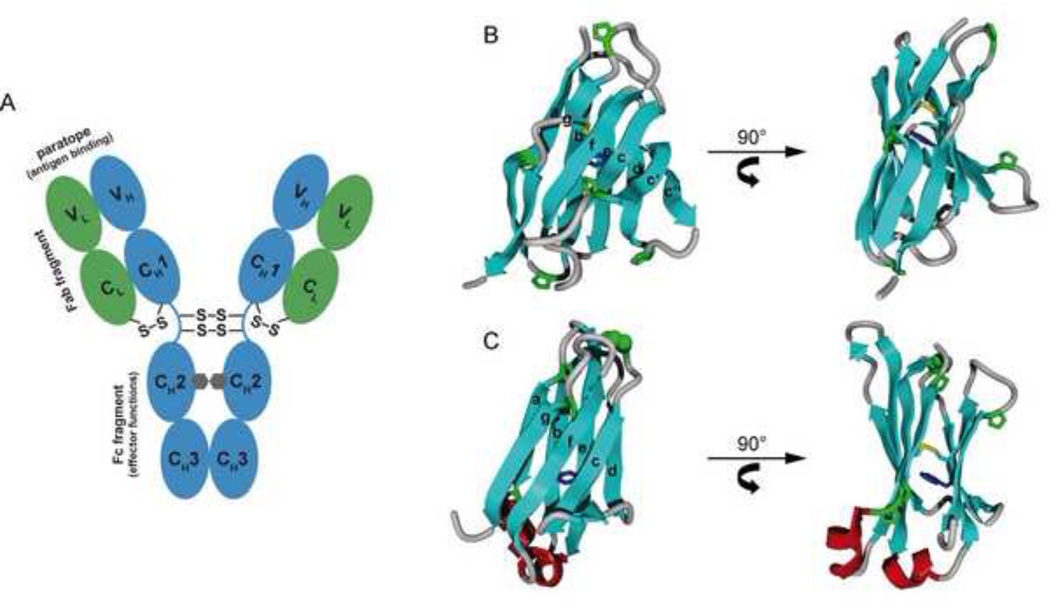
Figure 1. overall antibody structure and domain architecture.
(A) Domain arrangement of an IgG antibody molecule. The light chains are shown in green, the heavy chains in blue. The oligosaccharides between the CH2 domains are depicted as grey hexagons. Interchain disulfide bridges and important functional elements of the antibody (antigen binding paratope, Fab fragment, Fc fragment) are indicated. Domain architecture of the light chain variable (VL) (B) and constant (CL) domains (C). The strand nomenclature is indicated. The intrachain disulfide bridge (yellow) and the proximal conserved tryptophan residue (blue) are shown. The proline residues of the two domains are shown in green with the highly conserved cis-proline residue between strands b and c of CL highlighted in a CPK representation. Small helices (red) connect strands a and b and strands e and f of the CL domain.
The development of progenitor cells committed to the B cell lineage is characterized by the sequential expression of the HC and LC subunits. In preB cells, a unique HC variable region is created by combining a single variable (VH) gene segment with one diversity (DH) and one joining (JH) gene segment at the DNA level on one allele2,3, which is initially spliced to the IgM constant region at the mRNA level4. However, later during development, class switching can occur to juxtapose the rearranged VH domain to other downstream constant regions. The DNA rearrangements that give rise to HC variable regions involve imprecise joining of these three gene segments, the addition of non-templated bases at the site of joining of these gene segments, and finally, during later stages of differentiation, the directed hypermutation of the variable region exons5. Once a functional HC is made, similar gene rearrangements commence to form the VL domain of the LC6. These mechanisms are essential to generate antibody diversity and allow affinity maturation of the immune response, yet they clearly increase the likelihood of producing a protein that is incapable of folding and assembling properly, being transported to the cell surface or secreted, or engaging the appropriate signaling molecules thus compromising the functioning of the immune system. Therefore, B lineage cells are particularly dependent on the endoplasmic reticulum (ER) quality control system to ensure that only correctly assembled Ig molecules are transported to the cell surface. In light of this, it is not surprising that many of the major components of the mammalian ER quality control machinery were first identified by virtue of their association with antibody chains, and that Ig molecules were some of the earliest identified substrates of ER folding enzymes (Table 1).
Table 1.
Members of the ER protein folding machinery.
| Folding helper |
Additional names |
Molecular mass (kDa) |
Function | Involved in Ig biosynthesis |
Referencesa |
|---|---|---|---|---|---|
| BiP | Grp78, Kar2 (yeast) | 78 | Molecular chaperone, Hsp70 homologue | + |
58 59 |
| GRP94 | Gp96, Hsp90b1 | 94 | Molecular chaperone, Hsp90 homologue | + | 87 |
| Calnexin/ Calreticulin | 65/47 | Quality control, ER retention | + | 88 | |
| ERdj1 | Mtj1 | 64 | Co-chaperone of BiP, J-protein, Hsp40 protein membrane-bound | ? | 89 |
| ERdj2 | Sec63 | 83 | Co-chaperone of BiP, J-protein, Hsp40 protein membrane-bound | ? | 90 |
| ERdj3 | DnaJB11 | 43 | Co-chaperone of BiP, J-protein, Hsp40 protein | + | 91 |
| ERdj4 | DnaJB9 Mdj1 | 25 | Co-chaperone of BiP, J-protein, Hsp40 protein membrane-bound | + | 92 |
| ERdj5 | DnaJC10 | 91 | Co-chaperone of BiP, J-protein, reductase | ? | 93 |
| ERdj6 | P58-IPK, DnajC3 | 58 | Co-chaperone of BiP, J-protein, Hsp40 protein | ? | 94 |
| ERdj7 | Gng10, DnajC25 | 40 | Co-chaperone of BiP, J-protein, Hsp40 protein | ? | 95 |
| GRP170 | Lhs1 (yeast) | 110 | Nucleotide exchange factor for BiP | + | 96 |
| BAP | Sil1 | 50 | Nucleotide exchange factor for BiP | ? | 97 |
| Sig1R | Cofactor of BiP | ? | 98 | ||
| PDI | 55 | Oxido-reductase, disulfide bond formation, isomerisation | + | 99 | |
| Perp1 | 20 | Oxido-reductase, chaperone? | + |
100 101 |
|
| ERp72 | CaBP2 | 72 | Oxido-reductase | + | 102 |
| P5 | CaBP1 | 55 | Oxido-reductase | + | 102 |
| ERp57 | 60 | Oxido-reductase | + | 103 | |
| CypB | 20 | Peptidyl prolyl isomerase | + | 91 | |
| ERp29 | 25 | Chaperone? | ? | 104 | |
| UDP-GT | 53 | Glucosyl-Transferase | ? | 105 |
The references refer either to the first description of the protein, or to the first demonstration of a role in antibody biosynthesis.
HCs and LCs are co-translationally translocated into the ER, and folding begins even before the polypeptide chains are completely translated7. Most IgGs assemble first as HC dimers to which LCs are added covalently via a disulfide bond between the CL and CH1 domains8. IgG HC mutants with a deleted CH3 domain do not form HC dimers readily and are often secreted as HC-LC ‘hemimers’9. Indeed, Fc fragment dimerization is largely mediated by interactions between the CH3 domains and stabilized by disulfide bonds in the hinge region. The CH2 domains only interact via N-linked glycans (Figure 1A), which are covalently linked to this domain co-translationally10. They determine the orientation and spacing of the two CH2 domains, which is crucial for the binding of downstream effectors11–13. The heavily glycosylated HCs of IgM require the glycans for assembly and transport suggesting that they likely guide the folding of IgM μ heavy chains14, whereas the monoglycosylated γ HCs of IgG mature properly in their absence15. The analysis of the basic steps of antibody biosynthesis in cells, together with in vitro folding studies, now can provide a molecular understanding of Ig folding and assembly processes.
Antibody structure and the evolution of the immunoglobulin fold
In the case of IgG, for which most of the in vitro work has been done, the “Y”-shaped molecule is composed of two four-domain HCs and two two-domain LCs (Figure 1A). However, the orientation of the two arms of the Y is flexible due to an unstructured hinge region between the first (CH1) and the second (CH2) constant domain of the heavy chain. The IgG molecule can be proteolytically cleaved in the hinge region, which subdivides it into three functional segments; each of which is a dimer16,17. The two N-terminal fragments (composed of a LC associated with the VH-CH1 domains of the HC) are termed Fab fragments (for fragment antigen binding). The remaining Fc fragment (for fragment crystallizable) comprises two identical, two domain (CH2-CH3) segments that are covalently linked via disulfide bonds in the hinge region. The Fc fragment is important for connecting antigen binding to antibody effector functions. Each of the Ig domains forms a highly similar beta sandwich structure, known as the Ig fold. The Ig fold is characterized by a greek-key β-barrel topology in which the barrel is not continuously hydrogen-bonded, but instead composed of two sheets forming a sandwich-like structure. The variable domains (Figure 1B) comprise nine strands (abcc’c”defg) and the constant domains (Figure 1C) seven strands (abcdefg)18. In most antibody domains, a buried disulfide bridge, which spans ~60–70 residues, connects strands b and f11,18. It is orientated roughly perpendicular to the individual sheets and significantly stabilizes the folded domain19. Another characteristic feature shared among antibody domains is a conserved tryptophan residue that is located in proximity to the internal disulfide bridge. As its fluorescence is quenched only in the native state by the adjacent disulfide bond, it can be used as a reporter group for the conformational state of antibody domains19. Additionally, proline residues are unusually abundant in antibody domains, contributing up to 10% of the amino acids. Particularly important is a conserved cis-proline residue in the loop connecting strands b and c of the constant domains (Figure 1B).
Although the Ig fold was first identified in antibodies, it is in fact one of the most widely used protein topologies in nature, giving rise to the Ig superfamily (IgSF). The origin of the Ig fold dates back to ~750 million years of evolutionary history, with the identification of IgSF members as early as sponges20,21; by contrast, the ability to produce antibodies is a more recent development (~500 million years), first appearing in cartilaginous fish, such as sharks, skates and rays21,22. In vertebrates, the Ig fold is the major building block of extracellular recognition systems23,24, whereas in invertebrates, expression of IgSF members is mostly limited to the neural system25. The Ig fold has also been detected in prokaryotic and viral proteins, albeit less frequently, suggesting that it might have been acquired in these cases by horizontal gene transfer26. The evolutionary success of the IgSF can likely be attributed to its robust fold, which provides stability against proteases and harsh environments, and the ability to build highly diverse binding loops or edge strands on this core structure.
From the folding of antibody domains to complete IgG molecules
Dissection of the Ig protein into individual domains or fragments was necessary to detect differences in the folding of structurally similar domains. The pioneering studies on antibody folding were performed on secreted LCs that were denatured and allowed to refold in vitro19. Further studies examined isolated constant LC domains (CL)27,28 and IgG HC CH3 domains29, which revealed that these individual Ig domains can fold autonomously. A common characteristic in the folding of antibody domains are slow proline-isomerization reactions (Box 1)27,28,30–32, that often provide the rate limiting step33,34. Recent in vitro studies have addressed the folding of each of the IgG domains in more detail30–39. These studies found that although all Ig domains are very similar in terms of their final structure, they can be grouped into three different folding categories (Figure 2).
Box 1. Peptidyl-prolyl isomerization reactions.
Within proteins, the individual amino acids are covalently linked by the planar peptide bond, the product of the reaction between the carboxyl group of the amino acid number i and the amine group of the amino acid number i+1. For 19 out of the 20 natural amino acids, this bond populates almost exclusively a trans state, i.e. the angle between the CO group of amino acid i and the NH group of amino acid i+1 is ω=180° and the Cα atoms of amino acid i and i+1 are on opposite sites of the CO-NH bond (Figure I). The only exception is proline, where, due to its cyclic side chain, the cis state (ω=0°) of the peptide bond is energetically only slightly less favourable than the trans state (Figure I). In mature protein structures, ~10% of all bonds between proline and its preceding amino acid (Xaa-Pro bond) are found in the cis state. The conversion to the cis state is influenced by the side chain of the amino acid preceding the proline residue75. The cis conformation is particularly pronounced in bends and turns of proteins suggesting a structural role76. As the activation energy of the peptidyl-prolyl isomerization reaction is rather high, ~80 kJ/mol, it is an intrinsically slow reaction taking several minutes at room temperature. Furthermore, all cis Xaa-Pro bonds leave the ribosome in a trans state after polypeptide synthesis. Therefore, peptidyl-prolyl isomerization reactions are important rate limiting steps in protein folding and were among the first to be identified77,78. Due to their unusual structural properties, prolines can be used as molecular timers and switches in protein conformational changes79,80. In the cell, peptidyl-prolyl isomerization reactions are catalyzed by the diverse and ubiquitous family of peptidyl-prolyl isomerases (PPIases)81,82.
Box 1 / Figure I – Schematic representation of an alanyl-prolyl isomerization reaction.
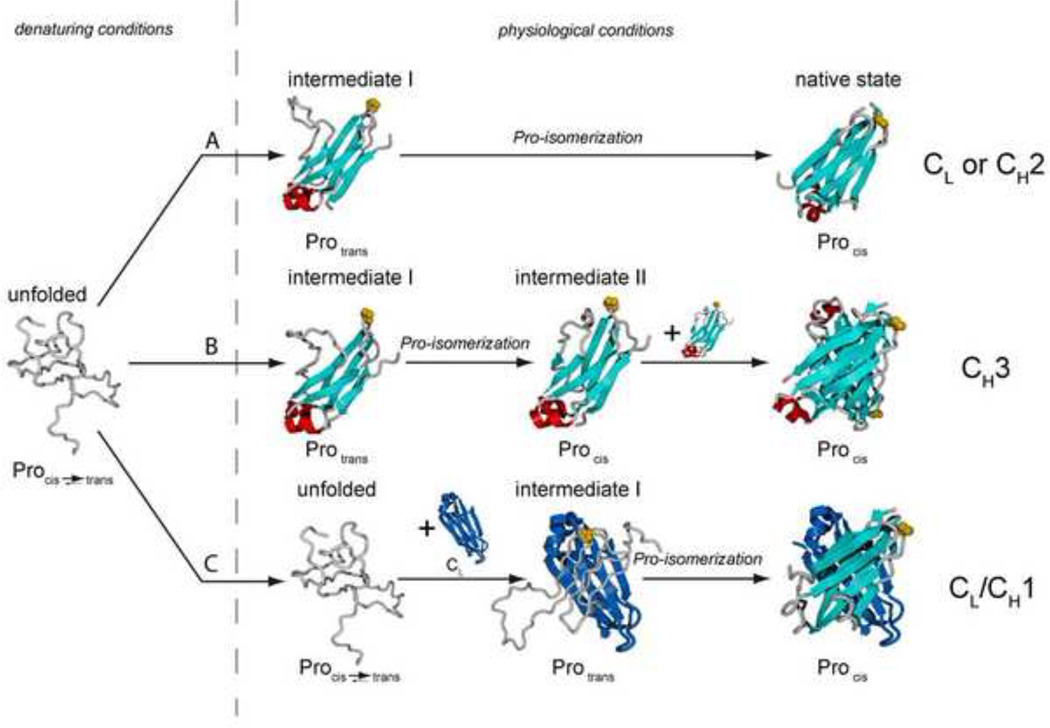
Figure 2. three pathways of antibody domain folding.
(i) CL and CH2 fold via a highly structured on-pathway intermediate that is trapped by the trans state of a proline residue in the loop connecting strands b and c (highlighted in yellow). In the intermediate, the core β-sheet structure and the two short helices connecting strands a and b and strands e and f are fully formed (shown in red). (ii) The obligate dimer CH3 folds via two intermediates, both most likely similar in structure to those of the CL and CH2 domains. In a first, rapidly formed intermediate, a critical proline residue (highlighted in yellow) must isomerize to its native cis state, leading to a second intermediate which can dimerize and thereby complete folding. (iii) CH1 is intrinsically disordered in isolation. Upon association with CL, it forms a loosely folded intermediate. In this complex, isomerization of the conserved proline residue between strands b and c (highlighted in yellow) limits the complete folding to the native state and formation of the interchain disulfide bridge between CH1 and CL.
In the first category, exemplified by the well-studied CL protein, domains are able to fold autonomously into a monomeric state. The chemically denatured protein exhibits no significant residual structure, regardless of whether or not its internal disulfide bridge is present34,36. However, once folding is initiated, the presence of an internal disulfide bridge exerts an important guiding impact on the folding pathway27,28,30,36. This is because the initial folding nucleus for Ig domains involves the clustering of hydrophobic residues in strands b, c, e and f, which establish the overall topology of the protein (Figure 2)35,40,41. The covalent linkage of the cysteines in strands b and f facilitates the establishment of this folding nucleus and the formation a structured, on-pathway intermediate thus preventing unproductive interactions (Figure 2). Indeed, a population of misfolded, aggregation-prone off-pathway folding intermediates for CL was detected in the absence of the internal disulfide bridge36.
Although folding intermediates are usually transient and therefore elusive, they can be populated for longer times if a slow reaction limits the subsequent folding step thus allowing their characterization. This is the case for CL, where the major on-pathway folding intermediate is relatively long-lived due to the non-native trans isomerization state of a proline residue between strands b and c, which must isomerize to its cis state before folding can proceed (Figure 2)27,34. Using NMR combined with molecular dynamics simulations, it was possible to follow the changes in the chemical environment of most amino acids and to obtain an atomic resolution view of the intermediate structure. In the intermediate, the core β-strands are almost completely formed, whereas the flanking strands, particularly strand d, remain highly flexible (Figure 2)34, in keeping with data on other IgSF members42–44. The analysis of the structural changes of individual amino acids in the course of folding unexpectedly revealed that two small helices linking strands a and b and strands e and f (Figure 1C, Figure 2) are important guiding elements in Ig domain folding. They become natively structured very early34 and can act as an organizing center, stabilizing the orientation and spacing of the β-strands of the Ig fold. Furthermore they correctly position bulky hydrophobic residues in the core of the protein34. Thus, these helices render the folding of the CL domain more robust, as they stabilize the conformation of a highly-structured on-pathway intermediate that is poised for subsequent productive folding. Consistent with their important role in folding, these small helices are highly conserved in most constant region domains as well as in other members of the Ig superfamily18,23,24. The structural insights gained for the major CL folding intermediate seem to be readily transferable to other HC constant domains, in particular CH231, where the effect of the sugar moieties on the folding reaction is currently unknown. Interestingly, no such helices are found in variable antibody domains (Figure 1B) or in several IgSF members that are prone to misfolding and amyloid formation43–45, suggesting that structural differences in folding intermediates might help determine whether or not IgSF members reliably fold (Box 2).
Box 2. Alternatively folded states and antibody deposition diseases.
For most proteins, the accessible conformations in equilibrium include the native state, the unfolded state and unspecific aggregates; some polypeptides might additionally adopt oligomeric fibrillar structures. For antibodies, the situation is different. Antibodies can adopt a specific additional conformation at low pH (below pH 3) where many other proteins would be largely unfolded. The low pH antibody conformation had been termed “alternatively folded state” as it exhibits characteristics of the folded state, such as remarkable stability against unfolding, but the available spectroscopic information suggests that it is structurally significantly different from the native state. It was first described for a complete IgG antibody83, but single domains, such as CH3, are also able to adopt this state84. The biological significance of this process remains enigmatic. However, there are biotechnological implications for this state, as antibody manufacturing processes often include low pH steps which can induce the alternatively folded state.
Another accessible state for some antibodies is the fibrillar amyloid structure which is associated with a number of protein folding diseases. In this cross-beta structure, fibrils are formed by β-strand exchange of the individual subunits. Whereas antibodies certainly evolved to robustly form and maintain their β-sheet structure in the human body, both secreted isolated LCs and also truncated HCs have been found to form fibrils which are deposited in organs, such as kidneys. As antibodies are produced and can thus be deposited in large quantities, these deposits can strongly interfere with physiological functions and thus these diseases can have fatal consequences. The most prevalent of the fatal diseases is light chain amyloidosis (AL), resulting from the over-production of monoclonal LCs which are prone to misfolding and the formation of amyloid deposits. In the case of AL, certain VL domains seem to be particularly susceptible to amyloid formation85, consistent with the idea that the CL domain is protected against misfolding by helical elements which are missing in VL34,38,45 and the fact that all CL domains are the same, whereas all VL domains are different. Although the precise fibrilization mechanism remains incompletely understood, it seems that the mechanisms that give rise to the production of variable domains might, at times, generate less stable domains that are able to pass ER quality control but that have a propensity outside the cell to misfold.
The second category of antibody domain folding pathways (Figure 2) is represented by the CH3 domain of the IgG HC29,33. In addition to folding slower than the domains of the first category, this domain forms an obligate homodimer with the internal disulfide bridge being dispensable for folding and self-association39. As with the CL domain, a partially folded species was observed, which was trapped by a non-native prolyl isomerization state33. Interestingly, the monomeric intermediate could not dimerize until the native proline isomer was formed. Thus, proline switches can regulate not only folding but also dimerization in antibodies.
The third, and most unexpected, category of antibody domain folding is the recently discovered, template-assisted folding of the CH1 domain, which interacts with the CL domain in the intact antibody (Figure 1A). Surprisingly, the isolated CH1 domain is intrinsically disordered as determined by various spectroscopic techniques37. This is in marked contrast to all antibody domains previously studied and completely unexpected from its structural similarity to other Ig domains11. To induce its folding, the CH1 domain strictly requires interaction with key residues in the dimerization interface of the folded CL domain. Unlike antibody domains where the intramolecular disulfide bond only enhances the folding process, the covalent linkage of the two cysteines in the CH1 domain is a prerequisite for its folding. Another unique twist is that the rate-limiting proline isomerization between strand b and c and subsequent productive folding can only occur after association with the CL domain. These observations are in agreement with previous studies on the Fab fragment where CH1 folding was proposed to be the slowest step, occurring after association of the HC and the LC32.
Together, the data reveal that the β-barrel topology of antibody domains is reached by an overall conserved mechanism, although strikingly different pathways are employed. The biological significance of these differences becomes clear when one considers that different domains of antibodies are used for significantly different purposes in the cell such as guiding dimerization, like CH3, or as a quality control sensor for assembly, like CH1.
Quality control of antibody folding and assembly in vivo
As the secretion of incompletely folded or assembled antibodies would be deleterious to the immune response, a number of quality control checkpoints are required during B cell development and differentiation that monitor the integrity of the antibody (Figure 3). One of the first quality control measures after HC variable gene rearrangements centers on the ability of the HC protein to associate with the “surrogate LC”, which is assembled from the VpreB protein (contributing the “variable domain”)46 and the λ5 protein (supplying the “constant domain”)47. This LC-mimetic tests the ability of HC to properly fold and assemble with a LC-like protein. If this occurs correctly, the preB cell receptor is transported to the cell surface along with signaling proteins, and provides the stimulus for the further development of the pre-B cell48. The CH1 domain constitutes a crucial aspect of this quality control step, as HCs that lack the CH1 domain can be transported to the cell surface and signal without assembling with a surrogate LC49, and remains a critical focus of Ig quality control efforts throughout B cell development and plasma cell differentiation. Once the HC is judged functional by successfully completing these steps, conventional LC gene rearrangements commence. Unlike LC, which can be secreted alone, HCs are retained in the ER and eventually degraded unless they assemble with LC (Figure 3)50. LC loss variants of plasmacytomas are very rarely observed, whereas HC loss variants occur much more frequently51. This was argued to be due to the “toxicity” of free HC, which could be neutralized by LC52. Exceptions to this rule occur in the rare B cell lymphoproliferative disorder known as Heavy Chain Disease, where truncated Ig HCs are secreted from cells without LCs (Box 2). Notably, although these short HC have been identified for a number of different isotypes (i.e., IgA, IgG, and IgM), the deletions nearly always involve portions of the VH and CH1 domains53. Similarly, mouse plasmacytoma lines expressing HC with deletions of the CH1 domain can secrete free HC54, whereas deletion of any of the other constant region domains does not permit this. Finally, the serum of Camelidae contains a significant fraction of antibodies that are naturally devoid of LC. These “HC-only” antibodies do not possess a CH1 domain55 further underscoring the evolutionarily conserved importance of this domain in regulating Ig transport and quality control.
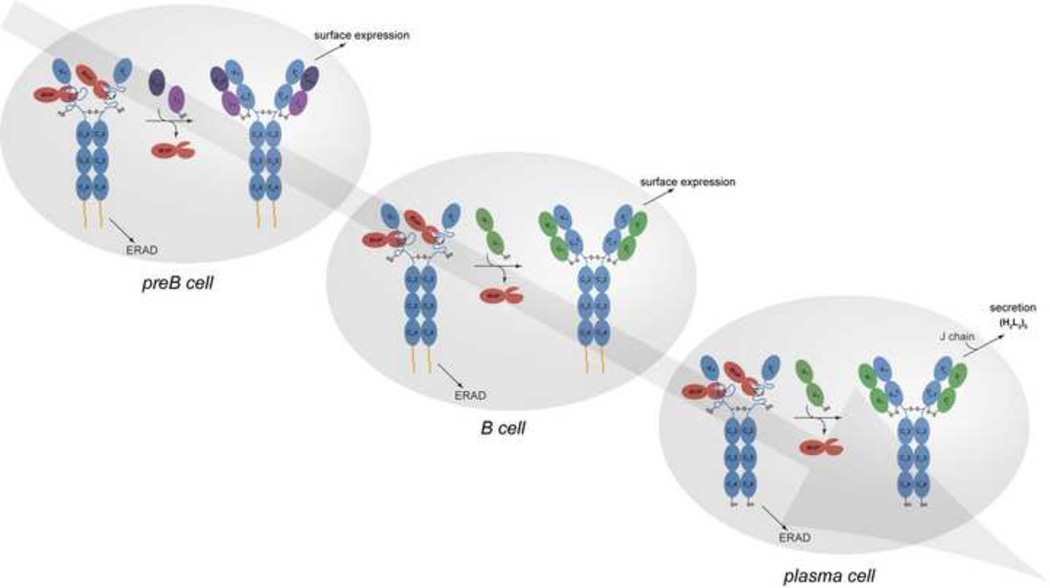
Figure 3. Immunoglobulin quality control checkpoints at various stages in B cell development.
After HC gene rearrangements, preB cells produce IgM HCs (μ HCs) (blue) bound to BiP (red). If their association with the surrogate LC, which is assembled from the VpreB (deep purple) and λ5 (light purple) proteins, induces BiP release and folding of the CH1 domain, and if the other Ig domains fold properly, the HC can traffic to the plasma membrane and engage signalling molecules (HC membrane anchor shown in yellow). If there is a failure in any of these steps, the μ HCs become substrates for ER associated degradation (ERAD) and are retrotranslocated to the cytosol for degradation by the 26S proteasome. Once conventional LCs (green) are produced in the B cell, they assemble with μ HCs, displace BiP from the CH1 domain, and induce its folding. As the ability of all domains of the HC to fold properly upon assembly was tested at the preB cell stage, quality control at this stage monitors the pairing and folding of the V domains. Plasma cell differentiation leads to the synthesis of extremely high levels of antibodies. Because the ability of the specific HC and LC combination to assemble and fold properly was verified at the B cell stage of development, quality control at this point involves monitoring the completeness of Ig assembly, focusing on the LC-induced release of BiP from the CH1 domain and its concomitant folding. There is a shift to production of the secretory form of μ HC in plasma cells, which possess a terminal cysteine that is involved in assembly with J chain and pentamer formation. Thiol-mediated retention mechanisms monitor the redox state of this cysteine and prevent IgM monomers from being secreted86.
The term “ER quality control” refers to the process of monitoring the maturation of nascent secretory proteins and allowing only properly folded and assembled proteins to transit further along the secretory pathway. Proteins that fail to mature correctly are retained and eventually retrotranslocated to the cytosol for degradation by the 26S proteasome in a process known as ER associated degradation (ERAD)56,57. Immunoglobulin heavy chain binding protein (BiP), the first component of the eukaryotic ER quality control apparatus to be identified, was found by virtue of its association with the unassembled, non-transported HCs produced in pre-B cell lines58. It interacts transiently with Ig assembly intermediates but not with completely assembled H2L2 molecules59. BiP is the ER orthologue of the Hsp70 family of chaperones60 and is retained in the ER, along with any associated proteins, by virtue of its C-terminal KDEL tetrapeptide61. Similar to the differences detected by in vitro folding experiments, the folding requirements and dependence on BiP are quite different for the various Ig domains in cells. BiP binds transiently to some Ig domains (i.e., VL, VH, and some CH domains), but other domains, such as CL, appear to fold rapidly without ever interacting with BiP62,63, even though they possess potential BiP binding sites64. Only the CH1 domain interacts stably with BiP in the absence of LC65 (Figure 3). The association of this domain with BiP is crucial for controlling Ig assembly and transport, because its deletion, and the resulting ablation of BiP binding, leads to the secretion of incompletely assembled Ig intermediates. In vivo studies o determine the folding status of proteins possessing intrachain disulfide bonds rely on the analysis of the oxidation status of cysteine residues, as disulfide bonds make proteins more compact and faster migrating on non-reducing SDS PAGE gels66. Unlike other Ig domains, the CH1 domain remains reduced in the absence of LC63, suggesting that it is not completely folded. However, in contrast to the in vitro studies described above, there is no evidence that BiP can associate oxidized CH1 domains in cells67, it might well be that in vivo the association with LC, oxidation, and folding of CH1 are more tightly coupled.
In keeping with in vitro studies, the ATP-mediated release of BiP from isolated HC resulted in CH1 domain oxidation, but this is not sufficient for proper folding67,37. This finding argues that LC association is also required for the folding of the CH1 domain in vivo. Only LC in which both domains (VL and CL) were folded could assemble with HC and induced CH1 domain oxidation and secretion from cells. In vivo experiments also confirmed the requirement for proline isomerization in the CH1 domain in these processes, as a mutation of the critical proline inhibited oxidation, assembly with LC and secretion37. Thus, the evolution of a unique CH1 domain that absolutely requires assembly with a CL domain for its folding, allows the cell to ensure that newly rearranged HCs in preB and cells will be retained unless they are able to pass an important test: their ability to combine with a surrogate or conventional LC respectively. In addition, it also ensures that plasma cells, which have been estimated to synthesize up to 103 antibody molecules a second68, are not releasing partially assembled subunits that cannot properly bind to the selected antigen or perform effector functions.
It appears that a comparable folding-based retention mechanism also operates on some LCs. Many LCs fold readily and can be secreted by themselves as either monomers69 or dimers70, suggesting that the VL domains of these LCs are likely to belong to folding categories 1 and 2 described above (Fig. 2). However, LCs exist that are not secreted without HC71 due to a failure of the VL domain to fold properly by itself72, suggesting that these VL domains might belong to category 3. The requirement for assembly-assisted folding of some VL, and presumably VH domains, would limit which VL and VH pairings could pass ER quality control. It is likely that only self-folding V regions on one chain could complement and fold the assembly-dependent V regions on the other chain, in much the same way that a folded CL domain is required to induce folding of the CH1 domain. This possibility could explain the fact that only certain possible HC and LC pairings are observed in cell lines and immune responses73.
Concluding remarks and future perspectives
In summary, the combination of biophysical and in vivo studies on individual Ig domains, antibody fragments and complete antibodies have identified common themes that together now provide a detailed picture of IgG folding (Figure 4). Once the internal disulfide bridge is formed, most domains will autonomously fold in at least a three step reaction. The first observable step is the formation of an on-pathway folding intermediate whose lifetime is increased by incorrect peptidyl-prolyl isomerization states. Subsequent peptidyl-prolyl isomerization reactions control folding to the native state, assembly and formation of interchain disulfide bridges and might also play a role in inhibiting aggregation. In the case of most IgG subclasses, once the CH3 domain folds it induces dimerization of the HC, which is further stabilized by the formation of disulfide bonds in the hinge region (Figure 4). At this stage, all the constant region domains, except CH1, are folded. The folding of the LCs will generally occur independently and in parallel. Association of a folded LC with HC will induce CH1 domain folding, and once CH1 is completely folded the assembly of HCs and LCs will be stabilized by an interchain disulfide bond (Figure 4). In the cell, the individual steps are attended by the ER chaperone machinery, which associates co-translationally with precursor HC and LC and allows high concentrations of unfolded domains to exist without aggregating and might be further supported by the co-translational folding of the individual domains74. Future work must therefore focus on further integrating the role of the complex ER folding network in modulating, synchronizing and controlling the folding and assembly of antibodies and other IgSF members.
Figure 4. A comprehensive view of IgG folding and assembly.
Folding, formation of disulfide bridges and glycosylation of the HC (blue) and LC (green) begins cotranslationally in the ER. The molecular chaperone BiP (red) interacts with most of the domains transiently before folding is completed. All constant domains except CH1 and most variable domains fold autonomously, populating an on-pathway intermediate on the way to the native state. CL is known to fold particularly fast in the cell. Once CH3 is folded, it induces HC dimerization which will be solidified by disulfide bridges in the hinge region. CH1 remains unfolded, unoxidized and stably bound to BiP until the LC displaces BiP and CL induces folding of the CH1 domain. Once the important CH1 prolines are in the correct isomerization state and CH1 is folded, a disulfide bridge between the LC and the HC forms rendering the IgG molecules ready for secretion. Most of these steps are likely to hold for other Ig classes. Chaperones and folding catalysts, such as Grp94, protein disulfide isomerase (PDI) and the peptidyl-prolyl isomerase CyclophilinB contribute to the individual steps in immunoglobulin biogenesis.
Acknowledgments
We thank Moritz Marcinowski and Dr. Roger Müller for helpful comments on the manuscript and Julia Behnke for help with preparing Box 1. Funding of MJF by the Studienstiftung des deutschen Volkes, of JB by the DFG SFB 749 and the Fonds der chemischen Industrie and of LMH by by NIH Grant GM54068, the Cancer Center CORE Grant CA21765, and the American Lebanese Syrian Associated Charities of St. Jude Children's Research Hospital is gratefully acknowledged.
Glossary
- Ig domain
a folding unit of approximately 100 amino acids with a highly conserved twisted barrel-like β-sheet structure that is in most cases stabilized by a buried intrachain disulfide bond.
- Hypervariable regions
highly diverse portions of the heavy and light chain variable regions that form the antigen binding site. They arise in part due to the genetic mechanisms used to produce variable regions.
- Heavy/light chain
constituent polypeptide chains of antibody molecules. Light chains are made up of two Ig domains and therefore possess a lower molecular weight than the heavy chains which are made up of a minimum of three Ig domains.
- Isotypes
refers to the antibody class, IgM, IgG, IgA, IgD, and IgE, which are named for the heavy chain constant region used; µ, γ, α, δ, and ε respectively. A single heavy chain variable region can be sequentially associated with different constant regions via a process known as class switching.
- Ig superfamily
refers to a large group of proteins that are composed of Ig domains. It is one of the most widespread protein topologies observed in nature and is often involved in extracellular binding and recognition processes.
- Pre-B cell
an early B cell developmental stage that is characterized by the production of heavy chain proteins but not light chains.
- B cell
refers to a mature developmental stage in which both heavy and light chains are synthesized and expressed at the cell surface via a transmembrane region at the C-terminus of the heavy chain that is produced by alternative splicing of the heavy chain mRNA.
- Plasma cell
the terminal stage of B cell differentiation that occurs after mitogen or antigen stimulation of B cells. These normally short-lived cells produce tremendous quantities of a single type of antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy KM, et al. Janeway's Immunobiology. Garland Science; 2008. [Google Scholar]
- 2.Maki R, et al. Exon shuffling generates an immunoglobulin heavy chain gene. Proc. Natl. Acad. Sci. U. S. A. 1980;77:2138–2142. doi: 10.1073/pnas.77.4.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early P, et al. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH D and JH. Cell. 1980;19:981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- 4.Calame K, et al. Mouse Cmu heavy chain immunoglobulin gene segment contains three intervening sequences separating domains. Nature. 1980;284:452–455. doi: 10.1038/284452a0. [DOI] [PubMed] [Google Scholar]
- 5.Alt FW, et al. Development of the primary antibody repertoire. Science. 1987;238:1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- 6.Bernard O, et al. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978;15:1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- 7.Bergman LW, Kuehl WM. Formation of an intrachain disulfide bond on nascent immunoglobulin light chains. J. Biol. Chem. 1979;254:8869–8876. [PubMed] [Google Scholar]
- 8.Baumal R, et al. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. 3. Assembly of the three subclasses of IgG. J. Exp. Med. 1971;134:1316–1334. doi: 10.1084/jem.134.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zack DJ, et al. Somatically generated mouse myeloma variants synthesizing IgA half-molecules. J. Exp. Med. 1981;154:1554–1569. doi: 10.1084/jem.154.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman LW, Kuehl WM. Temporal relationship of translation and glycosylation of immunoglobulin heavy and light chains. Biochemistry. 1978;17:5174–5180. doi: 10.1021/bi00617a017. [DOI] [PubMed] [Google Scholar]
- 11.Huber R, et al. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976;264:415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- 12.Krapp S, et al. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. Journal of Molecular Biology. 2003;325:979–989. doi: 10.1016/s0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 13.Feige MJ, et al. Structure of the murine unglycosylated IgG1 Fc fragment. J. Mol. Biol. 2009;391:599–608. doi: 10.1016/j.jmb.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 14.Sitia R, et al. The role of glycosylation in secretion and membrane expression of immunoglobulins M and A. Mol. Immunol. 1984;21:709–719. doi: 10.1016/0161-5890(84)90023-3. [DOI] [PubMed] [Google Scholar]
- 15.Hickman S, Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J. Immunol. 1978;121:990–996. [PubMed] [Google Scholar]
- 16.Kalmanson GM, Bronfenbrenner J. The reversal of Pneumococcus Quellung by digestion of the antibody with papain. Science. 1942;96:21–22. doi: 10.1126/science.96.2479.21. [DOI] [PubMed] [Google Scholar]
- 17.Porter RR. The formation of a specific inhibitor by hydrolysis of rabbit antiovalbumin. Biochem. J. 1950;46:479–484. doi: 10.1042/bj0460479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bork P, et al. The Immunoglobulin Fold - Structural Classification, Sequence Patterns and Common Core. Journal of Molecular Biology. 1994;242:309–320. doi: 10.1006/jmbi.1994.1582. [DOI] [PubMed] [Google Scholar]
- 19.Goto Y, Hamaguchi K. Role of the Intrachain Disulfide Bond in the Conformation and Stability of the Constant Fragment of the Immunoglobulin Light Chain. Journal of Biochemistry. 1979;86:1433–1441. doi: 10.1093/oxfordjournals.jbchem.a132661. [DOI] [PubMed] [Google Scholar]
- 20.Du PL, et al. Immunoglobulin superfamily receptors in protochordates: before RAG time. Immunol. Rev. 2004;198:233–248. doi: 10.1111/j.0105-2896.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- 21.Hsu E, et al. The plasticity of immunoglobulin gene systems in evolution. Immunol. Rev. 2006;210:8–26. doi: 10.1111/j.0105-2896.2006.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dooley H, Flajnik MF. Antibody repertoire development in cartilaginous fish. Dev. Comp Immunol. 2006;30:43–56. doi: 10.1016/j.dci.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Williams AF, Barclay AN. The immunoglobulin superfamily--domains for cell surface recognition. Annu. Rev. Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 24.Barclay AN. Membrane proteins with immunoglobulin-like domains--a master superfamily of interaction molecules. Semin. Immunol. 2003;15:215–223. doi: 10.1016/s1044-5323(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 25.Rougon G, Hobert O. New insights into the diversity and function of neuronal immunoglobulin superfamily molecules. Annu. Rev. Neurosci. 2003;26:207–238. doi: 10.1146/annurev.neuro.26.041002.131014. [DOI] [PubMed] [Google Scholar]
- 26.Halaby DM, Mornon JP. The immunoglobulin superfamily: an insight on its tissular, species, and functional diversity. J. Mol. Evol. 1998;46:389–400. doi: 10.1007/pl00006318. [DOI] [PubMed] [Google Scholar]
- 27.Goto Y, Hamaguchi K. Unfolding and Refolding of the Constant Fragment of the Immunoglobulin Light Chain. Journal of Molecular Biology. 1982;156:891–910. doi: 10.1016/0022-2836(82)90146-2. [DOI] [PubMed] [Google Scholar]
- 28.Goto Y, Hamaguchi K. Unfolding and Refolding of the Reduced Constant Fragment of the Immunoglobulin Light Chain - Kinetic Role of the Intrachain Disulfide Bond. Journal of Molecular Biology. 1982;156:911–926. doi: 10.1016/0022-2836(82)90147-4. [DOI] [PubMed] [Google Scholar]
- 29.Isenman D, et al. Folding Pathways of Immunoglobulin Domains. The Folding Kinetics of the C3 Domain of Human IgG. Biochemsitry. 1979;18:3327–3336. doi: 10.1021/bi00582a020. [DOI] [PubMed] [Google Scholar]
- 30.Ramm K, et al. Removal of the conserved disulfide bridges from the scFv fragment of an antibody: effects on folding kinetics and aggregation. J. Mol. Biol. 1999;290:535–546. doi: 10.1006/jmbi.1999.2854. [DOI] [PubMed] [Google Scholar]
- 31.Feige MJ, et al. Folding mechanism of the C(H)2 antibody domain. Journal of Molecular Biology. 2004;344:107–118. doi: 10.1016/j.jmb.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 32.Lilie H, et al. Association of antibody chains at different stages of folding: prolyl isomerization occurs after formation of quaternary structure. J. Mol. Biol. 1995;248:190–201. doi: 10.1006/jmbi.1995.0211. [DOI] [PubMed] [Google Scholar]
- 33.Thies MJW, et al. Folding and association of the antibody domain C(H)3: Prolyl isomerization preceeds dimerization. Journal of Molecular Biology. 1999;293:67–79. doi: 10.1006/jmbi.1999.3128. [DOI] [PubMed] [Google Scholar]
- 34.Feige MJ, et al. The structure of a folding intermediate provides insight into differences in immunoglobulin amyloidogenicity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13373–13378. doi: 10.1073/pnas.0802809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freund C, et al. Folding nuclei of the scFv fragment of an antibody. Biochemistry. 1996;35:8457–8464. doi: 10.1021/bi952764a. [DOI] [PubMed] [Google Scholar]
- 36.Feige MJ, et al. Influence of the internal disulfide bridge on the folding pathway of the C-L antibody domain. Journal of Molecular Biology. 2007;365:1232–1244. doi: 10.1016/j.jmb.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 37.Feige MJ, et al. An unfolded CH1 domain controls the assembly and secretion of IgG antibodies. Mol. Cell. 2009;34:569–579. doi: 10.1016/j.molcel.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson ER, et al. The Folding Pathway of the Antibody V(L) Domain. J. Mol. Biol. 2009 doi: 10.1016/j.jmb.2009.07.075. [DOI] [PubMed] [Google Scholar]
- 39.Thies MJ, et al. Folding and oxidation of the antibody domain C(H)3. J. Mol. Biol. 2002;319:1267–1277. doi: 10.1016/S0022-2836(02)00375-3. [DOI] [PubMed] [Google Scholar]
- 40.Geierhaas CD, et al. Comparison of the transition states for folding of two Ig-like proteins from different superfamilies. J. Mol. Biol. 2004;343:1111–1123. doi: 10.1016/j.jmb.2004.08.100. [DOI] [PubMed] [Google Scholar]
- 41.Lappalainen I, et al. Plasticity within the obligatory folding nucleus of an immunoglobulin-like domain. J. Mol. Biol. 2008;375:547–559. doi: 10.1016/j.jmb.2007.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizuguchi M, et al. Folding of a beta-sheet protein monitored by real-time NMR spectroscopy. Journal of Molecular Biology. 2003;328:1161–1171. doi: 10.1016/s0022-2836(03)00349-8. [DOI] [PubMed] [Google Scholar]
- 43.Kameda A, et al. Nuclear magnetic resonance characterization of the refolding intermediate of beta(2)-microglobulin trapped by non-native prolyl peptide bond. Journal of Molecular Biology. 2005;348:383–397. doi: 10.1016/j.jmb.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 44.Jahn TR, et al. Amyloid formation under physiological conditions proceeds via a native-like folding intermediate. Nature Structural & Molecular Biology. 2006;13:195–201. doi: 10.1038/nsmb1058. [DOI] [PubMed] [Google Scholar]
- 45.Qin ZJ, et al. Structural characterization of the partially folded intermediates of an immunoglobulin light chain leading to amyloid fibrillation and amorphous aggregation. Biochemistry. 2007;46:3521–3531. doi: 10.1021/bi061716v. [DOI] [PubMed] [Google Scholar]
- 46.Kudo A, Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 1987;6:2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi N, Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986;324:579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- 48.Karasuyama H, et al. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J. Exp. Med. 1990;172:969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaffer AL, Schlissel MS. A truncated heavy chain protein relieves the requirement for surrogate light chains in early B cell development. J. Immunol. 1997;159:1265–1275. [PubMed] [Google Scholar]
- 50.Mains PE, Sibley CH. The requirement of light chain for the surface deposition of the heavy chain of immunoglobulin M. J. Biol. Chem. 1983;258:5027–5033. [PubMed] [Google Scholar]
- 51.Coffino P, Scharff MD. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc. Natl. Acad. Sci. U. S. A. 1971;68:219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohler G. Immunoglobulin chain loss in hybridoma lines. Proc. Natl. Acad. Sci. U. S. A. 1980;77:2197–2199. doi: 10.1073/pnas.77.4.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seligmann M, et al. Heavy chain diseases: current findings and concepts. Immunol. Rev. 1979;48:145–167. doi: 10.1111/j.1600-065x.1979.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 54.Morrison SL. Murine heavy chain disease. Eur. J. Immunol. 1978;8:194–199. doi: 10.1002/eji.1830080311. [DOI] [PubMed] [Google Scholar]
- 55.Hamers-Casterman C, et al. Naturally-Occurring Antibodies Devoid of Light-Chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 56.Lippincott-Schwartz J, et al. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell. 1988;54:209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- 57.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haas IG, Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983;306:387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- 59.Bole DG, et al. Posttranslational Association of Immunoglobulin Heavy-Chain Binding-Protein with Nascent Heavy-Chains in Nonsecreting and Secreting Hybridomas. Journal of Cell Biology. 1986;102:1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haas IG, Meo T. cDNA cloning of the immunoglobulin heavy chain binding protein. Proc. Natl. Acad. Sci. U. S. A. 1988;85:2250–2254. doi: 10.1073/pnas.85.7.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 62.Hellman R, et al. The in vivo association of BiP with newly synthesized proteins is dependent on the rate and stability of folding and not simply on the presence of sequences that can bind to BiP. J. Cell Biol. 1999;144:21–30. doi: 10.1083/jcb.144.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee YK, et al. BiP and immunoglobulin light chain cooperate to control the folding of heavy chain and ensure the fidelity of immunoglobulin assembly. Molecular Biology of the Cell. 1999;10:2209–2219. doi: 10.1091/mbc.10.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knarr G, et al. BiP binding sequences in antibodies. J. Biol. Chem. 1995;270:27589–27594. doi: 10.1074/jbc.270.46.27589. [DOI] [PubMed] [Google Scholar]
- 65.Hendershot L, et al. Assembly and Secretion of Heavy-Chains That do Not Associate Posttranslationally with Immunoglobulin Heavy-Chain Binding-Protein. Journal of Cell Biology. 1987;104:761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braakman I, et al. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992;11:1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanhove M, et al. Unassembled Ig heavy chains do not cycle from BiP in vivo but require light chains to trigger their release. Immunity. 2001;15:105–114. doi: 10.1016/s1074-7613(01)00163-7. [DOI] [PubMed] [Google Scholar]
- 68.Hendershot LM, Sitia R. Antibody synthesis and assembly. Molecular Biology of B cells. 2004:261–273. [Google Scholar]
- 69.Dul JL, et al. Ig light chains are secreted predominantly as monomers. J. Immunol. 1996;157:2969–2975. [PubMed] [Google Scholar]
- 70.Leitzgen K, et al. Assembly of immunoglobulin light chains as a prerequisite for secretion. A model for oligomerization-dependent subunit folding. J. Biol. Chem. 1997;272:3117–3123. doi: 10.1074/jbc.272.5.3117. [DOI] [PubMed] [Google Scholar]
- 71.Horibata K, Harris AW. Mouse myelomas and lymphomas in culture. Exp. Cell Res. 1970;60:61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- 72.Skowronek MH, et al. The variable domain of nonassembled Ig light chains determines both their half-life and binding to the chaperone BiP. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1574–1578. doi: 10.1073/pnas.95.4.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiens GD, et al. Harmful somatic mutations: lessons from the dark side. Immunol. Rev. 1998;162:197–209. doi: 10.1111/j.1600-065x.1998.tb01442.x. [DOI] [PubMed] [Google Scholar]
- 74.Komar AA. A pause for thought along the co-translational folding pathway. Trends Biochem. Sci. 2009;34:16–24. doi: 10.1016/j.tibs.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Reimer U, et al. Side-chain effects on peptidyl-prolyl cis/trans isomerisation. J. Mol. Biol. 1998;279:449–460. doi: 10.1006/jmbi.1998.1770. [DOI] [PubMed] [Google Scholar]
- 76.Stewart DE, et al. Occurrence and role of cis peptide bonds in protein structures. J. Mol. Biol. 1990;214:253–260. doi: 10.1016/0022-2836(90)90159-J. [DOI] [PubMed] [Google Scholar]
- 77.Brandts JF, et al. Consideration of the Possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry. 1975;14:4953–4963. doi: 10.1021/bi00693a026. [DOI] [PubMed] [Google Scholar]
- 78.Schmid FX, Baldwin RL. Acid catalysis of the formation of the slow-folding species of RNase A: evidence that the reaction is proline isomerization. Proc. Natl. Acad. Sci. U. S. A. 1978;75:4764–4768. doi: 10.1073/pnas.75.10.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eckert B, et al. Prolyl isomerization as a molecular timer in phage infection. Nat. Struct. Mol. Biol. 2005;12:619–623. doi: 10.1038/nsmb946. [DOI] [PubMed] [Google Scholar]
- 80.Lu KP, et al. Prolyl cis-trans isomerization as a molecular timer. Nat. Chem. Biol. 2007;3:619–629. doi: 10.1038/nchembio.2007.35. [DOI] [PubMed] [Google Scholar]
- 81.Fischer G, et al. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989;337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 82.Lang K, et al. Catalysis of protein folding by prolyl isomerase. Nature. 1987;329:268–270. doi: 10.1038/329268a0. [DOI] [PubMed] [Google Scholar]
- 83.Buchner J, et al. Alternatively folded states of an immunoglobulin. Biochemistry. 1991;30:6922–6929. doi: 10.1021/bi00242a016. [DOI] [PubMed] [Google Scholar]
- 84.Thies MJ, et al. The alternatively folded state of the antibody C(H)3 domain. J. Mol. Biol. 2001;309:1077–1085. doi: 10.1006/jmbi.2001.4707. [DOI] [PubMed] [Google Scholar]
- 85.Bellotti V, et al. Review: Immunoglobulin light chain amyloidosis - The archetype of structural and pathogenic variability. Journal of Structural Biology. 2000;130:280–289. doi: 10.1006/jsbi.2000.4248. [DOI] [PubMed] [Google Scholar]
- 86.Anelli T, et al. Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J. 2003;22:5015–5022. doi: 10.1093/emboj/cdg491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melnick J, et al. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- 88.Hochstenbach F, et al. Endoplasmic reticulum resident protein of 90 kilodaltons associates with the T- and B-cell antigen receptors and major histocompatibility complex antigens during their assembly. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4734–4738. doi: 10.1073/pnas.89.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brightman SE, et al. Isolation of a mouse cDNA encoding MTJ1, a new murine member of the DnaJ family of proteins. Gene. 1995;153:249–254. doi: 10.1016/0378-1119(94)00741-a. [DOI] [PubMed] [Google Scholar]
- 90.Tyedmers J, et al. Homologs of the yeast Sec complex subunits Sec62p and Sec63p are abundant proteins in dog pancreas microsomes. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7214–7219. doi: 10.1073/pnas.97.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meunier L, et al. A sub-set of chaperones and folding enzymes form multi-protein complexes in the ER to bind nascent proteins. Molecular Biology of the Cell. 2002;12:488A. doi: 10.1091/mbc.E02-05-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen Y, et al. Identification and characterization of a novel endoplasmic reticulum (ER) DnaJ homologue, which stimulates ATPase activity of BiP in vitro and is induced by ER stress. J. Biol. Chem. 2002;277:15947–15956. doi: 10.1074/jbc.M112214200. [DOI] [PubMed] [Google Scholar]
- 93.Cunnea PM, et al. ERdj5, an endoplasmic reticulum (ER)-resident protein containing DnaJ and thioredoxin domains, is expressed in secretory cells or following ER stress. J. Biol. Chem. 2003;278:1059–1066. doi: 10.1074/jbc.M206995200. [DOI] [PubMed] [Google Scholar]
- 94.Rutkowski DT, et al. The role of p58IPK in protecting the stressed endoplasmic reticulum. Mol. Biol. Cell. 2007;18:3681–3691. doi: 10.1091/mbc.E07-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zahedi RP, et al. Analysis of the membrane proteome of canine pancreatic rough microsomes identifies a novel Hsp40, termed ERj7. Proteomics. 2009;9:3463–3473. doi: 10.1002/pmic.200800722. [DOI] [PubMed] [Google Scholar]
- 96.Chen X, et al. The 170 kDa glucose regulated stress protein is a large HSP70-, HSP110-like protein of the endoplasmic reticulum. FEBS Lett. 1996;380:68–72. doi: 10.1016/0014-5793(96)00011-7. [DOI] [PubMed] [Google Scholar]
- 97.Chung KT, et al. BAP, a mammalian BiP-associated protein, is a nucleotide exchange factor that regulates the ATPase activity of BiP. J. Biol. Chem. 2002;277:47557–47563. doi: 10.1074/jbc.M208377200. [DOI] [PubMed] [Google Scholar]
- 98.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 99.Roth RA, Pierce SB. In vivo cross-linking of protein disulfide isomerase to immunoglobulins. Biochemistry. 1987;26:4179–4182. doi: 10.1021/bi00388a001. [DOI] [PubMed] [Google Scholar]
- 100.van Anken E, et al. Efficient IgM assembly and secretion require the plasma cell induced endoplasmic reticulum protein pERp1. Proc. Natl. Acad. Sci. U. S. A. 2009 doi: 10.1073/pnas.0903036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimizu Y, et al. pERp1 is significantly up-regulated during plasma cell differentiation and contributes to the oxidative folding of immunoglobulin. Proc. Natl. Acad. Sci. U. S. A. 2009 doi: 10.1073/pnas.0811591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rupp K, et al. Effects of CaBP2, the rat analog of ERp72, and of CaBP1 on the refolding of denatured reduced proteins. Comparison with protein disulfide isomerase. J. Biol. Chem. 1994;269:2501–2507. [PubMed] [Google Scholar]
- 103.Lindquist JA, et al. ER-60, a chaperone with thiol-dependent reductase activity involved in MHC class I assembly. EMBO J. 1998;17:2186–2195. doi: 10.1093/emboj/17.8.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mkrtchian S, et al. A stress-inducible rat liver endoplasmic reticulum protein, ERp29. Eur. J. Biochem. 1998;251:304–313. doi: 10.1046/j.1432-1327.1998.2510304.x. [DOI] [PubMed] [Google Scholar]
- 105.Rao AK, et al. Biosynthesis of the carbohydrate units of immunoglobulins. 1. Purification and properties of galactosyltransferases from swine mesentary lymph nodes. Biochemistry. 1976;15:5001–5009. doi: 10.1021/bi00668a009. [DOI] [PubMed] [Google Scholar]