Abstract
Background
Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism has been speculated to be and extensively investigated as a risk factor for various vascular diseases, including intracerebral hemorrhage (ICH). However, results from published studies regarding the role of C677T polymorphism in ICH risk in Chinese populations were contradictory rather than conclusive.
Material/Methods
In this study, a total of 180 ICH patients and 180 matched controls of Chinese Han ethnicity were enrolled. The MTHFR C677T polymorphism was genotyped by polymerase chain reaction-ligation detection reaction (PCR-LDR). A meta-analysis was conducted by combining our data with previous relevant studies in Chinese populations.
Results
In our case-control study, similar allele frequency (p=0.492) and genotype distribution (p=0.748) of MTHFR C677T polymorphism were detected between ICH patients and controls. Further analysis based on hematoma location did not show a significant association. When combined with previous studies, however, C677T polymorphism was found to be significantly associated with an increased risk for ICH in Chinese populations (recessive model: OR=1.57, 95%CI=1.29–1.91). When focusing on the Han ethnicity, carriers of the TT genotype had an increased risk of ICH (recessive model: OR=1.36, 95%CI=1.05–1.75).
Conclusions
In this case-control study we did not observe that the MTHFR C677T polymorphism was associated with ICH risk in people of Chinese Han ethnicity. However, when combined with previous published studies, a significant association of C677T polymorphism with an increased risk of ICH was detected in Chinese populations, and also in the subgroup analysis focusing on Han ethnicity.
MeSH Keywords: Cerebral Hemorrhage; China; Methylenetetrahydrofolate Reductase (NADPH2); Polymorphism, Single Nucleotide
Background
Spontaneous intracerebral hemorrhage (ICH) is a devastating disease with an overall incidence of 24.6 per 100 000 person-years [1]. It accounts for 10–15% of all strokes, leading to a catastrophic consequence. The mortality of ICH at 1 month was 35–52% and only 20% regained functional independence by 6 months [2]. Hypertension, low cholesterol levels, heavy alcohol intake, advanced age, and male gender have been identified as important risk factors for ICH [3]. However, these conventional risk factors could not explain all cases of ICH. On the other hand, genome-wide association studies and large-scale genetic association studies have indicated an important role of genetic factors in ICH pathogenesis [4–6].
Homocysteine (Hcy) is a metabolite of the essential amino acid methionine. Elevated Hcy has been associated with various vascular diseases, including coronary artery disease, venous thrombosis, and stroke [7,8]. Although not fully understood, endothelial dysfunction related to hyperhomocysteinemia has been suggested as one of the mechanisms [9].
Methylenetetrahydrofolate reductase (MTHFR), encoded by MTFHR gene, is a crucial enzyme regulating intracellular Hcy and folate metabolism. MTHFR catalyzes the transformation of 5,10-methylentetrahydrofolate to 5-methyltetrahydrofolate, which serves as the methyl group donor for converting homocysteine into methionine [10,11]. The most investigated polymorphism in MTHFR gene is C677T, which can lead to the replacement at codon 222 for alanine to valine, causing increased thermolability and reduced activity of enzyme MTHFR and, subsequently, an elevated plasma level of Hcy [12]. MTHFR C677T polymorphism has been speculated to be and extensively investigated as a risk factor for various vascular diseases, including ICH [13,14]. However, results from published genetic-association studies were contradictory rather than conclusive. Some reported a significant impact of C677T polymorphism on ICH risk [15, 16] while some failed to replicate these findings [17,18].
Here, we conducted a case-control study to evaluate the influence of MTHFR C677T polymorphism on ICH risk. Furthermore, since ICH incidence is heterogeneous among different Chinese populations [19,20], this case-control study included subjects of Chinese Han ethnicity only. In addition, a meta-analysis was performed integrating data from the present samples and those available from previous studies focusing on Chinese populations.
Material and Methods
Study subjects
Consecutive adult CT-proved ICH patients of Chinese Han ethnicity admitted to West China Hospital of Sichuan University were enrolled in this study from November 2011 to September 2012. We excluded patients with secondary ICH due to brain tumor, aneurysm, vascular malformation, head trauma, hemorrhagic transformation of cerebral infarction, coagulation disorder, or concurrent use of anticoagulation. Age-, sex-, and ethnicity-matched control subjects without history of ICH were recruited from those undergoing routine health examinations in our hospital. The following demographic and clinical data were collected from medical records of each participant: age, sex, hypertension, diabetes mellitus, hyperlipidemia, alcohol consumption, and smoking. Hematoma location was classified as lobar or deep (basal ganglia, thalamus, brain stem and cerebellum). This study was approved by the Ethics Committee of West China Hospital of Sichuan University. Written informed consents were obtained from all participants or their legal surrogates.
Genotyping
DNA was isolated from peripheral whole blood using the DNA Blood Kit (Bioteke Corp., China). The C677T polymorphism was genotyped by the polymerase chain reaction- ligase detection reaction (PCR-LDR) sequencing method, as described previously [21]. Briefly, the PCR primers used were: 5′-AGGCCAGCCTCTCCTGACTGT-3′ (forward) and 5′-CCATGTCGGTGCATGCCTTCA-3′ (reverse). The PCR protocol was: an initial denaturation step of 5 min at 95°C, 35 cycles of 94°C for 15 s, 55°C for 15 s, and 72°C for 30 s, and a final extension step of 72°C for 10 min.
The following LDR was carried out in a total volume of 10μl, containing 3 μl PCR product, 1 μl 10×Taq DNA ligase buffer, 2 U of Taq DNA ligase (NEB), and 0.1 pmol of each probe. LDR probes were composed of 1 common probe and 2 discriminating probes. The LDR protocol was: 95°C for 2 min, 35 cycles of 94°C for 30 s, and 60°C for 2 min. The fluorescent products of LDR were differentiated using ABI 3730xl (Applied Biosystems, USA). Genotype was confirmed by DNA sequencing of randomly selected PCR products. The results were 100% concordant.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) in the control subjects was tested by a goodness-of-fit chi-squared test. Differences in baseline characteristics, genotype distribution, and allele frequency between ICH patients and controls were evaluated using the t test or χ2 test, as appropriate. Multiple regression analysis was used to assess the independent impact of C677T polymorphism after adjusting for age, sex, hypertension, diabetes, hyperlipidemia, smoking, and alcohol consumption. The alternative genetic models for the C677T polymorphism included alleles (T vs. C) and genotypes for codominant, dominant, and recessive models. Stratified analyses according to the hematoma location (lobar or deep) were also conducted. Statistical analysis was performed with SPSS for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA). Results were considered significant when P<0.05 (2-sided).
Meta-analysis
A meta-analysis was performed combining our data with previous published studies to further investigate the association of MTFHR C677T polymorphism with ICH risk in Chinese populations. We searched PubMed, Embase, and China National Knowledge Infrastructure (CNKI) databases until July 2015 to screen for relevant genetic association articles. The following search terms were used: intracerebral hemorrhage, hemorrhagic stroke, MTHFR, methylenetetrahydrofolate reductase, polymorphism, genotype, variant, and mutation. The search result was supplemented by screening references of retrieved articles and relevant reviews.
The inclusion criteria were: (1) a case-control design; (2) conducted in a Chinese population; (3) provided information on genotype frequencies or sufficient data to calculate them; (4) genotype distribution in control group was in accordance with HWE. For studies with overlapping subjects, only the one with the most complete dataset was included. For each included study, the following data were extracted: authors, year of publication, province, age, sex, sample size, source of control, matching criteria, and genotype distribution.
The association of C677T polymorphism with ICH risk in Chinese populations was evaluated by calculating pooled ORs with 95% CIs. We used Cochran’s Q test and I2 statistic to assess between-study heterogeneity. When substantial heterogeneity was detected (P<0.05 for Q test or an I2>50%), we used a random-effects model to assess the influence of genotype on ICH risk [22]. Otherwise, a fixed-effects model was applied [23]. To test the robustness of the findings, we performed sensitivity analysis by sequentially omitting each study. We assessed the potential publication bias visually by estimating the possible skewness in a funnel plot [24] and statistically with the methods described by Egger [25]. The latter is a weighted linear regression of standard normal deviates against the inverse of the standard error. The intercept of the regression is applied to measure the degree of funnel plot asymmetry [23].
Results
Case-control study
In total, we enrolled 180 patients and 180 healthy controls. Baseline characteristics are summarized in Table 1. Mean age was 57.7±14.1 years for ICH patients and 57.0±9.7 years for controls.
Table 1.
Baseline data in ICH patients and controls.
| Characteristics | ICH patients (n=180) | Controls (n=180) | p |
|---|---|---|---|
| Age, mean (SD), y | 57.7 (14.1) | 57.0 (9.7) | 0.575 |
| Male, n (%) | 126 (70.0) | 120 (66.7) | 0.497 |
| Hypertension, n (%) | 94 (52.2) | 56 (31.1) | <0.001 |
| Diabetes mellitus, n (%) | 16 (8.9) | 8 (4.4) | 0.091 |
| Hyperlipidemia, n (%) | 5 (2.8) | 2 (1.1) | 0.449 |
| Smoking, n (%) | 59 (32.8) | 37 (20.6) | 0.009 |
| Drinking, n (%) | 35 (19.4) | 27 (15.0) | 0.264 |
Genotype distribution in the controls was in accordance with HWE (p=0.903). No significant difference was observed regarding the genotype distribution (p=0.748) or T allele frequency (p=0.492) between the ICH patients and healthy controls (Table 2). When stratified by hematoma location, no significant difference in genotype frequencies between ICH patients and controls was detected in lobar ICH (p=0.193) or in deep ICH (p=0.682). In the multiple logistic regression model adjusted for age, sex, hypertension, diabetes, hyperlipidemia, smoking, and alcohol consumption, no significant association of C677T polymorphism with ICH risk was detected in allelic, codominant, dominant, or recessive models (Table 3).
Table 2.
Association between MTHFR C677T polymorphism and ICH risk.
| Genotype | Control (n=180) | Total ICH (n=180) | Lobar ICH (n=47) | Deep ICH (n=133) | OR (95% CI) Total ICH vs. control | OR (95% CI) Lobar ICH vs. control | OR (95% CI) Deep ICH vs. control |
|---|---|---|---|---|---|---|---|
| CC | 64 (35.6) | 71 (39.4) | 20 (42.6) | 51 (38.3) | 1.00 | 1.00 | 1.00 |
| CT | 86 (47.8) | 81 (45.0) | 24 (51.1) | 57 (42.9) | 0.865 (0.54, 1.39) | 0.89 (0.45, 1.76) | 0.84 (0.50, 1.42) |
| TT | 30 (16.7) | 28 (15.6) | 3 (6.4) | 25 (18.8) | 0.77 (0.41, 1.47) | 0.29 (0.08, 1.10) | 1.05 (0.53, 2.07) |
| C | 214 (59.4) | 223 (61.9) | 64 (68.1) | 159 (59.8) | 1.00 | 1.00 | 1.00 |
| T | 146 (40.6) | 137 (38.1) | 30 (31.9) | 107 (40.2) | 0.87 (0.64, 1.19) | 0.66 (0.41, 1.08) | 0.98 (0.70, 1.38) |
| Dominant | 116 (64.4) | 109 (60.6) | 27 (57.4) | 82 (61.7) | 0.84 (0.54, 1.31) | 0.71 (0.36, 1.37) | 0.89 (0.55, 1.46) |
| Recessive | 150 (83.3) | 152 (84.4) | 44 (93.6) | 108 (81.2) | 0.84 (0.47, 1.51) | 0.31 (0.09, 1.10) | 1.15 (0.62, 2.14) |
Dominant: TT+CT vs. CC; Recessive: TT vs. CT+CC; OR were adjusted for age, sex, hypertension, diabetes, hyperlipidemia, smoking, and alcohol consumption.
Table 3.
Characteristics of studies included in the meta-analysis.
| Author | Year | Province | Han ethnicity | No. (case/control) | Source of control | Match criteria | Case | Control | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C/C | C/T | T/T | C/C | C/T | T/T | |||||||
| Fang [15] | 2004 | Jilin | Yes | 27/96 | PB | NA | 7 | 10 | 10 | 40 | 37 | 19 |
| Fu [35] | 2005 | Shanghai | NA | 26/50 | PB | Age, gender | 10 | 13 | 3 | 22 | 25 | 3 |
| Hu [36] | 2007 | Inner Mongolia | No | 32/115 | PB | Age, gender | 11 | 12 | 9 | 61 | 42 | 12 |
| Xiao [37] | 2006 | Shanghai | NA | 61/100 | PB | NA | 12 | 33 | 16 | 49 | 41 | 10 |
| Zhang [38] | 2004 | Shanghai | NA | 94/100 | PB | Gender | 21 | 59 | 14 | 40 | 49 | 11 |
| Zhang [39] | 2004 | Beijing | Yes | 156/239 | PB | Smoking, drinking | 37 | 68 | 51 | 65 | 123 | 51 |
| Zhang [40] | 2008 | Beijing | Yes | 222/282 | PB | Current smoking, alcohol drinking | 57 | 103 | 62 | 74 | 140 | 68 |
| Zhao [16] | 2001 | Northern | NA | 202/190 | NA | Age, gender, blood pressure | 29 | 85 | 88 | 48 | 87 | 55 |
| Zheng [34] | 2000 | Hunan | NA | 30/122 | PB | Gender | 17 | 10 | 3 | 62 | 45 | 15 |
| This study | 2015 | Sichuan | Yes | 180/180 | PB | Age, gender | 71 | 81 | 28 | 64 | 86 | 30 |
NA – not available; PB – population-based.
Meta-analysis
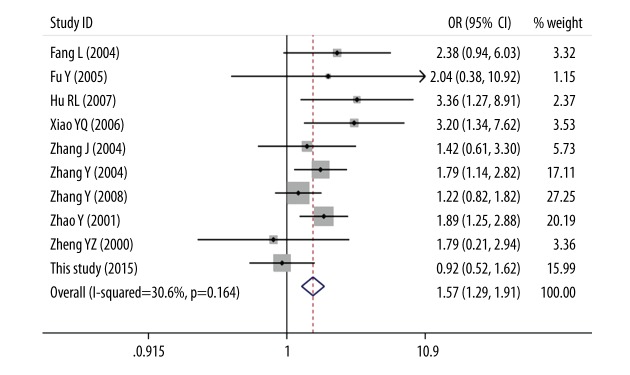
After a comprehensive literature search and records screening, full texts of 16 studies were included for further evaluation. Eight studies were then excluded: five for departure from HWE [26–30], one for insufficient data [31], one [32] for duplication with another study [28], and one for Mongolian population [33]. One more eligible study was detected through screening reference lists of achieved studies and reviews [34]. Finally, nine previous studies together with the current study, forming a total of 1030 ICH patients and 1474 controls, were enrolled in this meta-analysis [15,16,34–40] (Table 3). Overall, a significant association between C677T polymorphism and ICH risk in Chinese populations were observed (dominant model: OR=1.57, 95%CI=1.29–1.91, Table 4, Figure 1). When focusing on the Chinese Han population, a significant association was detected in the recessive model (dominant model: OR=1.36, 95%CI=1.05–1.75). When combining studies with population-based controls, C677T polymorphism was found to be significantly associated with an increased ICH risk (OR=1.39, 95%CI=1.09–1.77). When stratified by match status, we also detected a significant role of C677T polymorphism in ICH susceptibility (OR=1.29, 95%CI=1.05–1.59). In sensitivity analysis, we repeated the meta-analysis by omitting each study sequentially and found that the results did not change substantially under the genetic models except for the heterozygous codominant model. The funnel plots (Figure 2) and Egger’s test (data not shown) suggested that there was no publication bias in the present meta-analysis.
Table 4.
Meta-analysis of the association between MTHFR C677T polymorphism with ICH risk in Chinese population.
| Variables | N | Case/control | T vs. C | TT vs. CC | CT vs. CC | TT+CT vs. CC | TT vs. CT+CC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | |||
| Total | 10 | 1030/1474 | 1.42 (1.14, 1.77) | 0.001 | 1.97 (1.31, 2.95) | 0.009 | 1.23 (1.01, 1.49) | 0.054 | 1.49 (1.09, 2.05) | 0.005 | 1.57 (1.29, 1.91) | 0.164 |
| Han ethnicity | 4 | 585/797 | 1.18 (0.92, 1.51) | 0.080 | 1.31 (0.97, 1.77) | 0.147 | 0.95 (0.74, 1.23) | 0.791 | 1.06 (0.83, 1.34) | 0.365 | 1.36 (1.05, 1.75) | 0.177 |
| Population-based | 9 | 828/1284 | 1.39 (1.09, 1.77) | 0.002 | 1.89 (1.20, 2.96) | 0.012 | 1.26 (0.92, 1.73) | 0.049 | 1.44 (1.02, 2.02) | 0.008 | 1.49 (1.19, 1.86) | 0.148 |
| Match | 8 | 942/1278 | 1.29 (1.05, 1.59) | 0.018 | 1.57 (1.23, 2.01) | 0.052 | 1.13 (0.92, 1.39) | 0.203 | 1.31 (0.98, 1.74) | 0.049 | 1.48 (1.21, 1.82) | 0.227 |
N – number of datasets in the meta-analysis; Phet – P value of Q-test for heterogeneity test.
Figure 1.
Forest plot for association of MTHFR C677T polymorphism with ICH risk (TT vs. CT+CC).
Figure 2.
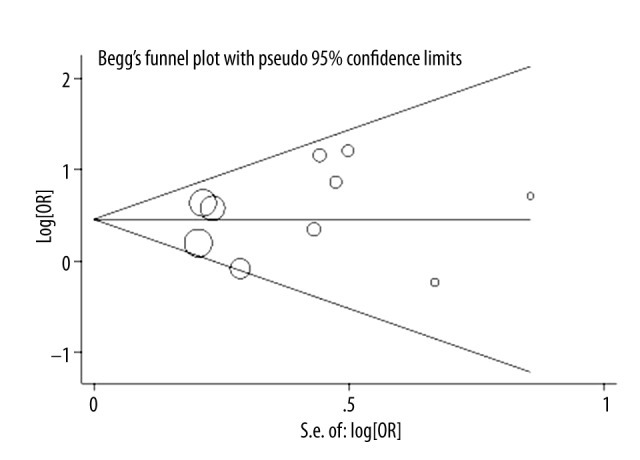
Funnel plot for publication bias test (TT vs. CT+CC).
Discussion
Despite advances in neurocritical care in recent decades, ICH remains a devastating disease causing substantial death and chronic disability. This status highlights the critical role of primary and secondary preventive strategies, which is now most promising for reducing the burden imposed by ICH. However, the current limited understanding of ICH pathogenesis remains a formidable barrier to the development of these strategies [41]. Data on familial aggregation suggests the role of genetics in ICH pathogenesis [42].
In our case-control study, we detected a similar genotype distribution regarding MTHFR C677T polymorphism between ICH patients and healthy controls in the Chinese Han population. However, when combined with previously published data, we found a significant association between this polymorphism and an increased ICH risk in Chinese populations. The association remained significant when confined to Han ethnicity, studies using matched controls, and studies including population-based controls. The impact of MTHFR C677T polymorphism on ICH risk has also been evaluated in other populations, with conflicting results. Hultdin et al., investigating Swedish cohorts, reported that MTHFR TT carriers had a 3.62-fold higher risk of hemorrhagic stroke when compared with CC carriers after adjusting for other confounding factors [43]. However, Dikmen et al. did not detect any significant role of C677T polymorphism in hemorrhagic stroke risk in a Turkish population [44]. Ethnic, environmental, and socioeconomic factors might partially contribute to this discrepancy.
In vivo and in vitro studies suggested that subjects homozygous for the T allele have significantly elevated Hcy levels [12,45]. It is generally accepted that elevated Hcy status can induce endothelial dysfunction. Interestingly, some studies, although still controversial, observed significant associations of MTHFR C677T polymorphism with increased risk of ischemic stroke [46,47]. Moreover, elevated tHcy has also been associated with development of ischemic stroke [48,49]. The explanation underlying this phenomenon was suggested to be that elevated Hcy can cause either ischemic stroke through its coagulative effect or ICH through promoting plaque rupture [28].
One plausible application of genetic information on ICH susceptibility is its potential to improve risk assessment and thus to provide immediate clinical impact, such as decision-making regarding coagulation. However, the further application of MTHFR polymorphism, at least for coagulation adjustment, seems to be hampered by its dual effect on both ischemic stroke and ICH. Notably, ICH is a polygenic disease, with many genes thought to be involved, while a specific gene might make only a small contribution to ICH risk. We could not exclude the possibility that the ability to predict ICH would overwhelm ischemic stroke when incorporating C677T polymorphism into other specific genetic or environmental risk factors. Therefore, MTHFR C677T polymorphism still has the potential to contribute to the construction of future risk assessment algorithms.
Several limitations should be addressed when interpreting our results. Firstly, some studies, including one multicenter case-control study, were excluded due to deviation from HWE. Secondly, although it was mentioned in all studies that participants were Chinese, it was not clearly if they were of Han ethnicity. Thus, only four studies were combined in the subgroup analysis focusing on the Chinese Han population. Thirdly, adjusted pooled ORs could not be calculated due to the limited information available in the included studies. Fourthly, although it is suggested that cerebral amyloid angiopathy might contribute to lobar ICH, while deep ICH is related to hypertensive vasculopathy, it remains unknown whether genes play different roles in lobar vs. deep ICH. However, the included studies did not perform further analysis based on hematoma location. Therefore, we could not calculate pooled ORs estimating the potential role of MTHFR C677T polymorphism in lobar ICH and deep ICH, respectively. Fifthly, the sample size in our case-control study was relatively small. However, this study has some strengths. Firstly, this is the first meta-analysis evaluating the role of MTHFR C677T polymorphism in ICH risk in Chinese populations. Secondly, we performed detailed subgroup analysis, including focusing on Chinese Han ethnicity.
Conclusions
In the present case-control study, we did not observe that MTHFR C677T polymorphism is associated with ICH risk in the Chinese Han population. However, when combined with previously published studies, a significant association of C677T polymorphism with an increased risk of ICH was detected in Chinese populations, and also in the subgroup analysis focusing on Han ethnicity.
Footnotes
Competing interests
The authors declared no competing interests.
Source of support: The study was supported by the National Key Technology R&D Program for the 12th Five-year Plan of P.R. China (No. 2011BAI08B05) and the Science and Technology Support Program of the Department of Science and Technology of Sichuan Province (2014SZ0043)
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.European Stroke Initiative Writing C, Writing Committee for the EEC. Steiner T, et al. Recommendations for the management of intracranial haemorrhage – part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis. 2006;22:294–316. doi: 10.1159/000094831. [DOI] [PubMed] [Google Scholar]
- 3.Rincon F, Mayer SA. Clinical review: Critical care management of spontaneous intracerebral hemorrhage. Crit Care. 2008;12:237. doi: 10.1186/cc7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson CD, Biffi A, Nalls MA, et al. Common variants within oxidative phosphorylation genes influence risk of ischemic stroke and intracerebral hemorrhage. Stroke. 2013;44:612–19. doi: 10.1161/STROKEAHA.112.672089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo D, Falcone GJ, Devan WJ, et al. Meta-analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. Am J Hum Genet. 2014;94:511–21. doi: 10.1016/j.ajhg.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68:934–43. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan U, Crossley C, Kalra L, et al. Homocysteine and its relationship to stroke subtypes in a UK black population: The south London ethnicity and stroke study. Stroke. 2008;39:2943–49. doi: 10.1161/STROKEAHA.107.513416. [DOI] [PubMed] [Google Scholar]
- 9.Castro R, Rivera I, Blom HJ, et al. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: An overview. J Inherit Metab Dis. 2006;29:3–20. doi: 10.1007/s10545-006-0106-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim YI. 5,10-Methylenetetrahydrofolate reductase polymorphisms and pharmacogenetics: A new role of single nucleotide polymorphisms in the folate metabolic pathway in human health and disease. Nutr Rev. 2005;63:398–407. doi: 10.1111/j.1753-4887.2005.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 11.Joachim E, Goldenberg NA, Bernard TJ, et al. The methylenetetrahydrofolate reductase polymorphism (MTHFR c.677C>T) and elevated plasma homocysteine levels in a U.S. pediatric population with incident thromboembolism. Thromb Res. 2013;132:170–74. doi: 10.1016/j.thromres.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–13. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 13.Klerk M, Verhoef P, Clarke R, et al. MTHFR 677C-->T polymorphism and risk of coronary heart disease: A meta-analysis. JAMA. 2002;288:2023–31. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 14.Sazci A, Ergul E, Tuncer N, et al. Methylenetetrahydrofolate reductase gene polymorphisms are associated with ischemic and hemorrhagic stroke: Dual effect of MTHFR polymorphisms C677T and A1298C. Brain Res Bull. 2006;71:45–50. doi: 10.1016/j.brainresbull.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Fang L, Wu YQ, Wang TW. Correlation of polymorphism of gene methylenetetrahydrofolate reductase and cystathionine beta-synthase with heredity of cerebral infarction and cerebral hemorrhage in northern Chinese Han people. Zhong Guo Lin Chuang Kang Fu. 2004;8:4654–56. [Google Scholar]
- 16.Zhao Y, Ma LY, Wang XY, et al. Relationship between MTHFR gene C677T polymorphism and hemorrhagic stroke. Zhong Guo Ji Jiu Yi Xue. 2001;21:21–22. [Google Scholar]
- 17.Li Z, Sun L, Zhang H, et al. Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke: A multicenter case-control study in China. Stroke. 2003;34:2085–90. doi: 10.1161/01.STR.0000086753.00555.0D. [DOI] [PubMed] [Google Scholar]
- 18.Nakata Y, Katsuya T, Takami S, et al. Methylenetetrahydrofolate reductase gene polymorphism: Relation to blood pressure and cerebrovascular disease. Am J Hypertens. 1998;11:1019–23. doi: 10.1016/s0895-7061(98)00046-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Yao Z, D’Souza W, et al. An epidemiological survey of stroke in Lhasa, Tibet, China. Stroke. 2010;41:2739–43. doi: 10.1161/STROKEAHA.110.586669. [DOI] [PubMed] [Google Scholar]
- 20.Zhu LC, Zhao D, Xu SZ, et al. Clinical investigation of 3550 patients with hypertensive cerebral hemorrhage of three nationalities in Xinjiang. Zhong Hua Shen Jing Wai Ke Za Zhi. 2015;31:912–17. [Google Scholar]
- 21.Hu X, Li Y, Li H, et al. Is Alpha-1 antichymotrypsin gene polymorphism a risk factor for primary intracerebral hemorrhage? A case-control study and meta-analysis. Med Sci Monit. 2015;21:2149–55. doi: 10.12659/MSM.894365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai YM, Lin L, Zhou WZ, Lu Q. Analysis between plasma homocysteine level and the gene polymorphism of MTHFR in cerebrovascular disease patients. Shen Jing Bing Xue Yu Shen Jing Kang Fu Xue Za Zhi. 2005;2:196–99. [Google Scholar]
- 27.LI RR, Pang X. Correlation betwen MTHFR gene polymorphism and cerebral hemorhage in esential hypertensive patients. Zhong Hua Lao Nian Xin Nao Xue Guan Bing Za Zhi. 2013;15:941–44. [Google Scholar]
- 28.Li Z, Sun L, Zhang H, et al. Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke: A multicenter case-control study in China. Stroke. 2003;34:2085–90. doi: 10.1161/01.STR.0000086753.00555.0D. [DOI] [PubMed] [Google Scholar]
- 29.Ou W, Liu X, Shen Y, et al. Association of CVD candidate gene polymorphisms with ischemic stroke and cerebral hemorrhage in Chinese individuals. PLoS One. 2014;9:e105516. doi: 10.1371/journal.pone.0105516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye H, Yan JT, Shao JM, et al. [A case-control study on the relationship between stroke and plasma homocysteine level and the mutation of MTHFR gene]. Zhong Hua Liu Xing Bing Xue Za Zhi. 2004;25:44–47. [in Chinese] [PubMed] [Google Scholar]
- 31.Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–35. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 32.Shen CD, Zhang WL, Sun K, et al. Interaction of genetic risk factors confers higher risk for thrombotic stroke in male Chinese: A multicenter case-control study. Ann Hum Genet. 2007;71:620–29. doi: 10.1111/j.1469-1809.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- 33.Fang X, Namba H, Akamine S, Sugiyama K. Methylenetetrahydrofolate reductase gene polymorphisms in patients with cerebral hemorrhage. Neurol Res. 2005;27:73–76. doi: 10.1179/016164105X18313. [DOI] [PubMed] [Google Scholar]
- 34.Zheng YZ, Tong J, Do XP, et al. Prevalence of methylenetetrahydrofolate reductase C677T and its association with arterial and venous thrombosis in the Chinese population. Br J Haematol. 2000;109:870–74. doi: 10.1046/j.1365-2141.2000.02112.x. [DOI] [PubMed] [Google Scholar]
- 35.Fu Y, Liu JR, Ni PH, et al. The relationship of plasma homocysteine levels and polymorphism in homocysteine metabolism related enzymes with brain stroke. Zhong Guo Lao Nian Yi Xue Za Zhi. 2005;24:413–17. [Google Scholar]
- 36.Hu RL, Zhao SG, Niu GM, et al. The association between gene polymorphism of N5, 10-methylenetetrahydrofolate reductase (MTHFR) and mongol nation patients with primarily hypertension disease and hypertension complicating cerebrovascular disease. Cu Zhong Yu Shen Jing Ji Bing. 2007;14:13–15. [Google Scholar]
- 37.Xiao YQ, LJL, Lu Q, et al. [The relationship between the gene polymorphism of methylenetetrahydrofolate reductase and plasma homocysteine level with cerebrovascular disease]. Jian Yan Yi Xue. 2006;21:201–4. [in Chinese] [Google Scholar]
- 38.Zhang J, Lu L, Shi H, et al. The relationship between MTHFR gene polymorphism and cerebral hemorrhage. Lin Chuang Shen Jing Bing Xue Za Zhi. 2004;17:267–69. [Google Scholar]
- 39.Zhang Y, Xie R, Chen DF, Fu Y. Association of methylenetetrahydrofolate reductase polymorphism with cerebral hemorrhage, a case-control study. Beijing Yi Xue. 2004;26:219–21. [Google Scholar]
- 40.Zhang Y, Xie RP, Shen Y, Fan DS. Interaction between methylenetetrahydrofolate reductase C677T gene polymorphism and sleep duration on risk of stroke pathogenesis. Beijing Da Xue Xue Bao. 2008;40:262–69. [PubMed] [Google Scholar]
- 41.Rost NS, Greenberg SM, Rosand J. The genetic architecture of intracerebral hemorrhage. Stroke. 2008;39:2166–73. doi: 10.1161/STROKEAHA.107.501650. [DOI] [PubMed] [Google Scholar]
- 42.Alberts MJ, McCarron MO, Hoffmann KL, Graffagnino C. Familial clustering of intracerebral hemorrhage: A prospective study in North Carolina. Neuroepidemiology. 2002;21:18–21. doi: 10.1159/000048609. [DOI] [PubMed] [Google Scholar]
- 43.Hultdin J, Van Guelpen B, Winkvist A, et al. Prospective study of first stroke in relation to plasma homocysteine and MTHFR 677C>T and 1298A>C genotypes and haplotypes – evidence for an association with hemorrhagic stroke. Clin Chem Lab Med. 2011;49:1555–62. doi: 10.1515/CCLM.2011.234. [DOI] [PubMed] [Google Scholar]
- 44.Dikmen M, Ozbabalik D, Gunes HV, et al. Acute stroke in relation to homocysteine and methylenetetrahydrofolate reductase gene polymorphisms. Acta Neurol Scand. 2006;113:307–14. doi: 10.1111/j.1600-0404.2005.00556.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhou BS, Bu GY, Li M, et al. Tagging SNPs in the MTHFR gene and risk of ischemic stroke in a Chinese population. Int J Mol Sci. 2014;15:8931–40. doi: 10.3390/ijms15058931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moe KT, Woon FP, De Silva DA, et al. Association of acute ischemic stroke with the MTHFR C677T polymorphism but not with NOS3 gene polymorphisms in a Singapore population. Eur J Neurol. 2008;15:1309–14. doi: 10.1111/j.1468-1331.2008.02308.x. [DOI] [PubMed] [Google Scholar]
- 47.Isordia-Salas I, Barinagarrementeria-Aldatz F, Leanos-Miranda A, et al. The C677T polymorphism of the methylenetetrahydrofolate reductase gene is associated with idiopathic ischemic stroke in the young Mexican-Mestizo population. Cerebrovasc Dis. 2010;29:454–59. doi: 10.1159/000289349. [DOI] [PubMed] [Google Scholar]
- 48.Han L, Wu Q, Wang C, et al. Homocysteine, ischemic stroke, and coronary heart disease in hypertensive patients: A population-based, prospective cohort study. Stroke. 2015;46:1777–86. doi: 10.1161/STROKEAHA.115.009111. [DOI] [PubMed] [Google Scholar]
- 49.Sacco RL, Anand K, Lee HS, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: The Northern Manhattan study. Stroke. 2004;35:2263–69. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]