Abstract
The prognostic heterogeneity of multiple myeloma (MM) is largely due to different genetic abnormalities. Cytogenetic analysis has revealed that most of MM harbor chromosome aberrations. Amplification of 1q21 is one of the most common chromosomal aberrations. Interphase fluorescence in situ hybridization was applied to detect the 1q21 amplification in 86 Chinese patients with newly diagnosed MM. Amp(1q21) was found in totally 40 of 86 (46.5%) cases, among which 29 with three copies of 1q21 and eleven with at least four copies of 1q21. Further analysis revealed a significant difference of overall survival and progression-free survival among the three arms (P<0.05). Bortezomib could not significantly improve the overall survival for patients with 1q21 amplification (P>0.05). These findings suggest that 1q21 amplification with four copies or more is prognostic factor for adverse outcomes of MM patients. Furthermore, chromosome 1q21 gains predicted a poor overall survival even in those receiving bortezomib-based regimens.
Keywords: multiple myeloma, 1q21 amplification, I-FISH, prognosis
Introduction
Multiple myeloma (MM) is a plasma cell malignancy characterized by the accumulation of clonal plasma cells in the bone marrow. Although in recent years, treatment strategies have improved,1,2 MM remains largely an incurable disease. One difficulty in clinical management of MM is the high heterogeneity of the disease. In spite of the same diagnosis, MM patients display variable clinical manifestations and courses. The heterogeneity of this disease is largely due to different genetic and epigenetic abnormalities involved.3–5 These genetic and epigenetic alterations affect cell proliferation and/or apoptosis through different molecular pathways that converge on the malignant transformation of B-lymphocyte.
Amplification of 1q21 is one of the most common chromosomal aberrations in MM, which has been detected in 36%–47% of newly diagnosed MM patients by fluorescence in situ hybridization (FISH).6–10 It is still unclear for the relevant genes on 1q21. CKS1B and PMSD4 genes are supposed targets of this amplification, which increases tumor aggressiveness and mediating resistance to bortezomib.11,12 Overexpression of CKS1B was associated with poor prognosis in MM patients.13 Although a series of reports considered 1q21 amplification as a significant and independent poor prognostic factor, some other studies have failed to demonstrate the prognostic value of 1q21 amplification in MM.7,9,14
In the present study, we examined the amplification of 1q21 in Chinese patients with newly diagnosed MM by interphase FISH and analyzed the relationship between this genetic event and clinical features of patients. The resultant data could provide useful information for the prognosis and treatment of MM patients.
Materials and methods
Patients
This study was approved by the Hospital Review Board of First Affiliated Hospital, Nanjing Medical University. Signed consent forms were obtained from all the patients included in the study. A total of 86 newly diagnosed Chinese MM patients from the First Affiliated Hospital of Nanjing Medical University were included between October 2007 and May 2014. All patients were followed up for mortality and survival until August 2014.
Interphase FISH
The plasma cells were purified by CD138 MicroBeads (MiltenyiBiotec, Auburn, CA, USA) before FISH as previously described.15 It is verified that plasma cell purity was routinely at least 85% by CD38/CD45 flow cytometry. We hybridized the slides with fluorescent labeled probes according to the manufacturer’s instruction. The GLP 1q21 probe (Beijing GP Medical Technology Co., Ltd, People’s Republic of China) was used to detect 1q21 amplification.
Fluorescent images were captured with epifluorescence microscope (Leica DRMA2, Solms, Germany) equipped with charge-coupled device camera (AI company, UK), and using proper filters. We scored 200 nucleus for each probe and estimated the positive results based on European Myeloma Network recommendations: more than 20% of at least three copies of 1q21 for the gains.16
Statistical analysis
The statistical analysis was conducted using SPSS 13.0 software (SPSS, Chicago, IL, USA). Correlations between 1q21 amplification results and sex, age, immunoglobulin isotype, International Staging System (ISS), Durie and Salmon (DS) staging and mortality were performed by Pearson chi-square test. Correlations between FISH and β2-microglobulin (β2-MG), C-reactive protein, erythrocyte sedimentation rate, hemoglobin, platelet and albumin were performed using one-way analysis of variance. The survival time was counted from the date of diagnosis to the date of death, progression, or last follow-up. Overall survival (OS) and progression-free survival (PFS) distributions were estimated using the Kaplan–Meier method, and log-rank test was used in analyzing the differences between survival curves. Cox proportional hazards regression analysis was used to evaluate the prognostic impact with prognostically relevant chromosomal aberrations. P<0.05 indicated a statistical significant and all tests were two-sided.
Results
Characteristics of patients
The clinical data and biological characteristics of 86 newly diagnosed MM patients are summarized in Table 1. Median age was 58 (33–79) years and male/female ratio was 1.77 (55/31) (Table 1). The M-protein types found in the 86 patients were: IgG in 40, IgA in 25, IgD in one, and light chain in 18, nonsecretory in two. According to ISS, 13 patients were stage I, 29 were stage II, and 44 were stage III. Based on DS staging, five patients were stage I, eleven were stage II, and 70 were stage III. Of all patients, there were 43 patients treated with two to four courses of bortezomib-based regimens including VTD (bortezomib, thalidomide, and dexamethasone), VD (bortezomib and dexamethasone), PCD (bortezomib, cyclophosphamide, and dexamethasone), and PAD (bortezomib, doxorubicin, and dexamethasone); 43 patients with other treatments including VAD, MPT (melphalan, prednisone, and thalidomide), MTD (mel-phalan, thalidomide, and dexamethasone), TD (thalidomide and dexamethasone), TAD (thalidomide, doxorubicin, and dexamethasone), and CTD (cyclophosphamide, thalidomide, and dexamethasone). The median follow-up of all patients was 28.6 (0.5–80.23) months (m) and the median PFS was 14.0 (0.5–52.27) months. During the follow-up, 23 deaths occurred (Table 2).
Table 1.
Baseline clinical and biological characteristics of the analyzed MM patients
| Characteristics | All patients | Groups
|
|||
|---|---|---|---|---|---|
| 1q21 gain negative | Patients with three copies of 1q21 | Patients with four copies of 1q21 | P-value | ||
| No of patients (%) | 86 | 46 (53.5) | 29 (33.7) | 11 (12.8) | |
| Sex | |||||
| Female (%) | 31 (36.0) | 16 (18.6) | 11 (12.8) | 4 (4.7) | 0.962* |
| Male (%) | 55 (64.0) | 30 (34.9) | 18 (20.9) | 7 (8.1) | |
| Age median (years); range | 58 (33–79) | 59.5 (33–79) | 56 (42–78) | 58 (49–72) | 0.680** |
| Ig-isotype | |||||
| IgA (%) | 25 (29.1) | 9 (10.5) | 11 (12.8) | 5 (5.8) | 0.677* |
| IgG (%) | 40 (46.5) | 25 (29.1) | 10 (11.6) | 5 (5.8) | |
| IgD (%) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 1 (1.1) | |
| Nonsecretory (%) | 2 (2.3) | 1 (1.2) | 1 (1.2) | 0 (0.0) | |
| Light chain only (%) | 18 (20.9) | 10 (11.6) | 7 (8.1) | 1 (1.2) | |
| ISS stage | |||||
| I (%) | 13 (15.1) | 6 (7.0) | 6 (7.0) | 1 (1.2) | 0.213* |
| II (%) | 29 (33.7) | 14 (16.3) | 13 (15.1) | 2 (2.3) | |
| III (%) | 44 (51.2) | 26 (30.2) | 10 (11.6) | 8 (9.3) | |
| DS stage | |||||
| I (%) | 5 (5.8) | 4 (4.7) | 1 (1.1) | 0 (0.0) | 0.410* |
| II (%) | 11 (12.8) | 6 (7.0) | 5 (5.8) | 0 (0.0) | |
| III (%) | 70 (81.4) | 36 (41.9) | 23 (26.7) | 11 (12.8) | |
| Treatment | |||||
| Bortezomib (%) | 43 (47.8) | 25 (29.1) | 15 (17.6) | 3 (3.5) | 0.265* |
| Other (%) | 43 (47.8) | 21 (24.4) | 14 (16.3) | 8 (9.3) | |
Notes: Statistical tests used:
Pearson chi-square,
ANOVA.
Abbreviations: ANOVA, analysis of variance; Ig, immunoglobulin; ISS, International Staging System; DS, Durie and Salmon; MM, multiple myeloma.
Table 2.
1q21 amplification and correlation with laboratory findings and patients survival
| Characteristics | All patients | Groups
|
|||
|---|---|---|---|---|---|
| 1q21 gain negative | Patients with three copies of 1q21 | Patients with four copies of 1q21 | P-value | ||
| ESR (mm/h) mean; range | 94.00 (3–168) | 94.21 (6–157) | 92.25 (3–168) | 99.00 (44–125) | 0.947** |
| CRP (mg/L) mean; range | 9.99 (2.1–113) | 9.90 (2.1–58.2) | 8.13 (3.16–58.7) | 15.40 (2.97–113) | 0.501** |
| β2-microglobulin (mg/L) mean; range | 9.94 (1.67–134) | 11.84 (1.67–134) | 5.86 (2–20.5) | 11.63 (2.73–24.9) | 0.272** |
| Hemoglobin (g/L) mean; range | 86.62 (41–162) | 88.15 (43–137) | 86.76 (41–162) | 79.82 (59–123) | 0.629** |
| Platelet (109/L) mean; range | 168.88 (43–481) | 174.33 (43–481) | 172.46 (67–312) | 137.45 (49–337) | 0.406** |
| Albumin (g/L) mean; range | 31.64 (15.4–60.8) | 31.02 (15.4–45.4) | 33.14 (15.8–60.8) | 30.0 (21.4–44.4) | 0.493** |
| Mortality (dead/alive) | 21/65 | 9/37 | 8/21 | 4/7 | 0.450* |
Notes: Statistical tests used:
Pearson chi-square,
one-way ANOVA.
Abbreviations: ANOVA, analysis of variance; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Interphase FISH studies
The FISH results showed that 1q21 amplification was detected in 40 of 86 patients (46.5%), 29 with three copies of 1q21 (33.7%) and eleven with at least four copies of 1q21 (12.8%). There was no significant difference in sex, age, ISS, DS stage, immunoglobulin subtype, C-reactive protein, erythrocyte sedimentation rate, β2-MG, hemoglobin, platelet number, and albumin concentrations among those patients without 1q21 amplification, with three copies of 1q21 and with at least four copies of 1q21 (P>0.05) (Table 2).
Correlation of 1q21 amplification with OS
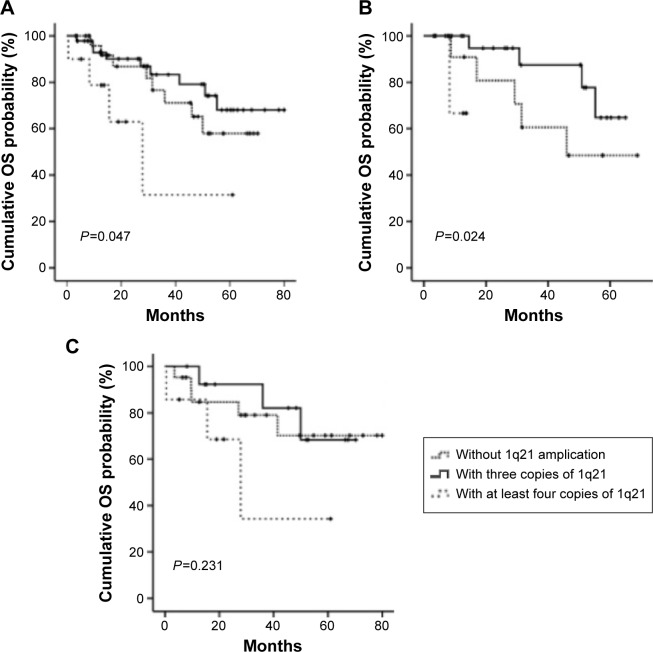
The OS was analyzed among the three arms: patients without 1q21 amplification (arm A), those with three copies of 1q21 (arm B), and those with at least four copies of 1q21 (arm C). There was significant difference among three arms (P=0.047) (Figure 1A). The OS was shorter in patients with at least four copies of 1q21 than those with two or three copies of 1q21 (14.6 vs 31.0 or 31.63 months). Similar results were found in the multivariate analysis, which was analyzed in combination with other chromosome aberrations including Del(13q14), Del(17p13), and IGH translocation. While the OS was not significantly shorter in patients with three copies of 1q21 than those without 1q21 gains (hazard ratio =1.613, 95% confidence interval: 0.593–4.386, P=0.349) (Table 3).
Figure 1.
Kaplan–Meier survival curves for OS.
Notes: (A) All patients, (B) patients treated with bortezomib, and (C) patients treated with other regimens.
Abbreviation: OS, overall survival.
Table 3.
Cox (multivariate) analysis of risk factors for PFS and OS
| Variable | PFS
|
OS
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Copy number of amp(1q21) | 0.001 | 0.041 | ||||
| Three copies vs two copies | 2.344 | 0.882–6.233 | 0.088 | 1.613 | 0.593–4.386 | 0.349 |
| Four copies vs two copies | 11.341 | 3.037–42.348 | 0.000 | 6.209 | 1.504–25.635 | 0.012 |
| Del(13q14) | 0.850 | 0.342–2.109 | 0.726 | 0.364 | 0.114–1.165 | 0.089 |
| Del(17p13) | 4.864 | 1.403–16.862 | 0.013 | 1.334 | 0.307–5.798 | 0.701 |
| IGH translocation | 1.738 | 0.655–4.612 | 0.267 | 2.353 | 0.803–6.893 | 0.119 |
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; vs, versus.
In a stratified analysis by therapeutic schedule, no significant difference of OS between bortezomib-treated patients with 1q21 amplification and without amplification (15.33 vs 30.78 months, P=0.086). However, the OS of patients with at least four copies of 1q21 is remarkably shorter than those with two or three copies of 1q21 (12.70 vs 30.76 or 29.23 months, P=0.024) by treating with bortezomib (Figure 1B). On the other hand, in those treated without bortezomib, there is no significant difference of OS among the three arms (P=0.231) (Figure 1C).
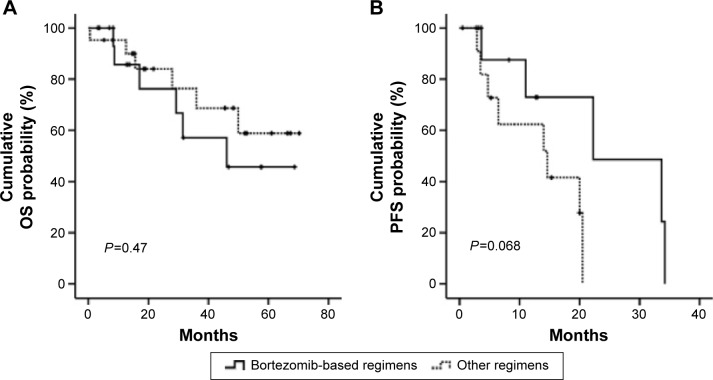
We further investigated the OS of patients with 1q21gains between treatment with or without bortezomib. Out of 39 patients, 18 patients were treated with bortezomib-based regimens. No significant difference was found between the patients treated with and without bortezomib (P=0.470 and 0.578, respectively) (Figure 2).
Figure 2.
Bortezomib could not improve the OS (A) or PFS (B) of patients with 1q21 gains.
Abbreviations: OS, overall survival; PFS, progression-free survival.
Correlation of 1q21 amplification with PFS
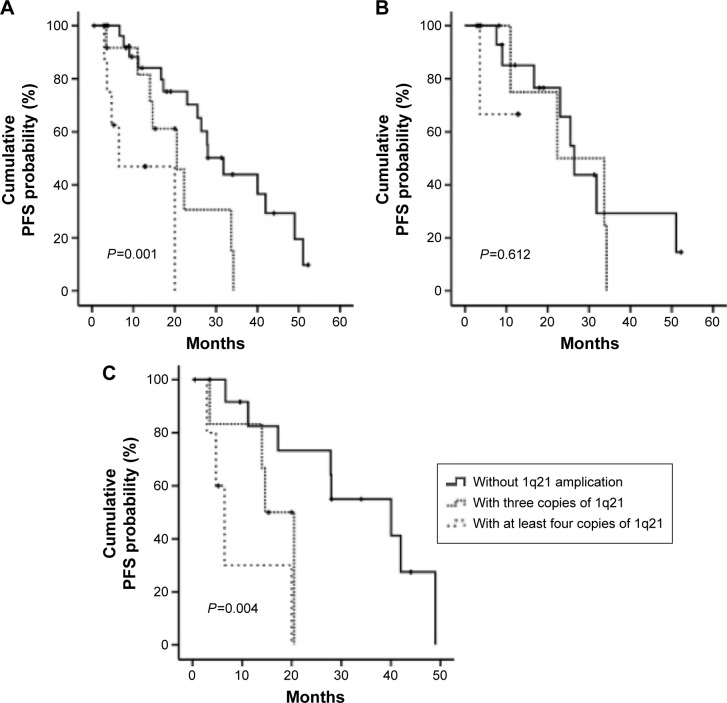
The PFS was also analyzed among the three arms, and significant difference was found (P=0.001). With a median follow-up of 14 months, the PFS was shorter in patients with three or at least four copies of 1q21 than those without 1q21 gains (14.0 or 5.72 vs 21.0 months, P=0.042 or 0.001, respectively). However, there was no significant difference between patients with three copies and at least four copies of 1q21 (P=0.067) (Figure 3A).
Figure 3.
Kaplan–Meier survival curves for PFS.
Notes: (A) All patients; (B) patients treated with bortezomib; (C) patients treated with other regimens.
Abbreviations: OS, overall survival; PFS, progression-free survival.
The PFS for the patients treated with and without bortezomib, respectively, was also analyzed among the three arms. For patients treated with bortezomib, there was no significant difference of PFS among the three arms (12.7 vs 18.5 or 9.62 months, P=0.612) (Figure 3B). Whereas, in those treated without bortezomib, the PFS of patients with at least four copies of 1q21 is remarkably shorter than those with two or three copies of 1q21 (4.98 vs 27.9 or 14.9 months, P=0.004) (Figure 3C).
We further investigated the PFS of patients with 1q21 gains between treatment with or without bortezomib-based regimens. No significant difference was found between the patients treated with or without bortezomib. And the PFS for patients treated with bortezomib was similar as those treated without bortezomib (11.0 vs 10.23 months, P=0.068) (Figure 2).
Discussion
In the current study, the rate of 1q21 gain (46.7%) appeared to be similar as that found in previous studies, suggesting that 1q21 gain aberration may represent the same character for Chinese patients when compared to Western population. Somatic genetic aberrations of chromosome 1 are frequently observed in the malignant cells of MM patients. In particular, the long arm of chromosome 1 is preferentially involved in amplification and the short arm in deletions.17 The amplification lead to a dosage effect of genes that may contribute to the progression of MM. One of the genes located at 1q21 is CKS1B, which codes an essential cofactor for SKP2-dependent ubiquitination of p27Kip1.10,18,19 p27Kip1, encoded by tumor suppressor gene CDKN1B, is capable of binding and inactivating cyclin/CDK2 complex, which lead to cell cycle arrest at G1/S transition. Assessment of the expression levels of the oncogene CKS1B and its target gene CDKN1B may be one direction for better understanding the functional implication of 1q21 amplification. An inverse correlation between CKS1B overexpression and decreased levels of p27Kip1 has been observed in the plasma cells from MM patients20 and CKS1B nuclear expression was found to be a predictor for adverse survival of MM patients. Besides, among other genetic and epigenetic mechanisms leading to the overexpression of CKS1B, increased copy number of CKS1B through amplification of 1q21 has also been observed in MM patients.10,21 However, confirmation on the significance of chromosome 1 amplification for the prognosis of MM also warrant the study efforts in identification of the gene(s) and relevant pathways involved in the progression of MM.
Previous studies showed the PFS and OS were significantly low in patients with 1q21 amplification.8,20 In a study of 286 cases with newly diagnosed MM, An et al22 demonstrate that copy numbers of 1q21 increased with progression of myeloma. Our study did not find the significant difference of OS between patients with three copies of 1q21 and those without 1q21 amplification. However, we found that the patients with more than three copies of 1q21 had the shortest OS as well as PFS. These data suggested that patients with 1q21 gains especially at least three copies of 1q21 may represent a special category of MM characterized by a more aggressive course and a faster progression. Our results are in agreement with the conclusion of the previous publication that increased copy numbers of 1q21 gains are linked with adverse outcome in MM. And more than three copies of 1q21 have been added to the group of high-risk aberrations.23 The possible reason could be a dosage effect of genes located at chromosome lq21, particularly CKS1B and the interleukin-6 receptor genes.14,24 However, given the relative small sample in our study, further confirmation should be addressed in the future.
Chang et al8 reported that patients with 1q21 gain had a significantly shorter OS and PFS than patients without such abnormality in relapsed or refractory MM treated with bortezomib. In the stratification analysis of the newly diagnosed patients treated with bortezomib, our results also showed that patients with 1q21 gains suffered from the shorter OS than those without 1q21 gains. On the other hand, for patients treated with bortezomib, no differences were found in PFS between patients with and without 1q21 amplification, which suggest that bortezomib could only overcome the poor prognosis of 1q21 gain in PFS rather than OS. For all patients with more than four copies of 1q21, our study failed to suggest a better PFS or OS in patients treated with bortezomib than those treated without bortezomib. Similar findings have been reported by An et al22 that patients with 1q21 amplification hardly benefit significantly from regimens including bortezomib.
Conclusion
We conducted interphase FISH to assess 1q21 amplification in 86 newly diagnosed MM patients and found that 1q21 amplification with four copies or more is prognostic factor for adverse outcomes of MM patients. Furthermore, chromosome 1q21 gains predicted poor OS even in those receiving bortezomib-based regimens.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81071946, 81241074, 81372540); The Scientific Research Starting Foundation for Returned Overseas Chellonese Scholars, Ministry of Education (2012); Jiangsu Province’s Medical Elite Program (RC201148); “333” project of Jiangsu Province (BRA2011217); National Public Health Grand Research Foundation (201202017); Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institute (JX10231801); Program for Development of Innovative Research Teams in the First Affiliated Hospital of Nanjing Medical University; Project of National Key Clinical Specialty; National Science & Technology Pillar Program (2014BAI09B12), and project funded by Jiangsu Provincial Special Program of Medical Science (BL2014086).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Avet-Loiseau H, Soulier J, Fermand JP, et al. Impact of high-risk cytogenetics and prior therapy on outcomes in patients with advanced relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone. Leukemia. 2010;24(3):623–628. doi: 10.1038/leu.2009.273. [DOI] [PubMed] [Google Scholar]
- 2.Prince HM, Schenkel B, Mileshkin L. Assessing response rates in clinical trials of treatment for relapsed or refractory multiple myeloma: a study of bortezomib and thalidomide. Leukemia. 2007;21(4):818–820. doi: 10.1038/sj.leu.2404573. author reply 821. [DOI] [PubMed] [Google Scholar]
- 3.Terpos E, Eleutherakis-Papaiakovou V, Dimopoulos MA. Clinical implications of chromosomal abnormalities in multiple myeloma. Leuk Lymphoma. 2006;47(5):803–814. doi: 10.1080/10428190500464104. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor P, Fonseca R, Rajkumar SV, et al. Evidence for cytogenetic and fluorescence in situ hybridization risk stratification of newly diagnosed multiple myeloma in the era of novel therapie. Mayo Clin Proc. 2010;85(6):532–537. doi: 10.4065/mcp.2009.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23(12):2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang H, Qi X, Trieu Y, et al. Multiple myeloma patients with CKS1B gene amplification have a shorter progression-free survival post-autologous stem cell transplantation. Br J Haematol. 2006;135(4):486–491. doi: 10.1111/j.1365-2141.2006.06325.x. [DOI] [PubMed] [Google Scholar]
- 7.Hanamura I, Stewart JP, Huang Y, et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood. 2006;108(5):1724–1732. doi: 10.1182/blood-2006-03-009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang H, Trieu Y, Qi X, et al. Impact of cytogenetics in patients with relapsed or refractory multiple myeloma treated with bortezomib: adverse effect of 1q21 gains. Leuk Res. 2011;35(1):95–98. doi: 10.1016/j.leukres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Shaughnessy JD, Jr, Haessler J, van Rhee F, et al. Testing standard and genetic parameters in 220 patients with multiple myeloma with complete data sets: superiority of molecular genetics. Br J Haematol. 2007;137(6):530–536. doi: 10.1111/j.1365-2141.2007.06586.x. [DOI] [PubMed] [Google Scholar]
- 10.Shaughnessy J. Amplification and overexpression of CKS1B at chromosome band 1q21 is associated with reduced levels of p27Kip1 and an aggressive clinical course in multiple myeloma. Hematology. 2005;10(Suppl 1):117–126. doi: 10.1080/10245330512331390140. [DOI] [PubMed] [Google Scholar]
- 11.Shaughnessy JD, Jr, Qu P, Usmani S, et al. Pharmacogenomics of bortezomib test-dosing identifies hyperexpression of proteasome genes, especially PSMD4, as novel high-risk feature in myeloma treated with total therapy 3. Blood. 2011;118(13):3512–3524. doi: 10.1182/blood-2010-12-328252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhan F, Colla S, Wu X, et al. CKS1B, overexpressed in aggressive disease, regulates multiple myeloma growth and survival through SKP2- and p27Kip1-dependent and -independent mechanisms. Blood. 2007;109(11):4995–5001. doi: 10.1182/blood-2006-07-038703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi L, Wang S, Zangari M, et al. Over-expression of CKS1B activates both MEK/ERK and JAK/STAT3 signaling pathways and promotes myeloma cell drug-resistance. Oncotarget. 2010;1(1):22–33. doi: 10.18632/oncotarget.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca R, Van Wier SA, Chng WJ, et al. Prognostic value of chromosome 1q21 gain by fluorescent in situ hybridization and increase CKS1B expression in myeloma. Leukemia. 2006;20(11):2034–2040. doi: 10.1038/sj.leu.2404403. [DOI] [PubMed] [Google Scholar]
- 15.Avet-Loiseau H, Facon T, Grosbois B, et al. Oncogenesis of multiple myeloma: 14q32 and 13q chromosomal abnormalities are not randomly distributed, but correlate with natural history, immunological features, and clinical presentation. Blood. 2002;99(6):2185–2191. doi: 10.1182/blood.v99.6.2185. [DOI] [PubMed] [Google Scholar]
- 16.Ross F, Avet-Loiseau H, Drach J, Hernandez Rivas J. European Myeloma Network recommendations for FISH in myeloma. Haematologica. 2007;92(s2):100–101. [Google Scholar]
- 17.Shaughnessy JD, Jr, Zhan F, Burington BE, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109(6):2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 18.Kroger N, Schilling G, Einsele H, et al. Deletion of chromosome band 13q14 as detected by fluorescence in situ hybridization is a prognostic factor in patients with multiple myeloma who are receiving allogeneic dose-reduced stem cell transplantation. Blood. 2004;103(11):4056–4061. doi: 10.1182/blood-2003-12-4435. [DOI] [PubMed] [Google Scholar]
- 19.Shaughnessy J, Jr, Tian E, Sawyer J, et al. Prognostic impact of cytogenetic and interphase fluorescence in situ hybridization-defined chromosome 13 deletion in multiple myeloma: early results of total therapy II. Br J Haematol. 2003;120(1):44–52. doi: 10.1046/j.1365-2141.2003.03948.x. [DOI] [PubMed] [Google Scholar]
- 20.Nemec P, Zemanova Z, Greslikova H, et al. Gain of 1q21 is an unfavorable genetic prognostic factor for multiple myeloma patients treated with high-dose chemotherapy. Biol Blood Marrow Transplant. 2010;16(4):548–554. doi: 10.1016/j.bbmt.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt-Wolf IG, Glasmacher A, Hahn-Ast C, et al. Chromosomal aberrations in 130 patients with multiple myeloma studied by interphase FISH: diagnostic and prognostic relevance. Cancer Genet Cytogenet. 2006;167(1):20–25. doi: 10.1016/j.cancergencyto.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 22.An G, Xu Y, Shi L, et al. Chromosome 1q21 gains confer inferior outcomes in multiple myeloma treated with bortezomib but copy number variation and percentage of plasma cells involved have no additional prognostic value. Haematologica. 2014;99(2):353–359. doi: 10.3324/haematol.2013.088211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119(4):940–948. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- 24.Kim SY, Min HJ, Park HK, et al. Increased copy number of the interleukin-6 receptor gene is associated with adverse survival in multiple myeloma patients treated with autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):810–820. doi: 10.1016/j.bbmt.2011.01.002. [DOI] [PubMed] [Google Scholar]