Abstract
STUDY QUESTION
How does a genetic variant in the FSHB promoter, known to alter FSH levels, impact female reproductive health?
SUMMARY ANSWER
The T allele of the FSHB promoter polymorphism (rs10835638; c.-211G>T) results in longer menstrual cycles and later menopause and, while having detrimental effects on fertility, is protective against endometriosis.
WHAT IS KNOWN ALREADY
The FSHB promoter polymorphism (rs10835638; c.-211G>T) affects levels of FSHB transcription and, as a result, circulating levels of FSH. FSH is required for normal fertility and genetic variants at the FSHB locus are associated with age at menopause and polycystic ovary syndrome (PCOS).
STUDY DESIGN, SIZE, DURATION
We used cross-sectional data from the UK Biobank to look at associations between the FSHB promoter polymorphism and reproductive traits, and performed a genome-wide association study (GWAS) for length of menstrual cycle.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We included white British individuals aged 40–69 years in 2006–2010, in the May 2015 release of genetic data from UK Biobank. We tested the FSH-lowering T allele of the FSHB promoter polymorphism (rs10835638; c.-211G>T) for associations with 29, mainly female, reproductive phenotypes in up to 63 350 women and 56 608 men. We conducted a GWAS in 9534 individuals to identify genetic variants associated with length of menstrual cycle.
MAIN RESULTS AND THE ROLE OF CHANCE
The FSH-lowering T allele of the FSHB promoter polymorphism (rs10835638; MAF 0.16) was associated with longer menstrual cycles [0.16 SD (c. 1 day) per minor allele; 95% confidence interval (CI) 0.12–0.20; P = 6 × 10−16], later age at menopause (0.13 years per minor allele; 95% CI 0.04–0.22; P = 5.7 × 10−3), greater female nulliparity [odds ratio (OR) = 1.06; 95% CI 1.02–1.11; P = 4.8 × 10−3] and lower risk of endometriosis (OR = 0.79; 95% CI 0.69–0.90; P = 4.1 × 10−4). The FSH-lowering T allele was not associated with other female reproductive illnesses or conditions in our study and we did not replicate associations with male infertility or PCOS. In the GWAS for menstrual cycle length, only variants near the FSHB gene reached genome-wide significance (P < 5 × 10−9).
LIMITATIONS, REASONS FOR CAUTION
The data included might be affected by recall bias. Cycle length was not available for 25% of women still cycling (1% did not answer, 6% did not know and for 18% cycle length was recorded as ‘irregular’). Women with a cycle length recorded were aged over 40 and were approaching menopause; however, we did not find evidence that this affected the results. Many of the groups with illnesses had relatively small sample sizes and so the study may have been under-powered to detect an effect.
WIDER IMPLICATIONS OF THE FINDINGS
We found a strong novel association between a genetic variant that lowers FSH levels and longer menstrual cycles, at a locus previously robustly associated with age at menopause. The variant was also associated with nulliparity and endometriosis risk. These findings should now be verified in a second independent group of patients. We conclude that lifetime differences in circulating levels of FSH between individuals can influence menstrual cycle length and a range of reproductive outcomes, including menopause timing, infertility, endometriosis and PCOS.
STUDY FUNDING/COMPETING INTEREST(S)
None.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: FSH β subunit, menstrual cycle, menopause, endometriosis, fertility
Introduction
FSH is a key pituitary hormone, which stimulates maturation of oocytes and is a biomarker of ovarian reserve. FSH is a heterodimer comprised a hormone-specific β-chain (FSH-β) associated with an α-chain shared by other members of the glycoprotein hormone family (Nagirnaja et al., 2010). The anterior pituitary produces FSH, with transcription of FSHB being the rate-limiting step for FSH production. FSH stimulates target cells by binding to the FSH receptor (FSHR), a G-protein-coupled receptor (Fan and Hendrickson, 2005), promoting follicle maturation and estrogen production in women, and Sertoli cell proliferation and spermatogenesis in men (Nagirnaja et al., 2010).
Rare mutations in the FSHB gene cause truncation of the FSH-β protein and result in hypogonadism and primary amenorrhoea in females (Layman et al., 1997; Matthews and Chatterjee, 1997; Kottler et al., 2010) and, in a male, delayed puberty with azoospermia (Phillip et al., 1998). Mouse models suggest that FSH is required for normal fertility. Female Fshb knockout mice are infertile and fail to complete normal folliculogenesis, while male knockouts remain fertile but have reduced sperm counts, and infertility is observed in both male and female transgenic mice overexpressing human FSH (Kumar et al., 1997, 1999).
A polymorphism in the promoter of FSHB (rs10835638; c.-211G>T) −211 bp upstream of the transcription start site is associated with reduced FSH-β production in vitro and in human genetic studies. In vitro, the T allele of the promoter polymorphism reduces expression of a luciferase reporter gene (Hoogendoorn et al., 2003) and decreases FSHB transcription in gonadotroph cells as a result of reduced LHX3 homeodomain transcription factor binding (Benson et al., 2013). The T allele of rs10835638 (c.-211G>T) is associated with lower FSH levels in men and women, and with higher LH and lower testicular volume, sperm count, FSH/LH ratio, inhibin B and testosterone in men, and has been found at a higher prevalence in infertile men (Grigorova et al., 2008, 2010, 2011; Tuttelmann et al., 2012; La Marca et al., 2013; Schuring et al., 2013; Simoni and Casarini, 2014; Ruth et al., 2015). Genetic association studies have identified signals at the FSHB locus associated with age at menopause (Stolk et al., 2012; Day et al., 2015), polycystic ovary syndrome (PCOS) (Hayes et al., 2015) and levels of LH (Hayes et al., 2015; Ruth et al., 2015).
Using the unique resource of the UK Biobank (Allen et al., 2014), we show that a common genetic variant known to alter FSH levels impacts a wide range of traits important to female reproductive health, including fertility, endometriosis and menstrual cycle length. In the first genome-wide association study (GWAS) for menstrual cycle length, we identified the FSHB locus as the only signal associated with this trait.
Materials and Methods
Source of data
The UK Biobank includes data for 503 325 people aged 40–69 years recruited in 2006–2010 from across the UK (Allen et al., 2014). We analysed data from the May 2015 interim release of imputed genetic data from UK Biobank, which contains 73 355 667 single-nucleotide polymorphisms (SNPs), short insertion/deletions and large structural variants in 152 249 individuals [http://www.ukbiobank.ac.uk/wp-content/uploads/2014/04/imputation_documentation_May2015.pdf (17 December 2015, date last accessed)]. UK Biobank invited 9.2 million people to participate, giving a response rate of 5.47% (Allen et al., 2012). Participants were registered with the UK National Health Service and lived within 25 miles of one of the 22 assessment centres. Participants answered detailed questions about themselves, had measurements taken and provided blood, urine and saliva samples. Two arrays with over 95% common marker content were used to genotype the individuals. Approximately 50 000 people were genotyped on the UK BiLEVE array, and the remainder were genotyped on the UK Biobank Axiom array.
Phenotypes
We derived reproductive phenotypes from the UK Biobank data (Supplementary data). Continuous phenotypes were age at birth of first and last child (females only), age at menarche, age at natural menopause, length of menstrual cycle, number of live births and number of children fathered (included to test the association with male fertility). Menstrual cycle length was only recorded in women who were still cycling and they were asked ‘How many days is your usual menstrual cycle? (The number of days between each menstrual period)’ (excluding those answering <7 or >365; and if the answer was <12 or >60, then the participant was asked to confirm). Cycle length was not available for 25% of women still cycling (1% did not answer, 6% did not know and for 18% cycle length was recorded as ‘irregular’).
To test assumptions of linearity, we analysed the binary outcomes early menarche (lower 5% tail), early menopause (20–44 years), long menstrual cycle (>31 days), short menstrual cycle (≤20 days) and multiple pregnancy loss (>1 case).
We defined two infertility-related binary phenotypes; never pregnant (females) and never fathered a child (males). We analysed female medical conditions as binary outcomes, comparing people reporting a condition (case) with those who did not (control). Medical conditions included dysmenorrhoea, endometriosis, fibroids, irregular menstrual cycles, menopausal symptoms, menorrhagia, ovarian cysts, PCOS, uterine polyps, vaginal/uterine prolapse and breast, endometrial and ovarian cancer. As more general indicators of gynaecological health, we included the medical interventions bilateral oophorectomy or hysterectomy in our analysis.
Participants
In our analysis, we included individuals who both self-identified as white British and were confirmed as ancestrally Caucasian by UK Biobank from genetic information (n = 128 266). We calculated principal components (PCs) for inclusion as covariates in our analyses using FlashPCA (Abraham and Inouye, 2014). PCs were calculated in 120 286 unrelated participants (as identified by UK Biobank) based on 95 535 independent, directly genotyped SNPs (pairwise r2 < 0.1). These SNPs had a minor allele frequency (MAF) ≥2.5% and missing-ness <1.5% across all participants in the May 2015 interim release of genetic data, and had a Hardy–Weinberg equilibrium (HWE) P > 1 × 10−6 within the white British participants.
Testing for associations of the FSHB promoter polymorphism with reproductive phenotypes
We tested the FSH-lowering T allele of the FSHB promoter polymorphism (rs10835638; c.-211G>T) for associations with reproductive phenotypes (up to 63 350 women and 56 608 men). SNP rs10835638 was well imputed in the data (imputation quality 0.995; HWE P = 0.16; missing rate = 0.3%). All analyses were carried out in males or females as appropriate (based on self-defined sex) using Stata (v13) (StataCorp LP, College Station, TX, USA).
For continuous phenotypes, we transformed the phenotype by adjusting for recruitment centre, age at recruitment and the first five PCs prior to inverse-normalization. We performed linear regression of transformed phenotype on imputed minor-allele dosages at SNP rs10835638 with genotyping chip as a covariate. We carried out a sensitivity analysis of the effect of different transformations, e.g. inverse normalizing the trait prior to calculating the residuals; however, this did not materially affect our results. Since the data on length of menstrual cycle included a wide range of values (Supplementary data, Figs S1 and S2), we carried out analyses on cycles from 21 to 35 days and in women aged <45 and ≥45 years at recruitment. We validated our results for length of menstrual cycle by carrying out analyses in two randomly chosen, equally sized groups. For age at menopause and age at menarche, we also ran analysis using the phenotype definition from the ReproGen Consortium GWAS (www.reprogen.org) (untransformed age at menopause between 40 and 60 years not adjusted for age, untransformed age at menarche) to allow comparisons with published data (Stolk et al., 2012; Perry et al., 2014a,b; Day et al., 2015).
For binary outcomes, we performed logistic regression of the phenotype on minor-allele dosages at SNP rs10835638 including the first five PCs, recruitment centre, age at recruitment and genotyping chip as covariates.
GWAS of length of menstrual cycle
We conducted a GWAS to identify genetic variants associated with length of menstrual cycle (n = 9534) using the BOLT-LMM algorithm (described in Loh et al., 2015) from the freely available BOLT-LMM software package [version 2.2, https://data.broadinstitute.org/alkesgroup/BOLT-LMM/ (17 December 2015, date last accessed)] to account for relatedness and population structure. This allowed us to include related individuals who were excluded from the association analysis of the FSHB promoter polymorphism (Supplementary data, Table SI). We transformed length of menstrual cycle by adjusting for recruitment centre and age at recruitment prior to inverse-normalization, and performed association testing while adjusting for genotype chip. We filtered results on imputation quality >0.4, HWE P > 1 × 10−5, and MAF >0.1%, resulting in ∼16.8 million variants that were tested. As the UK Biobank GWAS included more variants than a standard GWAS and we did not have a replication sample available, we chose a threshold of P < 5 × 10−9, based on a Bonferroni correction for the number of variants tested, rather than the conventional P < 5 × 10−8.
Results
A common allele in the FSHB gene, known to lower FSH levels, is associated with longer length of menstrual cycle
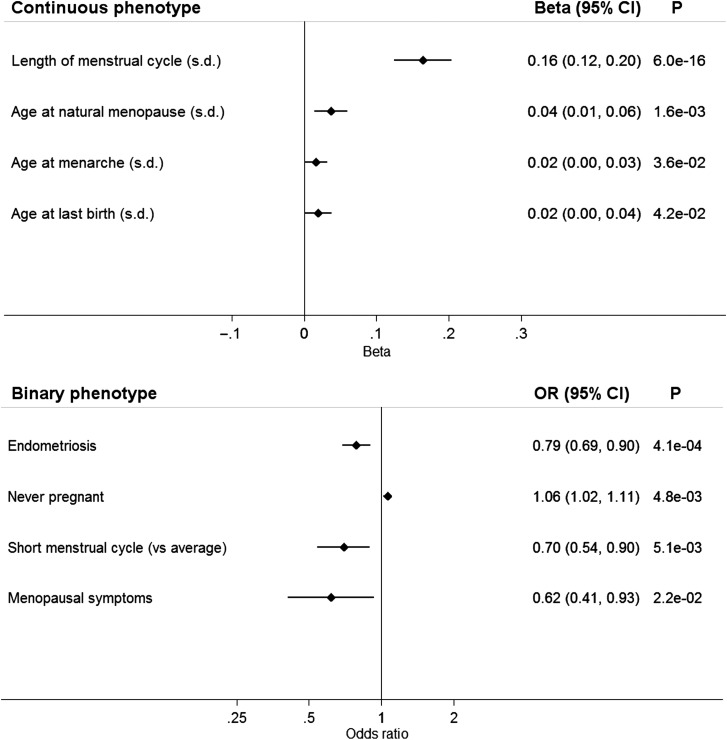
The FSH-lowering T allele of the FSHB promoter polymorphism (rs10835638; MAF 0.16) was associated with longer menstrual cycles [0.16 SD (∼1 day) per minor allele; 95% confidence interval (CI) 0.12–0.20; P = 6 × 10−16]. Of the reproductive traits tested (Tables I and II), length of menstrual cycle was the most strongly associated with rs10835638 (Fig. 1 and Table III). The SNP was also associated with cycle length when we dichotomized data into women reporting a cycle length of ≤20 days compared with those reporting an average length of 28 days [odds ratio (OR) = 0.70; 95% CI 0.54–0.90; P = 5.1 × 10−3] (Fig. 1). There was no evidence for an association with a cycle >31 days compared with the average (OR = 1.16; 95% CI 0.92–1.47; P = 0.21). Results remained consistent when we analysed cycle lengths of 21–35 days and when we split our analysis into women aged <45 or ≥45 years (Supplementary data, Fig. S3). Analysis after randomly dividing the sample into two equal parts supported these results (Supplementary data, Fig. S3).
Table I.
Description of cohort of unrelated individuals for continuous outcome measures.
| Phenotype | n | Min | Max | Mean | SD | Lower quartile | Median | Upper quartile |
|---|---|---|---|---|---|---|---|---|
| Age at first birth (years)1 | 43 066 | 10 | 50 | 25.1 | 4.6 | 22 | 25 | 28 |
| Age at last birth (years)1 | 43 008 | 15 | 50 | 30.0 | 4.8 | 27 | 30 | 33 |
| Age at menarche (years)1 | 61 306 | 9 | 17 | 12.9 | 1.6 | 12 | 13 | 14 |
| Age at natural menopause (years)1 | 27 996 | 18 | 65 | 49.9 | 4.5 | 48 | 50 | 53 |
| Length of menstrual cycle (days)1 | 8870 | 7 | 300 | 26.8 | 6.2 | 25 | 28 | 28 |
| Number of children fathered2 | 56 508 | 0 | 28 | 1.8 | 1.2 | 1 | 2 | 2 |
| Number of live births1 | 63 306 | 0 | 22 | 1.8 | 1.2 | 1 | 2 | 2 |
Min, minimum; Max, maximum.
1Females only.
2Males only.
Table II.
Number of people included in binary outcome measures.
| Phenotype | Description | Cases | Controls | n |
|---|---|---|---|---|
| Bilateral oophorectomy1 | Yes versus no | 5118 | 57 177 | 62 295 |
| Dysmenorrhoea1 | Yes versus none recorded | 78 | 63 272 | 63 350 |
| Breast cancer1 | Breast cancer recorded on cancer registry versus none recorded | 2810 | 60 540 | 63 350 |
| Early menarche1 | Youngest 5% age at menarche versus oldest 5% | 3050 | 3050 | 6100 |
| Early menopause1 | Natural menopause at 20–45 versus 50–60 years | 3058 | 17 805 | 20 863 |
| Endometrial cancer1 | Endometrial cancer recorded on cancer registry versus none recorded | 342 | 63 008 | 63 350 |
| Endometriosis1 | Yes versus none recorded | 993 | 62 357 | 63 350 |
| Fibroids1 | Yes versus none recorded | 1819 | 61 531 | 63 350 |
| Hysterectomy1 | Yes versus no | 4753 | 50 932 | 55 685 |
| Irregular menstrual cycles1 | Irregular menstrual cycles versus regular cycle | 2490 | 10 316 | 12 806 |
| Long menstrual cycle (versus average)1 | Menstrual cycle >31 versus 28 days | 237 | 3889 | 4126 |
| Menopausal symptoms1 | Yes versus none recorded | 126 | 63 224 | 63 350 |
| Menorrhagia1 | Yes versus none recorded | 348 | 63 002 | 63 350 |
| Multiple pregnancy loss1 | More than one pregnancy loss versus none | 4047 | 33 191 | 37 238 |
| Never fathered child2 | Never fathered a child versus one or more children fathered | 11 729 | 44 779 | 56 508 |
| Never pregnant1 | Never pregnant versus one or more pregnancies | 9247 | 52 966 | 62 213 |
| Ovarian cancer1 | Ovarian cancer recorded on cancer registry versus none recorded | 247 | 63 103 | 63 350 |
| Ovarian cysts1 | Yes versus none recorded | 1 015 | 62 335 | 63 350 |
| Polycystic ovary syndrome1 | Yes versus none recorded | 153 | 63 197 | 63 350 |
| Short menstrual cycle (versus average)1 | Menstrual cycle ≤20 versus 28 days | 288 | 3889 | 4 177 |
| Uterine polyps1 | Yes versus none recorded | 359 | 62 991 | 63 350 |
| Vaginal/uterine prolapse1 | Yes versus none recorded | 653 | 62 697 | 63 350 |
1Females only.
2Males only.
Figure 1.
Forest plot of phenotypes associated (P < 0.05) with the FSH-lowering T allele of rs10835638 (c.-211G>T). For continuous variables, effects (β) are in standard deviations of the inverse-normally transformed variable to enable effect size comparisons. CI, confidence interval; OR, odds ratio.
Table III.
Associations with the FSH-lowering T allele of rs10835638 (c.-211G>T).
| Phenotype | Statistic | Effect(95% CI) | SE | P-value |
|---|---|---|---|---|
| Length of menstrual cycle (SD) | β | 0.16 (0.12, 0.20) | 0.02 | 6.0E−16 |
| Endometriosis | OR | 0.79 (0.69, 0.90) | 0.05 | 4.1E−04 |
| Age at natural menopause (SD) | β | 0.04 (0.01, 0.06) | 0.01 | 1.6E−03 |
| Never pregnant | OR | 1.06 (1.02, 1.11) | 0.02 | 4.8E−03 |
| Short menstrual cycle (versus average) | OR | 0.70 (0.54, 0.90) | 0.09 | 5.1E−03 |
| Menopausal symptoms | OR | 0.62 (0.41, 0.93) | 0.13 | 2.2E−02 |
| Age at menarche (SD) | β | 0.02 (0.00, 0.03) | 0.01 | 3.6E−02 |
| Age at last birth (SD) | β | 0.02 (0.00, 0.04) | 0.01 | 4.2E−02 |
| Age at first birth (SD) | β | 0.02 (0.00, 0.03) | 0.01 | 7.9E−02 |
| Number of live births (SD) | β | −0.01 (−0.03, 0.00) | 0.01 | 8.1E−02 |
| Never fathered a child | OR | 1.03 (0.99, 1.08) | 0.02 | 1.2E−01 |
| Early menopause | OR | 0.95 (0.88, 1.02) | 0.04 | 1.6E−01 |
| Early menarche | OR | 0.94 (0.85, 1.04) | 0.05 | 2.1E−01 |
| Fibroids | OR | 0.94 (0.86, 1.03) | 0.04 | 2.1E−01 |
| Long menstrual cycle (versus average) | OR | 1.16 (0.92, 1.47) | 0.14 | 2.1E−01 |
| Polycystic ovary syndrome | OR | 1.18 (0.88, 1.59) | 0.18 | 2.7E−01 |
| Ovarian cysts | OR | 0.94 (0.83, 1.07) | 0.06 | 3.6E−01 |
| Number of children fathered (SD) | Beta | 0.01 (−0.01, 0.02) | 0.01 | 4.1E−01 |
| Menorrhagia | OR | 0.92 (0.74, 1.13) | 0.10 | 4.2E−01 |
| Irregular menstrual cycles | OR | 0.97 (0.89, 1.06) | 0.04 | 4.6E−01 |
| Multiple pregnancy loss | OR | 0.98 (0.91, 1.04) | 0.03 | 4.6E−01 |
| Dysmenorrhoea | OR | 0.87 (0.56, 1.38) | 0.20 | 5.6E−01 |
| Breast cancer | OR | 1.02 (0.95, 1.10) | 0.04 | 6.4E−01 |
| Ovarian cancer | OR | 0.94 (0.74, 1.21) | 0.12 | 6.4E−01 |
| Vaginal/uterine prolapse | OR | 0.97 (0.83, 1.13) | 0.08 | 6.7E−01 |
| Uterine polyps | OR | 0.98 (0.80, 1.20) | 0.10 | 8.6E−01 |
| Endometrial cancer | OR | 1.00 (0.81, 1.23) | 0.11 | 9.7E−01 |
Note: For continuous variables, effects (β) are in standard deviations of the inverse-normally transformed variable to enable effect size comparisons. Results significant at P < 5E−08 are in bold; results significant at P < 5E−02 are underlined.
CI, confidence interval; OR, odds ratio; SD, standard deviations.
Variants in or near the FSHB gene were the only ones that reached a conservative level of genome-wide significance in the GWAS for menstrual cycle length (Fig. 2). The strongest association was for rs564036233G>GA, a 1 bp insertion which was associated with longer cycles by 1 day (0.16 SD) per minor allele (95% CI 0.12–0.20; P = 1.30 × 10−16). The rs564036233 variant is in strong linkage disequilibrium (LD) with the promoter polymorphism rs10835638 (r2 = 0.82) and conditional analysis indicated that rs564036233 and rs10835638 represent the same signal. The next strongest signal in the GWAS was on Chromosome 9 in the NOTCH1 gene, but did not meet our genome-wide significance threshold and would require further replication (rs3124592A>G; MAF 0.45; 0.08 SD per minor allele; 95% CI 0.05–0.11; P = 1.9 × 10−8).
Figure 2.
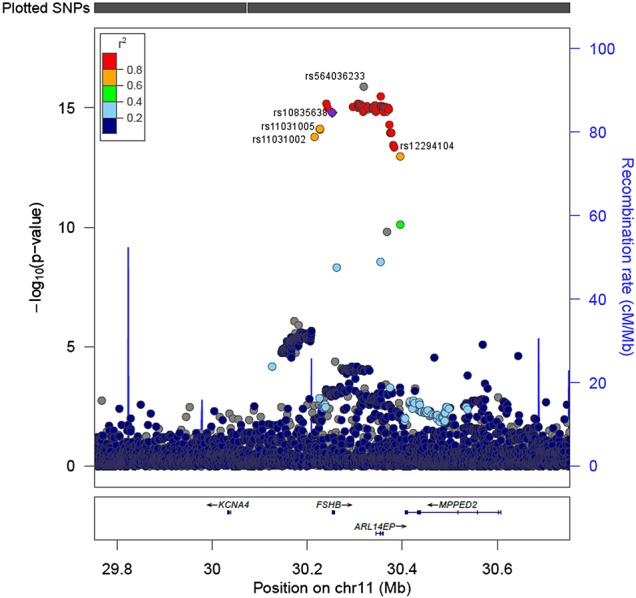
LocusZoom plot showing variants associated with length of menstrual cycle. The most strongly associated variant for cycle length is rs564036233. LD (1000 Genomes Nov 2014 EUR) shown is with rs10835638, the FSHB promoter polymorphism. Other SNPs indicated were the variants most significantly associated with FSH (rs11031005) and LH (rs11031002) in a GWAS of hormone levels (Ruth et al., 2015), and with age at natural menopause (rs12294104) in a meta-analysis (Stolk et al., 2012). KCNA4: potassium channel, voltage-gated shaker-related subfamily A, member 4. ARL14EP: ADP-ribosylation factor-like GTPase 14 effector protein. MPPED2: metallophosphoesterase domain containing 2. Note: LD values are not available for all SNPs since they are not included in 1000 Genomes Nov 2014 EUR. Position is in build hg19/GRCh37.
The FSHB allele associated with longer cycle length is associated with later menopause
The FSH-lowering T allele of rs10835638 was associated with later age at menopause for those in the UK Biobank [0.13 years per minor allele (ReproGen definition); 95% CI 0.04–0.22; P = 5.7 × 10−3]. There was no association between rs10835638 and menopause age when we dichotomized the phenotype into early menopause compared with later menopause (Table III). The FSHB locus is known to be associated with timing of menopause: in a GWAS conducted by the ReproGen consortium, the signal at this locus (rs12294104) increases age at menopause by 0.23 years (95% CI 0.16–0.29; P = 1.5 × 10−11) (Stolk et al., 2012). Later menopause has been shown to be associated with later age at last birth (Ayatollahi et al., 2003; Dorjgochoo et al., 2008) and rs10835638 was also associated with later age at last birth [0.02 SD (∼0.1 years) per T allele; 95% CI 0.00–0.04; P = 4.2 × 10−2].
Longer cycle length is not a general feature of alleles associated with later age at menopause
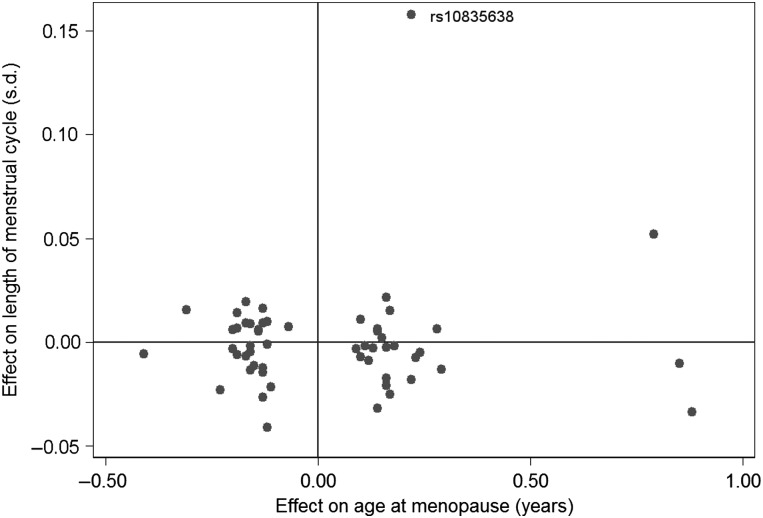
We next tested the role of all 56 genetic variants associated with age at menopause. In addition to the age at menopause signal at the FSHB locus (rs12294104), only one of the other 55 published age at menopause signals was nominally associated with cycle length (P > 0.05): rs10734411 was associated at P = 0.005 (Stolk et al., 2012; Perry et al., 2014a,b; Day et al., 2015). For the 56 published menopause SNPs, there was no correlation between the published effect estimates for age at menopause and the effect estimates from our GWAS for menstrual cycle length (R = 0.064, P = 0.63) (Fig. 3). The FSHB SNP was an outlier in this plot, but removing it did not substantially affect the correlation (R = −0.027; P = 0.84).
Figure 3.
Comparison of the published effect size of the 56 known age at menopause variants (Stolk et al., 2012; Perry et al., 2014a,b) and their effect size in the GWAS for menstrual cycle length. There was no significant correlation between the effects on age at menopause and cycle length (R = 0.064, P = 0.63). The FSHB promoter polymorphism (rs10835638) is indicated.
The FSHB allele associated with lower FSH is also associated with an indicator of female infertility
The FSH-lowering T allele of the FSHB promoter polymorphism (rs10835638) was associated with female nulliparity, i.e. greater odds of never being pregnant (OR = 1.06; CI 1.02–1.11; P = 4.8 × 10−3) (Fig. 1). The FSH-lowering allele was not associated with other possible indicators of female infertility (later age at first birth and fewer live births) or male infertility (number of children fathered) (P > 0.05) (Table III).
The FSHB allele associated with higher FSH is also associated with higher odds of endometriosis and surgical intervention
The more common G allele was associated with increased odds of endometriosis (OR = 1.27; CI 1.11–1.45; P = 4.1 × 10−4) (Fig. 1). Of the seven published GWAS variants associated with endometriosis risk (Nyholt et al., 2012), the variant on chromosome 12 was nominally associated with cycle length, with the allele associated with an increased risk of endometriosis also associated with shorter cycles (P = 0.02).
The G allele of rs10835638 was also associated with increased odds of having the medical interventions bilateral oophorectomy (OR = 1.12; 95% CI 1.06–1.19; P = 1.4 × 10−4) and hysterectomy (OR = 1.13; 95% CI 1.06–1.20; P = 1.0 × 10−4), which are used as treatments for a range of gynaecological conditions including endometriosis.
The common FSHB variant, associated with FSH levels, is not associated with reproductive traits more generally
There was no consistent evidence that the FSHB variant (rs10835638) was associated with age at menarche. There was a 0.03-year increase in age at menarche (ReproGen definition) per T allele of rs10835638 (95% CI 0.01–0.05; P = 1.4 × 10−2) and the binary phenotype of early menarche was associated at P > 0.05 (Table III). None of 122 published GWAS signals for menarche (Perry et al., 2014a,b) were associated with length of menstrual cycle at P < 0.008.
The FSHB promoter polymorphism (rs10835638) was not associated with other reproductive illnesses or conditions at P < 0.05 (Table III), except for menopausal symptoms (OR = 0.62; 95% CI 0.41–0.93; P = 0.02) (Fig. 1). No associations were found with dysmenorrhoea, fibroids, irregular menstrual cycles, menorrhagia, multiple pregnancy loss, ovarian cysts, PCOS, uterine polyps or vaginal/uterine prolapse, or with female breast, ovarian or endometrial cancer.
Discussion
In the first GWAS of menstrual cycle length, we found a strong association between an FSH lowering, likely functional, variant in the FSHB promoter and longer cycles (Hoogendoorn et al., 2003; Grigorova et al., 2008, 2010; Tuttelmann et al., 2012; Benson et al., 2013; La Marca et al., 2013; Simoni and Casarini, 2014; Ruth et al., 2015). This locus has been previously robustly associated with age at menopause in the ReproGen consortium GWAS of menopause timing (Stolk et al., 2012; Day et al., 2015) and the allele associated with longer cycle length is also associated with later age at menopause. We did not observe associations for the majority of age at menopause GWAS signals with length of menstrual cycle, including the four signals with effects of over one-third of a year per allele on menopause timing, implying that the association is specific to FSHB: either FSH-β has independent effects on both cycle length and menopause or changes in cycle length are causally influencing menopause timing.
Our results are consistent with the observed epidemiological relationship between longer menstrual cycles and later age at menopause (Whelan et al., 1990; Kaczmarek, 2007). It is possible that there is a biological limit on the lifetime number of menstrual cycles; hence, women with longer cycles would have later menopause. Alternatively, they may have reduced follicle recruitment per cycle, depleting their ovarian reserve more slowly. Women with longer cycles have more waves of folliculogenesis during each cycle (Baerwald et al., 2003, 2012) but may recruit fewer antral follicles per wave. Oocyte loss due to ovulation is unlikely to be driving the relationship, since this contributes much less to overall oocyte depletion than atresia, and there is no robust evidence that preventing ovulation by the use of the combined oral contraceptive pill influences menopause timing (van Noord et al., 1997; de Vries et al., 2001; Gold et al., 2001, 2013; Ayatollahi et al., 2003; Palmer et al., 2003; Kaczmarek, 2007; Dorjgochoo et al., 2008; OlaOlorun and Lawoyin, 2009; Pokoradi et al., 2011; Stepaniak et al., 2013) and both longer and shorter cycles are more likely to be anovulatory (Mihm et al., 2011). More work is needed to understand the molecular mechanism that explains the association between cycle length and menopause timing.
The FSH-reducing allele was associated with nulliparity, perhaps indicating increased female infertility. Although we were unable to distinguish those unable to have children from those not wishing to, the sample of nulliparous women will be enriched for both female and male factor infertility. The FSH-lowering allele has previously been found to be associated with male infertility (Grigorova et al., 2008, 2010; Tuttelmann et al., 2012; Simoni and Casarini, 2014), but we found no association with males who had never fathered a child suggesting a female-specific effect, although this may because the phenotype includes males who chose not to have children in addition to infertile males. Using nulliparity as a proxy for infertility is unlikely to generate a false-positive association, but may have reduced our power to detect a true association. The relationship between FSH and fertility over a woman's lifetime may differ from the age-related changes in FSH around menopause. In contrast to our genetic association between lower FSH and infertility, women nearing menopause have higher FSH concentrations, poorer ovarian reserve and decreased fertility (Waller et al., 1998; Mihm et al., 2011). FSH is required for follicle development and it is proposed that an FSH threshold is required to achieve ovulation (Kumar et al., 1997, 1999). Ovulation increases with increasing FSH in transgenic mice with FSH levels that increase with age independently of follicle depletion (McTavish et al., 2007). A high baseline level of FSH, determined by genetic variation, may promote ovulation and explain our association with parity.
The FSH-increasing allele increased the risk of endometriosis in our study. Several GWAS of endometriosis have been performed; however, none have reported a signal at the 11p14.1 locus and there was no evidence that the genome-wide significant endometriosis variants were associated with cycle length in our study (Adachi et al., 2010; Uno et al., 2010; Painter et al., 2011; Nyholt et al., 2012; Albertsen et al., 2013). Drug treatments for endometriosis aim to prevent ovulation and menstruation, and to stabilize hormone levels, since estrogens fuel ectopic endometrial growth (Vercellini et al., 2014). The FSH-increasing allele may similarly stimulate abnormal growth of endometrium. Endometriosis is associated with earlier menopause (Pokoradi et al., 2011; Yasui et al., 2011) and shorter menstrual cycles (Vercellini et al., 2014), consistent with our findings. The FSH-increasing variant associated with increased risk of endometriosis was also associated with parity; however, endometriosis can cause infertility as a result of endometriotic lesions and chronic pelvic inflammation. Therefore, the association of the FSHB polymorphism with infertility appears to be independent of the association with endometriosis.
We found a modest association of the FSH-lowering allele with increased age at menarche, but the published age at menarche GWAS signals were not associated with length of menstrual cycle. The closest GWAS menarche signal to FSHB (rs16918636) is 1.13 Mb away and is not in LD (r2 = 0.001) with the FSHB promoter polymorphism SNP (Perry et al., 2014a,b). Although FSH is important for normal puberty, the role of variation in baseline FSH levels on puberty timing is uncertain.
The UK Biobank recruited individuals over 40 years old, and many of the women still cycling will be approaching menopause; however, if the association with cycle length was being driven by peri-menopausal changes, we would expect all menopause-associated variants to be associated with cycle length. In addition, our sensitivity analysis suggested a stronger effect of the FSHB promoter polymorphism in younger women. We were unable to replicate an association between the FSH-lowering allele and increased odds of PCOS (Hayes et al., 2015). However, we had only a small number of cases (n = 153) limiting our power to detect this association. Other illnesses had relatively small sample sizes and may have been similarly under-powered. We might have also under-ascertained cases, as most illnesses will be subject to recall bias as they are self-reported and collected retrospectively, while controls might include people not reporting an illness.
Our study provides evidence that a likely functional variant in the FSHB promoter is strongly associated with longer menstrual cycles, and to a lesser extent with female infertility and lower risk of endometriosis. There is considerable evidence that the T allele of the FSHB promoter polymorphism decreases FSH levels (Hoogendoorn et al., 2003; Grigorova et al., 2008, 2010; Tuttelmann et al., 2012; Benson et al., 2013; La Marca et al., 2013; Simoni and Casarini, 2014; Ruth et al., 2015), but it has also been associated with increased LH levels (Hayes et al., 2015; Ruth et al., 2015). While we cannot rule out that the variant may be having direct or indirect effects on other hormone levels, a change in FSH is the most likely primary mechanism. In conclusion, we suggest that lower FSH levels result in longer menstrual cycles and as a consequence later menopause and, while having detrimental effects on female fertility, are protective against endometriosis.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
A.M. and K.S.R. designed the study, carried out analysis and drafted the article. All authors were involved in designing and performing analysis of the UK Biobank data, revising and approving the manuscript.
Funding
A.R.W., H.Y. and T.M.F. are supported by the European Research Council grant: 323195:GLUCOSEGENES-FP7-IDEAS-ERC. R.M.F. is a Sir Henry Dale Fellow (Wellcome Trust and Royal Society grant: 104150/Z/14/Z). R.N.B. is funded by the Wellcome Trust and Royal Society grant: 104150/Z/14/Z. J.T. is funded by the ERDF and a Diabetes Research and Wellness Foundation Fellowship. S.E.J. is funded by the Medical Research Council (grant: MR/M005070/1). M.A.T., M.N.W. and A.M. are supported by the Wellcome Trust Institutional Strategic Support Award (WT097835MF) (323195). The funders had no influence on study design, data collection and analysis, decision to publish or preparation of the manuscript. Funding to pay the Open Access publication charges for this article was provided by The Wellcome Trust.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We thank Dr A.M. Godwin (DRCOG, MRCGP) for identifying medications influencing length of menstrual cycle. This research has been conducted using the UK Biobank Resource.
References
- Abraham G, Inouye M. Fast principal component analysis of large-scale genome-wide data. PLoS One 2014;9:e93766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Tajima A, Quan J, Haino K, Yoshihara K, Masuzaki H, Katabuchi H, Ikuma K, Suginami H, Nishida N et al. Meta-analysis of genome-wide association scans for genetic susceptibility to endometriosis in Japanese population. J Hum Genet 2010;55:816–821. [DOI] [PubMed] [Google Scholar]
- Albertsen HM, Chettier R, Farrington P, Ward K. Genome-wide association study link novel loci to endometriosis. PloS One 2013;8:e58257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, Gallacher J, Green J, Matthews P, Pell J et al. UK Biobank: current status and what it means for epidemiology. Health Policy Technol 2012;1:123–126. [Google Scholar]
- Allen NE, Sudlow C, Peakman T, Collins R, Biobankobo U. UK biobank data: come and get it. Sci Transl Med 2014;6:224ed4. [DOI] [PubMed] [Google Scholar]
- Ayatollahi SM, Ghaem H, Ayatollahi SA. Menstrual-reproductive factors and age at natural menopause in Iran. Int J Gynaecol Obstet 2003;80:311–313. [DOI] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril 2003;80:116–122. [DOI] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update 2012;18:73–91. [DOI] [PubMed] [Google Scholar]
- Benson CA, Kurz TL, Thackray VG. A human FSHB promoter SNP associated with low FSH levels in men impairs LHX3 binding and basal FSHB transcription. Endocrinology 2013;154:3016–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, Stolk L, Finucane HK, Sulem P, Bulik-Sullivan B et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet 2015;47:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E, den Tonkelaar I, van Noord PA, van der Schouw YT, te Velde ER, Peeters PH. Oral contraceptive use in relation to age at menopause in the DOM cohort. Hum Reprod 2001;16:1657–1662. [DOI] [PubMed] [Google Scholar]
- Dorjgochoo T, Kallianpur A, Gao YT, Cai H, Yang G, Li H, Zheng W, Shu XO. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women's Health Study. Menopause 2008;15:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature 2005;433:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold E, Bromberger J, Crawford S, Samuels S, Greendale G, Harlow S, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001;153:865–874. [DOI] [PubMed] [Google Scholar]
- Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow SD. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013;178:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova M, Punab M, Ausmees K, Laan M. FSHB promoter polymorphism within evolutionary conserved element is associated with serum FSH level in men. Hum Reprod 2008;23:2160–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova M, Punab M, Poolamets O, Kelgo P, Ausmees K, Korrovits P, Vihljajev V, Laan M. Increased prevalence of the -211T allele of follicle stimulating hormone (FSH) beta subunit promoter polymorphism and lower serum FSH in infertile men. J Clin Endocrinol Metab 2010;95:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova M, Punab M, Zilaitiene B, Erenpreiss J, Ausmees K, Matulevicius V, Tsarev I, Jorgensen N, Laan M. Genetically determined dosage of follicle-stimulating hormone (FSH) affects male reproductive parameters. J Clin Endocrinol Metab 2011;96:E1534–E1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun 2015;6 doi:10.1038/ncomms8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendoorn B, Coleman SL, Guy CA, Smith K, Bowen T, Buckland PR, O'Donovan MC. Functional analysis of human promoter polymorphisms. Hum Mol Genet 2003;12:2249–2254. [DOI] [PubMed] [Google Scholar]
- Kaczmarek M. The timing of natural menopause in Poland and associated factors. Maturitas 2007;57:139–153. [DOI] [PubMed] [Google Scholar]
- Kottler ML, Chou YY, Chabre O, Richard N, Polge C, Brailly-Tabard S, Chanson P, Guiochon-Mantel A, Huhtaniemi I, Young J. A new FSHbeta mutation in a 29-year-old woman with primary amenorrhea and isolated FSH deficiency: functional characterization and ovarian response to human recombinant FSH. Eur J Endocrinol 2010;162:633–641. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 1997;15:201–204. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Palapattu G, Wang P, Woodruff TK, Boime I, Byrne MC, Matzuk MM. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol Endocrinol (Baltimore, MD) 1999;13:851–865. [DOI] [PubMed] [Google Scholar]
- La Marca A, Papaleo E, Alviggi C, Ruvolo G, De Placido G, Candiani M, Cittadini E, De Michele F, Moriondo V, Catellani V et al. The combination of genetic variants of the FSHB and FSHR genes affects serum FSH in women of reproductive age. Hum Reprod 2013;28:1369–1374. [DOI] [PubMed] [Google Scholar]
- Layman LC, Lee EJ, Peak DB, Namnoum AB, Vu KV, van Lingen BL, Gray MR, McDonough PG, Reindollar RH, Jameson JL. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone beta-subunit gene. N Engl J Med 1997;337:607–611. [DOI] [PubMed] [Google Scholar]
- Loh PR, Tucker G, Bulik-Sullivan BK, Vilhjalmsson BJ, Finucane HK, Salem RM, Chasman DI, Ridker PM, Neale BM, Berger B et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet 2015;47:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C, Chatterjee VK. Isolated deficiency of follicle-stimulating hormone re-revisited. N Engl J Med 1997;337:642. [DOI] [PubMed] [Google Scholar]
- McTavish KJ, Jimenez M, Walters KA, Spaliviero J, Groome NP, Themmen AP, Visser JA, Handelsman DJ, Allan CM. Rising follicle-stimulating hormone levels with age accelerate female reproductive failure. Endocrinology 2007;148:4432–4439. [DOI] [PubMed] [Google Scholar]
- Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci 2011;124:229–236. [DOI] [PubMed] [Google Scholar]
- Nagirnaja L, Rull K, Uuskula L, Hallast P, Grigorova M, Laan M. Genomics and genetics of gonadotropin beta-subunit genes: unique FSHB and duplicated LHB/CGB loci. Mol Cell Endocrinol 2010;329:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt DR, Low S-K, Anderson CA, Painter JN, Uno S, Morris AP, MacGregor S, Gordon SD, Henders AK, Martin NG et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet 2012;44:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OlaOlorun F, Lawoyin T. Age at menopause and factors associated with attainment of menopause in an urban community in Ibadan, Nigeria. Climacteric 2009;12:352–363. [DOI] [PubMed] [Google Scholar]
- Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, Lambert A, Zhao ZZ, Roseman F, Guo Q et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet 2011;43:51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JR, Rosenberg L, Wise LA, Horton NJ, Adams-Campbell LL. Onset of natural menopause in African American women. Am J Public Health 2003;93:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JR, Day F, Elks CE, Sulem P, Thompson DJ, Ferreira T, He C, Chasman DI, Esko T, Thorleifsson G et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 2014a;514:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JR, Hsu YH, Chasman DI, Johnson AD, Elks C, Albrecht E, Andrulis IL, Beesley J, Berenson GS, Bergmann S et al. DNA mismatch repair gene MSH6 implicated in determining age at natural menopause. Hum Mol Genet 2014b;23:2490–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillip M, Arbelle JE, Segev Y, Parvari R. Male hypogonadism due to a mutation in the gene for the beta-subunit of follicle-stimulating hormone. N Engl J Med 1998;338:1729–1732. [DOI] [PubMed] [Google Scholar]
- Pokoradi AJ, Iversen L, Hannaford PC. Factors associated with age of onset and type of menopause in a cohort of UK women. Am J Obstet Gynecol 2011;205:34.e1–13. [DOI] [PubMed] [Google Scholar]
- Ruth KS, Campbell PJ, Chew S, Lim EM, Hadlow N, Stuckey BG, Brown SJ, Feenstra B, Joseph J, Surdulescu GL et al. Genome-wide association study with 1000 genomes imputation identifies signals for nine sex hormone-related phenotypes. Eur J Hum Genet 2015. doi:10.1038/ejhg.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuring AN, Busch AS, Bogdanova N, Gromoll J, Tuttelmann F. Effects of the FSH-beta-subunit promoter polymorphism -211G->T on the hypothalamic–pituitary–ovarian axis in normally cycling women indicate a gender-specific regulation of gonadotropin secretion. J Clin Endocrinol Metab 2013;98:E82–E86. [DOI] [PubMed] [Google Scholar]
- Simoni M, Casarini L. Mechanisms in endocrinology: genetics of FSH action: a 2014-and-beyond view. Eur J Endocrinol 2014;170:R91–R107. [DOI] [PubMed] [Google Scholar]
- Stepaniak U, Szafraniec K, Kubinova R, Malyutina S, Peasey A, Pikhart H, Pajak A, Bobak M. Age at natural menopause in three central and eastern European urban populations: the HAPIEE study. Maturitas 2013;75:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, Barbalic M, Broer L, Byrne EM, Ernst F et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet 2012;44:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttelmann F, Laan M, Grigorova M, Punab M, Sober S, Gromoll J. Combined effects of the variants FSHB -211G>T and FSHR 2039A>G on male reproductive parameters. J Clin Endocrinol Metab 2012;97:3639–3647. [DOI] [PubMed] [Google Scholar]
- Uno S, Zembutsu H, Hirasawa A, Takahashi A, Kubo M, Akahane T, Aoki D, Kamatani N, Hirata K, Nakamura Y. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet 2010;42:707–710. [DOI] [PubMed] [Google Scholar]
- van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril 1997;68:95–102. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261–275. [DOI] [PubMed] [Google Scholar]
- Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. Am J Epidemiol 1998;147:1071–1080. [DOI] [PubMed] [Google Scholar]
- Whelan EA, Sandler DP, McConnaughey DR, Weinberg CR. Menstrual and reproductive characteristics and age at natural menopause. Am J Epidemiol 1990;131:625–632. [DOI] [PubMed] [Google Scholar]
- Yasui T, Hayashi K, Mizunuma H, Kubota T, Aso T, Matsumura Y, Lee JS, Suzuki S. Association of endometriosis-related infertility with age at menopause. Maturitas 2011;69:279–283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.