Abstract
Premise of the study:
Low-copy nuclear gene primers were developed for phylogenetic studies across the Melastomataceae.
Methods and Results:
Total genomic libraries from eight species in the Melastomataceae along with one transcriptome were used for marker identification and primer design. Eight exon-primed intron-crossing markers were amplified with success in taxa of nine tribes in the Melastomataceae. The new markers were directly sequenced for eight samples of closely related species of Miconia (Chaenanthera clade) in the tribe Miconieae. The DNA sequences for the eight loci ranged from 660 to 818 aligned base pairs. Compared with four commonly used markers in other studies, the loci developed here had a higher number of variable sites than plastid spacers (7–16 vs. 26–45) and comparable variation to the ribosomal spacers (28–39).
Conclusions:
The novel primer pairs should be useful for a broad range of studies of systematics and evolution in the diverse Melastomataceae.
Keywords: Melastomataceae, Miconieae, phylogeny, systematics, single copy
The Melastomataceae Juss. are a diverse group of plants found mainly and throughout the tropical regions (Clausing and Renner, 2001). The family has ca. 5000 species showing great morphological and ecological variability distributed across different habitats (Clausing and Renner, 2001). Recently, several local radiations with great potential to add to our understanding of evolution in tropical regions have been uncovered by molecular studies (Goldenberg et al., 2012; Michelangeli et al., 2013; Michelangeli et al., in prep.). The plastid rbcL and ndhF genes and the rpl16 intron have been the popular choice for inferring relationships among major clades in the family (Clausing and Renner, 2001; Goldenberg et al., 2012; Michelangeli et al., 2014), while the plastid spacers accD-psaI, atpF-atpH, psbK-psbL, and trnS-trnG, along with the ribosomal spacers (nrETS and nrITS), have commonly being used within lineages of Melastomataceae (Bécquer-Granados et al., 2008; Reginato et al., 2010; Michelangeli et al., 2013; Kriebel et al., 2015). Low-copy nuclear genes have scarcely been explored, being restricted to partial sequences of the genes GapC and waxy (Stone, 2006; Reginato and Michelangeli, in prep.), very likely due to the lack of specific primers. Low levels of variation in plastid and ribosomal DNA sequences associated with incomplete lineage sorting and/or lateral gene transference have yielded poorly resolved and/or conflicting hypotheses to address evolutionary questions in several lineages within this family (Reginato and Michelangeli, in prep.). Low-copy nuclear markers will likely improve understanding of lineage evolution in Melastomataceae. Here, we developed primer pairs for eight putative single-copy nuclear genes. Amplification was tested in several genera from distinct tribes in the family, while further direct sequencing was performed for eight species of the Chaenanthera clade, a ca. 8-Myr-old lineage within the tribe Miconieae (Michelangeli et al., in prep). Phylogenetic information of the new markers along with previously sequenced markers for the same Chaenanthera samples is presented.
METHODS AND RESULTS
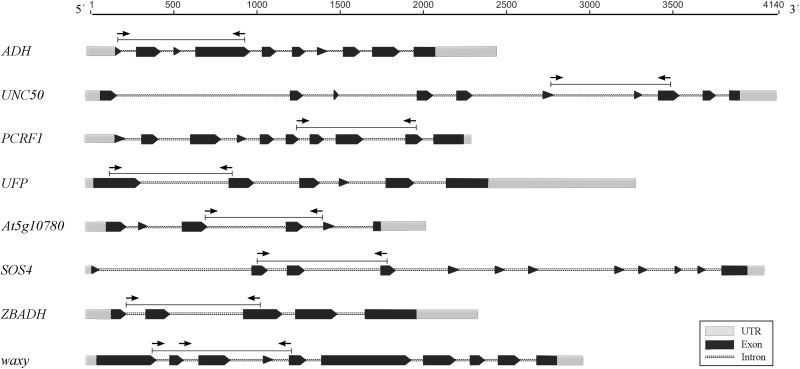
Genomic libraries from eight species in the Melastomataceae (five in the tribe Miconieae and three in the Melastomeae) generated for a phylogenomic study (Michelangeli et al., in prep.) along with the transcriptome of Tetrazygia biflora (Cogn.) Urb. (Miconieae) were used for primer design. Total genomic DNA was isolated from silica gel–dried tissue using the QIAGEN DNAeasy Plant Mini Kit (QIAGEN, Valencia, California, USA) following the protocol suggested by Alexander et al. (2007). Total DNA was then quantified using a NanoDrop Spectrophotometer (Thermo Scientific, Waltham, Massachusetts, USA). Total genomic libraries were generated and barcoding was performed at Cold Spring Harbor Laboratory (Cold Spring Harbor, New York, USA) on an Illumina HiSeq 2000 platform (Illumina, San Diego, California, USA) on a single multiplex lane. The number of total reads yielded was on average ca. 20 Gb per sample (SD = 3 Gb). Paired reads were imported into Geneious 7.1 (Biomatters Ltd., Auckland, New Zealand), trimmed by quality (at 0.05 probability), and de novo assembled (Geneious Assembler, “low sensitivity” option, default settings). The assembled T. biflora transcripts were downloaded from the oneKP transcriptome database (www.onekp.com). MegaBLAST searches were performed on the T. biflora transcripts using the default parameters and mapped against the COSII data set (Wu et al., 2006) along with the Arabidopsis adh and waxy genes. Mapping was performed in Geneious 7.1, using the medium sensitivity settings, with the “minimum overlap” parameter set to 500. Matches with a single hit and with more than 80% of coverage to the reference were selected for further analyses. Approximately 340 transcripts passed these criteria and were further mapped against the pool of total genomic de novo assembled contigs from all eight libraries. Mapping was performed using the high-sensitivity settings, with the “allowing gap size” parameter set to 1000. Then, we selected eight matches with high coverage, appropriate intronic-sized regions, and a single hit per sample. Primers flanking the target intronic regions were designed with Primer3, using the default settings (Rozen and Skaletsky, 1999). Primer sequences and their putative Arabidopsis homolog are presented in Table 1, and the gene models showing primer locations are shown in Fig. 1.
Table 1.
Primer sequences for the eight putative single-copy nuclear markers developed to amplify across Melastomataceae.
| Locus | Primer sequences (5′–3′) | Putative Arabidopsis homolog | Putative product | Ta (°C) |
| ADH | F: TGGCAAGCACYGCTGGTCAGG | At1g77120 | Alcohol dehydrogenase | 56 |
| R: GAGATGCCGCAGCTSAGGA | ||||
| UNC50 | F: CGGGAGGAGGCACCAAATAG | At2g15240 | UNC-50 family protein | 53 |
| R: AGARGCGGCCACCATGAAGA | ||||
| PCRF1 | F: RCTCAAGTTCGAGAGTGGAGT | At2g47020 | Peptide chain release factor 1 | 50 |
| R: YAGCTTTGACCGATCCRAGT | ||||
| UFP | F: AAGTGGAGCGGGAAGGAGTA | At4g06599 | Ubiquitin family protein | 53 |
| R: RGGAGMCTCCACTTGGTCCA | ||||
| At5g10780 | F: AGYGCCTTGCAGGGTGTTGG | At5g10780 | Unknown protein | 50 |
| R: RAGTAGGCCCAATGTGTTTA | ||||
| SOS4 | F: GCAACAAGTCGGCTGTCTTT | At5g37850 | Pyridoxal kinase | 51 |
| R: CGGAGCTTCTTGACAACTTCC | ||||
| ZBADH | F: TCCACGAGCTYGGGGGTRCC | At5g61510 | Zinc-binding alcohol dehydrogenase family | 53 |
| R: GGCCGGAAGAATCTGCTCTT | ||||
| waxy | F1: GRGGTCTTGGGGACGTGCTC | At1g32900 | Granule-bound starch synthase 1 | 54 |
| F2: ACACTTGCGTGGTCGTYCAG | ||||
| R: AGCAGTGTGCCARTCGTTGG |
Note: Ta = annealing temperature used in the PCR.
Fig. 1.
Gene models for the eight putative single-copy nuclear markers developed to amplify across Melastomataceae. Primer locations are indicated with arrows; intron/exon boundaries are based on the Tetrazygia biflora transcripts.
The primers were tested on total genomic DNA of eight species from different tribes across the family (PCR and electrophoresis only, voucher information in Appendix 1), while PCR and direct sequencing was performed for eight closely related species of Miconia Ruiz & Pav. within the Chaenanthera clade in the tribe Miconieae (voucher information and GenBank accessions in Appendix 2). PCR amplification was performed using the following mix: 7.5 μL of EconoTaq Plus Green Master Mix (Lucigen Corporation, Middleton, Wisconsin, USA), 2 μL of MgCl2 (25 mM), 2 μL of bovine serum albumin (BSA) (10 mg/mL), 1.2 μL of each primer (3 μM), and 0.7 μL of DNA template. A general amplification program was used, varying the annealing temperature for each marker (see Table 1): 2 min at 94°C; 36 cycles of 45 s at 94°C, 45 s at 50–56°C, 1 min and 15 s at 72°C; and a final extension of 10 min at 72°C. Direct sequencing was performed with the same forward and reverse primers used for amplification at the High-Throughput Genomics Unit sequencing service at the University of Washington (Seattle, Washington, USA). Contigs were assembled with Sequencher 4.9 (GeneCodes Corporation, Ann Arbor, Michigan, USA), and sequence alignment was performed with MAFFT version 7 using the strategy E-INS-i (Katoh, 2013).
The eight markers were successfully amplified in the majority of the nine genera tested (Appendix 1). The only three failures were registered for the genus Mouriri Aubl., a member of the small subfamily Olisbeoideae, which is sister to the remainder of the family (Clausing and Renner, 2001). Direct sequencing was successful for all samples of Miconia included in this study. However, sequencing was problematic for some other samples of the At5g10780 marker (also in the Chaenanthera clade, not included in this study), due to a repetitive sequence of ca. 15 Ts. Sequences of the eight amplified loci for the samples of the Chaenanthera clade of Miconia ranged from 660 to 818 aligned base pairs, while 20 to 45 variable and one to six parsimony informative sites were observed across the individual alignments. The level of polymorphism of most of the new markers is comparable to the ribosomal spacers nrETS and nrITS, and higher than the chloroplast spacers that are widely used in phylogenetic studies in the Melastomataceae (Table 2).
Table 2.
Summary statistics of the eight putative single-copy nuclear markers developed to amplify across Melastomataceae. Metrics are given for alignments including the same eight samples of the Chaenanthera clade and are compared with markers previously sequenced for these samples (indicated by an asterisk, accD-psaI and psbK-L are chloroplast spacers, and rETS and rITS are ribosomal spacers).
| Locus | Mean no. of bases (range) | Aligned bases | Variable sites (%) | PIS (%) |
| ADH | 768 (745–808) | 809 | 32 (4) | 3 (0.4) |
| At5g10780 | 799 (740–815) | 818 | 27 (3.3) | 1 (0.1) |
| PCRF1 | 671 (667–691) | 692 | 20 (2.9) | 5 (0.7) |
| SOS4 | 730 (728–732) | 732 | 26 (3.6) | 2 (0.3) |
| UFP | 654 (645–658) | 660 | 35 (5.3) | 5 (0.8) |
| UNC50 | 669 (664–670) | 670 | 26 (3.9) | 1 (0.1) |
| waxy | 767 (767–768) | 770 | 38 (4.9) | 6 (0.8) |
| ZBADH | 707 (697–764) | 776 | 45 (5.8) | 5 (0.6) |
| accD-psaI* | 675 (671–697) | 699 | 16 (2.3) | 3 (0.4) |
| psbK-L* | 350 (349–352) | 352 | 7 (2) | 2 (0.6) |
| rETS* | 586 (585–587) | 590 | 39 (6.6) | 6 (1) |
| rITS* | 802 (801–803) | 807 | 28 (3.5) | 8 (1) |
Note: PIS = parsimony informative sites.
CONCLUSIONS
Amplification of eight novel primer pairs was successful in samples from nine tribes across Melastomataceae for a majority of markers. Although the usefulness of the markers at a broader family scale still needs to be evaluated, the markers should be useful to increase resolution of phylogenetic relationships within lineages in the family.
Appendix 1.
List of genera across the Melastomataceae in which amplification of the eight putative single-copy nuclear markers was successful.
| Clade | Species | ADH | At5g10780 | PCRF1 | SOS4 | UFP | UNC50 | waxy | ZBADH | Voucher (Herbarium)a |
| Bertolonieae | Bertolonia mosenii Cogn. | + | + | + | + | + | + | + | + | Goldenberg, R. 804 (NY, UPCB) |
| Blakeeae | Blakea bracteata Gleason | + | + | + | + | + | + | + | + | Goldenberg, R. 964 (NY, UPCB) |
| Melastomeae | Brachyotum quinquenerve (Ruiz & Pav.) Triana | + | + | + | + | + | + | + | + | Michelangeli, F. A. 1979 (NY) |
| Marcetia alliance | Comolia microphylla Benth. | + | + | + | + | + | + | + | + | Michelangeli, F. A. 2499 (NY) |
| Henrietteeae | Henriettea trachyphylla (Triana) Penneys, Michelang., Judd & Almeda | + | + | + | + | + | + | + | + | Almeda, F. 10165 (CAS) |
| Cambessedesia alliance | Huberia consimilis Baumgratz | + | + | + | + | + | + | + | + | Michelangeli, F. A. 1618 (NY) |
| Merianieae | Meriania nobilis Triana | + | + | + | + | + | + | + | + | Clark, J. L. 13051 (UNA) |
| Olisbeoideae | Mouriri chamissoana Cogn. | — | — | + | + | + | + | + | — | Reginato, M. 1025 (UPCB) |
Note: + = successful amplification; — = no amplification.
Vouchers are deposited at the herbaria of the California Academy of Sciences (CAS), San Francisco, California, USA; New York Botanical Garden (NY), Bronx, New York, USA; University of Alabama (UNA), Tuscaloosa, Alabama, USA; or Universidade Federal do Paraná (UPCB), Curitiba, Paraná, Brazil.
Appendix 2.
Voucher information and GenBank accessions of the species in the Chaenanthera clade sequenced for the eight putative single-copy nuclear markers.
| Species | ADH | At5g10780 | PCRF1 | SOS4 | UFP | UNC50 | waxy | ZBADH | Voucher (Herbarium)a |
| Miconia caudigera DC. | KT377070 | KT377078 | KT377086 | KT377094 | KT377102 | KT377110 | KT377118 | KT377126 | Lima, J. 729 (NY) |
| Miconia dorsaliporosa R. Goldenb. & Reginato | KT377071 | KT377079 | KT377087 | KT377095 | KT377103 | KT377111 | KT377119 | KT377127 | Kollmann, L. 8572 (UPCB) |
| Miconia inaequidens (DC.) Naudin | KT377072 | KT377080 | KT377088 | KT377096 | KT377104 | KT377112 | KT377120 | KT377128 | Goldenberg, R. 732 (NY, UPCB) |
| Miconia longicuspis Cogn. | KT377073 | KT377081 | KT377089 | KT377097 | KT377105 | KT377113 | KT377121 | KT377129 | Kollmann, L. 8562 (UPCB) |
| Miconia paucidens DC. | KT377074 | KT377082 | KT377090 | KT377098 | KT377106 | KT377114 | KT377122 | KT377130 | UPCB 59855 |
| Miconia ramboi Brade | KT377075 | KT377083 | KT377091 | KT377099 | KT377107 | KT377115 | KT377123 | KT377131 | Goldenberg, R. 793 (NY, UPCB) |
| Miconia staminea (Desr.) DC. | KT377076 | KT377084 | KT377092 | KT377100 | KT377108 | KT377116 | KT377124 | KT377132 | Goldenberg, R. 784 (UPCB) |
| Miconia tristis Spring | KT377077 | KT377085 | KT377093 | KT377101 | KT377109 | KT377117 | KT377125 | KT377133 | Goldenberg, R. 812 (NY, UPCB) |
Vouchers are deposited at the herbaria of the New York Botanical Garden (NY), Bronx, New York, USA, and/or Universidade Federal do Paraná (UPCB), Curitiba, Paraná, Brazil.
LITERATURE CITED
- Alexander P. J., Rajanikanth G., Bacon C. D., Bailey C. D. 2007. Recovery of plant DNA using a reciprocating saw and silica-based columns. Molecular Ecology Notes 7: 5–9. [Google Scholar]
- Bécquer-Granados E. R., Neubig K. M., Judd W. S., Michelangeli F. A., Abbott J. R., Penneys D. S. 2008. Preliminary molecular phylogenetic studies in Pachyanthus (Miconieae, Melastomataceae). Botanical Review 74: 37–52. [Google Scholar]
- Clausing G., Renner S. S. 2001. Molecular phylogenetics of Melastomataceae and Memecylaceae: Implications for character evolution. American Journal of Botany 88: 486–498. [PubMed] [Google Scholar]
- Goldenberg R., de Fraga C. N., Fontana A. P., Nicolas A. N., Michelangeli F. A. 2012. Taxonomy and phylogeny of Merianthera (Melastomataceae). Taxon 61: 1040–1056. [Google Scholar]
- Katoh S. 2013. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel R., Michelangeli F. A., Kelly L. M. 2015. Discovery of unusual anatomical and continuous characters in the evolutionary history of Conostegia (Miconieae: Melastomataceae). Molecular Phylogenetics and Evolution 82: 289–313. [DOI] [PubMed] [Google Scholar]
- Michelangeli F. A., Guimaraes P. J., Penneys D. S., Almeda F., Kriebel R. 2013. Phylogenetic relationships and distribution of new world Melastomeae (Melastomataceae). Botanical Journal of the Linnean Society 171: 38–60. [Google Scholar]
- Michelangeli F. A., Ulloa C. U., Sosa K. 2014. Quipuanthus, a new genus of Melastomataceae from the foothills of the Andes in Ecuador and Peru. Systematic Botany 39: 533–540. [Google Scholar]
- Reginato M., Michelangeli F. A., Goldenberg R. 2010. Phylogeny of Pleiochiton (Melastomataceae, Miconieae): Total evidence. Botanical Journal of the Linnean Society 162: 423–434. [Google Scholar]
- Rozen S., Skaletsky H. 1999. Primer3 on the WWW for general users and for biologist programmers. In S. Misener and S. A. Krawetz [eds.], Methods in molecular biology, vol. 132: Bioinformatics methods and protocols, 365–386. Humana Press, Totowa, New Jersey, USA. [DOI] [PubMed] [Google Scholar]
- Stone R. D. 2006. Phylogeny of major lineages in Melastomataceae, subfamily Olisbeoideae: Utility of nuclear glyceraldehyde 3-phosphate dehydrogenase (GapC) gene sequences. Systematic Botany 31: 107–121. [Google Scholar]
- Wu F., Mueller L. A., Crouzillat D., Petiard V., Tanksley S. D. 2006. Combining bioinformatics and phylogenetics to identify large sets of single-copy orthologous genes (COSII) for comparative, evolutionary and systematic studies: A test case in the euasterid plant clade. Genetics 174: 1407–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]