Abstract
It has previously been suggested that reactive oxygen species (ROS) are involved in the pathogenesis of chronic inflammatory diseases, which entails the initial activation of pro-inflammatory cytokines to facilitate leukocyte transmigration. The present study investigated whether intracellular superoxide dismutase (SOD) suppressed monocyte endothelial trafficking and transmigration. Human umbilical vein endothelial cells (HUVECs) and THP-1 monocytes were activated by the cytokine tumor necrosis factor-α (TNF-α) in the absence and presence of cell-permeable transactivator of transcription (Tat)-SOD protein. External stimulation with SOD was conducted using endothelial cells and monocytes. Purified cell-permeable Tat-SOD, but not non-targeted SOD, at 1–3 µM was transduced into endothelial cells in a time- and dose-dependent manner. Non-toxic Tat-SOD at ≤0.5 µM, but not 1 µM SOD, blocked the monocyte-endothelium interactions by inhibiting the TNF-α-induced stimulation of vascular cell adhesion molecule-1 (VCAM-1) in HUVECs and integrin β1 in THP-1 cells. Endothelial VCAM-1 induction by TNF-α was responsible for superoxide anion production being quenched by N-acetyl-cysteine and Tat-SOD. SOD treatment markedly inhibited superoxide anion production induced by TNF-α, but no inhibition of endothelial transmigration was noted. Tat-SOD prevented transendothelial monocyte migration by firmly localizing occludin-1, platelet/endothelial cell adhesion molecule-1 (PECAM-1) and vascular endothelial-cadherin present in paracellular junctions and inhibiting endothelial induction and activation of matrix-degrading membrane type-1 (MT-1) matrix metalloproteinase (MMP), MMP-2 and MMP-9. By contrast, treatment with 1 µM SOD did not have such effects. Furthermore, transduced Tat-SOD hindered nuclear transactivation of nuclear factor-κB (NF-κB), modulating the induction of paracellular junction proteins and matrix-degrading MMP in TNF-α-stimulated HUVECs. Transduced Tat-SOD, but not external SOD, impeded cytokine-induced endothelial adhesion and the transmigration of monocytes. Thus, we suggest that transduced Tat-SOD qualifies as an atheroprotective agent against oxidation-driven and inflammation-associated atherosclerosis.
Keywords: atherosclerosis, nuclear factor-κB, occludin-1, superoxide anion, Tat-superoxide dismutase, vascular cell adhesion molecule-1
Introduction
It has previously been suggested that reactive oxygen species (ROS) are involved in the pathogenesis and progression of atherosclerotic diseases (1). Antioxidants protect against atherosclerosis by preventing ROS-induced injury to endothelial cells, which appears to be mediated by improving antioxidant defenses and preserving mitochondrial function (2). Atherosclerosis is a chronic inflammatory disease entailing an initial activation of pro-inflammatory cytokines, which facilitates leukocyte transmigration (3). Vascular endothelial growth factor is a key mediator in the development of T-cell priming, and vascular endothelial cells modulate the endothelial induction of many genes involved in immune cell transmigration (4,5). Various oxidative stress stimuli such as local hypoxia and vascular injury may cause endothelial cell dysfunction and stimulate increased permeability, and encourage leukocyte transmigration into areas of inflammation by enhancing the expression of cell adhesion molecules (6,7). Accordingly, the inhibition of endothelial adhesion molecule expression by drugs/agents with antioxidant effects may serve as a potential therapeutic strategy for clinical atherosclerosis (8).
Superoxide dismutase (SOD) is an enzyme that catalyzes the dismutation of superoxide anion radical (O2−), one of the ROS in cells, into oxygen and H2O2, and this is an important antioxidant defense in nearly all cells exposed to oxygen. SOD plays a critical role in inhibiting the oxidative inactivation of nitric oxide, thereby preventing peroxynitrite formation and endothelial and mitochondrial dysfunction (9). In addition, SOD exerts powerful anti-inflammatory effects. Treatment with SOD reduces peroxidation reactions in the inflamed colon and ameliorates colonic inflammatory changes in experimental colitis, which is related to a reduction in adhesion molecule expression and leukocyte recruitment into the inflamed intestine (10). Therefore, SOD may be an important new therapy for the treatment of inflammatory bowel disease. Since oxidative stress is critical to endothelial adhesiveness in atherogenesis (11), SOD therapy may prevent atherogenesis, which entails endothelial activation by cytokines and agonists. However, these atheroprotective effects require the targeted delivery of SOD into the cytoplasm of endothelial cells. A recent study has shown that SOD conjugated with antibodies alleviated endotoxin-induced leukocyte adhesion in the cerebral vasculature and protected the brain from ischemia-reperfusion injury (12).
The protein transduction domains or cell-penetrating peptides have been shown to be involved in the successful delivery of exogenous full-length fusion proteins into living cells in vitro and in vivo (13,14). In the present study, the delivery of SOD protein inside endothelial cells and monocytes in vitro was conducted using the cell-permeable transactivator of transcription (Tat) peptide in order to deliver exogenous proteins into cells, as has also been previously undertaken (15,16). We also examined the atheroprotective effect of transduced Tat-SOD in inflammatory cytokine-induced leukocyte-endothelial interaction and transmigration involving oxidative stress. We investigated whether leukocyte recruitment to inflamed human umbilical vein endothelial cells (HUVECs) was blocked by the antioxidant Tat-SOD. Monocyte extravasation was examined by studying the induction of intercellular junction proteins and matrix metalloproteinase (MMP) proteins in tumor necrosis factor-α (TNF-α)-activated and SOD-treated HUVECs. Furthermore, the blockade of nuclear factor-κB (NF-κB) signaling by transduced Tat-SOD was elucidated in relation to TNF-α-triggered monocyte transmigration.
Materials and methods
Materials
M199 and RPMI-1640 mediums, human epidermal growth factor (hEGF) and hydrocortisone were obtained from Sigma Chemical Co. (St. Louis, MO, USA), as were all other reagents, unless specifically stated. Fetal bovine serum (FBS), penicillin-streptomycin and trypsin-EDTA were purchased from Lonza (Walkersville, MD, USA). Restriction endonuclease and T4 DNA ligase were purchased from Promega Corporation (Madison, WI, USA). Oligonucleotide primers were synthesized from Gibco-BRL (Grand Island, NY, USA). Ni2+-nitrilotriacetic acid sepharose was purchased from Qiagen (Valencia, CA, USA). Human SOD cDNA was isolated using the polymerase chain reaction (PCR) technique. Isopropyl-β-D-thiogalactoside (IPTG) was obtained from Duchefa Biochemie (Haarlem, The Netherlands). Plasmid pET-15b and Escherichia coli strain BL21 (DE3) were obtained from Novagen (Darmstadt, Germany). Anti-vascular cell adhesion molecule-1 (VCAM-1; Cat. no. sc-8304) and anti-polyhistidine (Cat. no. sc-803) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-integrin β1 (Cat. no. MAB1778) was purchased from R&D Systems (Minneapolis, MN, USA). Anti-β-actin was purchased from Sigma Chemical Co. The NF-κB inhibitor SN50 (Cat. no. BML-P600-0005) was obtained from Enzo Life Sciences (Farmingdale, NY, USA).
Expression and purification of Tat-SOD protein
A Tat expression vector was prepared in our laboratory as described previously (17). A Tat-SOD expression vector was constructed to express the Tat peptides (RKKRRQRRR) as a fusion protein with human SOD. The cDNA sequence for human SOD was PCR amplified using the following sense and antisense primers (Fig. 1A). The SOD sense primer, 5′-CTCGAGGCGA CGAAGGCCGTGTGCGTG-3′ contains an XhoI site, and the SOD antisense primer, 5′-GGATCCTTATTGGGCGATCCCA ATTAC-3′, contains a BamHI restriction site. The PCR product was excised with XhoI and BamHI, eluted, ligated into a TA-cloning vector and a pTat vector using T4 DNA ligase, and cloned into Escherichia coli DH5α cells. The human SOD gene was fused with a 21 amino acid-Tat peptide in a bacterial expression vector in order to produce a genetic in-frame Tat-SOD fusion protein. Similarly, the PCR product excised with XhoI and BamHI was subcloned into the XhoI and BamHI sites of pET-15b in order to construct control SOD that expressed the SOD fusion protein without the Tat peptides.
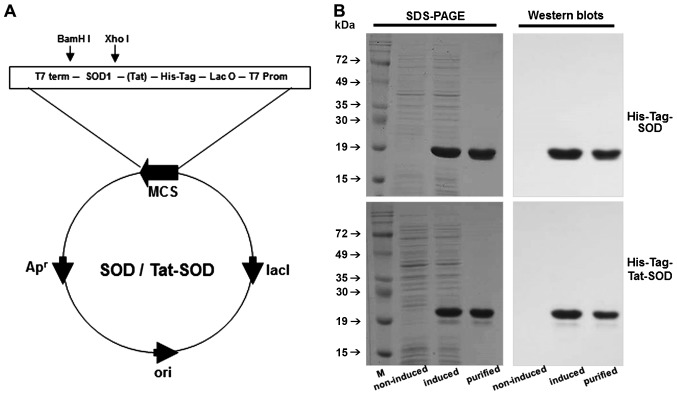
Figure 1.
Purification of transactivator of transcription (Tat)-superoxide dismutase (SOD) protein. Construction of the Tat-SOD expression vector system based on the vector pET-15b. Diagram of expressed Tat-SOD and control SOD proteins (A). The coding frame of human SOD is represented by an open box along with 6 histidine (His) residues and Tat peptide. Expression was induced by adding IPTG. After induction, purified SOD and Tat-SOD proteins were analyzed by 12% SDS-PAGE and subjected to western blot analysis with an anti-rabbit polyhistidine antibody (B). M, marker.
To produce the SOD fusion proteins (Tat-SOD and control SOD), the plasmid was transformed into E. coli BL21 cells, as previously described (15). Transformed bacterial cells grown in 100 ml LB media at 37°C to a D600 value of 0.5–1.0 were induced with 0.5 mM IPTG at 37°C for 4 h. Harvested cells were lysed by sonication at 4°C in a binding buffer (5 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9), and the formed recombinant Tat-SOD was purified using an Ni2+-nitrilotriacetic acid sepharose affinity column (Qiagen) under native conditions. After the column was washed with 10 volumes of a binding buffer and six volumes of a wash buffer (60 mM imidazole, 500 mM NaCl, and 20 mM Tris-HCl, pH 7.9), the fusion proteins were eluted in a buffer (250 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9). The fusion proteins containing Tat-SOD fractions were combined and the salts removed using PD-10 column chromatography (GE Healthcare Biosciences, Pittsburgh, PA, USA). Protein concentration was measured by the Bradford procedure using bovine serum albumin as a standard.
Transduction of Tat-SOD protein into HUVECs
HUVECs were isolated from the umbilical cords using collagenase (Worthington Biochemicals, Lakewood, NJ, USA) and were grown in M199 supplemented with 10% FBS, hEGF and hydrocortisone at 37°C in a humidified atmosphere of 5% CO2. Anonymous umbilical cord tissues were obtained from the Hallym University Hospital (Department of Obstetrics and Gynecology, Chuncheon Sacred Heart Hospital, Hallym University Medical Center). This study was approved by Hallym University Institutional Review Board (HIRB-2011-007-4). For the transduction of Tat-SOD, HUVECs grown to confluence on a 6-well plate were treated with 0.1–3 µM Tat-SOD or SOD for various durations, of 10–120 min. In addition, HUVECs were treated with 1 mM of antioxidant, N-acetyl-cysteine (NAC), or 10 µM of NF-κB inhibitor, SN50, in the absence or presence of 10 ng/ml TNF-α. The cells were harvested in order to prepare cell extracts to perform western blot analysis.
To measure HUVEC viability, cells were exposed to 10 ng/ml TNF-α and 0.1–1 µM Tat-SOD for 6 h. A 3-(4,5-Dimet-ylthiazol-yl)-diphenyl tetrazolium bromide (MTT; Duchefa Biochemie) assay was carried out to quantify cellular viability.
Western blot analysis
HUVECs and Tat-SOD-transfected HUVECs were lysed with a lysis buffer containing 1% β-mercaptoethanol, 1 M β-glycerophosphate, 0.1 M Na3VO4, 0.5 M NaF and protease inhibitor cocktail. Equal amounts of proteins from cell extracts were then electrophoresed on 8–12% SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking non-specific binding, the membrane was subsequently incubated overnight at 4°C with anti-polyhistidine, anti-VCAM-1, anti-integrin β1, anti-membrane type-1 (MT1)-MMP (Cat. no. sc-12367; Santa Cruz Biotechnology, Inc.), anti-MMP-2 (Cat. no. MAB902; R&D systems), anti-MMP-9 (Cat. no. MAB936; R&D systems), anti-occludin-1 (Cat. no. sc-5526; Santa Cruz Biotechnology, Inc.), anti-PECAM-1 (Cat. no. sc-1505; Santa Cruz Biotechnology, Inc.), anti-vascular endothelial (VE)-cadherin (Cat. no. ab-33168; Abcam, Cambridge, UK) and anti-NF-κB (Cat. no. sc-7151; Santa Cruz Biotechnology, Inc.). After washing with Tris-buffered saline-Tween-20, the membrane was incubated with an anti-rabbit IgG conjugated to horseradish peroxidase. The protein levels were subsequently determined using immobilon chemiluminescent horseradish peroxidase substrate (Millipore Corp., Billerica, MA, USA) and Agfa X-ray film (Agfa-Gevaert, Mortsel, Belgium). In addition, the bound antibodies were visualized by enhanced ECL chemilluminescence (Amersham, Pittsburgh, PA, USA). Incubation with monoclonal mouse β-actin antibody was also performed for comparative controls.
Cell staining
For the direct detection of fluorescein-labeled protein, purified Tat-SOD and control SOD were labeled using an EZ-labeled fluorescein isothiocyanate (FITC) protein labeling kit (Pierce, Rockford, IL, USA) according to the manufacturer's instructions. HUVECs grown on glass coverslips were treated with 3 µM Tat-SOD and control SOD fusion proteins. Following incubation for 2 h at 37°C, the cells were washed twice with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 10 min. The distribution of fluorescence was analyzed using a fluorescence microscope (Nikon, Tokyo, Japan).
Cell adhesion assay
The human monocytic leukemic cell line THP-1 (purchased from The American Type Culture Collection, Rockville, MD, USA) were labeled with 5 µM calcein AM (Molecular Probes, Eugene, OR, USA). HUVECs were treated with 0.1–0.5 µM Tat-SOD and 10 ng/ml TNF-α in a glass chamber (Nunc Lab-Tek II; Thermo Fisher Scientific Inc., Waltham, MA, USA) for 6 h. Thereafter, labeled THP-1 cells were added to the 6 h-stimulated HUVECs with 10 ng/ml TNF-α in the glass chamber, and these cells were co-cultured in RPMI-1640 medium containing 10% FBS for 1 h. After a thorough wash with PBS, the cultures were photographed with a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Measurement of superoxide anion production
Superoxide anions generated were detected using a commercial O2− assay kit (Sigma Chemical Co.), according to the manufacturer's instructions. This measurement is based on the oxidation of luminol by O2−, resulting in the formation of chemiluminescence light. HUVECs seeded on 96-well plates were treated with 10 ng/ml TNF-α in the absence and presence of Tat-SOD or control SOD. Simultaneously, the luminol solution and enhancer solution included in the kit were added, and the luminescence intensity was read every 10 min during a 4 h-period.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from HUVECs using a commercially available TRIzol reagent kit (Invitrogen, Carlsbad, CA, USA). RNA (5 µg) was then reverse transcribed with 200 units of reverse transcriptase and 0.5 mg/ml oligo(dT)15 primer (Bioneer, Daejeon, Korea). The expression levels of the mRNA transcripts of the following primers were subsequently evaluated by RT-PCR: MMP-2 forward, 5′-TGGCAAGTACGGCTGTC-3′ and reverse, 5′-TTCTTGTCGCGGTCGTAGTC-3′, 180 bp; MMP-9 forward, 5′-ACGTGGACATCTTCGACGC-3′ and reverse, 5′-CGAACCTCCAGAAGCTCTGC-3′, 709 bp; and β-actin forward, 5′-GACTACCTCATGAAGATC-3′ and reverse, 5′-GATCCACATCTGCTGGAA-3′, 513 bp. PCR was performed in 25 µl of 10 mM Tris-HCl (pH 9.0), 25 mM MgCl2, 10 mM dNTP, 5 units of TaqDNA polymerase, and 10 µM of each primer and terminated by heating at 94°C for 3 min. After 35 cycles of thermocycling at 94°C (30 sec), 55°C (45 sec), 72°C (90 sec), and 72°C (10 min), and electrophoresis of the PCR products (25 µl) on 1% agarose gel, the bands were visualized using a TFX-20 M model-UV transilluminator (Vilber Lourmat, Marne-la-Vallée, France) and gel photographs were obtained. The absence of contaminants was routinely checked by the RT-PCR assay of negative control samples without the addition of a primer.
Gelatin zymography
HUVECs were plated at 90–95% confluence for all experiments. To measure MMP-2 and MMP-9 activity, gelatin zymography was performed. Culture media were electrophoresed on 8% SDS-PAGE in Tris-HCl buffer [0.3 M Tris-HCl (pH 6.8), 4% SDS, 20% glycerol, and 0.03% bromophenol blue] co-polymerized with 0.1% gelatin as the substrate. The gel was incubated in a 2.5% Triton X-100 solution for 1 h. After three washes in 50 mM Tris-HCl (pH 7.5) for 30 min, the gel was incubated for 20 h in 50 mM Tris-HCl containing 10 mM CaCl2, 0.05% Brij-35, 200 mM NaCl at 37°C for 24 h. The gel was stained in a solution with 0.1% Coomassie brilliant blue G-250, 2% acetic acid and 45% methanol for 1 h, and destained in a solution with 30% methanol and 10% acetic acid, and the bands were visualized using a TFX-20 M model-UV transilluminator.
Measurement of THP-1 monocyte transmigration
The experimental models for monocyte transmigration employed 24-Transwell inserts with pore sizes of 8 µm (Corning Life Sciences, Corning, NY, USA). The lower surface of the insert filter was coated with 15 µl of 1 mg/ml gelatin solution and dried for 1 h. THP-1 cells (2×105) cultured in serum-free RPMI-1640 were loaded on gelatin-coated Transwell inserts. These cells were treated with 0.1–0.5 µM Tat-SOD and 1 µM SOD, and exposed to 10 ng/ml TNF-α for 6 h. Transmigrated THP-1 cells were collected for the 6 h-stimulation with TNF-α, plated in a glass chamber, and treated with 50 ng/ml phorbol 12-myristate 13-acetate for 24 h to attach suspended THP-1 cells. THP-1 cells attached on the slide glass were fixed with 4% paraformaldehyde for 20 min and dyed with toluidine blue for 10 min. Images were obtained using light microscopy.
Preparation of nuclear protein extracts
The cytosolic protein fraction and nuclear protein extract were prepared using a detergent lysis procedure to assay the translocation of NF-κB. HUVECs were lysed in a buffer of 20 mM HEPES (pH 7.9), 1 mM EDTA, 10 mM NaCl, 1 mM dithiothreitol, 1 mg/ml Nonidet P-40, 0.4 mM phenylmethylsulfonyl fluoride, 0.01 ng/ml leupeptin, and 200 units aprotinin and incubated on ice for 10 min. Proteins were extracted from nuclear pellets via incubation with a high-salt buffer containing 420 mM NaCl, 1 mM EDTA, 20 mM HEPES (pH 7.9), 25% glycerol, 1 mM dithiothreitol, 0.4 mM phenylmethylsulfonyl fluoride, 0.01 ng/ml leupeptin, and 200 units of aprotinin with vigorous shaking. The nuclear debris was pelleted by a brief centrifugation at 2,000 × g for 30 min to collect supernatants. For the measurement of protein levels of NF-κB, western blot analysis was conducted with nuclear protein extracts using a human NF-κB primary antibody.
Immunocytochemical analysis
Following cell culture protocols, HUVECs were fixed with 4% paraformaldehyde for 20 min. To make the cell membrane permeable, 0.1% Triton X-100 and 0.1% sodium citrate were used to treat the cells on ice for 1 min. After blocking for non-specific binding with 20% FBS in PBS, primary anti-NF-κB antibody was added and incubated overnight at 4°C. After washing with PBS-Tween-20, HUVECs were incubated with anti-rabbit IgG conjugated with fluorescent dye Cy3 and 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining of HUVECs. Immunocytochemical images of cells mounted on the slide were then observed by fluorescence microscopy at λ=550 nm excitation and λ=570 nm emission (Carl Zeiss).
Data analysis
The results are presented as the means ± SEM. Statistical analysis was conducted using the Statistical Analysis Statistical software package, version 6.12 (SAS Institute, Cary, NC, USA). One-way ANOVA was used to determine the inhibitory effects of Tat-SOD on endothelial trafficking and the migration of monocytes. Differences between the treatment groups were analyzed with Duncan's multiple range test, and a P-value <0.05 was considered to indicate a statistically significant difference.
Results
Tat-SOD purification and construction
For the generation of the Tat-SOD vector, human SOD cDNA was subcloned into a pET-15b plasmid reconstructed to contain the Tat peptide. The Tat-SOD expression vector contained consecutive cDNA sequences encoding human SOD, Tat peptide and six histidine residues at the amino terminus. A SOD expression vector was constructed to produce Tat peptide-free control SOD (Fig. 1A). Tat-SOD protein was purified using an Ni2+-nitrilotriacetic acid sepharose affinity column and PD-10 column chromatography, and was subsequently confirmed by SDS-PAGE and western blot analysis using an anti-rabbit polyhistidine antibody (Fig. 1B).
Transduction of Tat-SOD into HUVECs
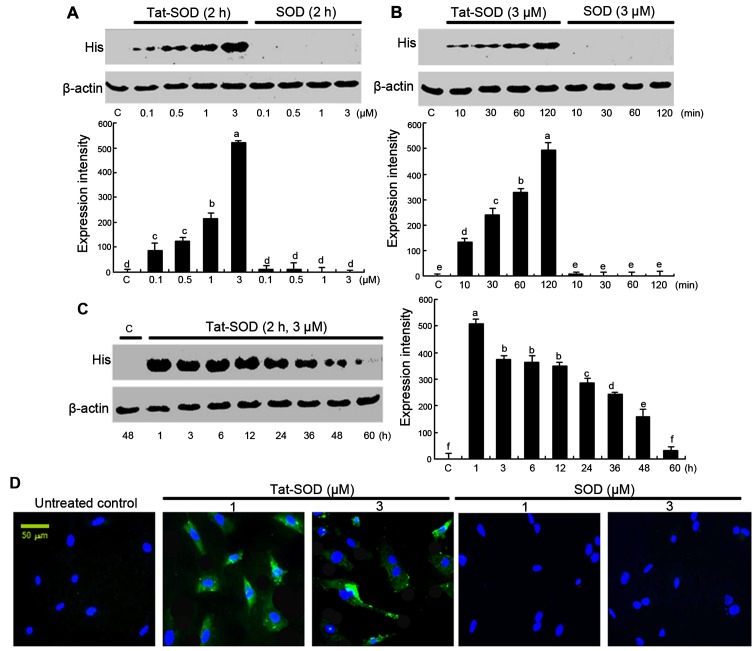
The transduction of Tat-SOD into HUVECs was performed by adding 0.1–3 µM Tat-SOD and control SOD to HUVEC culture medium for 10–120 min. The dose dependency of Tat-SOD transduction into HUVECs was observed at doses of 0.1–3 µM (Fig. 2A), as evidenced by western blot analysis with anti-histidine. The intracellular levels of Tat-SOD transduced at 3 µM in the cells were rapidly and markedly upregulated up to 120 min (Fig. 2B). By contrast, the control SOD was not transduced into the cells (Fig. 2A and B).
Figure 2.
Transduction of transactivator of transcription (Tat)-superoxide dismutase (SOD) proteins into human umbilical vein endothelial cells (HUVECs). Tat-SOD or control SOD (0.1–3 µM) was added to HUVECs for 2 h (A). Tat-SOD or control SOD (3 µM) was added to HUVECs for 10–120 min (B). After HUVECs were treated with 3 µM Tat-SOD for 2 h, Tat-SOD expression in HUVECs for 1–60 h was examined by western blot analysis (C). Data indicates the means ± SEM; n=5 experiments. Columns without a common letter differ, P<0.05. HUVECs were treated with various doses of Tat-SOD or SOD for 2 h. To identify cellular translocalization of Tat-SOD, cells were transduced with 1–3 µM FITC-stained Tat-SOD or SOD for 2 h. Cellular localization of Tat-SOD protein was observed by counterstaining with DAPI, using fluorescence microscopy (D). C, control.
To confirm the pharmacological effect of transduced Tat-SOD, the expression of transduced Tat-SOD in cells was determined (Fig. 2C). The intracellular level of transduced Tat-SOD protein in cells was initially detected after 1 h, and Tat-SOD protein gradually decreased up to 60 h. Furthermore, to identify the cellular translocalization of Tat-SOD, cells transduced with FITC-stained Tat-SOD were counter-stained with DAPI (Fig. 2D). Tat-SOD protein was detected in the cytoplasm of transduced cells.
Inhibition of cell adhesion molecules of activated HUVECs by Tat-SOD
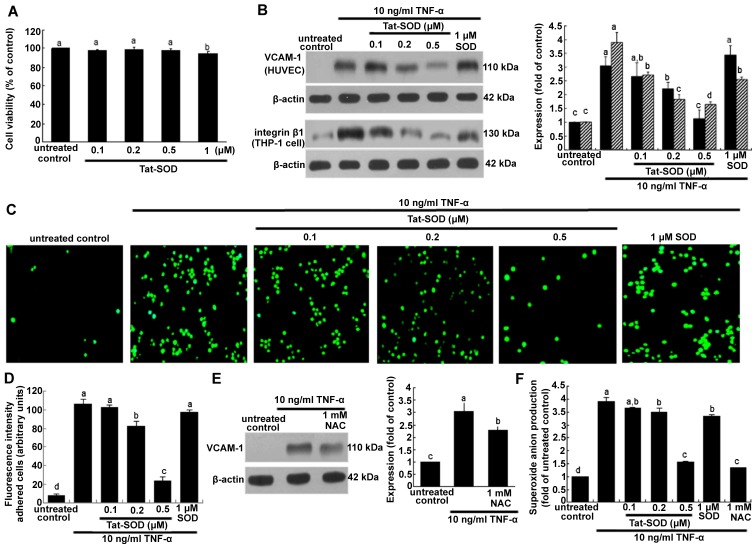
To examine the cytotoxicity to HUVECs of Tat-SOD, MTT analysis was performed. When Tat-SOD, at concentrations between 0.1 and 1 µM, was added to HUVECs, viability was not significantly influenced at ≤0.5 µM Tat-SOD (Fig. 3A). However, Tat-SOD at 1 µM caused cellular toxicity. Accordingly, non-toxic Tat-SOD at ≤0.5 µM was employed for the following experiments.
Figure 3.
Cell viability (A), vascular cell adhesion molecule-1 (VCAM-1) expression and monocyte adhesion in human umbilical vein endothelial cells (HUVECs) treated with transactivator of transcription (Tat)-superoxide dismutase (SOD) or control SOD (B-D), and N-acetyl-cysteine (NAC) (E), and the release of superoxide anion (F). HUVECs were treated with Tat-SOD, and cell viability was measured by MTT assay (A). Cell viability (3 separate experiments) was expressed as cell survival relative to untreated controls in percentage (viability, 100%). To measure the cellular protein expression of VCAM-1 and integrin β1 (n=5 experiments), total cell lysates were subjected to western blot analysis with a primary antibody against VCAM-1 or integrin β1 (B and E). To test whether oxidative stress was responsible for VCAM-1 expression, the antioxidant NAC was added to HUVECs activated by tumor necrosis factor-α (TNF-α) (E). β-actin was used as an internal control. The bar graph on the right represents quantitative results of blots obtained from densitometric analysis. To examine THP-1 cell adhesion to TNF-α-activated endothelial cells (C and D), HUVECs were treated with 10 ng/ml TNF-α in the presence or absence of Tat-SOD for 6 h and co-cultured with calcein-AM-labeled THP-1 monocytes for 1 h. Microscopic observation was conducted with a fluorescence microscope and fluorescence intensity was quantified (n=3 experiments). Magnification, x200. Superoxide anion radical production was measured with a commercial superoxide anion assay kit based on the oxidation of luminol (F). The columns represent the means ± SEM, and variables without a common letter differ; P<0.05.
The present study investigated Tat-SOD-inhibited monocyte trafficking onto activated HUVECs. As shown in Fig. 3B, VCAM-1 expression induced by 10 ng/ml TNF-α for 6 h was dose-dependently downregulated in the presence of 0.1–0.5 µM Tat-SOD. By contrast, we noted no marked inhibition of VCAM-1 expression triggered by 10 ng/ml TNF-α for 6 h in HUVECs treated with 1 µM control SOD. It should be noted that 0.5 µM Tat-SOD on its own did not stimulate VCAM-1 induction. We noted that Tat-SOD suppressed the cellular induction of integrin β1 in THP-1 monocytes exposed to 10 ng/ml TNF-α (Fig. 3B). Some inhibition of integrin β1 was observed with 1 µM control SOD. The monocyte adhesion assay was further conducted in the co-culture system of HUVECs and THP-1 monocytes. Monocyte adhesion onto endothelial cells was augmented by TNF-α stimulation, which was diminished by Tat-SOD in a dose-dependent manner (Fig. 3C and D). The inhibition of such adhesion was not observed in control SOD-treated HUVECs which were exposed to TNF-α. Thus, we suggest that Tat-SOD blocked the inflammatory interaction of HUVECs and monocytes, a process which may entail the induction of the adhesion molecules of endothelial VCAM-1 and monocyte integrin β1.
Involvement of oxidative stress in inducing endothelial adhesion molecules
The present study attempted to clarify that oxidative stress was responsible for inflammatory VCAM-1 expression. When the antioxidant NAC at 1 mM was added to HUVECs activated by TNF-α, VCAM-1 expression was significantly attenuated (Fig. 3E). This study demonstrated that 0.5 µM Tat-SOD abolished VCAM-1 induction (Fig. 3B). Treatment with ≥0.2 µM Tat-SOD decreased TNF-α-induced superoxide anion production, indicating that Tat-SOD moved to HUVEC cytoplasm and affected antioxidant activity (Fig. 3F). We suggest that external stimulation of HUVECs with SOD protein eliminates some of the extracellular superoxide anions secreted from cells (Fig. 3F). The inhibitory activity of Tat-SOD in relation to the adhesion of monocytes to activated endothelium may result from the antioxidant activity of intracellular Tat-SOD.
Suppression of TNF-α-induced MMP proteins by Tat-SOD
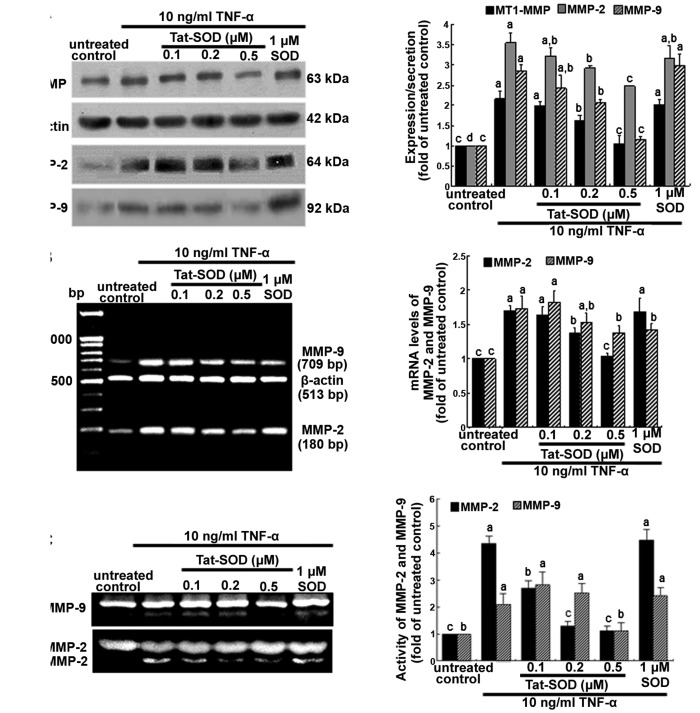
It has previously been mentioned that MMP proteins facilitate the passage of leukocytes across the matrix barriers of the vascular basement membrane (18,19). In the present study, we examined MMP induction in TNF-α-stimulated HUVECs using 0.1–0.5 µM Tat-SOD. Tat-SOD downregulated MT1-MMP expression in HUVECs which had been stimulated with 10 ng/ml TNF-α for 24 h (Fig. 4A). In addition, the secretory protein level of MMP-2 and MMP-9 were enhanced by 24 h-treatment of TNF-α, compared to the untreated control, and was dose-dependently attenuated by Tat-SOD (Fig. 4A). The diminution of protein production of MMP-2 and MMP-9 by Tat-SOD required the inhibition of these MMP proteins at the transcriptional levels (Fig. 4B). By contrast, external SOD had no such effects on the production and mRNA transcription of MMP-2 and MMP-9. Furthermore, we noted that the gelatinolytic activity of MMP-2 and MMP-9 was markedly enhanced by TNF-α, and this effect was attenuated by 0.5 µM Tat-SOD (Fig. 4C).
Figure 4.
Suppression of matrix proteins by transactivator of transcription (Tat)-superoxide dismutase (SOD). Human umbilical vein endothelial cells (HUVECs) were treated with 10 ng/ml tumor necrosis factor-α (TNF-α) for 24 h in the presence or absence of Tat-SOD and control SOD. For the measurement of cellular expression of membrane type-1 (MT-1) matrix metalloproteinase (MMP) and production of MMP-2 and MMP-9 at 24 h, total cell lysates and cell culture media were subjected to western blot analysis with a primary antibody against MT-1 MMP, MMP-2 and MMP-9 (A). β-actin was used as an internal control. To determine the transcriptional levels of MMP-2 and MMP-9, RT-PCR was performed (B). β-actin was used as a housekeeping gene for the co-amplification with MMP-2 and MMP-9. Gelatinolytic activity of MMP-2 and MMP-9 was measured using gelatin zymography (C). The bar graphs for blots in the right panels represent quantitative results obtained by densitometric analysis (means ± SEM), and variables without a common letter differ; P<0.05; n=3 experiments.
Effects of Tat-SOD on the cytokine induction of intercellular junction proteins
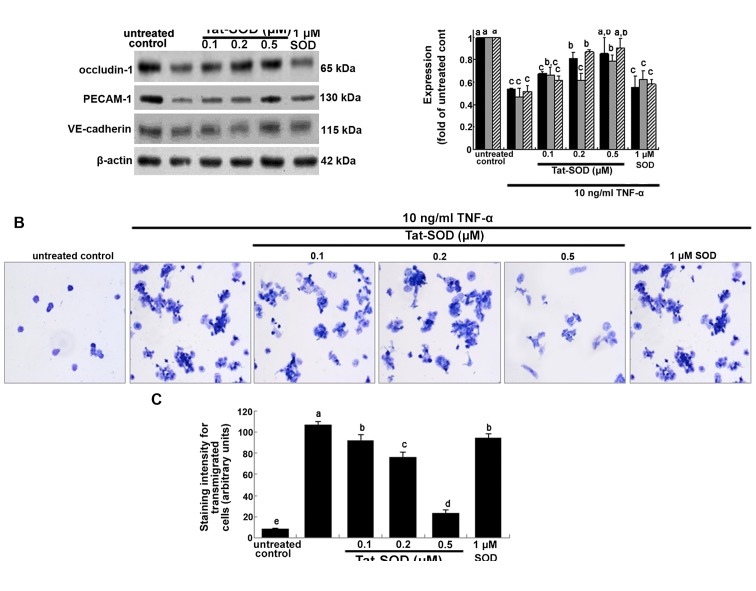
Occludins are integral plasma-membrane proteins located at the tight junctions and are distributed at low levels and in a discontinuous fashion at cell-cell contacts (20). In the present study, we investigated whether Tat-SOD-influenced occludin-1 expression was downregulated by pro-inflammatory TNF-α. Occludin-1 was downregulated at 24 h after treatment with TNF-α (Fig. 5A). By contrast, when HUVECs were treated with 0.1–0.5 µM Tat-SOD, occludin-1 expression rose, which is indicative of preserving the firm tight junction (Fig. 5A). However, 1 µM control SOD did not have such an effect. Platelet/endothelial cell adhesion molecule 1 (PECAM-1) and vascular endothelial (VE)-cadherin are responsible for the endothelial adhesive junction and are crucial to the process of leukocyte transmigration through intercel-lular junctions of vascular endothelial cells (22). Western blot analysis indicated that TNF-α suppressed the expression of PECAM-1 and VE-cadherin in HUVECs, and this suppression was reversed by treatment with 0.5 µM Tat-SOD but not 1 µM control SOD (Fig. 5A). This indicates that transduced Tat-SOD diminished the intercellular permeability of endothelial cells, leading to concrete cell-cell contacts.
Figure 5.
Inhibition of cellular induction of occludin-1, platelet/endothelial cell adhesion molecule 1 (PECAM-1) and vascular endothelial (VE)-cadherin and monocyte transmigration by transactivator of transcription (Tat)-superoxide dismutase (SOD). Human umbilical vein endothelial cells (HUVECs) were treated with 10 ng/ml tumor necrosis factor-α (TNF-α) for 24 h in the presence and absence of Tat-SOD or control SOD. To measure the cellular expression of occludin-1, PECAM-1 and VE-cadherin, total cell lysates were subjected to western blot analysis with a primary antibody against occludin-1, PECAM-1 and VE-cadherin (A). β-actin was used as an internal control. The bar graph in the right panel represents quantitative results of blots obtained by densitometric analysis. To examine the transmigration of TNF-α-treated THP-1 monocytes, THP-1 cells were loaded onto Transwell inserts and exposed to 10 ng/ml TNF-α for 24 h in the presence of Tat-SOD (B and C). Transmigrated monocytes were stained with toluidine blue and the staining intensity was quantified, and microphotograph images were taken with a light microscope (3 separate experiments). Magnification, x200. The columns represent the means ± SEM, and variables without a common letter differ, P<0.05; n=3 experiments.
It has previously been noted that MMPs facilitate the passage of leukocytes across the matrix barriers of the vascular basement membrane (21). When THP-1 cells in the upper compartment of Transwell plates were treated with TNF-α, a large number of toluidine blue-stained THP-1 cells appeared in their bottom compartments (Fig. 5B and C). This indicates that TNF-α increased monocyte transmigration. However, the TNF-α-elevated transmigration was markedly decreased in THP-1 cells treated with 0.5 µM Tat-SOD, almost to the level of the control. Accordingly, we suggest that Tat-SOD, unlike control SOD, inhibited cytokine-stimulated monocyte extrava-sation by suppressing MMP activity and intercellular junction protein induction (Figs. 4A and 5A).
Downregulation of NF-κB translocation by Tat-SOD
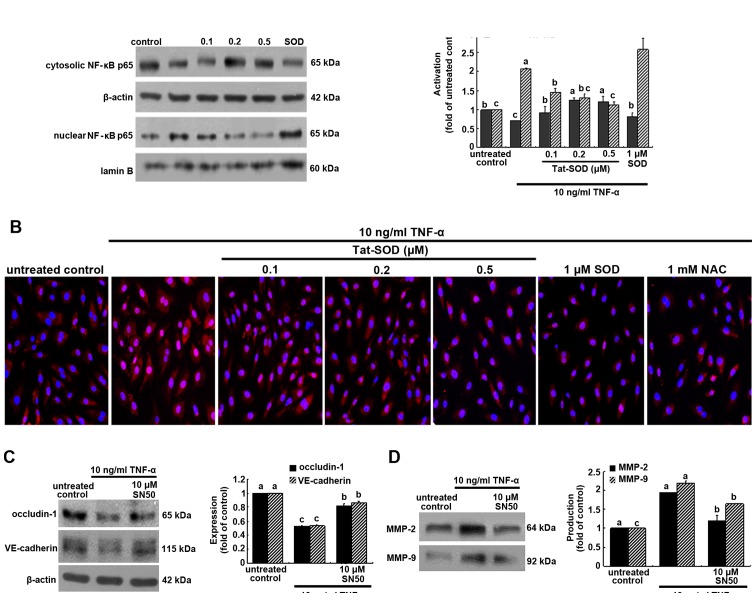
The NF-κB pathway was studied, and we noted that Tat-SOD protein regulated the cellular expression of intercellular junction proteins and MMP proteins, which are critical for regulating the endothelial migration of monocytes. NF-κB p65 was trans-located into the nucleus of HUVECs activated by 10 ng/ml TNF-α for 6 h, as evidenced by western blot analysis using the primary antibody for NF-κB p65 (Fig. 6A). When 0.1–0.5 µM Tat SOD was added to TNF-α-exposed HUVECs, cyotosolic NF-κB level rose, but nuclear NF-κB level declined in a dose-dependent manner. Thus, transduced Tat-SOD inhibited the NF-κB translocation and activation caused by inflammatory TNF-α. However, SOD used as a control against Tat-SOD had no such effect on NF-κB p65 activation (Fig. 6A).
Figure 6.
Inhibitory effects of transactivator of transcription (Tat)-superoxide dismutase (SOD) on the transactivation of nuclear factor-κB (NF-κB) (A and B), and the restoration of occludin-1, vascular endothelial (VE)-cadherin and matrix metalloproteinase (MMP) proteins by NF-κB inhibition (C and D). Human umbilical vein endothelial cells (HUVECs) were treated with 10 ng/ml tumor necrosis factor-α (TNF-α) for 6 h in the presence or absence of Tat-SOD or control SOD. Cytosolic NF-κB and nuclear NF-κB were examined by western blot analysis (A). Cytosolic and nuclear fractions were subjected to western blot analysis with a primary antibody against NF-κB. β-actin and lamin B were used as internal controls. For the immunocytochemical staining of NF-κB (B), HUVECs were immunostained and visualized using anti-NF-κB and Cy3-conjugated IgG. Fluorescence microphotographs were obtained with a fluorescence microscope. Each photograph is representative of 3 separate experiments. Magnification, x200. The expression levels of occludin-1, VE-cadherin, MMP-2 and MMP-9 were measured in the presence of 10 µM NF-κB inhibitor SN50 (C and D). Western blot analysis with a primary antibody against occludin-1, VE-cadherin, MMP-2 and MMP-9 was performed. The bar graphs (means ± SEM, n=3 experiments) for blots in the right panels represent quantitative results obtained by densitometric analysis. The columns represent the means ± SEM, and variables without a common letter differ, P<0.05; n=3 experiments.
This study further investigated NF-κB transactivation in HUVECs treated with 10 ng/ml TNF-α for 6 h, as measured by immunocytochemical staining. Heavy nuclear pinkish-red staining was observed in TNF-α-exposed HUVECs (Fig. 6B). When 0.1–0.5 µM Tat SOD or 1 mM NAC was applied to HUVECs treated with TNF-α, the cytosolic staining became stronger in a dose-dependent manner (blue nuclear staining). These data indicate that Tat-SOD retarded NF-κB transacti-vation of HUVECs. We also noted that 0.5 µM SOD did not block TNF-α-induced NF-κB activation (Fig. 6B).
In order to confirm that intercellular junction markers were mediated via NF-κB activation, we detected the induction of occludin-1 and VE-cadherin using the NF-κB inhibitor SN50. The inhibition of occludin-1 and VE-cadherin expression by TNF-α was restored in the presence of 10 µM SN50 (Fig. 6C). In addition, the enhanced production of MMP-2 and MMP-9 by TNF-α was attenuated by this NF-κB inhibitor (Fig. 6D).
Discussion
Seven major findings were observed in the present study. i) Tat-SOD protein was highly expressed as a major component of the total soluble proteins in cells, as determined by SDS-PAGE. ii) Purified Tat-SOD protein was efficiently transduced into HUVECs in a time- and dose-dependent manner. iii) Transduced Tat-SOD, which was non-toxic at ≤0.5 µM, inhibited the TNF-α-induced induction of endothelial VCAM-1 and monocyte integrin β1, indicating that intracellular SOD in the cytoplasm inhibited the tight adhesion mediated by the leukocyte integrins and endothelial adhesion molecules. iv) Endothelial VCAM-1 induction by TNF-α was attributed to oxidative stress that was blocked by the antioxidant Tat-SOD. v) Transduced Tat-SOD inhibited the matrix-degrading MMP activity of MT1-MMP, MMP-2 and MMP-9 in HUVECs. vi) The induction of intercellular occludin-1, PECAM-1 and VE-cadherin involved in transendothelial monocyte migration was enhanced by treatment with Tat-SOD. vii) Tat-SOD diminished the nuclear NF-κB transactivation responsible for paracellular junction proteins and matrix-degrading proteins in TNF-α-treated HUVECs. These results demonstrate that transduced Tat-SOD prevented cytokine-induced endothelial trafficking and the transmigration of monocytes. It should be noted that external stimulation with SOD did not inhibit endothelial adhesion or the transmigration of monocytes.
The SOD isoform enzymes are the major antioxidant defense systems against superoxide anion radicals in each subcellular location in mammals. SOD inhibits the oxidative inactivation of nitric oxide, thereby blocking peroxynitrite formation and endothelial dysfunction (9). Given the essential role which SOD plays in cardiovascular diseases such as atherosclerosis, hypertension and angiogenesis, the strengthening of endogenous antioxidant defenses with SOD-dependent antioxidant therapies, which will more effectively protect against oxidative stress, is of considerable interest (22,23). In addition, studying the role of manganese SOD, beyond its essential role for survival, may lead to the development of a novel strategy for an antioxidant approach to cancer intervention (24). Catalytic scavengers of peroxides and their potential uses as therapeutic agents for pulmonary, cardiovascular, neurodegenerative and inflammatory disorders have been previously noted (25). SOD liposomes and mimetics have been shown to be effective in animal models of cancer prevention (26). Therapeutic strategies which target oxidative stress with SOD mimetics such as tempol boost the endogenous levels of antioxidants and scavenging superoxide anions, as well as hydroxyl radical generation (27). However, clinical evidence remains controversial.
Although catalytic SOD enzymes are considered to be potential therapeutic agents for the treatement of atherosclerosis, hypertension and cancer, the inability of SOD to transduce into cells has limited their use in antioxidant therapy. Protein transduction domains or cell-penetrating peptides are employed for the successful delivery of exogenous full-length fusion proteins into living cells in vitro and in vivo (13,14). Our previous study showed that efficiently transduced Tat-glyoxalase protein protected against sodium nitroprusside-induced RINm5F cell death and streptozotocin-induced pancreatic β-cell destruction in diabetic mice (15). In the present study, Tat protein was used for SOD to become cell permeable, and Tat-SOD protein was highly and almost homogeneously expressed as a major component of the total soluble proteins in cells. Also, purified Tat-SOD protein was efficiently transduced into HUVEC in a time- and dose-dependent manner. Thus, we suggest that targeted SOD delivery into the HUVEC cytoplasm provides atheroprotective effects, with antioxidant activity against cytokine-induced endothelial activation and dysfunction. In a recent study, the targeted delivery of SOD to endothelium, but not non-targeted SOD, alleviated acute vascular inflammation (12).
Previous studies have demonstrated that endothelial activation, dysfunction and vascular inflammation occur when the endothelium is exposed to oxidative stress, leading to the pathogenesis of a range of disease states (28,29). Inflammatory atherosclerosis requires the initial activation of pro-inflammatory cytokines to facilitate leukocyte transmigration (3). Since oxidative stress is critical to the endothelial adhesiveness of immune cells, we suggest that SOD therapy hinders endothelial activation caused by cytokines and agonists. Accordingly, it has also been suggested that the inhibition of vascular cell-cell interaction by drugs/agents with antioxidant effects serves as a potential therapeutic strategy for clinical atherosclerosis (8). Although the detailed mechanism remains to be further elucidated, one may speculate that transduced Tat-SOD blocked monocyte trafficking onto endothelium through moderating the intracellular ROS production-associated induction of VCAM-1. As noted in the present study, external stimulation of HUVEC and THP-1 cells with non-targeted SOD did not result in such atheroprotection. In a similar study (10), SOD administration ameliorated inflammatory bowel disease by reducing VCAM-1 expression and leukocyte recruitment into the inflamed intestine in cases of experimental colitis. In addition, in mice, the injection of SOD conjugated with PECAM-directed antibody alleviated endotoxin-induced leukocyte adhesion in the cerebral vasculature, thus protecting the brain from ischemia-reperfusion injury (12). It has been previously demonstrated that SOD conjugated with PECAM antibody markedly alleviates abnormal endothelial permeability and endothelial barrier function caused by exogenous ROS and vascular endothelial growth factor (30). In the present study, we showed that Tat-SOD decreased the cytokine induction of occludin-1, PECAM-1, VE-cadherin and MMP in HUVECs, compared to the untreated control. These results suggest that intracellular SOD potentiated endothelial cell interaction and basement membrane matrix function. A previous investigation has shown that low-dose MnTBAP, a SOD mimetic and superoxide anion and peroxynitrite scavenger, effectively decreases the MMP-9 and MMP-9/TIMP-1 ratio, suggesting that MnTBAP exerts an anti-inflammatory effect in cases of angiogenesis and alveolarization in intermittent hypoxia of neonatal rats (31). However, how the cell adhesion molecule genes are selectively modulated in response to the antioxidant SOD and which signaling pathways are involved in the selective regulation of these genes remain unknown.
Several signaling pathways, particularly NF-κB-mediated signaling pathways, play crucial roles in a variety of pathophysiological processes. There is evidence that the SOD-quenched superoxide anion produced by endothelial cells in response to pro-inflammatory agents mediates NF-κB activation (12). Similarly, the present study found that transduced Tat-SOD inhibited TNF-α-triggered monocyte transmigration via the blockade of NF-κB signaling. Treatment with Tat-SOD decreased the cytokine induction of intercellular junction proteins and matrix-degrading proteins by deterring NF-κB activation. In fact, the concept of reciprocity of inflammation and oxidative stress is well-recognized. As ROS production and oxidative stress resulting from activation of immune cells lead to chronic inflammation through the activation of transcription factors including NF-κB, activator protein 1(AP-1) and peroxisome proliferator-activated receptor γ (PPAR-γ), inflammation itself has a reciprocal relationship with oxidative stress (32). Thus, the findings of the present study, bridging inflammation and oxidation, emphasized the novel mechanisms of Tat-SOD targeting inflammation- and oxidative stress-driven atherosclerosis. It has previously been noted that AMP-activated protein kinase activation is involved in the SOD reduction of high glucose-induced brain microvascular endothelial cell tight-junction proteins by suppressing the induction of NAD(P)H oxidase-derived superoxide anions (33). Overexpression of SOD1 but not SOD2 prevented the increase in hypoxia-induced transepithelial electrical conductance and reduction of the occluding junctions at the plasma membrane in alveolar epithelial cells though blocking protein kinase C, ζ (PKC-ζ) and protein phosphatase 2A (34).
In conclusion, this study demonstrated that transduced Tat-SOD blocked the inflammatory process, and this involved the expression of inducible adhesion proteins, the production of matrix-degrading proteins and the induction of intercellular junction proteins. Consequently, we suggest that Tat-SOD, but not external control SOD, is effective in hampering the trafficking and extravasation of circulating monocytes. Oxidative stress blocked by transduced Tat-SOD appeared to instigate NF-κB activation, which was responsible for monocyte extrav-asation-associated vascular inflammation and atherosclerosis. However, the specific mechanisms underlying the inhibition of inflammatory responses by the antioxidant Tat-SOD are not yet fully understood. Furthermore, the use of THP-1 cells versus monocytes or other leukocytes isolated from blood requires further study. Sufficient differences between these cell types exist to recommend confirmation of any critical results obtained with THP-1 cells and primary monocytes. Nevertheless, we suggest that transduced Tat-SOD has implications for strategies which attenuate monocyte/macrophage dysfunction-related inflammatory atherosclerosis.
Acknowledgments
This study was supported by the National Research Foundation of Korea (no. 2015R1A2A2A01006666) and by a Priority Research Centers Program grant (no. NRF-2009-0093812) through the National Research Foundation of Korea.
References
- 1.Magenta A, Greco S, Gaetano C, Martelli F. Oxidative stress and microRNAs in vascular diseases. Int J Mol Sci. 2013;14:17319–17346. doi: 10.3390/ijms140917319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen YD, Wang H, Kho SH, Rinkiko S, Sheng X, Shen HM, Zhu YZ. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One. 2013;8:e53147. doi: 10.1371/journal.pone.0053147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thromb Haemost. 2011;105(Suppl 1):S34–S42. doi: 10.1160/THS10-11-0717. [DOI] [PubMed] [Google Scholar]
- 4.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 5.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Immenschuh S, Schröder H. Heme oxygenase-1 and cardiovascular disease. Histol Histopathol. 2006;21:679–685. doi: 10.14670/HH-21.679. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1–9. doi: 10.1016/j.jdermsci.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Chen YH, Lin SJ, Chen YL, Liu PL, Chen JW. Anti-inflammatory effects of different drugs/agents with antioxidant property on endothelial expression of adhesion molecules. Cardiovasc Hematol Disord Drug Targets. 2006;6:279–304. doi: 10.2174/187152906779010737. [DOI] [PubMed] [Google Scholar]
- 9.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seguí J, Gironella M, Sans M, Granell S, Gil F, Gimeno M, Coronel P, Piqué JM, Panés J. Superoxide dismutase ameliorates TNBS-induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine. J Leukoc Biol. 2004;76:537–544. doi: 10.1189/jlb.0304196. [DOI] [PubMed] [Google Scholar]
- 11.Lubrano V, Balzan S. LOX-1 and ROS, inseparable factors in the process of endothelial damage. Free Radic Res. 2014;48:841–848. doi: 10.3109/10715762.2014.929122. [DOI] [PubMed] [Google Scholar]
- 12.Shuvaev VV, Han J, Tliba S, Arguiri E, Christofidou-Solomidou M, Ramirez SH, Dykstra H, Persidsky Y, Atochin DN, Huang PL, Muzykantov VR. Anti-inflammatory effect of targeted delivery of SOD to endothelium: mechanism, synergism with NO donors and protective effects in vitro and in vivo. PLoS One. 2013;8:e77002. doi: 10.1371/journal.pone.0077002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahid M, Robbins PD. Protein transduction domains: applications for molecular medicine. Curr Gene Ther. 2012;12:374–380. doi: 10.2174/156652312802762527. [DOI] [PubMed] [Google Scholar]
- 14.Bechara C, Sagan S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013;587:1693–1702. doi: 10.1016/j.febslet.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Kim MJ, Kim DW, Lee BR, Shin MJ, Kim YN, Eom SA, Park BJ, Cho YS, Han KH, Park J, et al. Transduced Tat-glyoxalase protein attenuates streptozotocin-induced diabetes in a mouse model. Biochem Biophys Res Commun. 2013;430:294–300. doi: 10.1016/j.bbrc.2012.10.134. [DOI] [PubMed] [Google Scholar]
- 16.Song HY, Ju SM, Goh AR, Kwon DJ, Choi SY, Park J. Suppression of TNF-alpha-induced MMP-9 expression by a cell-permeable superoxide dismutase in keratinocytes. BMB Rep. 2011;44:462–467. doi: 10.5483/BMBRep.2011.44.7.462. [DOI] [PubMed] [Google Scholar]
- 17.Kwon HY, Eum WS, Jang HW, Kang JH, Ryu J, Ryong Lee B, Jin LH, Park J, Choi SY. Transduction of Cu,Zn-superoxide dismutase mediated by an HIV-1 Tat protein basic domain into mammalian cells. FEBS Lett. 2000;485:163–167. doi: 10.1016/S0014-5793(00)02215-8. [DOI] [PubMed] [Google Scholar]
- 18.Kwon HM, Choi YJ, Choi JS, Kang SW, Bae JY, Kang IJ, Jun JG, Lee SS, Lim SS, Kang YH. Blockade of cytokine-induced endothelial cell adhesion molecule expression by licorice isoliquiritigenin through NF-kappaB signal disruption. Exp Biol Med (Maywood) 2007;232:235–245. [PubMed] [Google Scholar]
- 19.Kim MS, Kim DS, Kim HS, Kang SW, Kang YH. Inhibitory effects of luteolin on transendothelial migration of monocytes and formation of lipid-laden macrophages. Nutrition. 2012;28:1044–1054. doi: 10.1016/j.nut.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Hordijk PL. Endothelial signalling events during leukocyte transmigration. FEBS J. 2006;273:4408–4415. doi: 10.1111/j.1742-4658.2006.05440.x. [DOI] [PubMed] [Google Scholar]
- 22.Carillon J, Rouanet JM, Cristol JP, Brion R. Superoxide dismutase administration, a potential therapy against oxidative stress related diseases: several routes of supplementation and proposal of an original mechanism of action. Pharm Res. 2013;30:2718–2728. doi: 10.1007/s11095-013-1113-5. [DOI] [PubMed] [Google Scholar]
- 23.Wong GH. Protective roles of cytokines against radiation: induction of mitochondrial MnSOD. Biochim Biophys Acta. 1995;1271:205–209. doi: 10.1016/0925-4439(95)00029-4. [DOI] [PubMed] [Google Scholar]
- 24.Holley AK, Dhar SK, Xu Y, St Clair DK. Manganese superoxide dismutase: beyond life and death. Amino Acids. 2012;42:139–158. doi: 10.1007/s00726-010-0600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day BJ. Catalase and glutathione peroxidase mimics. Biochem Pharmacol. 2009;77:285–296. doi: 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St Clair D, Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta. 2012;1822:794–814. doi: 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samai M, Sharpe MA, Gard PR, Chatterjee PK. Comparison of the effects of the superoxide dismutase mimetics EUK-134 and tempol on paraquat-induced nephrotoxicity. Free Radic Biol Med. 2007;43:528–534. doi: 10.1016/j.freeradbiomed.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, Belkin N, Feinberg MW. Endothelial microRNAs and atherosclerosis. Curr Atheroscler Rep. 2013;15:372. doi: 10.1007/s11883-013-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varga ZV, Giricz Z, Liaudet L, Haskó G, Ferdinandy P, Pacher P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta. 2015;1852:232–242. doi: 10.1016/j.bbadis.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J, Shuvaev VV, Muzykantov VR. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J Pharmacol Exp Ther. 2011;338:82–91. doi: 10.1124/jpet.111.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang M, Bany-Mohammed F, Kenney MC, Beharry KD. Effects of a superoxide dismutase mimetic on biomarkers of lung angiogenesis and alveolarization during hyperoxia with intermittent hypoxia. Am J Transl Res. 2013;5:594–607. [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YW, West XZ, Byzova TV. Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med Berl. 2013;91:323–328. doi: 10.1007/s00109-013-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Wu J, Zou MH. Activation of AMP-activated protein kinase alleviates high-glucose-induced dysfunction of brain microvascular endothelial cell tight-junction dynamics. Free Radic Biol Med. 2012;53:1213–1221. doi: 10.1016/j.freeradbiomed.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caraballo JC, Yshii C, Butti ML, Westphal W, Borcherding JA, Allamargot C, Comellas AP. Hypoxia increases transepithelial electrical conductance and reduces occludin at the plasma membrane in alveolar epithelial cells via PKC-ζ and PP2A pathway. Am J Physiol Lung Cell Mol Physiol. 2011;300:L569–L578. doi: 10.1152/ajplung.00109.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]