Abstract
This review evaluates the current surgical options for the management of idiopathic macular holes (IMHs), including vitrectomy, ocriplasmin (OCP), and expansile gas use, and discusses key background information to inform the choice of treatment. An evidence-based approach to selecting the best treatment option for the individual patient based on IMH characteristics and patient-specific factors is suggested. For holes without vitreomacular attachment (VMA), vitrectomy is the only option with three key surgical variables: whether to peel the inner limiting membrane (ILM), the type of tamponade agent to be used, and the requirement for postoperative face-down posturing. There is a general consensus that ILM peeling improves primary anatomical hole closure rate; however, in small holes (<250 µm), it is uncertain whether peeling is always required. It has been increasingly recognized that long-acting gas and face-down positioning are not always necessary in patients with small- and medium-sized holes, but large (>400 µm) and chronic holes (>1-year history) are usually treated with long-acting gas and posturing. Several studies on posturing and gas choice were carried out in combination with ILM peeling, which may also influence the gas and posturing requirement. Combined phacovitrectomy appears to offer more rapid visual recovery without affecting the long-term outcomes of vitrectomy for IMH. OCP is licensed for use in patients with small- or medium-sized holes and VMA. A greater success rate in using OCP has been reported in smaller holes, but further predictive factors for its success are needed to refine its use. It is important to counsel patients realistically regarding the rates of success with intravitreal OCP and its potential complications. Expansile gas can be considered as a further option in small holes with VMA; however, larger studies are required to provide guidance on its use.
Keywords: ocriplasmin, vitrectomy, inner limiting membrane peel, posturing, tamponade agent, expansile gas
Introduction
The prevalence of idiopathic macular holes (IMHs) in the general population is estimated to be ~3.3 per 1,000 people.1,2 It usually affects individuals in their sixth or seventh decade of life, and approximately two-thirds are females.1,3–5 Until 1991, it was considered an untreatable condition, but now interventions are routinely carried out to close the hole and thereby improve the central visual defect. Initially, vitrectomy surgery with long-acting gas and postoperative face-down positioning for at least 1 week was the only option; however, nowadays, the surgeon and the patient have a number of possible treatment choices to select from. This review discusses these options and attempts to provide an evidence-based guide to aid decision making. First, we discuss some common features of IMHs, including their classification, progression, and fellow eye risk relevant to decision making. We then review the surgical options during vitrectomy surgery, followed by the new option of ocriplasmin (OCP) treatment and the more recent interest in intravitreal expansile gas injection. Finally, we suggest a pragmatic approach to choosing the optimum treatment in an individual case. The review confines itself to IMH and primary surgery.
Etiology and classification
There is a general consensus that vitreous traction plays an important role in macular hole formation.6 Johnson and Gass published a seminal article on its pathogenesis in 1988, in which they described the findings from careful slit lamp biomicroscopic examination of 158 eyes.7 Although now superseded with a greater insight into the pathogenesis provided by spectral domain optical coherence tomography (SD-OCT), it is still important to be aware of their findings. They described the earliest sign, during the evolution of IMH, as the presence of a yellowish foveal spot associated with flattening of the normal anatomic foveal depression (Stage 1A), which progressed into a yellow halo with a thinned reddish center (Stage 1B). This then enlarged into an early IMH (Stage 2), which could be either central or pericentric. If a vitreofoveal separation developed, this was termed Stage 3 and was detected by the presence of a suspended operculum anterior to the retinal plane. If the vitreous subsequently completely separated from the optic disc with the presence of a Weiss ring, they termed this as Stage 4. A size threshold of 400 µm was subsequently applied to distinguish stage 3 from Stage 4 holes.8 The original Gass classification is still widely quoted, but SD-OCT-based classification systems provide a more consistent nomenclature for diagnosis, monitoring, and surgical decision making in vitreomacular interface diseases (Table 1 and Figures 1 and 2). Perifoveal vitreous separation with persistent attachment and localized traction on the foveolar identified on SD-OCT is now known to be the major etiological factor in most IMH cases.9 The International Vitreomacular Traction Study (IVTS) Group developed an OCT-based anatomic classification of vitreomacular interface diseases in 2013.10 IMHs were subdivided by cause into primary (formerly referred to as idiopathic) or secondary and by the presence or absence of vitreous attachment. They were also classified into small (≤250 µm), medium (>250 µm and ≤400 µm), and large (>400 µm) based on the horizontally measured linear width at the narrowest point of the hole. More recently, a detailed classification of focal vitreomacular attachment (VMA) and traction (which includes Stage 1 macular holes) has been described with the acronym WISPERR (Width of vitreoretinal [VR] attachment, vitreoretinal Interface changes, Shape, Pigment epithelial changes, Elevation, and inner and outer Retinal changes).11
Table 1.
Comparison of the Gass and IVTS group classifications
| Gass stages in common use | Gass or Gass-derived classification | IVTS classification equivalent |
|---|---|---|
| 0 | VMA in the fellow eye of a patient with a known/previous IMH without any change in foveal architecture | VMA |
| 1 | Impending macular hole with outer retinal elevation from RPE at foveal center | VMT without IMH: can occur with outer or inner retinal changes or both |
| 2 | ≤400 µm IMH with VMA | Small- or medium-sized IMH with VMT |
| 3 | >400 µm IMH without VMA | Large IMH without VMT |
| 4 | IMH with complete vitreous separation including from optic disk | Not classified separately could be either small, medium, or large IMH without VMT |
Abbreviations: IVTS, International Vitreomacular Traction Study; VMA, vitreomacular attachment; IMH, idiopathic macular hole; VMT, vitreomacular traction; RPE, retinal pigment epithelium.
Figure 1.
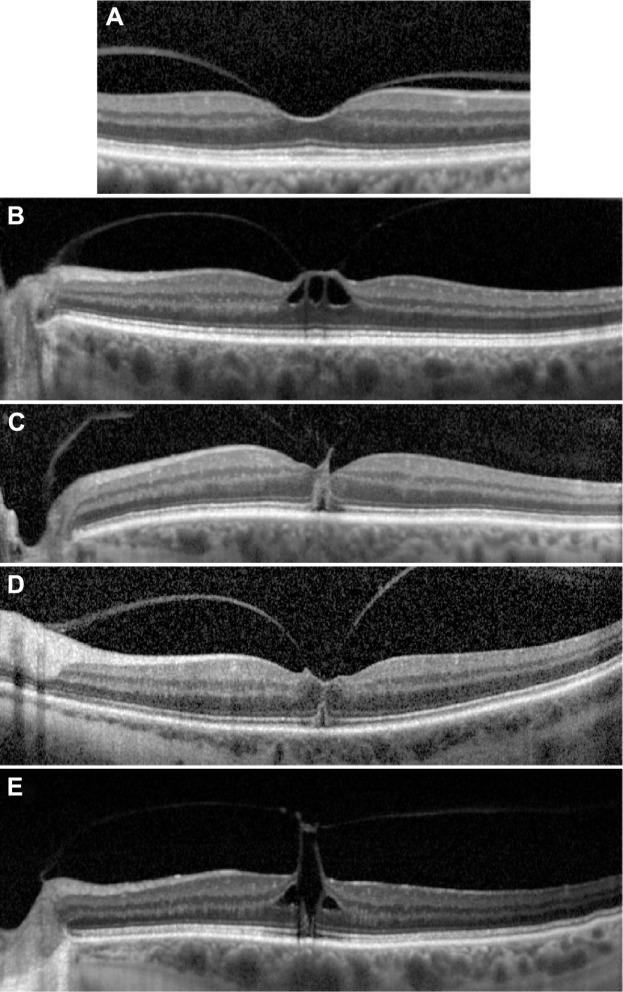
SD-OCT images of Stage 1 holes.
Notes: (A) Focal VMA without traction (Grade 0). (B) VMT with inner retinal changes, (C) VMT with outer retinal changes, (D) VMT with outer retinal changes, and (E) VMT with inner and outer retinal changes.
Abbreviations: SD-OCT, spectral-domain optical coherence tomography; VMA, vitreomacular traction; VMT, vitreomacular traction.
Figure 2.
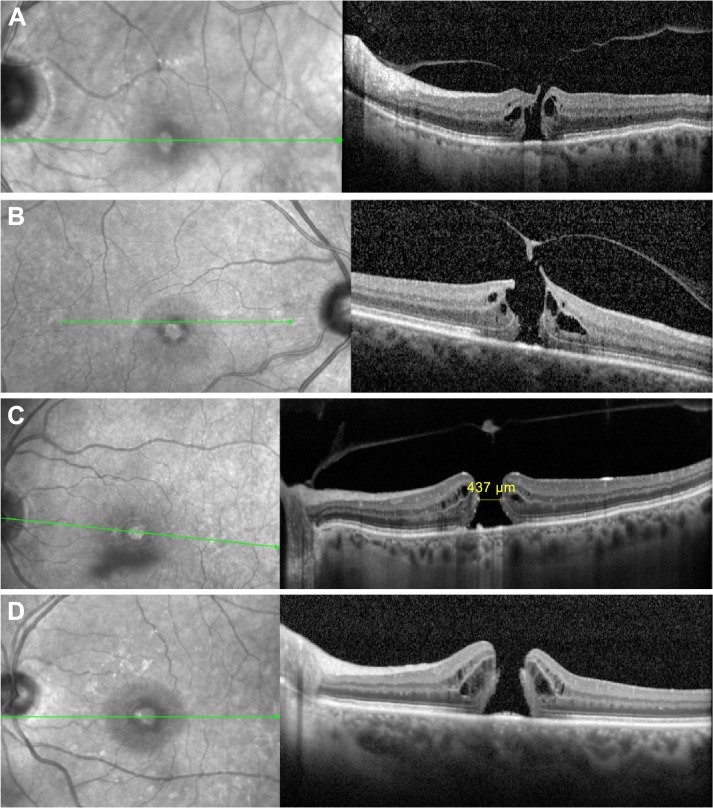
SD-OCT images of macular holes at different stages.
Notes: Left, fundus photography; right corresponding SD-OCT section, green line corresponds with level of OCT image, (A) Small IMH with VMA. (B) Small IMH with operculum VMA. (C) Large IMH with operculum and without VMT. (D) Large IMH with complete vitreous separation (Gass Stage 4).
Abbreviations: SD-OCT, spectral-domain optical coherence tomography; IMH, idiopathic macular hole; VMA, vitreomacular attachment; VMT, vitreomacular traction.
Gass Stage 4 is not separately covered by the IVTS classification. Stage 4 holes can be of any size and are defined by the presence of VR separation from the optic disk and fovea. Their significance relates to their likely chronicity and the extent of epiretinal vitreous and cellular remnants on the internal limiting surface (inner limiting membrane [ILM]) surface around the hole: Schumann et al found that extensive fibrocellular proliferation at the vitreal side of the ILM was seen more frequently in Stage 4 than in Stage 3 holes.12 They proposed that in eyes with spontaneous vitreous separation, remnants of the vitreous often remain attached to the ILM. Similarly, Steel et al found an incomplete staining pattern with Brilliant Blue G (BBG) dye, signifying residual vitreous material on the ILM confirmed histologically in 89% of patients with Stage 4 holes compared to only 24% of Stage 2 holes with VMA.13 Therefore, Gass Stage 4 holes generally require ILM peeling to achieve closure, regardless of the hole width.
Presentation grade
The case mix of patients with IMH presenting to a surgeon will vary by a range of factors, including the health care system and referral pathways. In a study in the UK, out of 106 eyes with IMH that underwent surgery by one surgeon, 36 were small, 40 were medium, and 30 were large. As shown in Table 2, 28% were initially <400 µm in diameter with VMA.14 In a similar study in Israel, only 6.7% were of the same stage at presentation.15
Table 2.
Case mix of macular holes presenting to a UK eye unit over a 1-year period14
| Size | VMA (%) | No VMA (%) | Total (%) |
|---|---|---|---|
| Small | 17 (16) | 19 (18) | 36 (34) |
| Medium | 13 (12) | 27 (25.5) | 40 (38) |
| Large | 3 (3) | 27 (25.5) | 30 (28) |
| Total | 33 (31) | 73 (69) | 106 (100) |
Note: Reproduced with permission from Karger © 2015, S. Karger AG. Madi HA, Dinah C, Rees J, et al. The case mix of patients presenting with full-thickness macular holes and progression before surgery: implications for optimum management. Ophthalmologica. 2015;233(3–4):216–221.14
Abbreviation: VMA, vitreomacular attachment.
Measurements
SD-OCT allows precise measurement of IMH dimensions allowing accurate staging and assisting in surgical decision making.16 Dense scanning protocols are needed to capture the true hole dimensions accurately. Currently available devices have a transverse image resolution of between 10 µm and 25 µm. The use of a real-time eye tracking system with precise multiple B-scan averaging reduces speckle noise artifact and results in very high repeatability and reproducibility.17,18
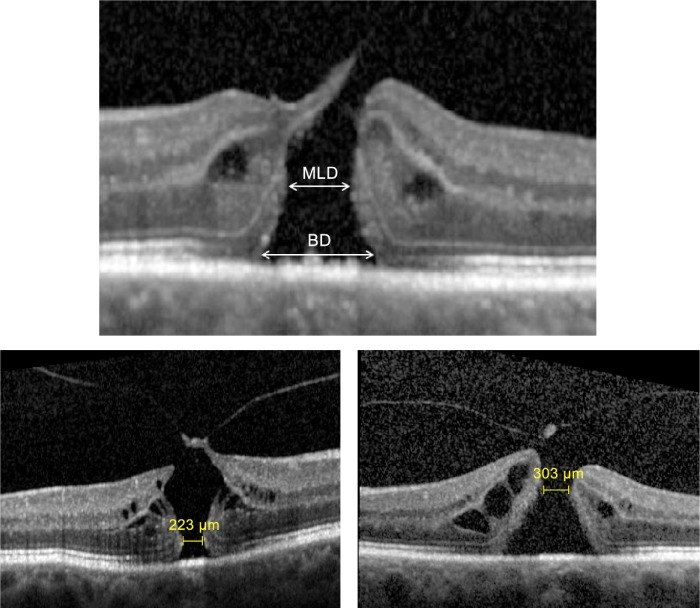
Several methods of IMH measurements using OCT scans have been described, including base diameter (BD) and minimum linear diameter (MLD), and the derived parameters, hole form factor, macular hole index, and tractional hole index.19,20 Studies have shown that there is no real advantage in terms of predicting prognosis by calculating derived indices from the basic ophthalmic measurements.21 The preoperative BD is easy to measure and is strongly associated with anatomical and visual outcomes.21 This finding seems rational, as BD is the linear dimension of IMH at the level of the retinal pigment epithelium layer, which is a reflection of the basic lesion being treated. However, MLD, which is defined as the minimum horizontal diameter in the scan with the widest hole dimensions, is more commonly used. It is important to select an accurate OCT image that represents the true extent of the hole with its maximum dimensions when measuring the MLD without involving the operculated component of a hole with VMA (Figure 3).
Figure 3.
Hole measurements (top), minimum linear diameter (MLD), and base diameter (BD) measurements.
Notes: The MLD is the minimum horizontal linear diameter in an area excluding the operculum and can be in the outer (left) or inner retina (right).
Progression and fellow eyes
The limited natural history data available indicate that in a small percentage of cases, IMHs, especially small ones and those with vitreomacular traction (VMT), may close spontaneously. The reported incidence of such spontaneously closing IMHs in the literature is 2.7% to 8.6%.22–24
Sometimes patients are seen with small (50–100 µm) full-thickness macular holes without VMA, which are termed macular microholes.25 Johnson reported a high rate of spontaneous resolution of these holes within weeks, albeit often with a residual defect in the photoreceptor layer.26 Presumably, they represent small holes with recent VMA separation and are in the process of closing when first seen. However, holes usually progress, and studies have shown a 21.7%–77% increase in hole size over 6 years of follow-up, up to 400–600 µm, although larger holes are also seen.1,24,27,28 In some studies, progression to Stage 3 or 4 holes has been reported to occur as early as 3 months after onset.3,7
The reported rate of development of macular holes in fellow eyes is variable, with an overall risk of 5%–10% over 5 years.24,29,30 A fellow eye with complete VR separation at the fovea appears to have a very low risk (<1%) of progressing to an IMH.31 In fellow eyes with VR attachment, the risk of progression to full-thickness macular hole is broadly related to the extent of findings on the OCT, although figures available from the literature are very variable. Eyes with no VR separation anywhere on the OCT have a lower risk of progression.32 Eyes with focal VMA but no change in foveal profile (termed as Stage 0 if in a fellow eye) have a rate of progression ranging from 3.8% to 42%,31,33 with higher rates of evolution to IMH progressively observed in the following order: those with inner retinal cystic change < outer retinal change with focal foveal dehiscence < both inner and outer retinal changes (22%–55.6%).29,32–35 A randomized controlled trial (RCT) was conducted in the pre-OCT era, in 1994, to study whether intervention with vitrectomy at Stage 1 could prevent the formation of IMH. The trial was terminated early because of recruitment problems, but out of 62 recruited patients, 37% in the vitrectomy group and 40% in the observation arm developed a full-thickness hole.36 Despite the risk of progression, there is also a high rate of resolution with spontaneous VR separation, and hence, unless symptoms are significant and/or prolonged, most surgeons advise observation initially unless IMH occurs.
Recently, there has been a debate as to the importance of tangential VR traction in the progression of macular holes once they form. The tangential separation of the outer retina as the macular hole enlarges is much greater than the tangential separation of the inner retinal layers. A bistable hypothesis of macular hole formation has been proposed to explain this phenomenon based on the “z”-shaped configuration of the Muller cells at the fovea.37
IMH characteristics can change very significantly in as short as a few weeks’ time, and if there is any gap between assessment and surgery, OCT assessment should be repeated immediately prior to the intervention to select the optimum management.14
General prognostic factors for treatment
Numerous studies have identified predictors for successful IMH surgery, among which preoperative visual acuity is the most important. In general, eyes with better preoperative acuity achieve higher rates of anatomical closure and visual gain.38,39 Smaller preoperative MLD and BD measurements are associated with better visual outcomes.20,21,39 Closure rates were found to be higher in patients with a shorter duration of symptoms and these patients similarly achieved better visual outcomes.40
Vitrectomy
IMHs had been considered untreatable until 1991, when Kelly and Wendel reported the first successful closure of a macular hole in 30 out of 52 (58%) patients with IMH using vitrectomy and gas.41 Subsequent to their publication, there was a rapid adoption of this basic technique, and it is now one of the commonest VR surgeries performed and accounted for >10% of all VR interventions performed in the UK from 2002 to 2010.4
Success rates in terms of closure of IMH with vitrectomy are now generally very high in the range of 85%–100%, depending on a number of prognostic predictors as mentioned above.21 In a recent analysis from a multicenter database study of real-world practice in the UK on a total of 1,045 patients, 48.6% achieved visual success at 12 weeks post-operatively (defined as ≥0.3 logarithm of minimal angle resolution [logMAR] units improvement); this proportion increased to 58.3% at 52 weeks, while 8% had deteriorated by >0.30 logMAR units. There is less evidence regarding changes in the quality of life following vitrectomy for IMH. However, in one study involving 45 patients, at 1 year following pars plana vitrectomy (PPV), statistically significant improvements in general vision, peripheral vision, near and distance activities, social functioning, mental health, and dependency were reported.42
Kelly and Wendel based the rationale for their surgery on Gass’s observation of macular hole formation and postulated that vitrectomy would remove traction around the hole and this, combined with gas tamponade, would result in the ring of perifoveal detachment flattening with some improvement in vision. Their technique fortunately resulted in hole apposition and closure in many patients with very significantly improved vision. In the concluding paragraph of their publication, they pointed out that although 58% of the patients had improved vision, 42% had persistently open holes, and encouraged further investigation into refinements that would improve the results and reduce the complications. Indeed during the intervening years, several variations and additions to the initial technique have been introduced with the aim of improving outcomes and safety. These include:
Tamponade type and posturing requirements;
ILM peeling;
combined lens surgery;
surgical adjuncts;
other advances in surgical technology and techniques.
Thus, a surgeon has a number of choices to select the operation he or she would perform on any individual patient with IMH. These choices are dependent to varying degrees on the characteristics of the IMH and will be discussed later.
Gas type and tamponade
After completion of vitrectomy and ILM peeling, fluid–air exchange is carried out with subsequent gas exchange, as originally described.41 Vitrectomy and posterior hyaloid face (PHF) separation act by removing the traction that is hypothesized to be causative. Gas tamponade is believed to additionally aid hole closure by:
Bridging the hole thereby preventing trans-hole fluid flow from the vitreous cavity, allowing the retinal pigment epithelium pump to remove the subretinal fluid, and also reducing trans-retinal uveal–scleral outflow with the resultant reduced retinal edema.
Creating interfacial surface tension forces between the gas bubble and the retina acting to pull the edges of the hole together.43
Acting as a surface to allow glial cell migration to bridge the gap between the retinal edges.44,45
The “buoyant force” of a gas bubble on a macular hole in the face-down position is thought to be less important (at least if all tangential force has been removed): it is the bridging of the gap that is the principal action.
Although Kelly and Wendel used sulfur hexafluoride (SF6) gas as a tamponade agent, when the procedure was subsequently adopted, most surgeons initially chose to use long-acting gas (C3F8) to maintain hole closure for as long as possible in an attempt to improve closure rates. Some surgeons have also used silicone oil for similar reasons. There has also been a dogma to position patients strictly face down for at least a week postoperatively to maximize tamponade apposition to the hole.46 However, there has been a gradual change in practice to increasing use of medium-(C2F6) and short-acting gases (SF6 or air) and a reduction in the duration and strictness of postoperative posturing requirements.20,47–50 Indeed, it has been increasingly recognized that face-down positioning is not always necessary, particularly with smaller holes.21,51
There is a relationship between these two choices – gas choice and posturing requirement – that relates to the amount of time that the gas bridges the hole. Gas can still bridge the defect of a macular hole without face-down positioning in an upright position if the gas fill exceeds 50%, and long-acting tamponades will maintain >50% gas fill for a longer time than air or short-acting gases.
One of the problems with making this decision is that the precise time period that gas needs to bridge a macular hole to result in closure is uncertain. Indeed, the known closure of IMH with spontaneous vitreous separation and the recent demonstration that hole closure can occur with OCP alone show that tamponade is not necessary at all in some holes. Preoperative macular hole size is the biggest single risk factor for surgical failure, and macular hole size appears to be a key factor dictating the type of gas used and the necessity to posture postoperatively.52 Hole chronicity is also important with likely reduced retinal compliance resisting closure. Most authors have reported reduced rates of hole closure with holes >1 year duration.40,53
We briefly review some of the evidence behind choosing one tamponade over another and the importance of posturing and then summarize the decision-making process.
Gas choice
Surgeons use a variety of different isovolumetric gas concentrations at the end of surgery, but the total duration of gas fills is usually in the range of 2–2.5 weeks for SF6, 4–6 weeks for C2F6, and 8–11 weeks for C3F8. The resultant 50% fill times will be approximately half of these. Silicone oil will obviously maintain its size until removed.
SF6 versus C3F8
In 2008, a retrospective cohort study of 79 eyes with postoperative face-down positioning reported a closure rate of 90% with SF6 and 91% with C3F8.49 In a prospective randomized trial published in 2015, 59 eyes were randomized to either SF6 or C3F8 with differing posturing regimes and closure was achieved in 93.3% and 92.9%, respectively. Mean best-corrected visual acuity improved by 17.7 letters in the SF6 and 16.9 letters in the C3F8 group.54
SF6 versus C2F6
In a prospective cohort study of 78 patients undergoing surgery, 39 eyes treated with SF6 were compared with 39 eyes treated with C2F6 both without face-down posturing after surgery. Closure was achieved in 87% and 90%, respectively. At 6 months after the procedure, mean visual acuity improved from 0.78 logMAR to 0.38 logMAR in the SF6 group and from 0.81 logMAR to 0.44 logMAR in the C2F6 group.50
Air versus SF6
In 2008, a prospective study by Eckardt et al reported closure rates of 55% and 76% at 24 hours and 48 hours, respectively, following PPV surgery with room air tamponade and strict face-down positioning. Face-down positioning was stopped as soon as hole closure was confirmed on OCT.55
In 2009, a retrospective study of 156 eyes using either 20% SF6 (91 eyes) or air (65 eyes) tamponade reported the primary hole closure rates of 90% and 92%, respectively. Face-down positioning was required for 7 days in the gas group and 4 days in the air group.56
Silicone oil versus C3F8
Silicone oil, both light and heavy, has also been used both as a tamponade agent and as a primary procedure.57,58
In a retrospective study published in 2003, Lai et al compared the outcomes using silicone oil (31 eyes) versus C3F8 gas (23 eyes) tamponade. Primary hole closure rates were 65% and 91%, respectively (P=0.022). The final median visual acuity values were 20/50 and 20/70, respectively (P=0.047).59
In a further retrospective study in 2005, 46 eyes with IMH received silicone oil tamponade (23 eyes) or C3F8 tamponade (23 eyes). Anatomic closure occurred in 83% and 87% of eyes, respectively. However, visual acuity improvement to 20/70 or better was achieved in 17% in the oil group compared to 73% in the gas group.60
Posturing
Face-down positioning is uncomfortable for patients, occasionally associated with a range of complications, including back pain, sinusitis, and ulnar nerve palsies, and in some instances is unfeasible even with the aid of specially designed supports.55,61 Studies using OCT in the immediate postoperative period suggest that IMH occurs early in the postoperative course in most holes and hence one approach is to monitor daily for hole closure and stop face-down positioning when this occurs.55 Most surgeons are unable to do this; furthermore, hole opening can occur after initial closure, and hence standardized postoperative posturing instructions usually are given.62 However, several recent studies have suggested that face-down positioning is not necessary at all in some cases.
A recent Cochrane review analyzed data from three RCTs that directly compared face-down posturing following IMH surgery with no face-down posturing.63–65 All the studies had quite different protocols other than posturing randomization. Guillaubey et al included 150 eyes and used ILM peeling and one of the three types of intraocular gases dependent on the size of the IMH (SF6, C2F6, or C3F8 gas for holes measuring <500 µm, 500–800 µm, and >800 µm, respectively).63 Tadayoni et al randomized 69 eyes with small- and medium-sized macular holes measuring <400 µm in diameter and used C2F6 gas for all the eyes, but ILM peeling was not undertaken.66 Lange et al randomized 30 eyes and included ILM peeling and used C3F8 gas in all cases.64 The authors of the Cochrane review tentatively concluded that for macular holes ≤400 µm, face-down posturing had no significant effect on successful hole closure. However, two of the studies found that there was a significant benefit of face-down posturing for successful closure when the diameter was >400 µm; this aspect is the subject of further ongoing RCTs.
Comment
It is possible that the results of nonposturing studies with long-acting tamponades may not apply to the use of short-acting tamponade agents – so that if short-acting gases are used with medium-sized holes, posturing may still be beneficial. Similarly, the duration of posturing is uncertain and has been variable in trials. The common recommendation is 8 hours a day for at least 5–7 days.
Other considerations also come into play when choosing the tamponade type. The presence of gas tamponade in the vitreous cavity severely limits visual function. A one-eyed patient may therefore opt for air or SF6 tamponade to allow quicker visual recovery. Similarly, air travel may be a consideration. Patients, if given a choice, may opt to do 5 days of posturing with air or a short-acting gas over nonposturing with a long-acting gas. Some patients may be unable to posture at all for various reasons, for whom long-acting gases would be the best option, especially in large holes.
The elimination of tangential traction is also important to achieve IMH closure. It is possible that holes with small degrees of persistent traction may still close if they are treated with prolonged gas tamponade as opposed to short-term tamponade. In addition, the small effect of the gas buoyant force with face-down posturing may be additive to overcome residual tangential forces. It is interesting that most of the aforementioned studies have used ILM peeling. Without ILM peeling, longer acting gases are probably more likely to result in closure.
Silicone oil seems to offer no advantages over the use of gas and has some possible disadvantages. Face-down posturing for at least 5 days postoperatively should be recommended for patients with macular holes >400 µm in size and holes >1 year in duration, until results of further RCTs are available.
ILM peeling
The removal of the ILM during MH surgery as a means to improve both anatomical and functional success was first described by Eckardt et al in 1997.67 Using ILM peeling, they described an anatomical success rate of 92% with an equally good functional success rate of 77%, and the procedure was quickly adopted.
Rationale
ILM peeling is believed to improve hole closure by:
Increasing retinal compliance – the ILM despite being only a few microns thick contributes very significantly to retinal rigidity and also has a higher rigidity on the retinal side, accounting for its tendency to scroll upward when peeled. Its removal results in increased retinal compliance that aids closure.68
Removing residual adherent vitreous cortex remnants on the ILM surface after PHF separation, which could exert persistent traction and prevent hole closure.
Preventing the associated fibrocellular proliferation that has been shown to occur surrounding the hole and extending out onto the ILM surface in a significant number of cases – the ILM thus acts as a scaffold for cells to grow on.69
Resulting in a retinal glial cell proliferation response that may paradoxically help IMH contraction and repair.44,68
Dyes to facilitate peeling
ILM peeling can be surgically challenging, and to facilitate its execution, various dyes have been introduced to help visualize this colorless thin membrane. Indocyanine green (ICG) was the first dye used in IMH surgery to stain ILM specifically in 2000.70 Subsequently, multiple reports debating the safety of ICG have been published. A wide variety of adverse effects have been described, including inner retinal changes,71 retinal pigment epithelium changes,72–74 and optic disk atrophy with associated detrimental effects on visual acuity,72,74,75 visual field,74,76,77 and electroretinogram changes.71,78 The concentration of ICG used is critical as are the application time and light exposure. ICG has known photosensitizing properties and its resultant decomposition products after illumination are believed to result in inner retinal toxic reactions, and it also results in large amounts of retinal side ILM cellular debris after peeling.76
Other dyes used for the same purpose include BBG, an ILM-specific dye; trypan blue, a vital dye that stains both ERM and ILM; and more recently Acid Violet 17.79 Particulate stains, such as triamcinolone, are also used that aid ILM identification during peeling by their physical presence on the peeled ILM. Both BBG and trypan blue appear to be safer dyes compared to ICG.78,80,81 Dyes heavier than water prepared by mixing with deuterium oxide, polyethylene glycol, or mannitol and avoiding the need for fluid–air exchange have been produced and are now in widespread use.13
Regardless of the dye used, the contact time, ie, the time that the dye is left on the retinal surface before being aspirated off, should be minimized, and contact times of 5–10 seconds can give adequate staining.13 ILM-specific dyes can also be used with this regime to assess the degree of fibrocellular material on the ILM, which could potentially be used to guide the extent or necessity of ILM peeling, especially in small holes.13
Technique and ILM peel size
Various techniques of ILM peeling have been described. Improved forceps design has allowed pinch peeling techniques to be used, thus avoiding the need for the potentially damaging use of picks to raise an ILM edge. Diamond-dusted membrane scrapers (DDMSs) have also been used to initiate and peel the ILM, although use of a DDMS to peel ILM has recently been shown to be associated with a greater degree of appearance of a dissociated optic nerve fiber layer post-operatively than that observed with forceps peeling with a deeper plane of retinal ILM separation.82 The extent of ILM peeled varies between surgeons, with no prescribed amount. A radius of peel of between 1.5- and 3-disk diameters has been described.83 Most surgeons peel with a 1- to 1.5-disk diameter radius, and wider areas are generally peeled in larger holes. Similarly, eyes that have persistent macular holes after surgery with ILM peeling can sometimes be closed if the ILM peel area is increased. D’Souza et al reported the results of 30 patients who had unsuccessful anatomical closure after initial surgery with ILM peeling. They underwent enlargement of the ILM rhexis and gas tamponade with C3F8, and closure was achieved in 14 (47%) out of 30 eyes.84 A technique of using autologous ILM folded into the hole itself to improve closure rates in cases of large primary or persistent macular holes, termed as the inverted ILM flap technique, has been described.85
On the other hand, in the pursuit of minimizing ILM peeling-related complications, Ho et al have described a foveola-sparing ILM peeling technique for Stage 2 IMH.86 Recently, a technique termed as ILM abrasion with a DDMS has been suggested as a suitable alternative to peeling, with high success rates.87
Side effects
Although ILM peeling has increased both anatomical and functional success rates, it can result in a number of sequelae. Various consequences have been described, including a characteristic dimpled appearance to the retina in the ILM-peeled area termed as a dissociated optic nerve fiber layer appearance,88,89 inner retinal defects,90 thinning of the ganglion cell complex,91 and migration of the fovea toward the disk after ILM peeling.92 Some authors have also reported functional deficits attributed to ILM peeling, including reduced retinal sensitivity and an increased incidence of perifoveal microscotomas, although no clear detrimental effect of ILM peeling on visual acuity has been demonstrated.93
Evidence base for ILM peeling to improve closure rate
Several randomized studies have been carried out on the efficacy of ILM peeling in IMH surgery. In one large RCT, closure was achieved in 84% of the patients undergoing ILM peeling compared to 48% who did not undergo ILM peeling (P<0.001) at 1 month postoperatively.83 A recent Cochrane review of four RCTs concluded that the available evidence supported ILM peeling in Stages 2, 3, and 4 IMHs.94 However, it should be noted that some of these studies were without OCT measurements of the hole size, and the evidence base for peeling small IMHs is less robust, where the closure rate without ILM peeling can be high.95
Summary of evidence for tamponade type, posturing, and ILM peeling
There are thus three key variables to vitrectomy surgery for IMH: which gas to use, whether to recommend posturing, and whether to peel the ILM. Some surgeons use the same regime for all patients; others adjust the regime according to IMH characteristics and individual patient features (eg, inability to posture). Each of the three choices are partly dependent on each other as already mentioned (eg, nonposturing surgery with air in medium/large holes without ILM peeling would likely have a low chance of success). Chronic macular holes >1 year in duration should probably be treated with ILM peeling and face-down positioning or at least longer acting gases in all cases. However, general recommendations are outlined in Table 3.
Table 3.
Recommendations for ILM peeling, gas use, and posturing necessity
| Size (MLD)/stage of hole | <250 µm | 250–400 µm | >400 µm | Stage 4 of any size |
|---|---|---|---|---|
| ILM peeling | Uncertain if required in all cases | Required | Required | Required regardless of size |
| Posturing | Not required | Not required (particularly if long-acting gas plus ILM peeling performed) | Probably improves closure rates | If ILM peeled based on size of hole |
| Long-acting gas | Not required | Not required | Probably improves closure rates | If peeled based on size of hole |
Abbreviations: ILM, inner limiting membrane; MLD, minimum linear diameter.
Combined phacovitrectomy versus sequential surgery
Cataracts are very common following vitrectomy, especially in the >60-year-old age group and when long-acting gases are used. Progression of existing cataracts in patients undergoing PPV for IMH has been reported to occur in 34% and 50% at 6 months and 12 months follow-up, respectively.39,96
Combined phacovitrectomy has been widely adopted during macular hole surgery to avoid the need for subsequent cataract surgery.97 However, potential adverse effects of the procedure can be a higher incidence of posterior synechiae formation, posterior capsule opacity, intraocular lens-related complications, and a small (~0.5 diopter) myopic shift in refraction.98,99 There is also the potential for cataract surgery-related complications, for example, corneal edema, which could make the primary aims of the vitrectomy surgery, including ILM peeling, more challenging to achieve. Several surgical tips and techniques can reduce the occurrence of these problems (Table 4).
Table 4.
Surgical tips for performing combined phacovitrectomy macular hole surgery
| Surgical problem | Surgical solution |
|---|---|
| Phacoemulsification wound leak if sclerostomy ports inserted after phacoemulsification has been carried out | Insert sclerostomy ports first prior to lens surgery. Use valved sclerostomies to avoid vitreous cavity volume loss and LIDRS precipitation |
| Corneal edema interfering with vitrectomy | Use a temporal wound to maximize distance of wound from visual axis. Keep IOP moderate and practice efficient in the bag phacoemulsification with minimum power necessary |
| Miosis after phacoemulsification prior to vitrectomy | Prevent hypotony at all times, maintain a deep anterior chamber and avoid any contact with iris. Use a suture to secure the wound if there is any leak at all after phacoemulsification prior to vitrectomy |
| Higher incidence of posterior capsule opacity | Perform a small central primary posterior capsulotomy with the vitrectomy cutter |
| Higher risk of IOL optic capture or displacement | Perform a central 5 mm maximum capsulorhexis to ensure the optic is held back and do not dilate the pupil after surgery for the 1st week. Use a large optic IOL ideally with plate style as opposed to single arm haptics |
| Posterior synechiae formation | Avoid dilatation for 1st week and use subconjunctival and frequent topical steroids postoperatively. Avoid AC shallowing during vitrectomy with corneal suture if needed (see miosis) |
Abbreviations: LIDRS, lens–iris diaphragm retropulsion syndrome; IOP, intraocular pressure; IOL, intraocular lens; AC, anterior chamber.
Conversely, sequential surgery has been associated with a higher rate of posterior capsule rupture and other operative difficulties during the subsequent cataract surgery, associated with the lens–iris diaphragm syndrome after vitrectomy.100,101
Combined phacovitrectomy has been compared with sequential vitrectomy and phacoemulsification, during the 1st year following vitrectomy in 120 eyes with IMH and preexisting cataract.102 Best-corrected visual acuity improved significantly at 6 months after phacovitrectomy, with no significant further improvement after this time. However, in the sequential procedure group, visual acuity improved only at the 1-year follow-up. IMH closure at 1 month after one surgical procedure was 100% in the phacovitrectomy and 96% in the sequential vitrectomy and phacoemulsification group. There is no clear evidence that combined phacovitrectomy affects the long-term results of PPV for IMH, but the visual recovery is quicker.
Adjunctive agents to improve closure rate
The use of a variety of agents at the time of surgery has been described to improve closure rate. Smiddy et al described the use of transforming growth factor beta 2 as a chorioretinal adhesive to assist in the closure of the IMH.103 Autologous serum and platelets have also been used.104,105 Currently, other than a few exceptions, these agents are reserved for patients with chronic or large (>650 µm) or nonclosure IMHs with initial surgery.106
Although outside the scope of this article, a variety of techniques have been described in cases of primary nonclosure, including simple enlargement of the peeled ILM area. The use of heavy silicone oil has also been advocated with a reported 87%–92% closure rate in these cases.57,107 A wide variety of other techniques have been reported, including ILM and capsular flaps, retinal relieving incisions, laser, mobilization of the edges of the hole, and drainage of chronic fluid from the center of the hole in cases of refractory holes.108–110
Other advances in surgical techniques and equipment
The most fundamental part of IMH surgery, namely PHF separation, has changed little since 1991. Aspiration is used to engage the PHF and then traction is exerted with an instrument movement anteriorly to strip the PHF from the retinal and optic disk surface. Surgeons generally start from the peripapillary area. Vitreous staining with particulate stains, including those based on triamcinolone and more recently lutein, has aided the visualization and hence the surgical ease of this phase.
Narrow-gauge vitrectomy surgery
In 1991, at the time of the first vitrectomy surgery for macular holes, 20 G surgery noncannulated sclerostomy systems were universally used. Since then, there have been a variety of advancements in the technology, including higher speed lower traction cutting, narrower gauge instrumentation with cannulated and valved entry systems, and improvements in intraoperative visualization with wide-field viewing and improved illumination. As a result, surgery has become safer and more efficient, thereby reducing perioperative complications such as iatrogenic retinal breaks and postoperative retinal detachment.
Narrow-gauge surgery has in particular been adopted widely, although with debate regarding its relative pros and cons (Table 5). Indeed, 27 G surgery has recently been introduced with an instrument diameter of ~0.35 mm compared to 20 G at 1.1 mm. However, there is no evidence that narrow-gauge surgery is more effective for macular hole surgery than 20 G in terms of visual outcome and closure although results appear comparable.111
Table 5.
Pros and cons of conventional sutured 20 G vitrectomy versus narrow-gauge transconjunctival systems with cannulated sclerostomies
| Pros | Cons | |
|---|---|---|
| 20 G | More rigid instruments Availability of angulated scissors Greater potential illumination Higher achievable flow rates Less prone to clogging with dense material |
Sutures needed to close conjunctiva and sclerostomies with postoperative surface discomfort and irritation Higher rate of sclerostomy related retinal breaks Requirements for plugs to close sclerostomies when instruments removed from eye Reduced globe stability with higher potential for surge |
| Narrow gauge | Less conjunctival disruption and faster surface recovery with less postoperative discomfort Quick entry and exit from eye Less potential for entry site trauma and lower incidence of entry site associated retinal breaks Lower flow rates with narrower field flow effects and less retinal traction Smaller cutter port size with more precise end cutting ability Valved sclerostomies eliminate unwanted sclerostomy leak |
Postoperative hypotony with sclerostomy leak in some cases Change in technique needed to use less rigid instruments Lower achievable flow rates Occasional requirement to open conjunctiva and enlarge sclerostomy to introduce larger instruments Lower infusion flow rates with higher resistance |
In case series and reviews of patients undergoing 20 G conventional PPV, the reported incidence of iatrogenic peripheral retinal breaks has been variable, ranging from 6% to 36%.83,111,112 The reported rates of peripheral retinal break formation appear to be lower with narrow-gauge surgery using cannulated sclerostomy systems with breaks reported in 11%–16% of procedures and only 3%–6% related to sclerostomies.111,113,114 Retinal detachment has been reported in ~2%–6% of patients undergoing PPV for IMH.83,113–115 The rate is possibly lower with narrow-gauge surgery, but there is no evidence from an RCT.116 There is no difference in the rate of cataract formation between narrow-gauge vitrectomy and 20 G.117
Hypotony related to wound leakage is a concern with sutureless PPV, occurring in a reported 3%–16% of eyes.115,118 In the majority of cases, this resolves spontaneously, and its incidence has been reducing as sclerostomy entry systems improve and gauge size reduces.
Endophthalmitis is very rare, occurring in 0.02%–0.05% of cases. Initially, narrow-gauge vitrectomy was believed to be associated with a higher incidence of endophthalmitis than 20 G PPV; however, this has not proven to be true.119,120
Limited vitrectomy and PHF separation
Some authors have suggested limiting PHF separation to the macular area during surgery. Kim et al suggested that partial posterior hyaloidectomy, as they had termed it, could minimize the traction on the vitreous base and therefore reduce the risk of iatrogenic retinal breaks.121 They reported a reduction in retinal break formation from 21% to 5% compared with conventional 23 G surgery, although they did note two postoperative retinal detachments in the modified technique group. Cullinane and Cleary also suggested a similar technique, but primarily to reduce the incidence of symptomatic field defects after surgery.122
Symptomatic peripheral field defects after vitrectomy for IMH have been the subject of a number of publications, although they are an uncommon finding (<1% of procedures).123 These are usually temporal and possibly related to vitreous separation, particularly from the nasal side of the optic disk or alternatively from the consequences of air/fluid exchange and secondary desiccation of the nasal retinal surface.122,124 Various modifications of the technique have been reported including using air humidifiers for air/fluid exchange, although it has not been the subject of many recent series.125 Cullinane and Cleary reported a reduction in the incidence of field defects from 22% to 0% with their technique, although the incidence of postoperative retinal detachment was 10% with the modified limited vitrectomy group technique compared to 3.6% in the conventional technique. Closure rates with both series of limited vitrectomy were similar to other reported cohorts.
Ocriplasmin
OCP has been recently approved for the nonsurgical treatment of symptomatic VMA, including when associated with IMH of <400 µm in minimum linear diameter.126 It is administered by a single 0.1 mL of 125 µg intravitreal injection. It is a recombinant truncated form of human plasmin and has proteolytic activity against fibronectin and laminin, two major components of the VR interface.126–128 This action coupled with the activation of endogenous matrix metalloproteinase-2 is believed to result in its ability to precipitate VR separation.129 By releasing VMA in cases with early IMH formation, it can result in hole closure in some patients.
Two multicenter, double-blinded, placebo-controlled Phase III trials on 652 patients provided pre-marketing clinical outcome data. Patients were randomized to OCP or an OCP vehicle injection without the drug as a control.126 In total, 153 patients with IMH <400 µm with VMA were included in the studies with 106 patients in the OCP group and 47 in the placebo group. The trial excluded patients with high myopia (>−8 diopters) from the study. Since the marketing of the drug, there have also been small series of real-world experience published.
Anatomical outcomes
IMH closure occurred in 43/106 (40.6%) of eyes in the OCP group compared with 5/47 (10.6%) in the placebo group (P<0.001).126 Closure was evident by a month postinjection in all cases. In a subset analysis, 48 patients with small IMH showed a higher closure rate with OCP (58.3% versus 16% with placebo, P<0.001) compared to the 38 patients with medium-sized holes (36.8% versus 5.3% with placebo, P=0.009). None of the 19 patients included in the study against protocol with large holes achieved closure.
Interestingly in the Phase III trials, 19 out of 43 patients with OCP-induced IMH closure had not had VMA release by day 28 or month 6 meaning macular hole closure occurred without VMA release.130 This could be related to the drugs liquefactant effect or local vitreous separation around the VMA point.131
The proportion of patients with IMH reopening after FTMH closure was low: four of 43 (9%) patients in the OCP group.132
Overall, the recent published postmarketing experiences with OCP have reported a lower rate of IMH closure than the MIVI studies (Table 6). The rate of closure in small holes was 35% (10/29), medium holes was 19% (6/32), and large holes was 25% (1/4).131,133–140 The overall rate of closure in the post launch studies was 26% (17/65), in contrast to the 40.6% rate achieved in the MIVI trials. The rate of VMA release was 33/61 (54%), indicating that in at least 16 cases OCP induced VR cleavage without resultant IMH closure.
Table 6.
Summary of post marketing published series of IMH treated with OCP
| IMH closure by size
|
VMA release by size
|
|||||
|---|---|---|---|---|---|---|
| ≤250 µm | 250–400 µm | >400 µm | ≤250 µm | 250–400 µm | >400 µm | |
| Study 1133 | 1/2 | 1/4 | 0/1 | 1/2 | 1/4 | 0/1 |
| Study 2134 | 2/6 | 1/3 | 1/1 | 6/6 | 2/3 | 1/1 |
| Study 3135 | 2/6 | 2/8 | 0/1 | 2/6 | 4/8 | 0 |
| Study 4136 | 0 | 0/2 | 0 | 0 | 0/2 | 0 |
| Study 5137 | 0 | 0/2 | 0 | 0 | 0/2 | 0 |
| Study 6138 | 1/3 | 0/3 | 0 | 3/6a | 0 | |
| Study 7139 | 0 | 2/2 | 0/1 | NR | NR | NR |
| Study 8140 | 1/6 | 0/2 | 0 | 6/8 | ||
| Study 9131 | 3/6 | 0/6 | 0 | 5/6 | 2/6 | 0 |
| Total (%) | 10/29 (34.5) | 6/32 (18.8) | 1/4 (25) | 61/62 (54) | ||
Notes:
The small IMH that closed had persistent VMA. In studies 138–140 VMA release data by hole size could not be extrapolated from published data.
Abbreviations: IMH, idiopathic macular hole; OCP, ocriplasmin; VMA, vitreomacular attachment; NR, not recorded.
Visual acuity
For the patients with OCP-induced hole closure, 72% patients gained at least two lines of vision (10 letters) at month 6 and 49% gained at least three lines of vision (15 letters) at month 6.132 Conversely in the 63 patients treated with OCP without hole closure, 14% lost at least two lines of vision and 11% lost at least three lines of vision by month 6. In the 42 patients without hole closure after vehicle injection, percentage declines were similar with 15% patients loosing at least two lines of vision and 12% loosing at least three lines of vision at month 6.
Macular hole formation/enlargement after OCP
The incidence of new or worsening IMH in the MIVI trials was reported as 7% in OCP-treated arm and 10% in the vehicle-treated arm and although IMH can be induced in patients with VMT treated with OCP, this does not appear to be at much higher rate than after placebo or indeed possibly natural history.
In patients with unsuccessful nonsurgical IMH closure at day 28, the hole diameter increased from baseline in both groups but the increase was greater in the OCP versus the placebo group at all time points. This observation has been confirmed post marketing, and the increase in size is particularly noticeable in BD (Figure 4).131 Indeed even in eyes with successful closure after OCP, it is quite common to observe the presence of subfoveal subretinal fluid with greater dimensions than the original hole BD (Figure 5).137,139
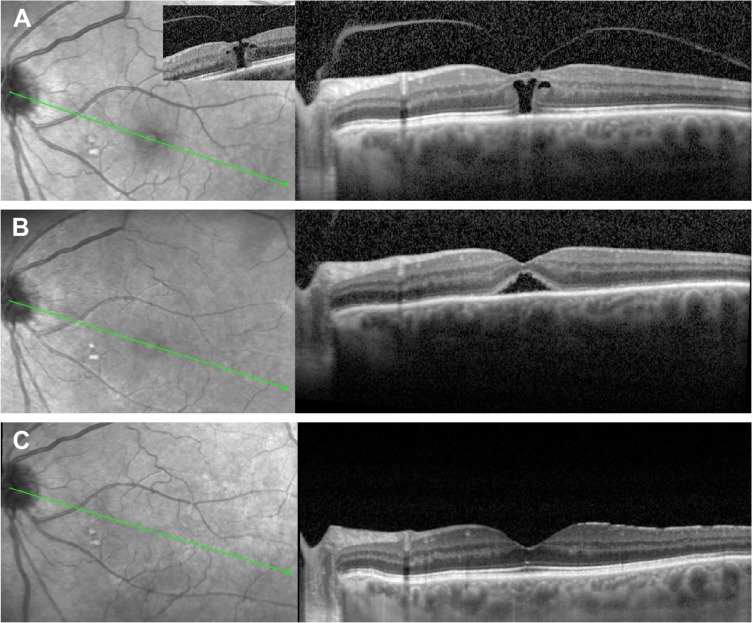
Figure 4.
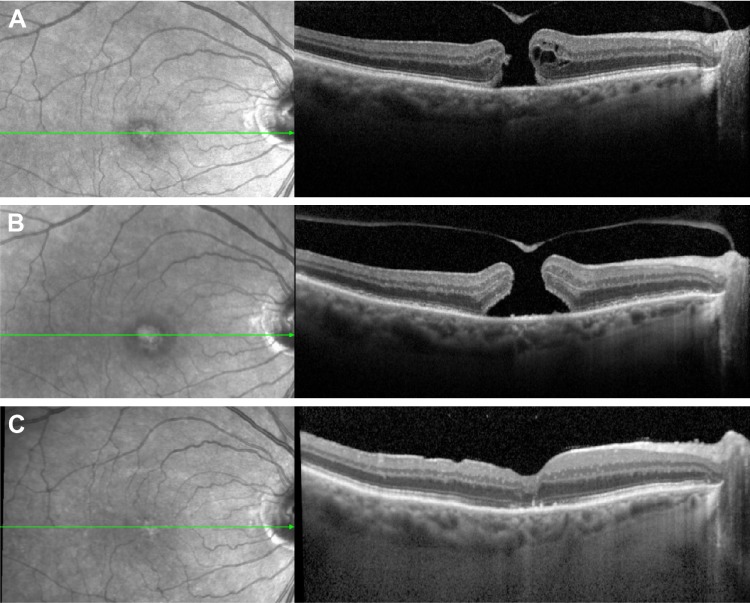
SD-OCT image of a macular hole pre- and post-Ocriplasmin injection without closure.
Notes: Left, fundus photography; right, SD-OCT, green line corresponds with level of OCT image, (A) immediately pre-ocriplasmin (OCP) injection (note area of VMA was on edge of hole, not shown), (B) 1 week post OCP injection showing widened base diameter, and (C) following successful hole closure after vitrectomy and ILM peel.
Abbreviations: VMA, vitreomacular attachment; ILM, inner limiting membrane; OCP, ocriplasmin; SD-OCT, spectral-domain optical coherence tomography.
Figure 5.
SD-OCT image of a macular hole pre- and post-Ocriplasmin injection with successful hole closure.
Notes: Left, fundus photography; right, SD-OCT, green line corresponds with level of OCT image, (A) small hole with VMA pre-ocriplasmin injection (note the full-thickness defect was eccentric and shown in insert picture), (B) closure of hole 1 week post ocriplasmin with VMA release but note the presence of subfoveal subretinal fluid, and (C) resolution of subretinal fluid 3 months later.
Abbreviations: VMA, vitreomacular attachment; SD-OCT, spectral-domain optical coherence tomography.
Visual outcomes in those with initial nonclosure
For the patients not achieving IMH closure after OCP or vehicle and who then underwent vitrectomy, the rates of IMH closure in both groups were high at 79% and 89%, respectively. Among the patients who required vitrectomy to achieve hole closure, 32% eyes had previously received OCP and 26% (P=0.622) had previously received vehicle gained at least two lines of vision. Both these results and subsequent studies have suggested no detrimental effect of OCP if it does not work and vitrectomy is required.131
Hager et al however detected abnormal subretinal fluid post vitrectomy following unsuccessful OCP use, which persisted for 7 months, although the hole remained closed and vision improved.137
Positive predictive factors for VMA release
In the Phase III trials, age <65 years, phakia, width of VMT <1,500 µm, and absence of ERM were identified as positive predictive factors for release of VMA but not specifically for IMH closure. Future studies with SD OCT might identify other factors predictive of IMH closure with OCP.
Adverse events
The Phase III trials concluded that OCP had an acceptable safety profile. The incidence of ocular adverse events (AEs) was similar in the OCP and control vehicle groups (77% and 69%, respectively). The majority of AE that occurred in the OCP group were minor and related to its vitreolytic effects. These included floaters, photopsia, and transiently blurred vision.141 Indeed, it is common for patients treated with OCP to experience a snowstorm effect to their vision in the first 24–48 hours with usual complete resolution. However, various other more serious AEs have been described some of which might be expected and related to increased traction as vitreous liquefies without VR release (increased VMT and MH formation) or conversely the adverse effects of vitreous separation (retinal tears and detachment). Conversely, some of the events reported were less expected including:
Reduced vision with a variety of outer retinal changes including the development of subretinal fluid, ellipsoid zone changes, ERG changes, and dyschromatopsia;
impaired pupillary reflex;
retinal vessel changes;
lens subluxation or phacodonesis.
Most of these were known from the Phase III trials but some have only been recognized since the product was marketed. In particular, the higher resolution of SD OCT led to the recognition that changes in the ellipsoid zone can occur transiently with OCP. None of the AEs have been reported to be more frequent in eyes with IMH than VMT alone.
Visual loss
Out of 465 patients treated with OCP in the Phase III trials, 36 (8%) experienced an acute reduction in visual acuity with a mean letter loss of almost three lines.141 Most were attributed to either progression of the associated macular pathology (VMT and/or IMH) or to onset of a subretinal fluid at the fovea despite VMA resolution. The onset of subretinal fluid was initially attributed to increased traction prior to VMT release but has also been postulated to be related to the effect of OCP on laminin substrates in the outer retina.
Thirty of the 36 cases resolved by the 6-month study end point but visual loss in six patients (17% of patients with vision loss and 1.3% of total patients) persisted to study end at 6 months. Five of these six patients were reported to have reasons related to the pathology or vitrectomy complications but one of the six patients had vision loss for unknown reasons, despite VMA resolution and anatomical improvement.
Dyschromatopsia, ERG changes, and anatomically observed outer retinal changes
To go along with an effect of OCP on the outer retina, dyschromatopsia (usually described as yellow-tinted vision) was reported in 4% of patients treated with OCP in the Phase III studies. The symptoms resolved in 75% of the cases with a median time of 3 months. Many cases of dyschromatopsia reported have also had ERG changes, and ERG changes have been described in other patients receiving OCP. They have principally been decreased in a- and b-wave amplitude. The incidence is uncertain but based on Phase II data may be as high as 70%, although many will be subclinical.
There have also been at least 36 reported cases in the literature of transient changes in the outer retinal layers on SD-OCT scans described as disruption/loss of the ellipsoid zone, and some associated transient visual loss.135,138–140,142 The mean time to onset ranged from 5 days to 7 days. The changes in the majority of cases appear to resolve by 2 months with a mean time in reported cases of 49 days; however in four reported cases, changes and symptoms have persisted throughout 6 months of follow-up.140 Interestingly, these changes have been more common in eyes with successful VMA release and in some cases have also been associated with the transient accumulation of subretinal fluid in the macular area.
All four of these outer retinal effects have been postulated to be due to the action of OCP on laminin and hence retinal disruption at the photoreceptor level. Recovery presumably occurs through laminin restoration, although the long-term sequelae of this are uncertain.
Pupillary changes
Miosis, pupillary inequality, and impaired pupillary reflexes have all been reported 1–2 days post OCP with an incidence of 1%–2% and usual resolution within 3 days.
Lens subluxation or phacodonesis
In the premarketing trials, there were two cases of lens subluxation or phacodonesis out of a total of 976 OCP-treated patients.141 After marketing, there have been further case reports and the effect is likely due to the action of OCP on the lens zonules.143 It is interesting to postulate that this is more likely with anterior intravitreal injection, and hence, deep mid cavity OCP injection is recommended away from the zonular area.
Retinal tears and detachment
Paradoxically the incidence of retinal breaks and detachments was lower in the OCP group than the placebo (1.9% versus 4.3%, respectively) in the Phase III trials. In both groups, however, the majority of retinal tears and detachments were noted during or shortly after vitrectomy.
Since launch a further 17 cases (0.4%) of retinal tears and/or breaks have been reported separately from vitrectomy surgery as might be expected with the creation of vitreous separation. The mean time to onset of these cases has been reported as 20 days (range 8–68 days).132
Comment
OCP is a new treatment option for small- and medium-sized IMH with VMA, although its exact clinical role is an evolving story. Its price is greater than vitrectomy surgery in some health care systems. It is important to counsel patients realistically, regarding the rates of success with intravitreal OCP which are much lower than with vitrectomy surgery particularly in medium-sized holes. It should not be used in large holes. Further factors predicting success in individual cases are needed to refine its optimum use. The incidence and characteristics of AEs have been generally similar between the premarketing and postmarketing periods, although more experience will be needed to assess the long-term morbidity in some patients particularly those with photoreceptor dysfunction and visual loss. In patients treated with OCP, careful monitoring and immediate evaluation of any symptomatic patient are paramount. Surgical treatment should not be delayed if closure does not occur by 4 weeks post OCP injection.
Pneumatic vitreolysis
Pneumatic vitreolysis was first described on animal models in 1984 by two groups lead by Thresher et al144 and Miller et al145. In 1995, Chan et al reported a series of 19 eyes with Stage 1–3 macular holes treated with injection of expansile gas bubble into the vitreous cavity to relieve vitreofoveal traction by inducing a PVD, which was achieved in 18 of 19 eyes within 2–9 weeks of treatment. Hole closure was achieved in three of the six Stage 2 IMH included but none of the Stage 3 holes.146 In 2007, a comparable study by Mori et al reported results for treatment of Stage 2 macular holes with intravitreal injection of SF6 gas bubble. PVD was achieved in 95% of eyes (19 of 20) with a 50% anatomical closure rate.147 In both series, no major complications were reported. In 2012, however a series by Chen et al of 12 patients with Stage 2 MH only achieved a 50% vitreomacular separation rate and a 25% hole closure rate. One case developed a rhegmatogenous retinal detachment.148 Jorge et al reported on six patients with Stage 2 IMH treated with expansile C3F8. Closure was achieved in five and VMT release in all.149 Overall of the full-thickness IMH treated in the abovementioned series, ~45% have closed comparable to the results with OCP.
Some of these series included postoperative prone positioning and some didn’t. Clearly, this is an area that requires further investigation with large controlled trials but reported results are encouraging.
Choosing the optimum management option
Thus, there are three current treatment choices for patients with IMH. The third, expansile gas has yet to undergo large scale RCTs and so no clear guidance can be given regarding its use. However, it is an option that could be considered in patients with small holes and VMA.
Of note, and applicable to all treatment choices is that macular hole characteristics can change in a short period of time, and therefore, repeat OCTs done on the day of treatment are important to ensure that the correct treatment is chosen particularly if using OCP.
For patients with small- or medium-sized holes and VMA, OCP is an option with significantly better results reported in small holes in the Phase III trials, albeit with far lower closure rates than surgery. For holes without VMA, vitrectomy surgery is the only option but the surgeon has choices about whether to peel the ILM, use a short-acting gas (or air) or long-acting gas, whether to advice the patient to posture face down or combine the surgery with phacoemulsification. The choice of treatment is summarized in Table 7. Many of the studies on posturing and gas choice were carried out combined with ILM peeling however as discussed, if not done, this may influence the gas and posturing requirement. It is also worth reiterating that patient-specific factors and patient choice will influence the actual treatment decided upon. Adjuvants, such as platelets and transforming growth factor beta 2, and other surgical techniques, such as retinal relieving incisions and ILM flaps, are generally reserved for revision cases and beyond the scope of this guidance.
Table 7.
Summary treatment choice based on IMH characteristics
| Focal VMA present | No VMA present | |
|---|---|---|
| ≤250 µm | Vitrectomy ± peel | Vitrectomy ± peel |
| Short-acting gas | Short-acting gas | |
| Nonposture or OCP | Nonposture | |
| 250–400 µm | Vitrectomy + peel | Vitrectomy + peel |
| Short-acting gas | Short-acting gas | |
| Nonposture or OCP | Nonposture | |
| >400 µm | Vitrectomy + peel | Vitrectomy + peel |
| Long-acting gas | Long-acting gas | |
| Posture | Posture | |
| Stage 4 | NA | Vitrectomy + peel |
| Long- or short-acting gas | ||
| Posture based on size |
Abbreviations: IMH, idiopathic macular hole; VMA, vitreomacular attachment; OCP, ocriplasmin; NA, not applicable.
Footnotes
Disclosure
David H Steel has received research funding from Alcon and attended advisory boards for Alcon. The authors report no other conflicts of interest in this work.
References
- 1.Casuso LA, Scott IU, Flynn HW, et al. Long-term follow-up of unoperated macular holes. Ophthalmology. 2001;108(6):1150–1155. doi: 10.1016/s0161-6420(01)00581-4. [DOI] [PubMed] [Google Scholar]
- 2.Ezra E. Idiopathic full thickness macular hole: natural history and pathogenesis. Br J Ophthalmol. 2001;85(1):102–108. doi: 10.1136/bjo.85.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim W, Freeman WR, El-haig W, et al. Baseline characteristics, natural history, and risk factors to progression in eyes with stage 2 macular holes results from a prospective randomized clinical trial. Vitrectomy for macular hole study group. Ophthalmology. 1995;102(12):1818–1829. doi: 10.1016/s0161-6420(95)30788-9. [DOI] [PubMed] [Google Scholar]
- 4.Jackson TL, Donachie PHJ, Sparrow JM, et al. United Kingdom national ophthalmology database study of vitreoretinal surgery: report 1; case mix, complications, and cataract. Eye (Lond) 2013;27(5):644–651. doi: 10.1038/eye.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risk factors for idiopathic macular holes. The eye disease case-control study group. Am J Ophthalmol. 1994;118(6):754–761. [PubMed] [Google Scholar]
- 6.Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106(5):629–639. doi: 10.1001/archopht.1988.01060130683026. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RN, Gass JD. Idiopathic macular holes. Observations, stages of formation, and implications for surgical intervention. Ophthalmology. 1988;95(7):917–924. doi: 10.1016/s0161-6420(88)33075-7. [DOI] [PubMed] [Google Scholar]
- 8.Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119(6):752–759. doi: 10.1016/s0002-9394(14)72781-3. [DOI] [PubMed] [Google Scholar]
- 9.Spaide RF. Macular hole hypotheses. Am J Ophthalmol. 2005;139(1):149–151. doi: 10.1016/j.ajo.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Duker JS, Kaiser PK, Binder S, et al. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. doi: 10.1016/j.ophtha.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 11.Steel D, Downey L, Greiner K, et al. The design and validation of an optical coherence tomography-based classification system for focal vitreomacular traction. Eye Press. doi: 10.1038/eye.2015.262. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schumann RG, Schaumberger MM, Rohleder M, et al. Ultrastructure of the vitreomacular interface in full-thickness idiopathic macular holes: a consecutive analysis of 100 cases. Am J Ophthalmol. 2006;141(6):1112–1119. doi: 10.1016/j.ajo.2006.01.074. [DOI] [PubMed] [Google Scholar]
- 13.Steel DHW, Dinah C, Madi HA, et al. The staining pattern of brilliant blue G during macular hole surgery: a clinicopathologic study. Invest Ophthalmol Vis Sci. 2014;55(9):5924–5931. doi: 10.1167/iovs.14-14809. [DOI] [PubMed] [Google Scholar]
- 14.Madi HA, Dinah C, Rees J, et al. The case mix of patients presenting with full-thickness macular holes and progression before surgery: implications for optimum management. Ophthalmologica. 2015;233(3–4):216–221. doi: 10.1159/000375378. [DOI] [PubMed] [Google Scholar]
- 15.Moisseiev J, Moroz I, Katz G. Effect of ocriplasmin on the management of macular holes: assessment of the clinical relevance of ocriplasmin. JAMA Ophthalmol. 2014;132(6):709–713. doi: 10.1001/jamaophthalmol.2013.8223. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi A, Yoshida A, Nagaoka T, et al. Idiopathic full-thickness macular holes and the vitreomacular interface: a high-resolution spectral-domain optical coherence tomography study. Am J Ophthalmol. 2012;154(5):881.e2–892.e2. doi: 10.1016/j.ajo.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Barak Y, Ihnen MA, Schaal S. Spectral domain optical coherence tomography in the diagnosis and management of vitreoretinal interface pathologies. J Ophthalmol. 2012;2012:876472. doi: 10.1155/2012/876472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiernan DF, Mieler WF, Hariprasad SM. Spectral-domain optical coherence tomography: a comparison of modern high-resolution retinal imaging systems. Am J Ophthalmol. 2010;149(1):18.e2–31.e2. doi: 10.1016/j.ajo.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Ip MS, Baker BJ, Duker JS, et al. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002;120(1):29–35. doi: 10.1001/archopht.120.1.29. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Moreno JM, Staicu C, Piñero DP, et al. Optical coherence tomography predictive factors for macular hole surgery outcome. Br J Ophthalmol. 2008;92(5):640–644. doi: 10.1136/bjo.2007.136176. [DOI] [PubMed] [Google Scholar]
- 21.Wakely L, Rahman R, Stephenson J. A comparison of several methods of macular hole measurement using optical coherence tomography, and their value in predicting anatomical and visual outcomes. Br J Ophthalmol. 2012;96(7):1003–1007. doi: 10.1136/bjophthalmol-2011-301287. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama A. Reappraisal of spontaneous closure rate of idiopathic full-thickness macular holes. Open Ophthalmol J. 2012;6(1):73–74. doi: 10.2174/1874364101206010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tadayoni R, Massin P, Haouchine B, et al. Spontaneous resolution of small stage 3 and 4 full-thickness macular holes viewed by optical coherence tomography. Retina. 2001;21(2):186–189. doi: 10.1097/00006982-200104000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Chew EY, Sperduto RD, Hiller R, et al. Clinical course of macular holes: the eye disease case-control study. Arch Ophthalmol. 1999;117(2):242–246. doi: 10.1001/archopht.117.2.242. [DOI] [PubMed] [Google Scholar]
- 25.Reddy CV, Folk JC, Feist RM. Microholes of the macula. Arch Ophthalmol. 1996;114(4):413–416. doi: 10.1001/archopht.1996.01100130409007. [DOI] [PubMed] [Google Scholar]
- 26.Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149(3):371.e1–382.e1. doi: 10.1016/j.ajo.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Kim JW, Freeman WR, Azen SP, et al. Prospective randomized trial of vitrectomy or observation for stage 2 macular holes. Vitrectomy for macular hole study group. Am J Ophthalmol. 1996;121(6):605–614. doi: 10.1016/s0002-9394(14)70625-7. [DOI] [PubMed] [Google Scholar]
- 28.Hikichi T, Yoshida A, Akiba J, et al. Natural outcomes of stage 1, 2, 3, and 4 idiopathic macular holes. Br J Ophthalmol. 1995;79(6):517–520. doi: 10.1136/bjo.79.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonnell PJ, Fine SL, Hillis AI. Clinical features of idiopathic macular cysts and holes. Am J Ophthalmol. 1982;93(6):777–786. doi: 10.1016/0002-9394(82)90474-3. [DOI] [PubMed] [Google Scholar]
- 30.Ezra E, Wells JA, Gray RH, et al. Incidence of idiopathic full-thickness macular holes in fellow eyes. A 5-year prospective natural history study. Ophthalmology. 1998;105(2):353–359. doi: 10.1016/s0161-6420(98)93562-x. [DOI] [PubMed] [Google Scholar]
- 31.Chan A, Duker JS, Schuman JS, et al. Stage 0 macular holes: observations by optical coherence tomography. Ophthalmology. 2004;111(11):2027–2032. doi: 10.1016/j.ophtha.2004.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi A, Yoshida A, Nagaoka T, et al. Macular hole formation in fellow eyes with a perifoveal posterior vitreous detachment of patients with a unilateral macular hole. Am J Ophthalmol. 2011;151(6):981.e4–989.e4. doi: 10.1016/j.ajo.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Niwa H, Terasaki H, Ito Y, et al. Macular hole development in fellow eyes of patients with unilateral macular hole. Am J Ophthalmol. 2005;140(3):370–375. doi: 10.1016/j.ajo.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 34.Akiba J, Kakehashi A, Arzabe CW, et al. Fellow eyes in idiopathic macular hole cases. Ophthalmic Surg. 1992;23(9):594–597. [PubMed] [Google Scholar]
- 35.Haouchine B, Massin P, Gaudric A. Foveal pseudocyst as the first step in macular hole formation: a prospective study by optical coherence tomography. Ophthalmology. 2001;108(1):15–22. doi: 10.1016/s0161-6420(00)00519-4. [DOI] [PubMed] [Google Scholar]
- 36.de Bustros S. Vitrectomy for prevention of macular holes. Results of a randomized multicenter clinical trial. Vitrectomy for prevention of macular hole study group. Ophthalmology. 1994;101(6):1055–1059. doi: 10.1016/s0161-6420(94)31218-8. discussion 1060. [DOI] [PubMed] [Google Scholar]
- 37.Woon WH, Greig D, Savage MD, et al. Movement of the inner retina complex during the development of primary full-thickness macular holes: implications for hypotheses of pathogenesis. Graefes Arch Clin Exp Ophthalmol. 2015 Feb 13; doi: 10.1007/s00417-015-2951-0. Epub. [DOI] [PubMed] [Google Scholar]
- 38.Jaycock PD, Bunce C, Xing W, et al. Outcomes of macular hole surgery: implications for surgical management and clinical governance. Eye (Lond) 2005;19(8):879–884. doi: 10.1038/sj.eye.6701679. [DOI] [PubMed] [Google Scholar]
- 39.Gupta B, Laidlaw DAH, Williamson TH, et al. Predicting visual success in macular hole surgery. Br J Ophthalmol. 2009;93(11):1488–1491. doi: 10.1136/bjo.2008.153189. [DOI] [PubMed] [Google Scholar]
- 40.Stec LA, Ross RD, Williams GA, et al. Vitrectomy for chronic macular holes. Retina. 2004;24(3):341–347. doi: 10.1097/00006982-200406000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109(5):654–659. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 42.Hirneiss C, Neubauer AS, Gass CA, et al. Visual quality of life after macular hole surgery: outcome and predictive factors. Br J Ophthalmol. 2007;91(4):481–484. doi: 10.1136/bjo.2006.102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang S, Reppucci V, Zimmerman NJ, et al. Perfluorocarbon liquids in the management of traumatic retinal detachments. Ophthalmology. 1989;96(6):785–791. doi: 10.1016/s0161-6420(89)32812-0. discussion 791–792. [DOI] [PubMed] [Google Scholar]
- 44.Smiddy WE, Flynn HW. Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol. 2004;137(3):525–537. doi: 10.1016/j.ajo.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Thompson JT, Smiddy WE, Glaser BM, et al. Intraocular tamponade duration and success of macular hole surgery. Retina. 1996;16(5):373–382. doi: 10.1097/00006982-199616050-00002. [DOI] [PubMed] [Google Scholar]
- 46.Berger JW, Brucker AJ. The magnitude of the bubble buoyant pressure: implications for macular hole surgery. Retina. 1998;18(1):84–86. doi: 10.1097/00006982-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 47.Passemard M, Yakoubi Y, Muselier A, et al. Long-term outcome of idiopathic macular hole surgery. Am J Ophthalmol. 2010;149(1):120–126. doi: 10.1016/j.ajo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Kellner L, Wimpissinger B, Stolba U, et al. 25-gauge vs 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trial. Br J Ophthalmol. 2007;91(7):945–948. doi: 10.1136/bjo.2006.106799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim SS, Smiddy WE, Feuer WJ, et al. Outcomes of sulfur hexafluoride (SF6) versus perfluoropropane (C3F8) gas tamponade for macular hole surgery. Retina. 2008;28(10):1408–1415. doi: 10.1097/IAE.0b013e3181885009. [DOI] [PubMed] [Google Scholar]
- 50.Rahman R, Madgula I, Khan K. Outcomes of sulfur hexafluoride (SF6) versus perfluoroethane (C2F6) gas tamponade for non-posturing macular-hole surgery. Br J Ophthalmol. 2012;96(2):185–188. doi: 10.1136/bjo.2010.201699. [DOI] [PubMed] [Google Scholar]
- 51.Nadal J, Delas B, Piñero A. Vitrectomy without face-down posturing for idiopathic macular holes. Retina. 2012;32(5):918–921. doi: 10.1097/IAE.0b013e318229b20e. [DOI] [PubMed] [Google Scholar]
- 52.Ullrich S, Haritoglou C, Gass C, et al. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002;86(4):390–393. doi: 10.1136/bjo.86.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roth DB, Smiddy WE, Feuer W. Vitreous surgery for chronic macular holes. Ophthalmology. 1997;104(12):2047–2052. doi: 10.1016/s0161-6420(97)30060-8. [DOI] [PubMed] [Google Scholar]
- 54.Briand S, Chalifoux E, Tourville E, et al. Prospective randomized trial: outcomes of SF6 versus C3F8 in macular hole surgery. Can J Ophthalmol. 2015;50(2):95–100. doi: 10.1016/j.jcjo.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Eckardt C, Eckert T, Eckardt U, et al. Macular hole surgery with air tamponade and optical coherence tomography-based duration of face-down positioning. Retina. 2008;28(8):1087–1096. doi: 10.1097/IAE.0b013e318185fb5f. [DOI] [PubMed] [Google Scholar]
- 56.Hasegawa Y, Hata Y, Mochizuki Y, et al. Equivalent tamponade by room air as compared with SF(6) after macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2009;247(11):1455–1459. doi: 10.1007/s00417-009-1120-8. [DOI] [PubMed] [Google Scholar]
- 57.Lappas A, Foerster AMH, Kirchhof B. Use of heavy silicone oil (Densiron-68) in the treatment of persistent macular holes. Acta Ophthalmol. 2009;87(8):866–870. doi: 10.1111/j.1755-3768.2008.01371.x. [DOI] [PubMed] [Google Scholar]
- 58.Schurmans A, Van Calster J, Stalmans P. Macular hole surgery with inner limiting membrane peeling, endodrainage, and heavy silicone oil tamponade. Am J Ophthalmol. 2009;147(3):495–500. doi: 10.1016/j.ajo.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Lai JC, Stinnett SS, McCuen BW. Comparison of silicone oil versus gas tamponade in the treatment of idiopathic full-thickness macular hole. Ophthalmology. 2003;110(6):1170–1174. doi: 10.1016/S0161-6420(03)00264-1. [DOI] [PubMed] [Google Scholar]
- 60.Couvillion SS, Smiddy WE, Flynn HW, et al. Outcomes of surgery for idiopathic macular hole: a case-control study comparing silicone oil with gas tamponade. Ophthalmic Surg Lasers Imaging. 2005;36(5):365–371. [PubMed] [Google Scholar]
- 61.Yamashita T, Sakamoto T, Yamashita T, et al. Individualized, spectral domain-optical coherence tomography-guided facedown posturing after macular hole surgery: minimizing treatment burden and maximizing outcome. Retina. 2014;34(7):1367–1375. doi: 10.1097/IAE.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 62.Chow DR, Chaudhary KM. Optical coherence tomography-based positioning regimen for macular hole surgery. Retina. 2015;35(5):899–907. doi: 10.1097/IAE.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 63.Guillaubey A, Malvitte L, Lafontaine PO, et al. Comparison of face-down and seated position after idiopathic macular hole surgery: a randomized clinical trial. Am J Ophthalmol. 2008;146(1):128–134. doi: 10.1016/j.ajo.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 64.Lange CAK, Membrey L, Ahmad N, et al. Pilot randomised controlled trial of face-down positioning following macular hole surgery. Eye (Lond) 2012;26(2):272–277. doi: 10.1038/eye.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solebo AL, Lange CA, Bunce C, et al. Face-down positioning or posturing after macular hole surgery. Cochrane Database Syst Rev. 2011;(12) doi: 10.1002/14651858.CD008228.pub2. Art No.:CD008228. [DOI] [PubMed] [Google Scholar]
- 66.Tadayoni R, Vicaut E, Devin F, et al. A randomized controlled trial of alleviated positioning after small macular hole surgery. Ophthalmology. 2011;118(1):150–155. doi: 10.1016/j.ophtha.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 67.Eckardt C, Eckardt U, Groos S, et al. Entfernung der Membrana limitans interna bei Makulalöchern Klinische und morphologische Befunde [Removal of the internal limiting membrane in macular holes. Clinical and morphological findings] Ophthalmologe. 1997;94(8):545–551. doi: 10.1007/s003470050156. [DOI] [PubMed] [Google Scholar]
- 68.Wollensak G, Spoerl E, Grosse G, et al. Biomechanical significance of the human internal limiting lamina. Retina. 2006;26(8):965–968. doi: 10.1097/01.iae.0000250001.45661.95. [DOI] [PubMed] [Google Scholar]
- 69.Kwok AK, Li WW, Pang CP, et al. Indocyanine green staining and removal of internal limiting membrane in macular hole surgery: histology and outcome. Am J Ophthalmol. 2001;132(2):178–183. doi: 10.1016/s0002-9394(01)00976-x. [DOI] [PubMed] [Google Scholar]
- 70.Burk SE, Da Mata AP, Snyder ME, et al. Indocyanine green-assisted peeling of the retinal internal limiting membrane. Ophthalmology. 2000;107(11):2010–2014. doi: 10.1016/s0161-6420(00)00375-4. [DOI] [PubMed] [Google Scholar]
- 71.Horio N, Horiguchi M. Effect on visual outcome after macular hole surgery when staining the internal limiting membrane with indocyanine green dye. Arch Ophthalmol. 2004;122(7):992–996. doi: 10.1001/archopht.122.7.992. [DOI] [PubMed] [Google Scholar]
- 72.Cheng S-N, Yang T-C, Ho J-D, et al. Ocular toxicity of intravitreal indocyanine green. J Ocul Pharmacol Ther. 2005;21(1):85–93. doi: 10.1089/jop.2005.21.85. [DOI] [PubMed] [Google Scholar]
- 73.Balaiya S, Brar VS, Murthy RK, et al. Comparative in vitro safety analysis of dyes for chromovitrectomy: indocyanine green, brilliant blue green, bromophenol blue, and infracyanine green. Retina. 2011;31(6):1128–1136. doi: 10.1097/IAE.0b013e3181fe543a. [DOI] [PubMed] [Google Scholar]
- 74.Wu Y, Zhu W, Xu D, et al. Indocyanine green-assisted internal limiting membrane peeling in macular hole surgery: a meta-analysis. PLoS One. 2012;7(11):e48405. doi: 10.1371/journal.pone.0048405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scupola A, Mastrocola A, Sasso P, et al. Assessment of retinal function before and after idiopathic macular hole surgery. Am J Ophthalmol. 2013;156(1):132.e1–139.e1. doi: 10.1016/j.ajo.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Haritoglou C, Gandorfer A, Gass CA, et al. Indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcome: a clinicopathologic correlation. Am J Ophthalmol. 2002;134(6):836–841. doi: 10.1016/s0002-9394(02)01816-0. [DOI] [PubMed] [Google Scholar]
- 77.Yamashita T, Uemura A, Kita H, et al. Long-term outcomes of visual field defects after indocyanine green-assisted macular hole surgery. Retina. 2008;28(9):1228–1233. doi: 10.1097/IAE.0b013e31817b6b2e. [DOI] [PubMed] [Google Scholar]
- 78.Machida S, Toba Y, Nishimura T, et al. Comparisons of cone electroretinograms after indocyanine green-, brilliant blue G-, or triamcinolone acetonide-assisted macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2014;252(9):1423–1433. doi: 10.1007/s00417-014-2594-6. [DOI] [PubMed] [Google Scholar]
- 79.Steel DHW, Karimi AA, White K. An evaluation of two heavier-than-water internal limiting membrane-specific dyes during macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2015 Oct 10; doi: 10.1007/s00417-015-3193-x. Epub. [DOI] [PubMed] [Google Scholar]
- 80.Lee KL, Dean S, Guest S. A comparison of outcomes after indocyanine green and trypan blue assisted internal limiting membrane peeling during macular hole surgery. Br J Ophthalmol. 2005;89(4):420–424. doi: 10.1136/bjo.2004.049684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schumann RG, Gandorfer A, Priglinger SG, et al. Vital dyes for macular surgery: a comparative electron microscopy study of the internal limiting membrane. Retina. 2009;29(5):669–676. doi: 10.1097/IAE.0b013e318196b1c8. [DOI] [PubMed] [Google Scholar]
- 82.Steel DHW, Dinah C, Habib M, et al. ILM peeling technique influences the degree of a dissociated optic nerve fibre layer appearance after macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2015;253(5):691–698. doi: 10.1007/s00417-014-2734-z. [DOI] [PubMed] [Google Scholar]
- 83.Lois N, Burr J, Norrie J, et al. Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: a pragmatic randomized controlled trial. Invest Ophthalmol Vis Sci. 2011;52(3):1586–1592. doi: 10.1167/iovs.10-6287. [DOI] [PubMed] [Google Scholar]
- 84.D’Souza MJJ, Chaudhary V, Devenyi R, et al. Re-operation of idiopathic full-thickness macular holes after initial surgery with internal limiting membrane peel. Br J Ophthalmol. 2011;95(11):1564–1567. doi: 10.1136/bjo.2010.195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michalewska Z, Michalewski J, Adelman RA, et al. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117(10):2018–2025. doi: 10.1016/j.ophtha.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 86.Ho T-C, Yang C-M, Huang J-S, et al. Foveola nonpeeling internal limiting membrane surgery to prevent inner retinal damages in early stage 2 idiopathic macula hole. Graefes Arch Clin Exp Ophthalmol. 2014;252(10):1553–1560. doi: 10.1007/s00417-014-2613-7. [DOI] [PubMed] [Google Scholar]
- 87.Almeida DRP, Chin EK, Tarantola RM, et al. Effect of internal limiting membrane abrasion on retinal tissues in macular holes. Invest Ophthalmol Vis Sci. 2015;56(5):2783–2789. doi: 10.1167/iovs.14-16355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miura M, Elsner AE, Osako M, et al. Dissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular hole. Retina. 2003;23(4):561–563. doi: 10.1097/00006982-200308000-00024. [DOI] [PubMed] [Google Scholar]
- 89.Ito Y, Terasaki H, Takahashi A, et al. Dissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular holes. Ophthalmology. 2005;112(8):1415–1420. doi: 10.1016/j.ophtha.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 90.Alkabes M, Salinas C, Vitale L, et al. En face optical coherence tomography of inner retinal defects after internal limiting membrane peeling for idiopathic macular hole. Invest Ophthalmol Vis Sci. 2011;52(11):8349–8355. doi: 10.1167/iovs.11-8043. [DOI] [PubMed] [Google Scholar]
- 91.Baba T, Yamamoto S, Kimoto R, et al. Reduction of thickness of ganglion cell complex after internal limiting membrane peeling during vitrectomy for idiopathic macular hole. Eye (Lond) 2012;26(9):1173–1180. doi: 10.1038/eye.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishida M, Ichikawa Y, Higashida R, et al. Retinal displacement toward optic disc after internal limiting membrane peeling for idiopathic macular hole. Am J Ophthalmol. 2014;157(5):971–977. doi: 10.1016/j.ajo.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 93.Tadayoni R, Svorenova I, Erginay A, et al. Decreased retinal sensitivity after internal limiting membrane peeling for macular hole surgery. Br J Ophthalmol. 2012;96(12):1513–1516. doi: 10.1136/bjophthalmol-2012-302035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spiteri Cornish K, Lois N, Scott N, et al. Vitrectomy with internal limiting membrane (ILM) peeling versus vitrectomy with no peeling for idiopathic full-thickness macular hole (FTMH) Cochrane Database Syst Rev. 2013;6:CD009306. doi: 10.1002/14651858.CD009306.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tadayoni R, Gaudric A, Haouchine B, et al. Relationship between macular hole size and the potential benefit of internal limiting membrane peeling. Br J Ophthalmol. 2006;90(10):1239–1241. doi: 10.1136/bjo.2006.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong SC, Clare G, Bunce C, et al. Cataract progression in macular hole cases: results with vitrectomy or with observation. J Cataract Refract Surg. 2012;38(7):1176–1180. doi: 10.1016/j.jcrs.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 97.Steel DHW. Phacovitrectomy: expanding indications. J Cataract Refract Surg. 2007;33(6):933–936. doi: 10.1016/j.jcrs.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 98.Manvikar S, Steel D, Pimenidis D. Refractive outcome of phacovitrectomy. J Cataract Refract Surg. 2008;34(12):2009–2010. doi: 10.1016/j.jcrs.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 99.Manvikar SR, Allen D, Steel DHW. Optical biometry in combined phacovitrectomy. J Cataract Refract Surg. 2009;35(1):64–69. doi: 10.1016/j.jcrs.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 100.Lee JY, Kim K-H, Shin KH, et al. Comparison of intraoperative complications of phacoemulsification between sequential and combined procedures of pars plana vitrectomy and cataract surgery. Retina. 2012;32(10):2026–2033. doi: 10.1097/IAE.0b013e3182561fab. [DOI] [PubMed] [Google Scholar]
- 101.Ghosh S, Best K, Steel DH. Lens-iris diaphragm retropulsion syndrome during phacoemulsification in vitrectomized eyes. J Cataract Refract Surg. 2013;39(12):1852–1858. doi: 10.1016/j.jcrs.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 102.Muselier A, Dugas B, Burelle X, et al. Macular hole surgery and cataract extraction: combined vs consecutive surgery. Am J Ophthalmol. 2010;150(3):387–391. doi: 10.1016/j.ajo.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Smiddy WE, Glaser BM, Green WR, et al. Transforming growth factor beta. A biologic chorioretinal glue. Arch Ophthalmol. 1989;107(4):577–580. doi: 10.1001/archopht.1989.01070010591036. [DOI] [PubMed] [Google Scholar]
- 104.Liggett PE, Skolik DS, Horio B, et al. Human autologous serum for the treatment of full-thickness macular holes. A preliminary study. Ophthalmology. 1995;102(7):1071–1076. doi: 10.1016/s0161-6420(95)30909-8. [DOI] [PubMed] [Google Scholar]
- 105.Gaudric A, Massin P, Paques M, et al. Autologous platelet concentrate for the treatment of full-thickness macular holes. Graefes Arch Clin Exp Ophthalmol. 1995;233(9):549–554. doi: 10.1007/BF00404704. [DOI] [PubMed] [Google Scholar]
- 106.Kapoor KG, Khan AN, Tieu BC, et al. Revisiting autologous platelets as an adjuvant in macular hole repair: chronic macular holes without prone positioning. Ophthalmic Surg Lasers Imaging. 2012;43(4):291–295. doi: 10.3928/15428877-20120426-03. [DOI] [PubMed] [Google Scholar]
- 107.Rizzo S, Genovesi-Ebert F, Vento A, et al. Heavy silicone oil (Densiron-68) for the treatment of persistent macular holes: Densiron-68 endotamponade for persistent macular holes. Graefes Arch Clin Exp Ophthalmol. 2009;247(11):1471–1476. doi: 10.1007/s00417-009-1131-5. [DOI] [PubMed] [Google Scholar]
- 108.Morizane Y, Shiraga F, Kimura S, et al. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol. 2014;157(4):861.e1–869.e1. doi: 10.1016/j.ajo.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 109.Chen S-N, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2015 Jul 21; doi: 10.1097/IAE.0000000000000674. Epub. [DOI] [PubMed] [Google Scholar]
- 110.Charles S, Randolph JC, Neekhra A, et al. Arcuate retinotomy for the repair of large macular holes. Ophthalmic Surg Lasers Imaging Retina. 2013;44(1):69–72. doi: 10.3928/23258160-20121221-15. [DOI] [PubMed] [Google Scholar]
- 111.Krishnan R, Tossounis C, Fung Yang Y. 20-gauge and 23-gauge phaco-vitrectomy for idiopathic macular holes: comparison of complications and long-term outcomes. Eye (Lond) 2013;27(1):72–77. doi: 10.1038/eye.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scartozzi R, Bessa AS, Gupta OP, et al. Intraoperative sclerotomy-related retinal breaks for macular surgery, 20- vs 25-gauge vitrectomy systems. Am J Ophthalmol. 2007;143(1):155–156. doi: 10.1016/j.ajo.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 113.Tarantola RM, Tsui JY, Graff JM, et al. Intraoperative sclerotomy-related retinal breaks during 23-gauge pars plana vitrectomy. Retina. 2013;33(1):136–142. doi: 10.1097/IAE.0b013e31825e1d62. [DOI] [PubMed] [Google Scholar]
- 114.Tan HS, Mura M, de Smet MD. Iatrogenic retinal breaks in 25-gauge macular surgery. Am J Ophthalmol. 2009;148(3):427–430. doi: 10.1016/j.ajo.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 115.Byeon SH, Chu YK, Lee SC, et al. Problems associated with the 25-gauge transconjunctival sutureless vitrectomy system during and after surgery. Ophthalmologica. 2006;220(4):259–265. doi: 10.1159/000093081. [DOI] [PubMed] [Google Scholar]
- 116.Pielen A, Guerra NIP, Böhringer D, et al. Intra- and postoperative risks and complications of small-gauge (23-G) versus conventional (20-G) vitrectomy for macular surgery. Eur J Ophthalmol. 2014;24(5):778–785. doi: 10.5301/ejo.5000461. [DOI] [PubMed] [Google Scholar]
- 117.Feng H, Adelman RA. Cataract formation following vitreoretinal procedures. Clin Ophthalmol. 2014;8:1957–1965. doi: 10.2147/OPTH.S68661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lommatzsch A, Heimes B, Trieschmann M, et al. Langzeitergebnisse nach Pars-plana-Vitrektomie mit 25-Gauge-Technik [Long-term results after pars plana vitrectomy with 25 gauge technique] Ophthalmologe. 2008;105(5):445–451. doi: 10.1007/s00347-007-1629-3. [DOI] [PubMed] [Google Scholar]
- 119.Wu L, Berrocal MH, Arévalo JF, et al. Endophthalmitis after pars plana vitrectomy: results of the Pan American collaborative retina study group. Retina. 2011;31(4):673–678. doi: 10.1097/IAE.0b013e318203c183. [DOI] [PubMed] [Google Scholar]
- 120.Scott IU, Flynn HW, Acar N, et al. Incidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2011;249(3):377–380. doi: 10.1007/s00417-010-1505-8. [DOI] [PubMed] [Google Scholar]
- 121.Kim JH, Kang SW, Kim YT, et al. Partial posterior hyaloidectomy for macular disorders. Eye (Lond) 2013;27(8):946–951. doi: 10.1038/eye.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cullinane AB, Cleary PE. Prevention of visual field defects after macular hole surgery. Br J Ophthalmol. 2000;84(4):372–377. doi: 10.1136/bjo.84.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gass CA, Haritoglou C, Messmer EM, et al. Peripheral visual field defects after macular hole surgery: a complication with decreasing incidence. Br J Ophthalmol. 2001;85(5):549–551. doi: 10.1136/bjo.85.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kokame GT. Visual field defects after vitrectomy with fluid-air exchange. Br J Ophthalmol. 2001;85(1):121. doi: 10.1136/bjo.85.1.121a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vote BJT, Newland A, Polkinghorne PJ. Humidity devices in vitreo-retinal surgery. Retina. 2002;22(5):616–621. doi: 10.1097/00006982-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 126.Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367(7):606–615. doi: 10.1056/NEJMoa1110823. [DOI] [PubMed] [Google Scholar]
- 127.Gandorfer A, Rohleder M, Sethi C, et al. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci. 2004;45(2):641–647. doi: 10.1167/iovs.03-0930. [DOI] [PubMed] [Google Scholar]
- 128.de Smet MD, Valmaggia C, Zarranz-Ventura J, et al. Microplasmin: ex vivo characterization of its activity in porcine vitreous. Invest Ophthalmol Vis Sci. 2009;50(2):814–819. doi: 10.1167/iovs.08-2185. [DOI] [PubMed] [Google Scholar]
- 129.Takano A, Hirata A, Inomata Y, et al. Intravitreal plasmin injection activates endogenous matrix metalloproteinase-2 in rabbit and human vitreous. Am J Ophthalmol. 2005;140(4):654–660. doi: 10.1016/j.ajo.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 130.Dugel PU, Regillo C, Eliott D. Characterization of anatomic and visual function outcomes in patients with full-thickness macular hole in ocriplasmin phase 3 trials. Am J Ophthalmol. 2015;160(1):94.e1–99.e1. doi: 10.1016/j.ajo.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 131.Steel DHW, Sandinha MT, White K. The plane of vitreoretinal separation and results of vitrectomy surgery in patients given ocriplasmin for idiopathic macular hole. Invest Ophthalmol Vis Sci. 2015;56(6):4038. doi: 10.1167/iovs.15-16409. [DOI] [PubMed] [Google Scholar]
- 132.Hahn P, Chung MM, Flynn HW, et al. Safety profile of ocriplasmin for symptomatic vitreomacular adhesion a comprehensive analysis of premarketing and postmarketing experiences. Retina. 2015;35(6):1128–1134. doi: 10.1097/IAE.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 133.Chatziralli I, Theodossiadis G, Parikakis E, et al. Real-life experience after intravitreal ocriplasmin for vitreomacular traction and macular hole: a spectral-domain optical coherence tomography prospective study. Graefes Arch Clin Exp Ophthalmol. 2015 May 5; doi: 10.1007/s00417-015-3031-1. Epub. [DOI] [PubMed] [Google Scholar]
- 134.Willekens K, Abegão Pinto L, Vandewalle E, et al. Improved efficacy of ocriplasmin for vitreomacular traction release and transient changes in optic disk morphology. Retina. 2015;35(6):1135–1143. doi: 10.1097/IAE.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 135.Sharma P, Juhn A, Houston SK, et al. Efficacy of intravitreal ocriplasmin on vitreomacular traction and full-thickness macular holes. Am J Ophthalmol. 2015;159(5):861.e2–867.e2. doi: 10.1016/j.ajo.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 136.Chin EK, Almeida DRP, Sohn EH, et al. Incomplete vitreomacular traction release using intravitreal ocriplasmin. Case Rep Ophthalmol. 2014;5(3):455–462. doi: 10.1159/000370024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hager A, Seibel I, Riechardt A, et al. Does ocriplasmin affect the RPE-photoreceptor adhesion in macular holes? Br J Ophthalmol. 2014;99(5):635–638. doi: 10.1136/bjophthalmol-2014-305620. [DOI] [PubMed] [Google Scholar]
- 138.Warrow DJ, Lai MM, Patel A, et al. Treatment outcomes and spectral-domain optical coherence tomography findings of eyes with symptomatic vitreomacular adhesion treated with intravitreal ocriplasmin. Am J Ophthalmol. 2015;159(1):20.e1–30.e1. doi: 10.1016/j.ajo.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 139.Singh RP, Li A, Bedi R, et al. Anatomical and visual outcomes following ocriplasmin treatment for symptomatic vitreomacular traction syndrome. Br J Ophthalmol. 2014;98(3):356–360. doi: 10.1136/bjophthalmol-2013-304219. [DOI] [PubMed] [Google Scholar]
- 140.Miller JB, Kim LA, Wu DM, et al. Ocriplasmin for treatment of stage 2 macular holes: early clinical results. Ophthalmic Surg Lasers Imaging Retina. 2014;45(4):293–297. doi: 10.3928/23258160-20140709-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaiser PK, Kampik A, Kuppermann BD, et al. Safety profile of ocriplasmin for the pharmacologic treatment of symptomatic vitreo-macular adhesion/traction. Retina. 2015;35(6):1111–1127. doi: 10.1097/IAE.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 142.Freund KB, Shah SA, Shah VP. Correlation of transient vision loss with outer retinal disruption following intravitreal ocriplasmin. Eye (Lond) 2013;27(6):773–774. doi: 10.1038/eye.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Keller J, Haynes RJ. Zonular dehiscence at the time of combined vitrectomy and cataract surgery after intravitreal ocriplasmin injection. JAMA Ophthalmol. 2015;133(9):1091–1092. doi: 10.1001/jamaophthalmol.2015.1680. [DOI] [PubMed] [Google Scholar]
- 144.Thresher RJ, Ehrenberg M, Machemer R. Gas-mediated vitreous compression: an experimental alternative to mechanized vitrectomy. Graefes Arch Clin Exp Ophthalmol. 1984;221(5):192–198. doi: 10.1007/BF02134139. [DOI] [PubMed] [Google Scholar]
- 145.Miller B, Lean JS, Miller H, et al. Intravitreal expanding gas bubble. A morphologic study in the rabbit eye. Arch Ophthalmol. 1984;102(11):1708–1711. doi: 10.1001/archopht.1984.01040031392034. [DOI] [PubMed] [Google Scholar]
- 146.Chan CK, Wessels IF, Friedrichsen EJ. Treatment of idiopathic macular holes by induced posterior vitreous detachment. Ophthalmology. 1995;102(5):757–767. doi: 10.1016/s0161-6420(95)30958-x. [DOI] [PubMed] [Google Scholar]
- 147.Mori K, Saito S, Gehlbach PL, et al. Treatment of stage 2 macular hole by intravitreous injection of expansile gas and induction of posterior vitreous detachment. Ophthalmology. 2007;114(1):127–133. doi: 10.1016/j.ophtha.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 148.Chen T-C, Yang C-H, Yang C-M. Intravitreal expansile gas in the treatment of early macular hole: reappraisal. Ophthalmologica. 2012;228(3):159–166. doi: 10.1159/000337840. [DOI] [PubMed] [Google Scholar]
- 149.Jorge R, Costa RA, Cardillo JA, et al. Optical coherence tomography evaluation of idiopathic macular hole treatment by gas-assisted posterior vitreous detachment. Am J Ophthalmol. 2006;142(5):869–871. doi: 10.1016/j.ajo.2006.05.062. [DOI] [PubMed] [Google Scholar]