Abstract
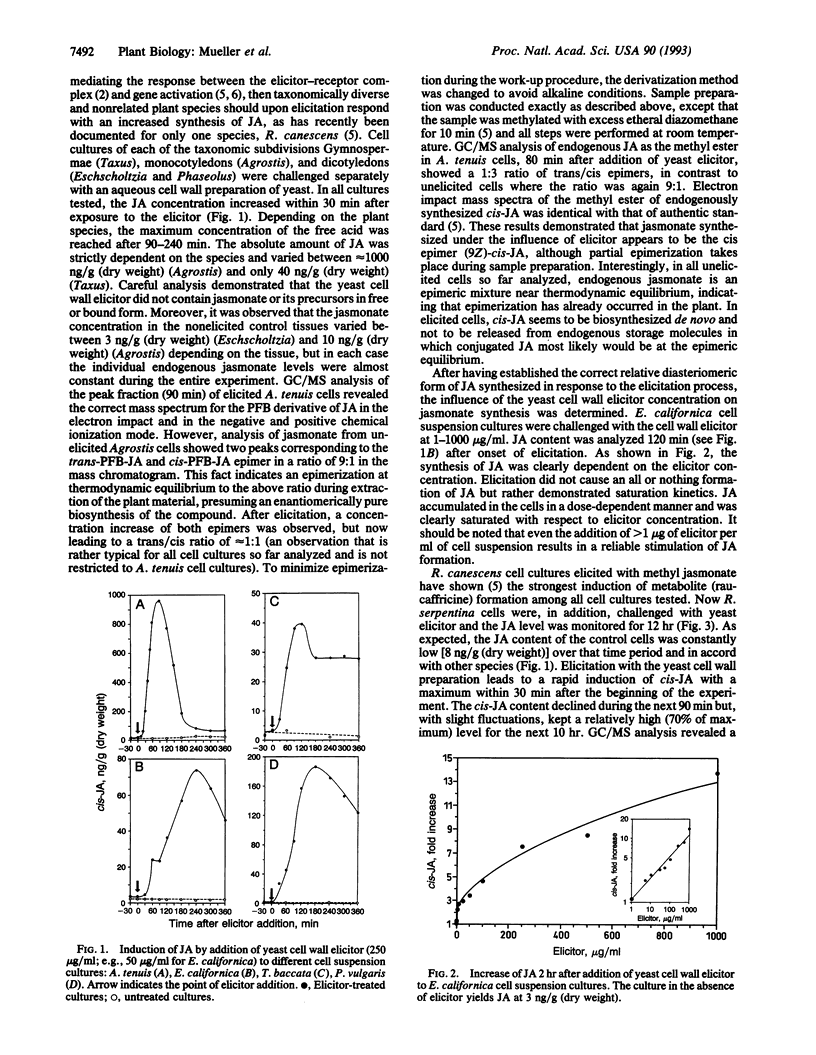
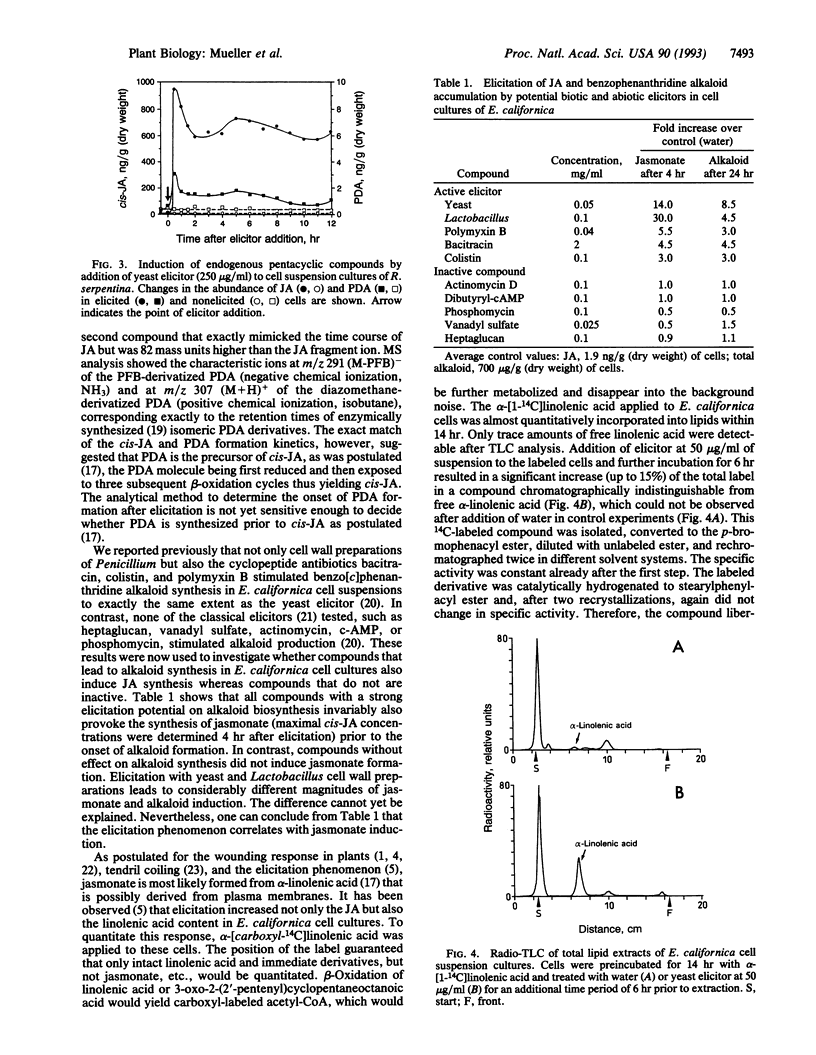
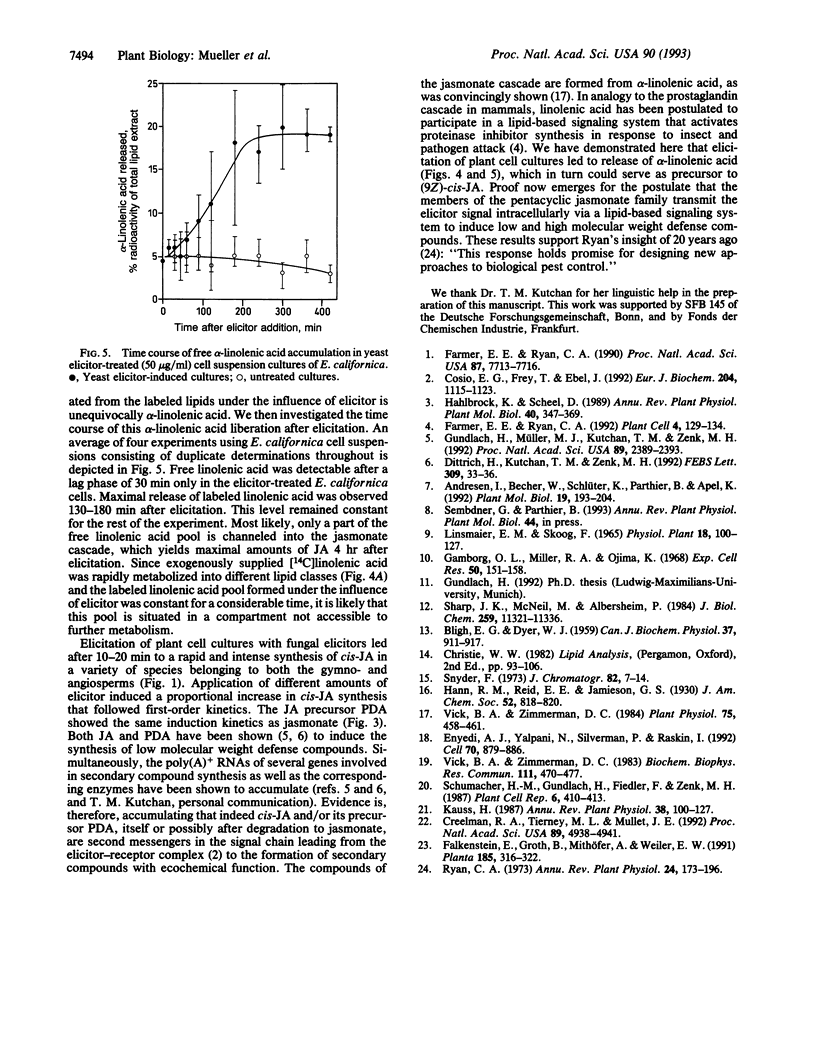
Fungal cell walls and fragments thereof (elicitors) induce the formation of low and high molecular weight defense compounds in plant cell suspension cultures. This induced synthesis requires a signal molecule transmitting the message between the elicitor plant cell wall receptor and gene activation. We demonstrate in this study that cis-jasmonic acid is rapidly synthesized in plant cell cultures of diverse taxonomic origin (gymnosperms and mono- and dicotyledonous plants) after challenge with a fungal elicitor preparation. The rapid decline of cis-jasmonic acid in some of these tissues is attributed to rapid metabolism of this pentacyclic acid. The induction of alkaloids by several different molecules provoking the elicitation process is strictly correlated with the synthesis of jasmonates. Elicitation leads to a rapid release of alpha-linolenic acid from the lipid pool of the plant cell. alpha-Linolenic acid and 12-oxophytodienoic acid, the formation of which is also induced, are known to be distant precursors of jasmonic acid. We assume cis-jasmonic acid and its precursors to be the signaling molecules in the elicitation process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andresen I., Becker W., Schlüter K., Burges J., Parthier B., Apel K. The identification of leaf thionin as one of the main jasmonate-induced proteins of barley (Hordeum vulgare). Plant Mol Biol. 1992 May;19(2):193–204. doi: 10.1007/BF00027341. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cosio E. G., Frey T., Ebel J. Identification of a high-affinity binding protein for a hepta-beta-glucoside phytoalexin elicitor in soybean. Eur J Biochem. 1992 Mar 15;204(3):1115–1123. doi: 10.1111/j.1432-1033.1992.tb16736.x. [DOI] [PubMed] [Google Scholar]
- Creelman R. A., Tierney M. L., Mullet J. E. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich H., Kutchan T. M., Zenk M. H. The jasmonate precursor, 12-oxo-phytodienoic acid, induces phytoalexin synthesis in Petroselinum crispum cell cultures. FEBS Lett. 1992 Aug 31;309(1):33–36. doi: 10.1016/0014-5793(92)80733-w. [DOI] [PubMed] [Google Scholar]
- Enyedi A. J., Yalpani N., Silverman P., Raskin I. Signal molecules in systemic plant resistance to pathogens and pests. Cell. 1992 Sep 18;70(6):879–886. doi: 10.1016/0092-8674(92)90239-9. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Octadecanoid Precursors of Jasmonic Acid Activate the Synthesis of Wound-Inducible Proteinase Inhibitors. Plant Cell. 1992 Feb;4(2):129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Gundlach H., Müller M. J., Kutchan T. M., Zenk M. H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. K., McNeil M., Albersheim P. The primary structures of one elicitor-active and seven elicitor-inactive hexa(beta-D-glucopyranosyl)-D-glucitols isolated from the mycelial walls of Phytophthora megasperma f. sp. glycinea. J Biol Chem. 1984 Sep 25;259(18):11321–11336. [PubMed] [Google Scholar]
- Snyder F. Thin-layer chromatographic behavior of glycerolipid analogs containing ether, ester, hydroxyl, and ketone groupings. J Chromatogr. 1973 Jul 18;82(1):7–14. doi: 10.1016/s0021-9673(01)80070-4. [DOI] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Biosynthesis of jasmonic Acid by several plant species. Plant Physiol. 1984 Jun;75(2):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun. 1983 Mar 16;111(2):470–477. doi: 10.1016/0006-291x(83)90330-3. [DOI] [PubMed] [Google Scholar]



