Abstract
The in vivo roles for even the most intensely studied microRNAs remain poorly defined. Here, analysis of mouse models revealed that let-7, a large and ancient microRNA family, performs tumor suppressive roles at the expense of regeneration. Too little or too much let-7 resulted in compromised protection against cancer or tissue damage, respectively. Modest let-7 overexpression abrogated MYC-driven liver cancer by antagonizing multiple let-7 sensitive oncogenes. However, the same level of overexpression blocked liver regeneration, while let-7 deletion enhanced it, demonstrating that distinct let-7 levels can mediate desirable phenotypes. let-7 dependent regeneration phenotypes resulted from influences on the insulin-PI3K-mTOR pathway. We found that chronic high-dose let-7 overexpression caused liver damage and degeneration, paradoxically leading to tumorigenesis. These dose-dependent roles for let-7 in tissue repair and tumorigenesis rationalize the tight regulation of this microRNA in development, and have important implications for let-7 based therapeutics.
DOI: http://dx.doi.org/10.7554/eLife.09431.001
Research Organism: Mouse
eLife digest
The development of animals is guided by the expression of certain genes at critical moments. Many different mechanisms control development; in one of them, the expression of genes can be decreased by molecules called microRNAs. In particular, the group of microRNAs called let-7 has been intensively studied in roundworms and fruit flies. Although mammals have extremely similar let-7 microRNAs they seem to be more important during adulthood.
Previous studies using cells grown in the laboratory have shown that mammalian let-7 microRNAs decrease cell proliferation and cell growth. Furthermore, in mouse models of various cancers, let-7 microRNAs often reduce tumour growth when they are supplied to adult mice. Therefore, overall the let-7 group has been classified as genes that act to suppress tumors, and thus protect mice (and most likely humans too) from cancers. However, in-depth analysis of let-7 microRNAs was still missing.
Wu and Nguyen et al. have now studied mice with liver cancer using strains where they were able to regulate the levels of let-7. These mice overproduce a strong cancer-inducing gene in the liver; half were used as controls and the other half were further engineered to have moderately elevated levels of let-7 expression. Most of the control mice got large cancerous tumors, but only a few mice in the other group developed cancers and the tumors were smaller. This confirmed that let-7 hinders tumor formation.
Wu and Nguyen et al. also observed that the protected mice were less able to regenerate their liver tissues. Further experiments showed that deleting just two out of ten let-7 microRNAs enhanced the mice’s ability to regenerate liver tissue after injury. These findings indicate that let-7 microRNAs slow down the growth of both cancerous and normal cells. Lastly, when let-7 levels were raised to very high levels for a prolonged amount of time this actually led to liver damage and subsequent tumor formation.
This last observation may have important consequences for possible cancer therapies. Some scientists have shown that providing extra let-7 can slow or even reverse tumour growth, but the findings here clearly point out that too much let-7 could actually worsen the situation. Since the let-7 family comprises a handful of microRNAs in mammals, in the future it will also be important to find out to what extent these molecules play overlapping roles and how much they differ.
Introduction
MicroRNAs are thought to control cellular responses to stresses such as tissue damage and transformation (Leung and Sharp, 2010; Chivukula et al., 2014), but the impact of this idea is unclear because microRNAs have been understudied in vivo. let-7 is one of the most ancient and omnipresent microRNAs, yet relatively little is known about its functional roles in mammalian development and physiology. let-7 was first identified as a gene that regulates the timing of developmental milestones in a C. elegans screen (Reinhart et al., 2000). In mammals, mature let-7 is undetectable in early embryos and embryonic stem cells, but becomes highly expressed in most adult tissues (Schulman et al., 2005; Thomson et al., 2006). A handful of previous studies have implicated let-7 in body size regulation, metabolism, stem cell self-renewal, and colon carcinogenesis (Zhu et al., 2011; Frost and Olson, 2011; Shyh-Chang, et al., 2013; Nishino et al., 2013; Madison, et al., 2013, but the core functions of let-7 in regeneration and disease remain incompletely understood.
In addition to questions about what let-7 does, it is unknown why so many let-7s are expressed at such high levels. In mice and humans, the let-7 family is comprised of 10 to 12 members who are thought to share a common set of mRNA targets. It has been thought that deep redundancy might make it difficult to discern any phenotypes that individual let-7s might have. Essential unanswered questions regarding let-7 biology include whether let-7 members are redundant, have unique functions, or are regulated to maintain a specific total dose. Our previous study of Lin28a, which inhibits the biogenesis of each let-7 member similarly (Heo et al., 2008; Nam et al., 2011), suggests that total let-7 dose alterations, rather than regulation of specific members, is important. In transgenic mice, modest increase in Lin28a and consequent 40% suppression of total let-7 levels promote increased glucose uptake and an overgrowth syndrome (Zhu et al., 2010).
In this study we examined the consequences of let-7 dose disruption in cancer and organ regeneration in genetic mouse models. While let-7s have been implicated as a tumor suppressor, this has predominantly been shown in cell lines and xenograft assays (Guo et al., 2006; Chang et al., 2009; Iliopoulos et al., 2009; Viswanathan et al., 2009; Wang et al., 2010; Lan et al., 2011), as well as using exogenous let-7 delivery to mouse cancer models (Esquela-Kerscher et al., 2008; Trang et al., 2010; Trang et al., 2011). Here, we confirmed the tumor suppressor activity of an endogenous transgenic let-7 in a MYC-driven hepatoblastoma model. However, we found that this same level of let-7 overexpression impaired liver regeneration after partial hepatectomy (PHx). Furthermore, chronic high-dose let-7 resulted in severe liver damage and paradoxical liver cancer development. Overall, we provide in vivo evidence that let-7 expression levels have been developmentally constrained to balance the need for regenerative proliferation against the need to antagonize malignant proliferation, findings with implications for let-7 based therapies.
Results
let-7g inhibits the development of MYC-driven hepatoblastoma
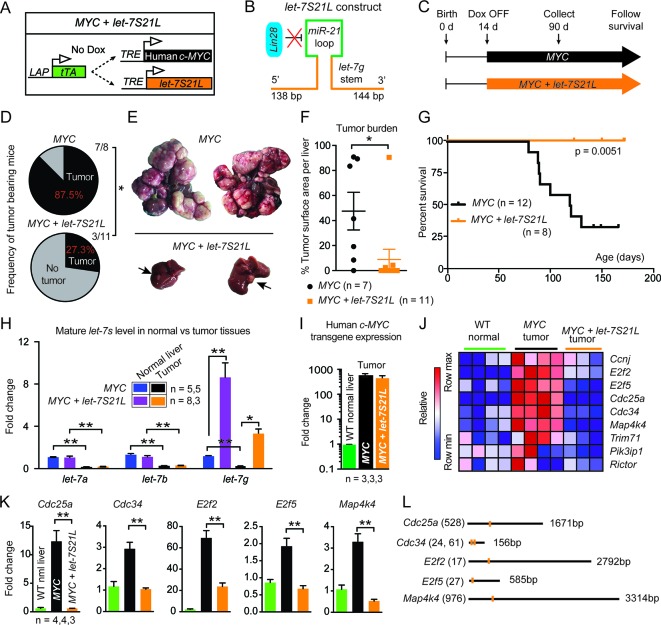
To study the effect of let-7 on carcinogenesis, we employed an inducible MYC-driven hepatoblastoma model (Shachaf et al., 2004). In this model, most let-7s are suppressed by more than 60% (Nguyen et al., 2014). However, MYC affects the expression of many other microRNAs (Chang et al., 2009; Kota et al., 2009). To test if let-7 suppression is specifically required for MYC’s oncogenic program, we simultaneously overexpressed let-7g and MYC using a triple transgenic, liver-specific, tet-off model (Figure 1A: LAP-tTA; TRE-MYC; TRE-let-7S21L transgenic mice). This transgenic form of let-7g is an engineered let-7 species called let-7S21L (let-7g Stem + miR-21 Loop) (Zhu et al., 2011), in which the precursor microRNA loop derives from mir-21 and cannot be bound and inhibited by Lin28b (Figure 1B), which is highly expressed in MYC-driven tumors (Chang et al., 2009; Nguyen et al., 2014).
Figure 1. let-7g inhibits the development of MYC-driven hepatoblastoma.
(A) Schema of the liver-specific inducible LAP-MYC +/- let-7S21L cancer model. (B) let-7S21L is a chimeric construct containing the let-7g stem, miR-21 loop, and let-7g flanking sequences. (C) Schema showing that LAP-MYC +/- let-7S21L mice were induced at 14 days of age, tissues were collected at 90 days of age, and survival was followed. (D) Ninety-day old mice bearing tumors in the LAP-MYC (87.5%, 7/8) and LAP-MYC + let-7S21L (27.3%, 3/11) mouse models. (E) Livers showing tumors from the above mice. (F) Liver surface area occupied by tumor. (G) Kaplan-Meier curve of LAP-MYC alone and LAP-MYC + let-7S21L mice. (H) Mature let-7 expression levels in as determined by RT-qPCR. (I) Human c-MYC mRNA expressionin tumors as determined by RT-qPCR. (J) Heat map of let-7 target gene expression in WT normal livers, MYC tumors, and MYC + let-7S21L tumors as measured by RT-qPCR. Red is higher and blue is lower relative mRNA expression. (K) Gene expression plotted as bar graphs to show relative changes. (L) Evolutionarily conserved let-7 target sites within 3’UTRs (Targetscan.org). All data in this figure are represented as mean ± SEM. *p < 0.05, **p < 0.01.
Figure 1—figure supplement 1. H&E staining of LAP-MYC and LAP-MYC + let-7S21L tumor-adjacent normal tissues and tumor tissues.
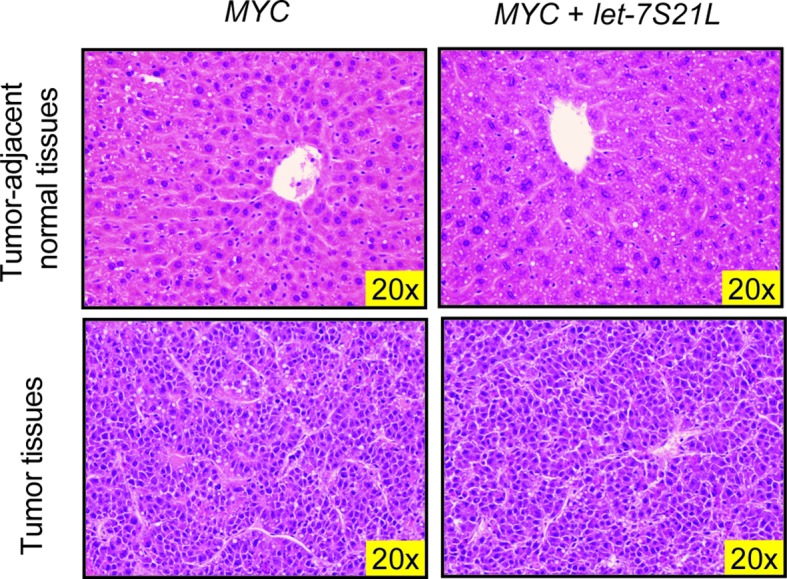
Induction of MYC with or without let-7S21L was initiated at 14 days of age (Figure 1C). By 90 days of age, large multifocal tumors had formed in 88% of the MYC alone group, whereas single small tumors appeared in only 27% the MYC + let-7S21L group (Figure 1D–F) and overall survival was dramatically improved (Figure 1G). The level of let-7g was increased more than eightfold in both non-tumor and tumor tissues (Figure 1H). Tumors from both groups showed similar histology (Figure 1—figure supplement 1) and MYC expression (Figure 1I). Gene-expression within tumors showed that previously validated let-7 targets involved in proliferation and growth including Cdc25a (Johnson et al., 2007), Cdc34 (Legesse-Miller et al., 2009), E2f2 (Dong et al., 2010), E2f5 (Kropp et al., 2015), and Map4k4 (Tan et al., 2015) were upregulated in MYC-tumors, but suppressed back down to normal levels in the context of let-7 overexpression (Figure 1J–L), suggesting that the repression of these targets restrains MYC-dependent tumorigenesis. These data indicated that let-7g has potent tumor suppressor activity in the context of MYC-driven hepatoblastoma.
let-7g overexpression inhibits liver regeneration after partial hepatectomy
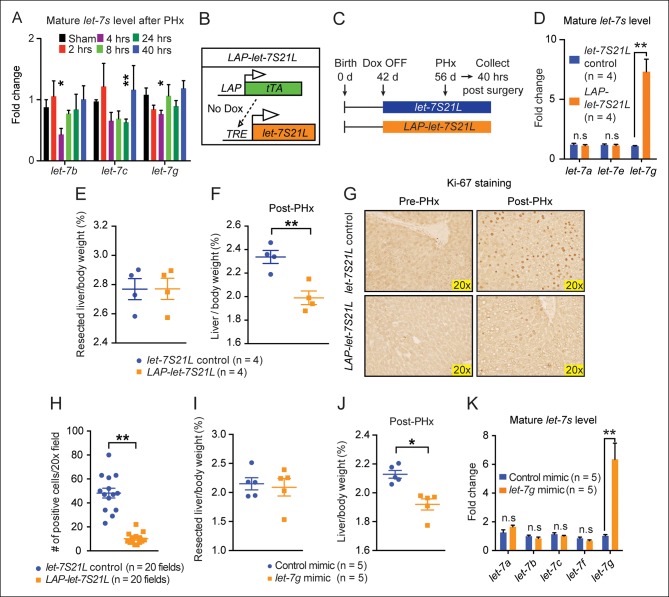
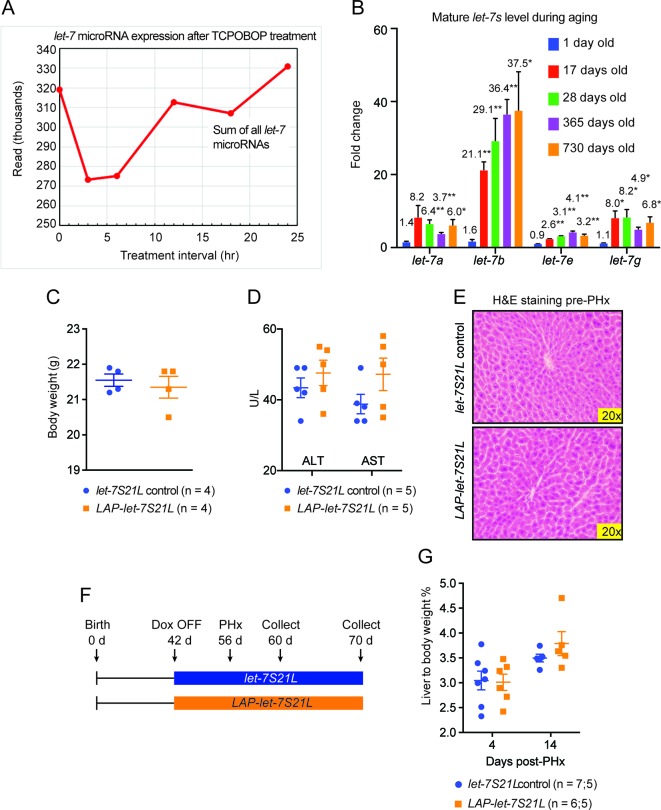
Since increasing let-7g was extremely effective at suppressing hepatoblastoma without compromising overall health, we asked if this increase in levels would impact tissue homestasis. We examined let-7g overexpression in the setting of liver injuries that drive rapid proliferation and growth. After PHx, let-7s in regenerating tissues fell, but returned to normal after forty hours (Figure 2A), findings consistent with a previous report (Chen et al., 2011). Similarly, let-7s also declined acutely after chemical injury with the xenobiotic TCPOBOP (1,4-bis-[2-[3,5-dichloropyridyloxy]] benzene) (Figure 2—figure supplement 1A). This shows that while let-7 increases in a temporally defined fashion during development (Figure 2—figure supplement 1B), it can transiently fluctuate after environmental perturbations. To test if the observed let-7 suppression is necessary for regeneration, we induced let-7g in LAP-let-7S21L mice and performed PHx (Figure 2B–D). The body weight (Figure 2—figure supplement 1C), liver function (Figure 2—figure supplement 1D), resected liver mass (Figure 2E) and histology (Figure 2—figure supplement 1E) were unaffected in LAP-let-7S21L mice compared to control mice. Forty hours after PHx, there was reduced liver mass and decreased Ki-67 in LAP-let-7S21L mice (Figure 2F–H). Liver mass was no different at four and fourteen days, indicating a kinetic delay but not a permanent impairment (Figure 2—figure supplement 1F,G).
Figure 2. let-7g overexpression inhibits liver regeneration after partial hepatectomy.
(A) Mature endogenous let-7 expression levels in WT C57Bl/6 mice at different time points after PHx as determined by RT-qPCR (n=4 and 4 for each time point). (B) Schema of the LAP-let-7S21L dox-inducible model. LAP-tTA single transgenic mice served as the controls. (C) Schema showing that let-7S21L control and LAP-let-7S21L mice were induced at 42 days of age, PHx was performed after 14 days of induction, and tissues were collected 40 hr post PHx. (D) Mature let-7 expression levels in let-7S21L and LAP-let-7S21L livers after 14 days of induction (n=4 and 4). (E) Resected liver/body weight ratios of LAP-tTA Control and LAP-let-7S21L mice at the time of PHx (n=4 and 4). (F) Liver/body weight ratios 40 hr after PHx (n=4 and 4). (G) Ki-67 staining on resected and post-PHx liver tissues. (H) Quantification of Ki-67-positive cells (n=2 and 2 mice; ten 20x fields for each mouse were quantified). (I) Resected liver/body weight ratios 2 days after intravenous injection of 0.5 mg/kg negative control or let-7g microRNA mimics packaged in C12-200 LNPs (n=5 and 5). (J) Liver/body weight ratios 40 hr after PHx (n=4 and 4). (K) Mature let-7g expression levels in mimic treated livers (n=5 and 5). All data in this figure are represented as mean ± SEM. *p<0.05, **p<0.01.
Figure 2—figure supplement 1. Data associated with Figure 2.
To rule out increasing demands on microRNA biogenesis machinery as a mechanism of proliferative suppression, we delivered mature control or let-7g microRNA mimics (0.5 mg/kg) into wild-type mice two days prior to hepatectomy using C12-200 lipidoid nanoparticles (LNPs) (Love et al., 2010). let-7g, but not control mimics, inhibited regeneration (Figure 2I–K). While let-7 overexpression blocked MYC-induced tumorigenesis, these data show that a similar increase in let-7 levels inhibited post-injury organ growth and regeneration.
Loss of let-7b and let-7c2 is sufficient to enhance liver regeneration
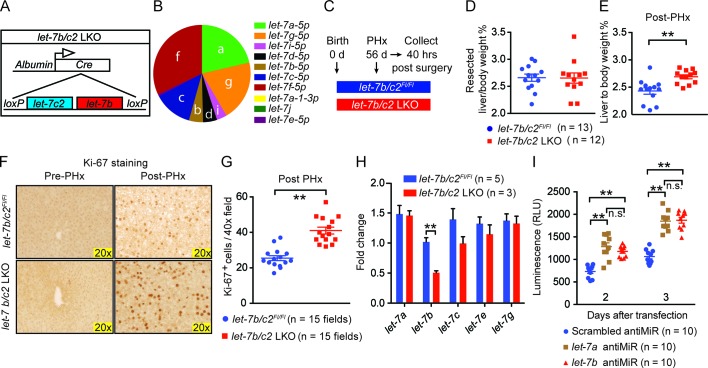
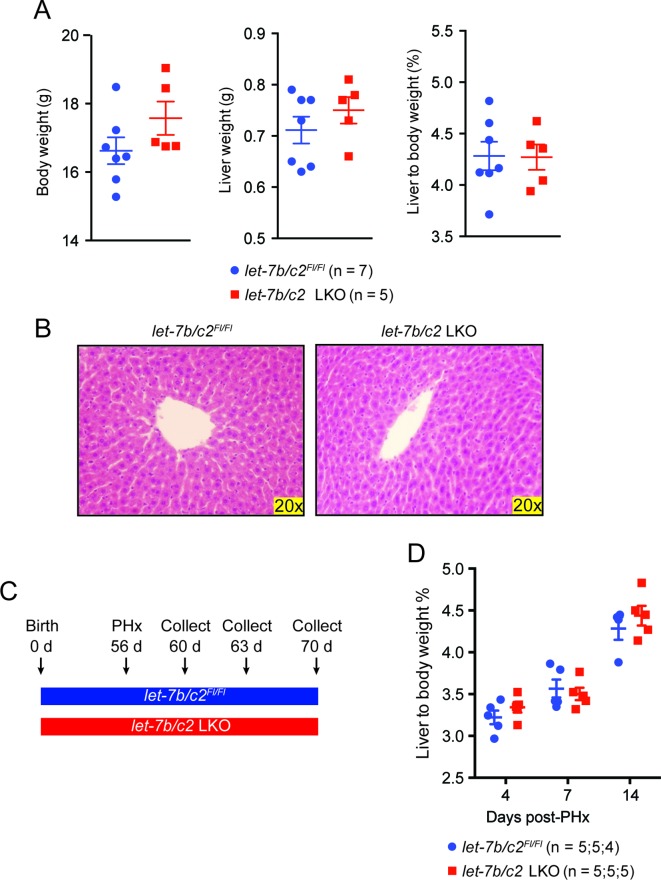
To assess the physiological relevance of our gain-of-function experiments, we examined knockout mice to determine if let-7 is a bona fide regeneration suppressor. Both let-7b and let-7c2 were conditionally deleted from the liver by crossing Albumin-Cre into a let-7b/c2 floxed mouse ("let-7b/c2 LKO" mice, Figure 3A). Small RNA-sequencing data from Xie et al. showed that let-7 is one of the most highly expressed microRNA families in the liver and that let-7b and let-7c2 together comprise approximately 18% of the let-7 total (Figure 3B) (Xie et al., 2012). Thus, let-7b/c2 LKO mice have substantial, but far from a complete reduction of total let-7 levels.
Figure 3. Loss of let-7b and let-7c2 is sufficient to enhance liver regeneration.
(A) Schema of liver-specific let-7b and let-7c2 knockout mice (let-7b/c2 LKO). Albumin-Cre excises loxPs in the embryonic liver of let-7b/c2Fl/Fl mice. Mice without Cre serve as the controls. (B) Small RNA sequencing showing the distribution of 10 let-7s in WT mice (n=2) (Data obtained from Xie et al. 2012). (C) Schema showing that PHx was performed on let-7b/c2Fl/Fl and let-7b/c2 LKO mice at 56 days of age and tissues were collected 40 hr post PHx. (D) Resected liver/body weight ratios at the time of PHx, and (E) Liver to body ratios of let-7b/c2Fl/Fl (n=11) and let-7b/c2 LKO mice (n=10) 40 hr after PHx. (F) Ki-67 staining and (G) Quantification of Ki-67-positive cells on resected and 40 hr post-PHx liver tissues (n=3 and 3 mice; total of five 40x fields/mouse were used for quantification). (H) RT-qPCR on let-7 family members from let-7b/c2Fl/Fl and let-7b/c2 LKO mice pre- and 40 hr post-PHx. (I) Viability of H2.35 immortalized human hepatocytes treated with either scrambled, let-7a, or let-7b antiMiRs, measured at two and three days after transfection (n=10 each). All data in this figure are represented as mean ± SEM. *p<0.05, **p<0.01.
Figure 3—figure supplement 1. Characterization of let-7b/c2 LKO mice.
Figure 3—figure supplement 2. Post-natal deletion of let-7b/c2 also enhances liver regeneration.
These LKO mice were healthy and showed normal liver/body weight ratios and histology at baseline (Figure 3—figure supplement 1A,B). An identical amount of liver mass was resected from let-7b/c2Fl/Fl control and let-7b/c2 LKO mice (Figure 3C,D), but LKO mice exhibited significant increases in liver mass and proliferation 40 hr after surgery (Figure 3E–G). Four and seven days after PHx, there were no differences in liver weights, indicating that other phases of regeneration were unaffected (Figure 3—figure supplement 1C,D). At fourteen days, the liver weight precisely achieved pre-surgery levels in control and LKO mice, indicating accelerated but not excessive regeneration (Figure 3—figure supplement 1C,D). There was no compensatory upregulation of other let-7s in pre- or post-PHx tissues (Figure 3H), supporting the concept that let-7 is a dose-dependent regeneration suppressor.
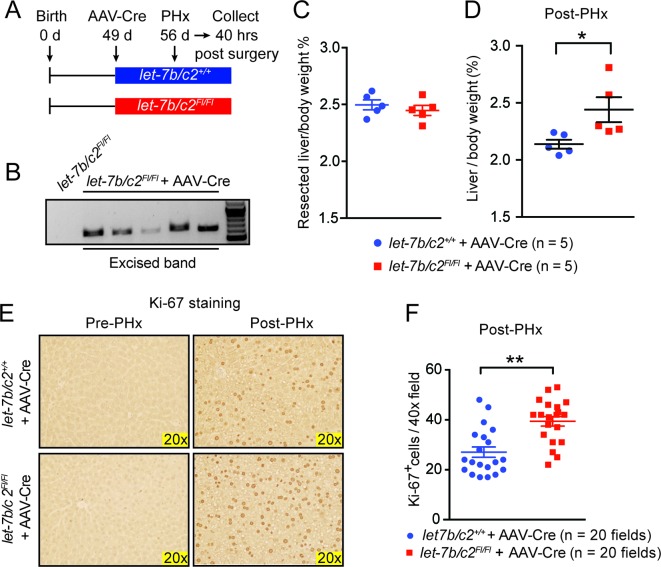
Cre under the Albumin promoter is expressed in embryonic hepatoblasts that give rise to both hepatocyte and bile duct compartments (Postic and Magnuson, 1999, 2000; Xu et al., 2006; Weisend et al., 2009; Malato et al., 2011), so developmental influences of let-7 loss could have led to adult phenotypes. To define cell- and temporal-specific roles of let-7b/c2, we used adeno-associated virus expressing Cre (AAV8.TBG.PI.Cre.rBG, hereafter called "AAV-Cre"), known to mediate efficient gene excision in hepatocytes but never in biliary epithelial cells (Yanger et al., 2013) (Figure 3—figure supplement 2A,B). These adult and hepatocyte-specific conditional knockout mice also exhibited significantly enhanced regenerative capacity (Figure 3—figure supplement 2C–F). To test if proliferative effects are specific to particular let-7 species, we knocked-down either let-7a or let-7b in SV40 immortalized hepatocytes (H2.35 cells) and found that both led to increased proliferation (Figure 3I). Collectively, our data shows that physiological let-7 levels regulate the kinetics of adult liver regeneration by hepatocytes.
let-7g suppresses liver regeneration through insulin-PI3K-mTOR
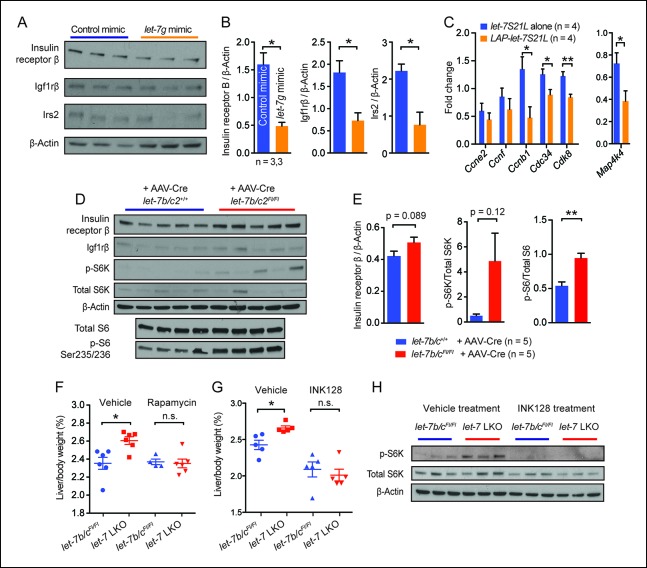
let-7 was previously demonstrated to regulate the insulin-PI3K-mTOR pathway (Zhu et al., 2011; Frost and Olson, 2011), which is also important in liver regeneration (Okano et al., 2003; Chen et al., 2009; Haga et al., 2009; Espeillac et al., 2011). To avoid auto-regulatory feedback and compensation as confounding factors, we focused on liver tissues exposed to acute let-7 gain or loss. In regenerating livers treated with let-7g mimic (Figure 2I–K), we found significant protein suppression of insulin receptor β, Igf1rβ, and Irs2, previously validated let-7 targets at the top of the insulin pathway (Figure 4A,B) (Zhu et al., 2011). In addition to insulin signaling components, the expression of cell cycle genes (Ccnb1, Cdc34, and Cdk8) and Map4k4 were also downregulated (Figure 4C). In mice with acute let-7b/c2 deletion by AAV-Cre (Figure 3—figure supplement 2), there was a small increase in insulin receptor β protein levels (Figure 4D,E). Increased mTOR signaling was also evident in the increased phospho-S6K/Total S6K and phospho-S6/Total S6 ratios (Figure 5E).
Figure 4. let-7g suppresses liver regeneration through insulin-PI3K-mTOR.
(A) Western blots of insulin receptor β, Igf1rβ, Irs2, and β-Actin in negative control or let-7g microRNA mimic treated liver tissues 40 hr after PHx. (B) Quantification of intensity of insulin receptor β, Igf1rβ, Irs2 (Image J). (C) Cell cycle gene expression in let-7S21L alone (n=4) and LAP-let-7S21L (n=4) livers before and 40 hr after PHx as determined by RT-qPCR. (D) Western blots of insulin receptor β, Igf1rβ, p-S6K, total S6K, β-Actin, p-S6 (Ser235/236), and total S6 in AAV-Cre treated let-7b/c2 +/+ and let-7b/c2Fl/Fl livers (n=5 and 5). (E) Quantification of intensity of insulin receptor β/β-Actin, p-S6K/total S6K, and p-S6/total S6, 40 hr after PHx (Image J). (F) Rapamycin treatment during and after PHx in let-7b/c2Fl/Fl control and let-7b/c2 LKO mice. Shown are liver weights 40 hr post PHx. (G) INK128 treatment during and after PHx in let-7b/c2Fl/Fl control and let-7b/c2 LKO mice. Shown are liver weights 40 hr post PHx. (H) Western blots of p-S6K, total S6K, and β-Actin in let-7b/c2Fl/Fl control and let-7b/c2 LKO livers treated with either vehicle or INK128 at 40 hr post PHx. All data in this figure are represented as mean ± SEM. *p<0.05, **p<0.01.
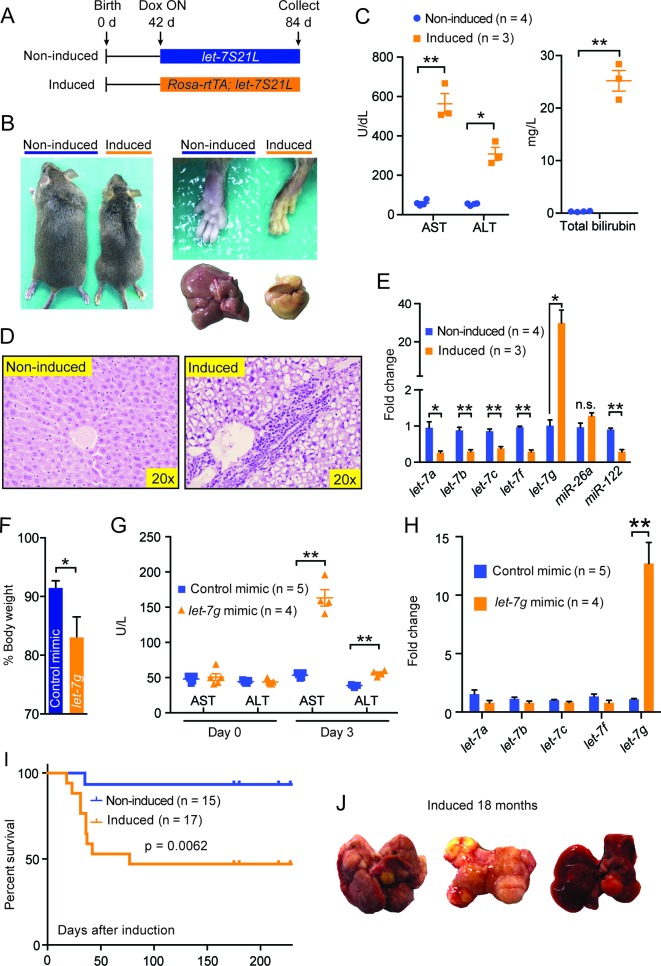
Figure 5. Chronic high-dose let-7g causes hepatotoxicity and liver carcinogenesis.
(A) Schema showing that let-7S21L control and Rosa-rtTA; let-7S21L mice were induced at 42 days of age and collected at 84 days. (B) Images showing the whole body, extremities, and livers of Rosa-rtTA (n=4) and Rosa-let-7S21L mice (n=3) given 1 mg/mL dox between 42 and 84 days of age. (C) Liver function tests: AST (U/L), ALT (U/L), and total bilirubin (mg/L) in these mice. (D) H&E staining of livers. (E) RT-qPCR of mature let-7s and other microRNAs in let-7g overexpressing mice (n=4 and 3). (F) Body weight 3 days after injection of 2.0 mg/kg negative control or let-7g microRNA mimics packaged in C12-200 LNPs relative to pre-injection weight (n=5 and 4). (G) Liver function tests: AST (U/L) and ALT (U/L) in WT C57Bl/6 mice before and 3 days after mimic injection (n=5 and 4). (H) Mature let-7 levels in wild-type C57Bl/6 mice treated with mimics as determined by RT-qPCR (n=5 and 4). (I) Kaplan-Meier curve for Rosa-let-7S21L induced with 1.0 g/L dox at 6 weeks old (n=15 and 17). (J) Gross images of the liver of Rosa-let-7S21L mice induced for 18 months. All data in this figure are represented as mean ± SEM. *p<0.05, **p<0.01.
Figure 5—figure supplement 1. Data associated with Figure 5.
To determine if mTOR signaling is functionally relevant in LKO mice, we treated mice with rapamycin two hours prior to and immediately after PHx. Rapamycin abrogated differences in regenerating liver weights between control and LKO mice (Figure 4F), demonstrating that let-7b/c2 loss promotes additional mTOR activation to enhance regeneration. Rapamycin’s allosteric inhibition of mTOR can lead to pleiotropic and unpredictable effects due to cell-type specific and feedback related phenomena (Thoreen et al., 2009). INK128 is a second generation mTOR inhibitor that directly competes with ATP at the catalytic domains of mTORC1/2, leading to more complete abrogation of 4EBP and S6K1 (Hsieh et al., 2012). INK128, similar to rapamycin, completely abrogated the regenerative enhancement associated with let-7b/c2 loss (Figure 4G). Analysis of p-S6K confirmed that mTOR is hyperactivated in LKO livers and that INK128 extinguishes the mTOR dependent phosphorylation of this substrate (Figure 4H). Similar results after rapamycin and INK128 indicated that mTOR and its substrates play an essential role in driving increased regeneration in the context of let-7 suppression.
Chronic high-dose let-7g causes hepatotoxicity and liver carcinogenesis
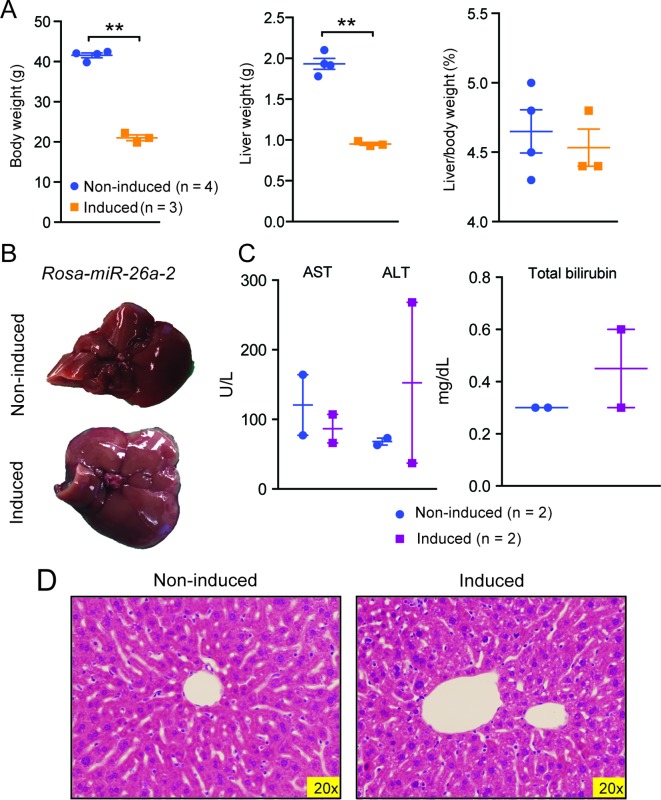
Since acute let-7g induction interferes with hepatocyte proliferation, we asked what the effects of chronic high-dose let-7g induction might be. To answer this question, we induced let-7g using rtTA under the control of the Rosa promoter, which drives higher expression than the LAP promoter (Figure 5A). These mice lost significant body weight and became jaundiced (Figure 5—figure supplement 1A and Figure 5). Liver function tests indicated severe liver injury (Figure 5C) and histology showed prominent microvesicular steatosis, characterized by intra-cytoplasmic lipid droplets (Figure 5D), a finding associated with drug-induced liver injury, acute fatty liver of pregnancy, or Reye’s syndrome in humans. Using this system, mature let-7g was overexpressed by more than twentyfold, as compared to ∼eightfold induction in the LAP-let-7S21L system (Figure 5E). Liver dysfunction was not seen after low-dose let-7 overexpression in LAP-let-7S21L mice (Figure 2—figure supplement 1) or after high-dose miR-26a-2 overexpression in Rosa-rtTA; TRE-miR-26a-2 mice (Figure 5—figure supplement 1B–D), suggesting a dose and let-7 microRNA specific effect.
Another possibility was that the miR-21 loop of the let-7S21L construct saturated the microRNA biogenesis machinery, thus causing non-specific toxicity independent of the let-7 seed sequence. To address this we again delivered a higher dose (2.0 mg/kg) of mature let-7g and control microRNA mimics, which do not harbor loops or tails, into wild-type mice. Mice receiving let-7g mimic lost significantly more weight (Figure 5F) and suffered hepatocyte destruction leading to increased AST/ALT levels (Figure 5G), while control mice remained healthy. Mimic delivery achieved a twelvefold increase of let-7g (Figure 5H). These results suggest that above certain doses let-7 is incompatible with hepatocyte survival, and that let-7’s anti-proliferative activities would interfere with normal tissue homeostasis.
When the Rosa-let-7S21L mice were induced chronically, approximately 50% of the mice survived the acute liver injury seen after dox induction (Figure 5I). Over the course of 18 months, 5 of 10 (50%) of these surviving mice developed large liver tumors, whereas only 1 of 12 (7.7%) of the non-induced mice had any tumors (Figure 5J). Chronic let-7 overexpression likely caused hepatocyte toxicity and selected for pre-malignant hepatocytes that eventually become cancer. Our long-term experiments revealed the potential dangers of chronic let-7 treatment, and the consequent disruption of the balance between tissue regeneration, degeneration, and cancer risk.
Discussion
The role of let-7s in adult animal physiology is unclear in part because the redundancy of this large microRNA family has made loss of function studies challenging. Deep redundancy of multiple highly conserved genes raises the possibility that dose regulation is important. Despite this, overexpression has been helpful in uncovering physiological functions of let-7 (Zhu et al., 2011; Frost and Olson, 2011; Shyh-Chang et al., 2013; Nishino et al., 2013; Madison et al., 2013). Using overexpression tools, we have shown that let-7 suppression is a fundamental requirement for MYC-mediated liver transformation, and that let-7 is capable of counteracting strong oncogenic drivers in vivo. However, one negative consequence of raising the level of let-7 expression is a limitation in the ability to regenerate after major tissue loss. More surprisingly, our knockout mouse model showed that the loss of two out of the ten let-7 members in the liver resulted in improved liver proliferation and regeneration. These data suggest a lack of complete redundancy between let-7 microRNA species, but rather a precisely regulated cumulative dose that when increased or decreased, leads to significant alterations in regenerative capacity.
The knowledge that let-7 suppresses both normal and malignant growth will have particular relevance to malignancies that arise from chronically injured tissues. In these tissues, winners of the competition between cancer and host cells might ultimately dictate whether organ failure or tumor progression ensues. It has been thought that one key advantage of using microRNAs therapeutically is that they are already expressed at high levels in normal tissues, thus making increased dosing likely to be safe and tolerable. Surprisingly, we showed that chronic let-7 overexpression caused hepatotoxicity, disrupted tissue homeostasis, ultimately leading to carcinogenesis. This is likely due to the high levels of overexpression achieved in the Rosa-rtTA transgenic system, as opposed to the lower dose in the LAP-tTA system. These high doses are likely to be toxic to hepatocytes, a phenomena compounded by the fact that excess let-7 impairs proliferation in surviving cells that might serve to replenish lost tissues. Eventually, certain clones must epigenetically or genetically evolve to evade let-7 growth inhibition in order to transform.
It is also interesting that let-7 overexpression led to dramatically different outcomes in distinct cancer contexts. While dose is most likely the critical variable between the Rosa-rtTA and LAP-tTA systems, Rosa-rtTA does induce expression in cells other than hepatocytes and bile duct epithelia, leaving the possibility that non-cell autonomous influences of let-7 overexpression play a role in liver injury and cancer development. A more interesting possibility would be if distinct genetic subtypes of cancer respond differently to let-7 overexpression. Since let-7 has been conceptualized as a general tumor suppressor, it is surprising that it can cause opposing phenotypes in distinct cancer models. MYC liver cancers show a dramatic suppression of let-7, rendering it especially sensitive to let-7 replacement. Tumors or tissues with more normal levels of let-7 might not respond to increases in let-7. Alternatively, the growth of other cancer models may not depend on the overproduction of let-7 target genes/proteins. let-7 overexpression in these contexts would probably not elicit growth suppression, but may instead exacerbate tissue injury. It would be interesting to evaluate the effects of let-7 overexpression in hepatocellular carcinomas caused by different driver mutations. Together, our data suggest that let-7 therapy directed at hepatocellular carcinomas could be risky, given that most of these cancers occur in severely compromised, cirrhotic livers (Yang et al., 2011).
We speculate that the total dose of let-7 is evolutionarily determined via regulation of the expression levels of individual let-7 members, and is postnatally maintained at a level that can suppress cancer, but which also allows for adequate levels of mammalian regenerative capacity. Clearly, let-7 levels are not static throughout life, since let-7 levels are dynamic after environmental perturbations. However, when baseline let-7 levels are altered permanently by genetic means, compromises in tumor suppression or tissue regeneration were revealed. Our study underscores the importance of regulating appropriate levels of this small RNA to maintain health during times of regenerative stress.
Materials and methods
Mice
All mice were handled in accordance with the guidelines of the Institutional Animal Care and Use Committee at UTSW. MYC tumor models and the LAP-let-7S21L inducible mice were carried on a 1:1 FVB/C57Bl/6 strain background. Please see (Nishino et al., 2013) for more details about the let-7b/c2 floxed mice, which are on a C57Bl/6 background. The chronically injured let-7 inducible mice were on a mixed B6/129 background. All experiments were done in an age and sex controlled fashion unless otherwise noted in the figure legends.
Partial hepatectomy
Two-thirds of the liver was surgically excised as previously described (Mitchell and Willenbring, 2008).
RNA extraction and RT-qPCR
Total RNA was isolated using Trizol reagent (Invitrogen). For RT-qPCR of mRNAs, cDNA synthesis was performed with 1 ug of total RNA using miScript II Reverse Transcription Kit (Cat. #218161, Qiagen). Gene expression levels were measured using the ΔΔCt method as described previously (Zhu et al., 2010).
Western blot assay
Mouse liver tissues were ground with a pestle and lysed in T-PER Tissue Protein Extraction Reagent (Thermo Scientific Pierce). Western blots were performed in the standard fashion. The following antibodies were used: Anti-Insulin receptor β (Cell Signaling #3025), Anti-Igf1rβ (Cell Signaling #9750), Anti-Irs2 (Cell Signaling #3089), Anti-total S6K (Cell Signaling #9202), Anti-p-S6K (Cell Signaling #9205), Anti-total S6 (Cell Signaling #2217), Anti-p-S6 Ser235/236 (Cell Signaling #2211), Anti-mouse β-Actin (Cell Signaling #4970), Anti-rabbit IgG, HRP-linked Antibody (Cell Signaling #7074) and Anti-mouse IgG, HRP-linked Antibody (Cell Signaling #7076).
Histology and immunohistochemistry
Tissue samples were fixed in 10% neutral buffered formalin or 4% paraformaldehyde (PFA) and embedded in paraffin. In some cases, frozen sections were made. Immunohistochemistry was performed as previously described (Zhu et al., 2010). Primary antibodies used: Ki-67 (Abcam #ab15580). Detection was performed with the Elite ABC Kit and DAB Substrate (Vector Laboratories), followed by Hematoxylin Solution counterstaining (Sigma).
Liver function tests
Blood samples (∼50 ul) were taken retro-orbitally in heparinized tubes. Liver function tests were analyzed by the UTSW Molecular Genetics core.
Viral Cre excision
100 μL of AAV8.TBG.PI.Cre.rBG (University of Pennsylvania Vector Core) was retro-orbitally injected at a titer of 5 x× 1010 genomic particles to mediate 90%-–100% Cre excision.
Cell culture and in vitro antiMiR experiments
The H2.35 cell line was directly obtained from ATCC and has been cultured for less than 6 months. The cells were authenticated by ATCC using Short Tandem Repeat (STR) DNA profiling. Cells were cultured in DMEM with 4% (vol/vol) FBS, 1x Pen/Strep (Thermo Scientific) and 200 nM Dexamethasone (Sigma). Cells were transfected with control (Life Technologies Cat. AM17010), let-7a (Life Technologies Cat. #4464084-Assay ID MH10050), or let-7g (Life Technologies Cat. #4464084-Assay ID MH11050) miRVana antiMiRs. AntiMiRs were packaged by RNAiMAX (Invitrogen) and transfected into H2.35 cells cultured in 96-well plates at a concentration of 50 nM. The number of viable cells in each well was measured at 2 and 3 days after transfection using CellTiter-Glo Luminescent Cell Viability Assay (Promega Cat. #G7570).
In vivo microRNA mimic experiments
For in vivo experiments, formulated C12-200 lipidoid nanoparticles (LNPs) were used to package either Control (Life Technologies Cat. #4464061) or let-7g (Life Technologies Cat. 364 #4464070-Assay ID MC11758) miRVana mimic at either 0.5 or 2 mg/kg and delivered intravenously through the tail vein. LNPs were formulated following the previously reported component ratios (Love et al., 2010) with the aid of a microfluidic rapid mixing instrument (Precision Nanosystems NanoAssemblr) and purified by dialysis in sterile PBS before injection.
In vivo drug treatments
Rapamycin (LC Biochem) was dissolved in 25% ethanol/PBS and then injected at 1.5 mg/kg 2 hr prior to and 20 hr after PHx. INK128 (LC Biochem) was formulated in 5% polyvinylpropyline, 15% NMP, 80% water and administered by oral gavage at 1 mg/kg 2 hr prior to and 20 hr after PHx.
MicroRNA sequencing
Female CD1 mice were treated with 3 mg/kg TCPOBOP in DMSO-corn oil by gavage (Tian et al., 2011), sacrificed at 3, 6, 9, 12, and 18 hr after treatment, and compared to untreated controls. Replicate libraries were made from two individual mice for each condition. RNA was purified with the Qiagen miRNeasy Mini kit. Small RNA libraries were constructed using an Illumina Truseq Small RNA Sample Prep Kit. 12 indexed libraries were multiplexed in a single flow cell lane and received 50 base single-end sequencing on an Illumina HiSeq 2500 sequencer. Sequence reads were aligned to mm9 using Tophat and quantified with Cufflinks by the FPKM method (Trapnell et al., 2012). Data for each experimental condition represent the average values from two libraries.
Statistical analysis
The data in most figure panels reflect multiple experiments performed on different days using mice derived from different litters. Variation is always indicated using standard error presented as mean ± SEM. Two-tailed Student's t-tests (two-sample equal variance) were used to test the significance of differences between two groups. Statistical significance is displayed as p<0.05 (*) or p<0.01 (**) unless specified otherwise. In all experiments, no mice were excluded form analysis after the experiment was initiated. Image analysis for the quantification of cell proliferation, cell death, and fibrosis were blinded.
Acknowledgements
LW was supported by The Science and Technology Program of Guangzhou, China (2012J5100031) and Youth Teachers Cultivation Project of Sun Yat-Sen University (12ykpy21). LHN was supported by the Howard Hughes Medical Institute (HHMI) Pre-doctoral International Student Fellowship. JTM was supported by the Cancer Prevention and Research Institute of Texas (CPRIT grant R1008) and NIH (R01CA120185). SJM is an HHMI Investigator, the Mary McDermott Cook Chair in Pediatric Genetics, the director of the Hamon Laboratory for Stem Cells and Cancer, and a CPRIT Scholar. SJM was also supported by a grant from the National Institute on Aging (AG024945). GQD is an investigator of HHMI and the Manton Center for Orphan Disease Research and was supported by NIGMS R01GM107536. DJS acknowledges support from CPRIT (grant R1212) and the Welch Foundation (I-1855). NSC was supported by a NSS Scholarship from the Agency for Science, Technology and Research, Singapore. HZ was supported by a American Cancer Society Postdoctoral Fellowship, a NIH/NCI K08 grant (5K08CA157727), a NIH/NCI R01 grant (1R01CA190525), the Pollack Foundation, a Burroughs Welcome Career Medical Award, and a CPRIT Recruitment Award (R1209).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
National Cancer Institute 1K08CA157727-02 to Hao Zhu.
Howard Hughes Medical Institute to Sean J Morrison, George Q Daley.
Howard Hughes Medical Institute 59108180 to Liem H Nguyen.
Cancer Prevention and Research Institute of Texas R1209 to Hao Zhu.
National Cancer Institute 1R01CA190525-01 to Hao Zhu.
National Institute of General Medical Sciences R01GM107536 to George Q Daley.
The Science and Technology Program of Guangzhou, China 2012J5100031 to Linwei Wu.
National Institute on Aging AG024945 to Sean J Morrison.
Welch Foundation I-1855 to Daniel J Siegwart.
Agency for Science, Technology and Research NSS Scholarship to Ng Shyh-Chang.
Youth Teachers Cultivation Project of Sun Yat-Sen University 12ykpy21 to Linwei Wu.
Burroughs Wellcome Fund Career Award for Medical Scientists to Hao Zhu.
Pollock Foundation to Hao Zhu.
Cancer Prevention and Research Institute of Texas to Joshua T Mendell, Sean J Morrison.
Cancer Prevention and Research Institute of Texas R1212 to Daniel J Siegwart.
American Cancer Society Postdoctoral Fellowship to Hao Zhu.
Cancer Prevention and Research Institute of Texas R1008 to Joshua T Mendell.
Cancer Prevention and Research Institute of Texa R01CA120185 to Joshua T Mendell.
Additional information
Competing interests
SJM: Senior editor, eLife.
The other authors declare that no competing interests exist.
Author contributions
LW, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
LHN, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
KZ, Conception and design, Acquisition of data, Contributed unpublished essential data or reagents.
TYdeS, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article, Contributed unpublished essential data or reagents.
LL, Acquisition of data, Analysis and interpretation of data, Contributed unpublished essential data or reagents.
JBM, Conception and design, Acquisition of data, Analysis and interpretation of data, Contributed unpublished essential data or reagents.
JT, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
JL, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
SZ, Conception and design, Acquisition of data, Analysis and interpretation of data.
GS, Conception and design, Acquisition of data, Analysis and interpretation of data, Contributed unpublished essential data or reagents.
MTS, Conception and design, Acquisition of data, Analysis and interpretation of data, Contributed unpublished essential data or reagents.
LRZ, Conception and design, Acquisition of data, Analysis and interpretation of data.
AA, Conception and design, Acquisition of data, Analysis and interpretation of data.
SCW, Conception and design, Drafting or revising the article.
JTM, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
XH, Analysis and interpretation of data, Drafting or revising the article.
JN, Analysis and interpretation of data, Contributed unpublished essential data or reagents.
SJM, Drafting or revising the article, Contributed unpublished essential data or reagents.
DJS, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article, Contributed unpublished essential data or reagents.
GQD, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article, Contributed unpublished essential data or reagents.
NSC, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article, Contributed unpublished essential data or reagents.
HZ, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
Ethics
Animal experimentation: This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All of the animals were handled according to approved institutional animal care and use committee (IACUC) protocols (#2012-0143) of the University of Texas Southwestern Medical Center. All surgery was performed under isoflurane anesthesia with appropriate analgesia, and every effort was made to minimize suffering.
Additional files
Major datasets
The following datasets were generated:
Xie J, Ameres SL, Friedline R, Hung JH et al,2011,AAV vector-mediated in vivo miRNA antagonism for studying miRNA function and treating dyslipidemia,http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25971,Publicly available at the NCBI Gene Expression Omnibus (Accession no: GSE25971).
References
- Chang T-C, Zeitels LR, Hwang H-W, Chivukula RR, Wentzel EA, Dews M, Jung J, Gao P, Dang CV, Beer MA, Thomas-Tikhonenko A, Mendell JT, et al. Lin-28B transactivation is necessary for myc-mediated let-7 repression and proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3384–3389. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Yan H, Chen Y, He Z. The variation of AkT/TSC1-TSC1/mTOR signal pathway in hepatocytes after partial hepatectomy in rats. Experimental and Molecular Pathology. 2009;86:101–107. doi: 10.1016/j.yexmp.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Chen X, Murad M, Cui Y-Y, Yao L-J, Venugopal SK, Dawson K, Wu J. MiRNA regulation of liver growth after 50% partial hepatectomy and small size grafts in rats. Transplantation. 2011;91:293–299. doi: 10.1097/TP.0b013e318204756c. [DOI] [PubMed] [Google Scholar]
- Chivukula RR, Shi G, Acharya A, Mills EW, Zeitels LR, Anandam JL, Abdelnaby AA, Balch GC, Mansour JC, Yopp AC, Maitra A, Mendell JT. An essential mesenchymal function for miR-143/145 in intestinal epithelial regeneration. Cell. 2014;157:1104–1116. doi: 10.1016/j.cell.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Meng P, Wang T, Qin W, Qin W, Wang F, Yuan J, Chen Z, Yang A, Wang H. MicroRNA let-7a inhibits proliferation of human prostate cancer cells in vitro and in vivo by targeting E2F2 and CCND2. PloS One. 2010;5:e09431. doi: 10.1371/journal.pone.0010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeillac C, Mitchell C, Celton-Morizur Séverine, Chauvin Céline, Koka V, Gillet C, Albrecht JH, Desdouets C, Pende M. S6 kinase 1 is required for rapamycin-sensitive liver proliferation after mouse hepatectomy. The Journal of Clinical Investigation. 2011;121:2821–2832. doi: 10.1172/JCI44203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Trang P, Wiggins JF, Patrawala L, Cheng A, Ford L, Weidhaas JB, Brown D, Bader AG, Slack FJ. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–764. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- Frost RJA, Olson EN. Control of glucose homeostasis and insulin sensitivity by the let-7 family of microRNAs. Proceedings of the National Academy of Sciences. 2011;108:21075–21080. doi: 10.1073/pnas.1118922109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Chen Y, Ito H, Watanabe A, Ge X, Kodama T, Aburatani H. Identification and characterization of lin-28 homolog b (lIN28B) in human hepatocellular carcinoma. Gene. 2006;384:51–61. doi: 10.1016/j.gene.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Haga S, Ozaki M, Inoue H, Okamoto Y, Ogawa W, Takeda K, Akira S, Todo S. The survival pathways phosphatidylinositol-3 kinase (pI3-K)/phosphoinositide-dependent protein kinase 1 (pDK1)/Akt modulate liver regeneration through hepatocyte size rather than proliferation. Hepatology. 2009;49:204–214. doi: 10.1002/hep.22583. [DOI] [PubMed] [Google Scholar]
- Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Molecular Cell. 2008;32:276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, Wang S, Ren P, Martin M, Jessen K, Feldman ME, Weissman JS, Shokat KM, Rommel C, Ruggero D. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J, Chin L, Brown D, Slack FJ. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Research. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang H-W, Chang T-C, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp J, Degerny C, Morozova N, Pontis J, Harel-Bellan A, Polesskaya A. MiR-98 delays skeletal muscle differentiation by down-regulating E2F5. The Biochemical Journal. 2015;466:85–93. doi: 10.1042/BJ20141175. [DOI] [PubMed] [Google Scholar]
- Lan F-F, Wang H, Chen Y-C, Chan C-Y, Ng SS, Li K, Xie D, He M-L, Lin MC, Kung H-F. Hsa-let-7g inhibits proliferation of hepatocellular carcinoma cells by downregulation of c-myc and upregulation of p16(INK4A) International Journal of Cancer. Journal International Du Cancer. 2011;128:319–331. doi: 10.1002/ijc.25336. [DOI] [PubMed] [Google Scholar]
- Legesse-Miller A, Elemento O, Pfau SJ, Forman JJ, Tavazoie S, Coller HA. Let-7 overexpression leads to an increased fraction of cells in G2/M, direct down-regulation of Cdc34, and stabilization of Wee1 kinase in primary fibroblasts. The Journal of Biological Chemistry. 2009;284:6605–6609. doi: 10.1074/jbc.C900002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AKL, Sharp PA. MicroRNA functions in stress responses. Molecular Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DWY, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG, Racie T, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison BB, Liu Q, Zhong X, Hahn CM, Lin N, Emmett MJ, Stanger BZ, Lee J-S, Rustgi AK. LIN28B promotes growth and tumorigenesis of the intestinal epithelium via let-7. Genes & Development. 2013;27:2233–2245. doi: 10.1101/gad.224659.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malato Y, Naqvi S, Schürmann N, Ng R, Wang B, Zape J, Kay MA, Grimm D, Willenbring H. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. The Journal of Clinical Investigation. 2011;121:4850–4860. doi: 10.1172/JCI59261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nature Protocols. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- Nam Y, Chen C, Gregory RI, Chou JJ, Sliz P. Molecular basis for interaction of let-7 microRNAs with Lin28. Cell. 2011;147:1080–1091. doi: 10.1016/j.cell.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Robinton DA, Seligson MT, Wu L, Li L, Rakheja D, Comerford SA, Ramezani S, Sun X, Parikh MS, Yang EH, Powers JT, Shinoda G, Shah SP, Hammer RE, Daley GQ, Zhu H. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell. 2014;26:248–261. doi: 10.1016/j.ccr.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino J, Kim S, Zhu Y, Zhu H, Morrison SJ. A network of heterochronic genes including Imp1 regulates temporal changes in stem cell properties. eLife. 2013;2:e09431. doi: 10.7554/eLife.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano Jun-Ichi, Shiota G, Matsumoto K, Yasui S, Kurimasa A, Hisatome I, Steinberg P, Murawaki Y. Hepatocyte growth factor exerts a proliferative effect on oval cells through the PI3K/AKT signaling pathway. Biochemical and Biophysical Research Communications. 2003;309:298–304. doi: 10.1016/j.bbrc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Postic C, Magnuson MA. [role of glucokinase (gK) in the maintenance of glucose homeostasis. specific disruption of the gene by the cre-loxP technique] Journées Annuelles De Diabétologie De l'Hôtel-Dieu. 1999:115–124. [PubMed] [Google Scholar]
- Postic C, Magnuson MA. DNA excision in liver by an albumin-cre transgene occurs progressively with age. Genesis. 2000;26:149–150. doi: 10.1002/(SICI)1526-968X(200002)26:2<149::AID-GENE16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Schulman BRM, Esquela-Kerscher A, Slack FJ. Reciprocal expression of lin - 41 and the microRNAs let - 7 and mir - 125 during mouse embryogenesis. Developmental Dynamics. 2005;234:1046–1054. doi: 10.1002/dvdy.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, Bachmann MH, Borowsky AD, Ruebner B, Cardiff RD, Yang Q, Bishop JM, Contag CH, Felsher DW. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- Shyh-Chang N, Zhu H, Yvanka de Soysa T, Shinoda G, Seligson MT, Tsanov KM, Nguyen L, Asara JM, Cantley LC, Daley GQ. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155 doi: 10.1016/j.cell.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Gao Y, Nan Y, Zhang J, di C, Wang X, Lian F, Cao Y, Hu Y, Xu L, Ma H, Hong Y, Liu T, Wu Y, Xu X, Yan Y, Yang L. Cellular MicroRNA let-7a suppresses KSHV replication through targeting MAP4K4 signaling pathways. PloS One. 2015;10:e09431. doi: 10.1371/journal.pone.0132148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes & Development. 2006;20:2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. The Journal of Biological Chemistry. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Huang H, Hoffman B, Liebermann DA, Ledda-Columbano GM, Columbano A, Locker J. Gadd45?β is an inducible coactivator of transcription that facilitates rapid liver growth in mice. The Journal of Clinical Investigation. 2011;121:4491–4502. doi: 10.1172/JCI38760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang P, Medina PP, Wiggins JF, Ruffino L, Kelnar K, Omotola M, Homer R, Brown D, Bader AG, Weidhaas JB, Slack FJ. Regression of murine lung tumors by the let-7 microRNA. Oncogene. 2010;29:1580–1587. doi: 10.1038/onc.2009.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, Weidhaas JB, Bader AG, Slack FJ. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Molecular Therapy. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and cufflinks. Nature Protocols. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O'Sullivan M, Lu J, Phillips LA, Lockhart VL, Shah SP, Tanwar PS, Mermel CH, Beroukhim R, Azam M, Teixeira J, Meyerson M, Hughes TP, Llovet JM, Radich J, Mullighan CG, Golub TR, Sorensen PH, Daley GQ. Lin28 promotes transformation and is associated with advanced human malignancies. Nature Genetics. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-C, Chen Y-L, Yuan R-H, Pan H-W, Yang W-C, Hsu H-C, Jeng Y-M. Lin-28B expression promotes transformation and invasion in human hepatocellular carcinoma. Carcinogenesis. 2010;31:1516–1522. doi: 10.1093/carcin/bgq107. [DOI] [PubMed] [Google Scholar]
- Weisend CM, Kundert JA, Suvorova ES, Prigge JR, Schmidt EE. Cre activity in fetal albCre mouse hepatocytes: utility for developmental studies. Genesis. 2009;47:789–792. doi: 10.1002/dvg.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Li X, Ling Y, Shen C, Wu X, Xu W, Gao X. Alpha-lipoic acid pre- and post-treatments provide protection against in vitro ischemia-reperfusion injury in cerebral endothelial cells via Akt/mTOR signaling. Brain Research. 2012;1482:81–90. doi: 10.1016/j.brainres.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Xu X, Kobayashi S, Qiao W, Li C, Xiao C, Radaeva S, Stiles B, Wang R-H, Ohara N, Yoshino T, Leroith D, Torbenson MS, Gores GJ, Wu H, Gao B, Deng C-X. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and pten in mice. The Journal of Clinical Investigation. 2006;116:1843–1852. doi: 10.1172/JCI27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JD, Kim WR, Coelho R, Mettler TA, Benson JT, Sanderson SO, Therneau TM, Kim B, Roberts LR. Cirrhosis is present in most patients with hepatitis b and hepatocellular carcinoma. Clinical Gastroenterology and Hepatology. 2011;9:64–70. doi: 10.1016/j.cgh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE, Stanger BZ. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes & Development. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, Takeuchi A, Grasemann C, Rinn JL, Lopez MF, Hirschhorn JN, Palmert MR, Daley GQ. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nature Genetics. 2010;42:626–630. doi: 10.1038/ng.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Altshuler D, Daley GQ, DIAGRAM Consortium. MAGIC Investigators The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]