Abstract
Autologous hematopoietic stem cell transplantation (ASCT) for multiple myeloma (MM) is associated with high symptom burden, particularly for older patients and those with amyloid light-chain (AL) amyloidosis. Symptom burden peaks during leukopenia. We hypothesized that higher doses of CD34+ stem cells would be associated with an improved symptom outcome. Patients undergoing ASCT for MM who were ≥60 years old or had AL amyloidosis were randomized to receive either a standard (4–6×106 cells/kg) or high dose (10–15×106 cells/kg) of CD34+ cells after melphalan 200 mg/m2. Symptom burden was assessed via the MD Anderson Symptom Inventory MM module (MDASI-MM). Eighty patients were enrolled. Median CD34+ cell doses were 5.1×106 cells/kg (standard dose) and 10.5×106 cells/kg (high dose). The most severe symptoms during the first week were fatigue, lack of appetite, drowsiness, disturbed sleep, and pain. The AUC for the mean composite severity score of these symptoms was similar between treatment arms (P = .819). Median times to neutrophil, lymphocyte, and platelet engraftment were also similar between groups. IL-6 increased similarly for both groups throughout the ASCT course. Infusion of higher autologous stem cell dose after high-dose chemotherapy does not yield a difference in symptom burden or engraftment time in the first few weeks post-ASCT.
Keywords: Myeloma, transplantation, symptom burden
INTRODUCTION
High-dose chemotherapy and autologous stem cell transplantation (ASCT) has become part of the standard of care for patients with multiple myeloma (MM). However, this process is often associated with a high symptom burden, including lack of appetite, fatigue, weakness, and disturbed sleep.[1, 2] The underlying etiology of these symptoms is not completely clear but may be dependent on an ongoing systemic inflammatory response and cytokine flux.[3]
We have previously shown that symptom burden during ASCT reaches a peak at the time of white blood cell (WBC) nadir.[3] This increased burden has been associated with elevated serum levels of interleukin (IL)-6 as well as other inflammatory markers.[4] Furthermore, prospective analysis from our institution has suggested that older patients (aged > 60 years) and those with albumin < 3 g/dL are more likely to have a higher symptom burden during their ASCT course.[5]
Because the greatest symptom burden is temporally associated with the WBC nadir, we were interested in the possible beneficial effects of a higher stem cell dose on severity of symptoms during transplant. Several studies have suggested a dose–response relationship between the number of CD34+ cells and rate of hematological recovery during allogeneic or autologous stem cell transplant.[6, 7] In addition, a higher CD34+ cells dose appears to correlate with rate of engraftment in patients with AL amyloidosis undergoing ASCT.[8] Finally, preliminary data from our institution has suggested that infusion of a higher stem cell dose during ASCT is associated with a lower symptom severity score.[9]
On the basis of these findings, we hypothesized that a higher dose of CD34+ stem cells in the autologous rescue graft would be associated with an improved symptom outcome, not only due to a potentially shorter time to engraftment, but also through cytokine modulation. In addition, we theorized that this effect would best be elucidated in the setting of a population at higher risk for symptom burden, such as older patients or those with AL amyloidosis. We therefore conducted a single-center, prospective, randomized controlled trial of two stem cell doses for MM patients older than 60 years or with AL amyloidosis undergoing ASCT after highdose melphalan, 200 mg/m2.
METHODS
Patients and treatment
Patients were recruited from the Department of Stem Cell Transplantation at The University of Texas MD Anderson Cancer Center. Patients with MM were eligible if they were more than 60 years old or had evidence of AL amyloidosis defined by a biopsy showing light chain amyloid deposition at any site. Patients had to be deemed by their treating physicians as candidates for high-dose melphalan and ASCT and were required to have collected > 10 × 106 CD34+ cells/kg. Patients who would be unable to complete the symptom inventory questionnaire were excluded.
At the time of enrollment, patients were randomized to receive either a standard dose of stem cells (4–6 × 106 CD34+ cells/kg) or a high dose of stem cells (10–15 × 106 CD34+ cells/kg). All patients were treated with melphalan 100 mg/m2 intravenously on day −3 and day −2, with autologous stem cell rescue (per assigned stem cell dose) on day 0. Standard supportive care measures, including granulocyte-colony stimulating factor and antimicrobial prophylaxis, were administered as per standard departmental practice. Patients were assessed by multiple modalities (detailed below) throughout the ASCT process. The study was approved by the MD Anderson Institutional Review Board and registered at clinicaltrials.gov (NCT00651937). All patients provided written informed consent to participate.
Measurement of symptom burden
Treatment-related symptom burden was measured using the MD Anderson Symptom Inventory (MDASI), a validated tool for symptom assessment.[10] Specifically, the MM module (MDASI-MM) was employed for this patient population.[11] The MDASI-MM assesses the 13 core symptoms contained in the basic MDASI, including pain, fatigue, sleep disturbance, and lack of appetite, and six items measuring symptom interference with function. In addition, the MDASI-MM assesses seven additional symptoms commonly associated with MM, including bone aches, muscle weakness, sore mouth/throat, rash, difficulty concentrating, constipation, and diarrhea. Symptom severity over the previous 24 hours is rated on a 0–10 scale ranging from “not present” to “as bad as you can imagine.” The MDASI-MM was administered at baseline, before treatment with chemotherapy, and then every other day during the first week of the ASCT. Thereafter, the MDASI-MM was administered twice weekly for 2 weeks and then once in the fourth week after ASCT.
Functional testing
Physical performance was measured by two methods: the 6-minute walk test and the sit-to-stand test. For the 6-minute walk test we measured the distance (in feet) that patients could walk in 6 minutes, with rest periods allowed. In the sit-to-stand test, patients were timed as they stood up from a sitting position and sat back down twice. This cycle was repeated and the average time of the two attempts was used. Both of these tests are believed to be complementary to cancer patients’ self-reporting of symptoms.[12]
Inflammatory markers
Serum was isolated from peripheral blood samples collected from patients before they received high-dose melphalan and then on days (−2), (0), (+3), (+5), (+7) and then twice weekly after ASCT until 4 weeks. Sera were stored frozen at −80°C. On the day of cytokine assay, serum samples were thawed and subjected to enzyme-linked immunosorbant assay (ELISA; R&D Systems Inc, Minneapolis, MN) to measure levels of IL-6, IL-1 receptor antagonist (IL-1RA), macrophage inflammatory protein (MIP)-1α, tumor necrosis factor (TNF)-α, IL-1β, soluble TNF receptor I (sTNF-RI), and soluble TNF receptor II (sTNF-RII). Results for each of the analytes were reported as pg/mL.
Statistical analysis
The primary objective of this study was to determine if a higher stem cell dose would result in a lower increase in symptom severity at 1 week post-ASCT. We defined the five most-severe symptoms as those that received the highest ratings during the first 7 days of transplant. A composite symptom score was obtained by averaging those five symptom levels. The area under the curve (AUC) for each stem cell group was calculated using the composite scores for these five most-severe symptoms over time, according to the trapezoidal rule.[13] AUCs were compared between treatment groups using a t-test. In addition, mean scores for these symptoms were calculated at each time point and compared between groups. Linear mixed models were applied to estimate the development of the five most-severe symptoms during the first week and first 28 days after transplant. Based on our previous experience we estimated that the severity score in the standard dose group would increase by 0.85 points more than the increase in the high dose group. We thus enrolled 50 patients per arm to have 80% power to detect this difference.
The other outcome measurements were also compared between the two groups. For functional tests, the mean scores for each test (time in seconds for sit-to-stand and feet for 6-minute walk) were compared between treatment groups at each time point. In addition, the mean change from baseline to day 28 was analyzed and compared between groups by linear mixed models. For cytokine analyses, we first normalized the values using natural-based log transformation. Linear mixed models were used to compare means of transformed values between treatment groups over 7 days post-ASCT. SAS version 9.3 statistical software (SAS Institute, Cary, NC) was used to perform all analyses. All statistical tests were two-sided, and P values < 0.05 were considered statistically significant.
RESULTS
Patient characteristics
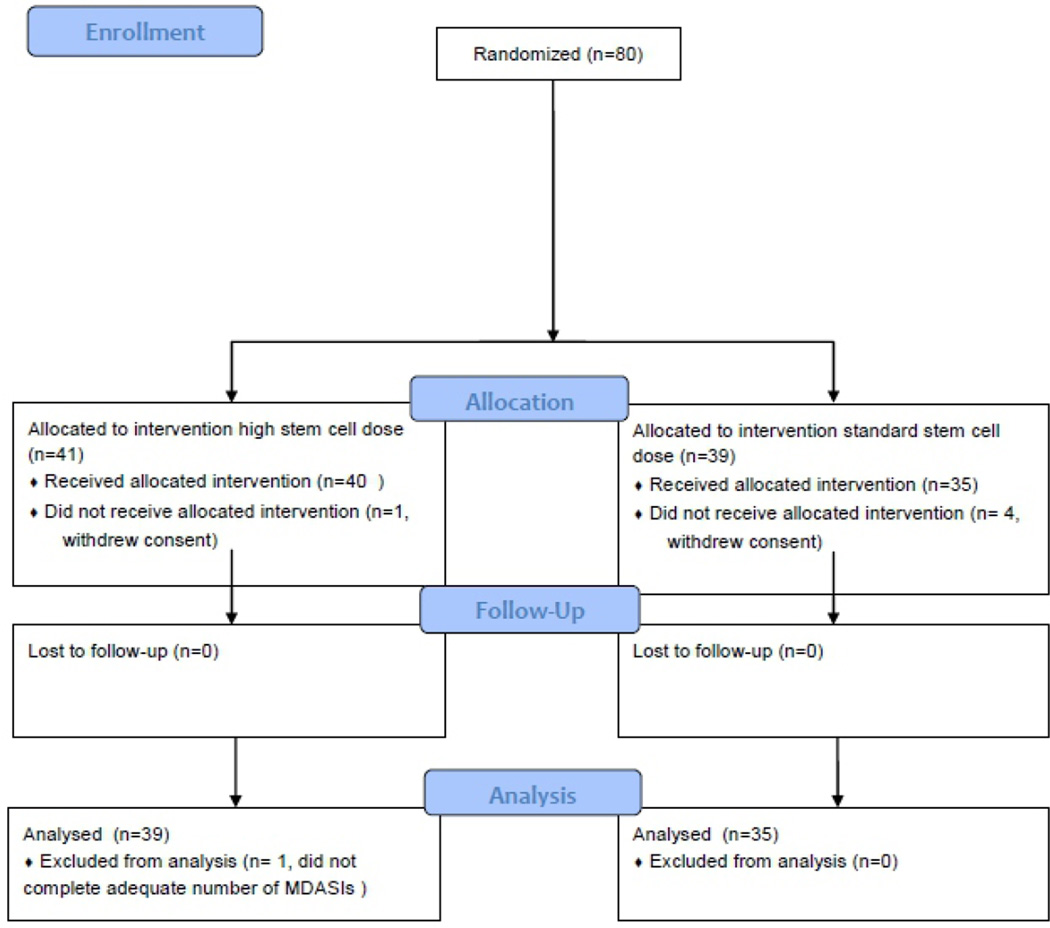
Between March 2008 and May 2013, 80 patients were enrolled. On the basis of preliminary data, we had planned to enroll 50 patients per group. Due to funding constraints, we performed an unplanned interim analysis for futility/superiority after 77% of the targeted number of patients had completed assessment. This analysis indicated that the predicted probability of demonstrating a significant difference between treatment groups would be 3% if the trial were to continue to full enrollment. Thus the trial was halted early with 41 patients in the high-dose group and 39 patients in the standard-dose group.
Patient characteristics are detailed in Table I. There were no significant differences between the two treatment groups with regard to demographic or clinical characteristics. Twenty-two patients had AL amyloidosis. The median CD34+ cell dose was 5.1 × 106 cells/kg in the standard-dose arm and 10.5 × 106 cells/kg in the high-dose arm. Median number of stem cells collected was also similar between the 2 arms (13.80 5.1 × 106 cells/kg in the standard dose arm and 13.93 5.1 × 106 cells/kg in the high dose arm). All patients collected at least 10 × 106 cells/kg as per eligibility criteria. Two patients in the high-dose arm were unable to receive the appropriate dose (in 1 case due to contamination and in another due to physician ordering error). One patient in the standard-dose arm received more than 6×106 cells/kg due to cryopreservation of all cells in 1 bag.
Table I.
Patient Characteristics
| Stem Cell Dose | ||||
|---|---|---|---|---|
| High (n = 41) | Standard (n = 39) | P-value | ||
| Median age at ASCT (range) | 65 (37–75) | 64 (42–79) | .82 | |
| Median cell dose, × 106 CD34+ cells/kg (range) | 10.5 (5.5–18.8) | 5.1 (3.5–11.1) | < .0001 | |
| Female (%) | 12 (29.3) | 13 (33.3) | .70 | |
| International Staging System (ISS) stage (%) | .95 | |||
| Unknown | 10 (24.4) | 10 (25.6) | ||
| I | 12 (29.3) | 13 (33.3) | ||
| II | 12 (29.3) | 11 (28.2) | ||
| III | 7 (17.0) | 5 (12.8) | ||
| ECOG performance status at baseline | .17 | |||
| 0 | 9 (22.0) | 13 (33.3) | ||
| 1 | 29 (77.7) | 25 (64.1) | ||
| 2 | 3 (7.3) | 0 (0) | ||
| 3 | 0 (0) | 1 (2.6) | ||
| Evidence of AL amyloidosis* (%) | 14 (34.15%) | 8 (21.05%) | .19 | |
| Poor risk cytogenetics (%) | 7 (17.95%) | 3 (8.57%) | .24 | |
| Induction regimen | ||||
| VD | 13 (31.7%) | 12 (30.8%) | .43 | |
| RD | 1 (2.4%) | 9 (23.1%) | .01 | |
| CyBorD | 7 (17.0%) | 5 (12.9%) | .35 | |
| VRD | 5 (12.2%) | 5 (12.9%) | 1.00 | |
| CVAD | 1 (2.4%) | 0 | NA | |
| VTD or TD | 6 (14.6%) | 1 (2.6%) | .104 | |
| Mobilization regimen | ||||
| G-CSF alone | 27 (65.8%) | 29 (74.3%) | .63 | |
| Cyclophosphamide-based chemomobilization | 8 (19.5%) | 7 (17.9%) | 1.00 | |
| Plerixafor and G-CSF | 6 (14.6%) | 4 (10.3%) | .74 | |
| Response prior to transplant | ||||
| CR | 1 (2.4%) | 1 (2.6%) | 1.00 | |
| VGPR | 13 (31.7%) | 20 (51.3%) | .11 | |
| PR | 19 (46.3 %) | 11 (28.2%) | .16 | |
| SD | 7 (17.05) | 6 (15.3%) | 1.00 | |
| PD | 1 (2.4%) | 1 (2.6%) | 1.00 | |
Abbreviations: ASCT, autologous hematopoietic stem cell transplant; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; G-CSF, granulocyte colony stimulating factor; VD, bortezomib-dexamethasone; RD, lenalidomide-dexamethasone; CyBorD, cyclophosphamide-bortezomib-dexamethasone; VRD, bortezomib-Lenalidomide-dexamethasone; CVAD, cyclophosphamide-vincristine-doxorubicin-dexamethasone; VTD, bortezomib-thalidomide-dexamethasone; TD, thalidomide-dexamethasone.
Evidence of AL amyloidosis was defined by a biopsy showing light chain amyloid deposition at any site.
Symptom data
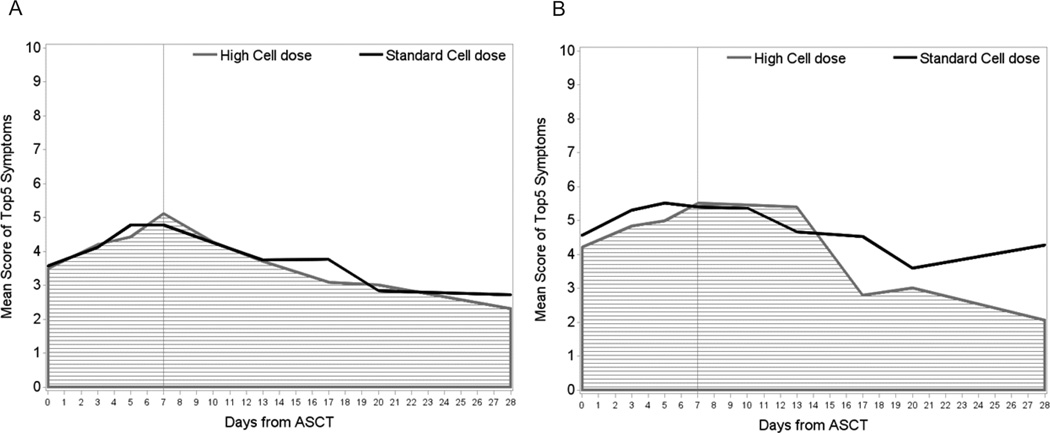
The primary endpoint of the study was symptom burden during the first week after ASCT. Of the 80 patients enrolled on the study, 74 were evaluable for the primary endpoint (Figure 1). The five most severe symptoms during the first 7 days post-ASCT were fatigue, lack of appetite, drowsiness, disturbed sleep, and pain. During this time, the AUC for the mean composite severity score of these five symptoms did not differ between the two treatment arms (Figure 2A, P = 0.819). Over the 28-day course of ASCT, the five symptoms with the greatest cumulative severity were fatigue, lack of appetite, drowsiness, disturbed sleep, and pain. Again, AUCs for these symptoms were not different between the two treatment arms (P = 0.863). When we looked specifically at patients with AL amyloidosis, the AUC of symptoms again did not differ between the two treatment arms, either over the first 7 days or the first 28 days (P = 0.685 and P = 0.609, respectively; Figure 2B).
Figure 1.
Patient enrollment and disposition.
Figure 2.
Symptom burden throughout ASCT course for (a) all patients (n = 74) and (b) the subset of patients with amyloidosis (n = 20). Curves represent mean composite severity scores of the five most-severe symptoms reported. As we have seen in previous studies, symptoms peaked in severity at WBC nadir. However, there were no significant differences between the AUCs of severity scores over the first 7 days (P = 0.819 for all patients and P = 0.685 for amyloid patients) or over the first 28 days (P = 0.863 for all patients and P = 0.609 for amyloid patients) during recovery after ASCT. ASCT, autologous hematopoietic stem cell transplant; AUC, area under the curve; WBC, white blood cell.
Functional data
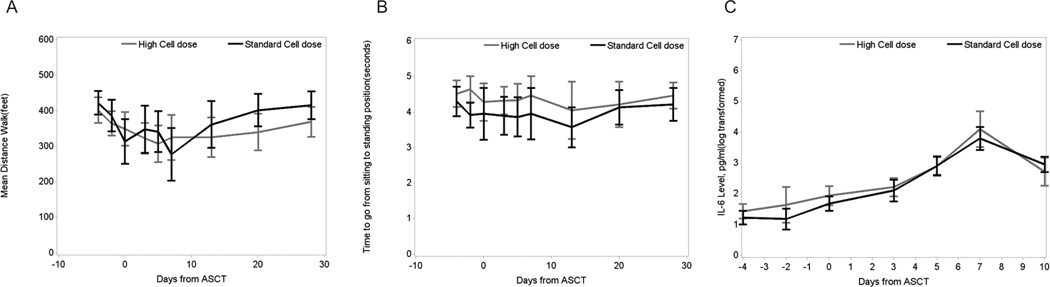
As would be expected, the mean (+/− standard deviation) distance that the patients could walk in 6 minutes decreased from 289.1 ± 198.8 feet to 174.7 ± 216.9 feet in the first week of ASCT (P < 0.0001) and increased from 174.7 ± 216.9 feet on day 7 to 350.8 ± 188.5 feet on day 28 (P < 0.0001). However, this increase was similar between the two treatment groups (Figure 3A; P = 0.221). Similarly, there were no significant differences between the groups in the time taken to go from a sitting to a standing position during the post-ASCT period (Figure 3B).
Figure 3.
Patient function and cytokine data. (a) Distance traveled during 6-minute walk test (feet) throughout the ASCT course. There were no significant differences noted between treatment groups (P = 0.221). (b) Time (seconds) to go from sitting to standing position throughout the ASCT course. No significant differences were noted between stem cell dose groups except at day −2 (2 days before stem cell infusion). (c) IL-6 levels throughout the ASCT course. Although both groups experienced an increase in serum IL-6 levels during the first week post-ASCT, there were no differences in these levels between treatment groups (P = 0.377). ASCT, autologous hematopoietic stem cell transplant; IL, interleukin.
Cytokines
Peripheral blood collection and analysis for correlative studies was successfully completed in approximately 80% of patients during the first week of the study. In the first 10 days after ASCT, mean serum IL-6 levels increased significantly from baseline in both groups (from 8.35 pg/mL to 33.25 pg/mL in the high-dose group and from 6.24 pg/mL to 22.87 pg/mL in the standard-dose group; P = 0.0009 for all patients between time points). However, we did not find any differences between the two groups for this increase (Figure 3C, P = 0.377). Similarly, there were no other significant differences between treatment groups for the levels of MIP-1α, sIL-1RI, sTNF-RI, or sTNF-RII during the first 10 days after ASCT. The high-dose group demonstrated a higher level of serum TNF-α at day 10 after ASCT (mean value of 26.9 pg/mL versus 17.46 pg/mL, respectively, P = 0.024), but this difference had also existed at baseline, before stem cell infusion (18.26 pg/mL versus 12.91 pg/mL, respectively; P = 0.028). Patients in the high-dose group also demonstrated a higher level of serum IL-1RA at day 10 post-ASCT (308.48 pg/mL versus 195.19 pg/mL; P = 0.025) but not at any other time point during the transplant course.
Clinical outcomes
By intention-to-treat analysis, the peritransplant clinical outcomes were relatively similar between treatment arms. Median time to neutrophil engraftment (absolute neutrophil count of 500/µL for the first of 3 consecutive days) was 10 days in both groups and did not differ between the groups (P = 0.078). Median time to platelet count ≥ 20,000/µL was also similar between the high-dose and standard-dose groups (10 days for both, P = 0.085). Median time to reach and absolute lymphocyte count of 500/µL was 11 days in the high-dose group and 12 days in the standard-dose group (P = 0.895). The incidence of grade ≥3 toxicities was 61% for the high-dose group and 41% for the standard-dose group (P = 0.074). One patient in the high-dose arm died during the transplant course on day 8 post-ASCT. Infectious workup was negative and the cause of death was deemed to be cardiac in nature. Of note this patient (age 65) did not have any evidence of AL amyloidosis.
At the 1-year follow-up, best responses in the high-dose group were: 24% complete response (CR), 32% very good partial response (VGPR), 34% partial response (PR), 7% stable disease (SD) (Table II). In the standard-dose group, best responses were: 50% CR, 34% VGPR, 11% PR, and 5% SD. These did not differ significantly between the two arms (P = 0.054) but did show a trend in favor of the standard stem cell dose arm with regards to reaching CR.
Table II.
Best Response at 1 Year After ASCT
| High stem cell dose (n = 41) | Standard stem cell dose (n = 39) | P value | |
|---|---|---|---|
| Best response | .054 | ||
| CR | 10 (24.40%) | 19 (50.00%) | |
| VGPR | 13 (31.71%) | 13 (34.21%) | |
| PR | 14 (34.15%) | 4 (11.00%) | |
| SD | 3 (7.32%) | 2 (5.26%) | |
| ED | 1 (2.44%) | 0 (0.00%) | |
Abbreviations: CR, complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; ED, early death.
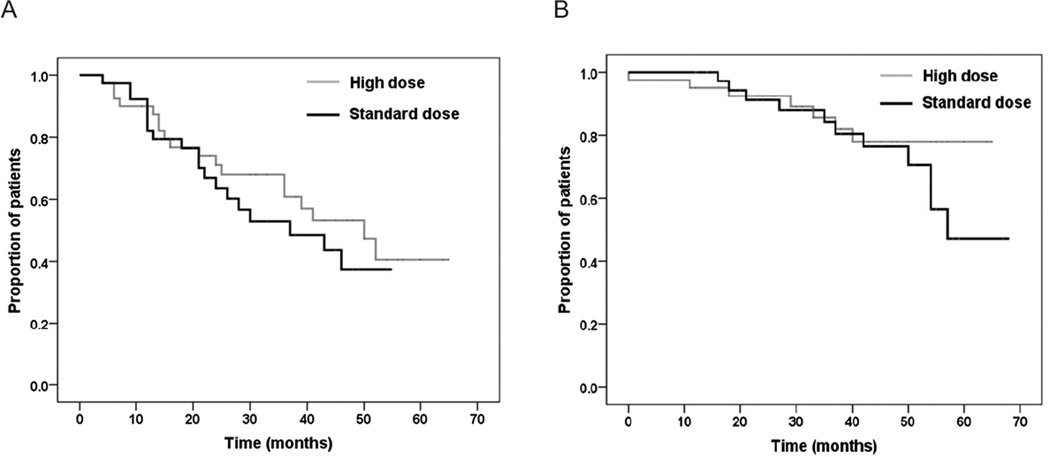
With a median follow-up time of 41 months for all patients, the median progression-free survival was similar between the groups: 50 months (95% CI, 35–65 months) versus 37 months (95% CI, 16–58 months) in the high versus low stem cell dose groups, respectively (P = 0.43). Mean overall survival was also similar between groups: 56.5 months versus 54.8 months in the high versus low stem cell groups, respectively (P = 0.363) (Figure 4A and 4B). Median survival time was not estimated for overall survival, as fewer than half of the patients in the high-dose group had died at time of analysis.
Figure 4.
Survival curves from time of autologous hematopoietic stem cell transplant. No differences between treatment groups were noted in either (a) progression-free survival or (b) overall survival (P = 0.43 and P = 0.363 respectively, by log-rank test).
DISCUSSION
Reducing the symptomatic impact of aggressive cancer treatment such as ASCT would be of major benefit to patients. This would be particularly important for older MM patients who are increasingly undergoing ASCT [14] as a clinically- and cost-effective therapy [15, 16] and for patients with AL amyloidosis who also appear benefit from ASCT.[17] This study demonstrated the feasibility of prospectively collecting frequent longitudinal data on multiple symptoms in conjunction with longitudinal functional and immunological correlative data in the setting of an intense treatment such as ASCT. We have previously shown an association between increasing inflammatory markers, especially IL-6, and increases in symptom severity during the WBC nadir period of stem cell transplant.[3, 4] We have also reported that higher stem cell doses may be associated with lower symptom severities.[9] We therefore expected that infusion of a high dose of stem cells would be associated with lower transplant symptom burden, and that this might be mediated through a reduction in treatment-induced inflammatory response, particularly IL-6.
In this prospective, randomized controlled trial we found no difference in symptoms during the ASCT course between the high and standard stem cell dose arms. Furthermore, we found no differences between the treatment groups in the expected increase in IL-6 over the transplant period. The results were unequivocal in terms of both self-reported symptoms and secondary functional outcomes. Our data suggest that there is no benefit of more stem cells for reduction of symptoms during the transplant period or for performance measures of functional capacity.
It should be noted that the top 5 symptoms reported as most severe were fatigue, lack of appetite, drowsiness, disturbed sleep, and pain. While it may seem counterintuitive that nausea is not among these symptoms we believe that this may be related to better anti-emetic prophylaxis and patient education, with a realistic expectation of this symptom. Indeed nearly all (91%) patients experienced some degree of nausea but the symptom inventory data indicated that it was not the most severe symptom. Similarly, mucositis was not among the most severe symptoms (though it was assessed as a symptom separate from general pain) and only occurred in 35% of patients. Again, this may reflect better supportive care, less toxic induction regimens and the effects of oral cryotherapy, which has been reported in other studies as well.[18, 19]
Interestingly, there were also no significant differences in time to neutrophil or platelet engraftment or clinical responses between the treatment groups. Although the general consensus in the myeloma community is that a dose of 2 × 106 CD34+ cells/kg is sufficient,[20] there are some data to suggest that a dose of 5 × 106 CD34+ cells/kg can further improve engraftment time.[21] However, our data seem to indicate that doses greater than this do not alter the kinetics of hematopoietic recovery. While there seemed to be a trend towards and improved CR in the standard dose arm this did not translate to an improvement in PFS and was likely due to limited patient numbers. The sample size may have also explained the slight trend toward increased ≥ grade 3 toxicities in the high stem cell dose group.
The discrepancy between our preliminary data and hypotheses and our actual findings may be explained by our choice in stem cell dose. If there is truly no difference in escalating beyond 5 × 106 CD34+ cells/kg, then perhaps a more clinically relevant question would be the difference between a dose of 2.5 × 106 CD34+ cells/kg and 5 × 106 CD34+ cells/kg. This would be a stem cell range in which there is a greater signal for dose-response and thus could yield differences in inflammatory markers and/or symptom burden. However, AL amyloidosis patients would not likely be studied in this situation, as the consensus is that at least 5 × 106 CD34+ cells/kg should be infused in these patients.[8, 22] Finally, it should be noted that eligibility criteria included collection of at least 10 × 106 CD34+ cells/kg during mobilization. Thus the effect of different stem cell doses could not be studied in all MM patients in our transplant center, only those who had collected enough cells and agreed to participate in the study.
The lack of differences in IL-6 leaves open the critical question of whether or not modulation of specific cytokines (especially IL-6) might be associated with lower transplant symptom burden. Although the dose of stem cells did not appear to affect IL-6 (or other inflammatory cytokines) it is possible that other more-direct modulation of inflammation would be more effective. A possible candidate for this includes siltuximab (anti-IL-6 monocolonal antibody), which has also been studied for its anti-myeloma effect.[23]
In conclusion, the infusion of a high stem cell dose for older MM patients undergoing high-dose chemotherapy and ASCT did not yield a reduction in symptom burden or change in functional outcomes. The concomitant lack of effect on inflammatory cytokines suggests that there is still a window of opportunity to intervene. Indeed, the changing demographics of myeloma mandates further efforts to improve the patient experience in this growing population.
Highlights.
We examined the effect of stem cell dose on symptom burden during auto-transplant.
Severe symptoms were fatigue, lack of appetite, drowsiness, disturbed sleep, pain.
There were no differences in symptom burden between the two groups during transplant.
Higher stem cell dose did not yield faster hematologic recovery or lower IL-6 levels.
ACKNOWLEDGEMENTS
NS, LAW, JMR, PMD, QB, MHQ, REC and SAG performed the research. LAW, TRM, XSW, PMD, REC, CSC and SAG designed the research study. NS, QS, TRM, XSW and CSC analyzed the data. NS, QS, XSW, JMR, QB, MHQ, REC, CSC and SAG wrote the paper. The authors acknowledge the editorial support of Jeanie F. Woodruff, BS, ELS.
Research support: This study was supported by grants from the National Cancer Institute of the National Institutes of Health, including NCI P01 CA124787 to Charles Cleeland and MD Anderson Cancer Center Support Grant NCI P30 CA016672.
Disclaimers: Nina Shah has a consulting role with Sanofi and research funding from Celgene. Qaiser Bashir has research funding from Celgene. Richard Champlin has consulting roles with Pharmacyclics, Inc. and Actinium Pharmaceuticals. Charles Cleeland has research funding from Bayer HealthCare Pharmaceuticals. Sergio Giralt has consulting roles with Celgene, Jazz Pharmaceuticals, Amgen, Onyx Pharmaceuticals, Sanofi, and Novartis and has received honoraria from these companies; he has research funding from Celgene and Sanofi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary results presented at: American Society of Hematology 55th Annual Meeting and Exposition, New Orleans LA, Dec 7–10, 2013
REFERENCES
- 1.Anderson KO, Giralt SA, Mendoza TR, Brown JO, Neumann JL, Mobley GM, et al. Symptom burden in patients undergoing autologous stem-cell transplantation. Bone marrow transplantation. 2007;39:759–766. doi: 10.1038/sj.bmt.1705664. [DOI] [PubMed] [Google Scholar]
- 2.Campagnaro E, Saliba R, Giralt S, Roden L, Mendoza F, Aleman A, et al. Symptom burden after autologous stem cell transplantation for multiple myeloma. Cancer. 2008;112:1617–1624. doi: 10.1002/cncr.23299. [DOI] [PubMed] [Google Scholar]
- 3.Wang XS, Shi Q, Shah ND, Heijnen CJ, Cohen EN, Reuben JM, et al. Inflammatory markers and development of symptom burden in patients with multiple myeloma during autologous stem cell transplantation. Clin Cancer Res. 2014;20:1366–1374. doi: 10.1158/1078-0432.CCR-13-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang XS, Shi Q, Williams LA, Cleeland CS, Mobley GM, Reuben JM, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008;113:2102–2109. doi: 10.1002/cncr.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campagnaro E, Saliba R, Anderson KO, Giralt S, Cleeland C. Risk factors for development of symptoms post aytologous transplant for multiple myeloma. Blood. 2005;106 abstract 1771. [Google Scholar]
- 6.Mavroudis D, Read E, Cottler-Fox M, Couriel D, Molldrem J, Carter C, et al. CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies. Blood. 1996;88:3223–3229. [PubMed] [Google Scholar]
- 7.Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–3969. [PubMed] [Google Scholar]
- 8.Oran B, Malek K, Sanchorawala V, Wright DG, Quillen K, Finn KT, et al. Predictive factors for hematopoietic engraftment after autologous peripheral blood stem cell transplantation for AL amyloidosis. Bone marrow transplantation. 2005;35:567–575. doi: 10.1038/sj.bmt.1704826. [DOI] [PubMed] [Google Scholar]
- 9.Williams LA, Giralt S, Mendoza TR, Anderson KO, Mobley GM, Saliba R, et al. Larger stem cell dose is associated with decreased symptom severity after autologous stem cell transplantation for multiple myeloma. Biology of Blood and Marrow Transplantation. 2008;14:98–99. [Google Scholar]
- 10.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Jones D, Vichaya EG, Wang XS, Williams LA, Shah ND, Thomas SK, et al. Validation of the M. D. Anderson Symptom Inventory multiple myeloma module. Journal of hematology & oncology. 2013;6:13. doi: 10.1186/1756-8722-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmonds MJ. Physical function in patients with cancer: psychometric characteristics and clinical usefulness of a physical performance test battery. Journal of pain and symptom management. 2002;24:404–414. doi: 10.1016/s0885-3924(02)00502-x. [DOI] [PubMed] [Google Scholar]
- 13.Yeh KC, Kwan KC. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978;6:79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
- 14.Costa LJ, Zhang MJ, Zhong X, Dispenzieri A, Lonial S, Krishnan A, et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19:1615–1624. doi: 10.1016/j.bbmt.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah GL, Winn A, Lin PJ, Klein A, Sprague KA, Smith HP, et al. Cost-Effectiveness of Autologous Hematopoietic Stem Cell Transplantation for Elderly Patients with Multiple Myeloma using the Surveillance, Epidemiology, and End Results-Medicare Database. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015 doi: 10.1016/j.bbmt.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma M, Zhang MJ, Zhong X, Abidi MH, Akpek G, Bacher U, et al. Older patients with myeloma derive similar benefit from autologous transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:1796–1803. doi: 10.1016/j.bbmt.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afrough A, Saliba RM, Hamdi A, El Fakih R, Varma A, Dinh YT, et al. Outcome of Patients with Immunoglobulin Light-Chain Amyloidosis with Lung, Liver, Gastrointestinal, Neurologic, and Soft Tissue Involvement after Autologous Hematopoietic Stem Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015 doi: 10.1016/j.bbmt.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lilleby K, Garcia P, Gooley T, McDonnnell P, Taber R, Holmberg L, et al. A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone marrow transplantation. 2006;37:1031–1035. doi: 10.1038/sj.bmt.1705384. [DOI] [PubMed] [Google Scholar]
- 19.Fleming S, Harrison SJ, Blombery P, Joyce T, Stokes K, Seymour JF, et al. The choice of multiple myeloma induction therapy affects the frequency and severity of oral mucositis after melphalan-based autologous stem cell transplantation. Clinical lymphoma, myeloma & leukemia. 2014;14:291–296. doi: 10.1016/j.clml.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114:1729–1735. doi: 10.1182/blood-2009-04-205013. [DOI] [PubMed] [Google Scholar]
- 21.Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C. Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:1360–1377. doi: 10.1200/JCO.2000.18.6.1360. [DOI] [PubMed] [Google Scholar]
- 22.Comenzo RL, Gertz MA. Autologous stem cell transplantation for primary systemic amyloidosis. Blood. 2002;99:4276–4282. doi: 10.1182/blood.v99.12.4276. [DOI] [PubMed] [Google Scholar]
- 23.Voorhees PM, Manges RF, Sonneveld P, Jagannath S, Somlo G, Krishnan A, et al. A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. British journal of haematology. 2013;161:357–366. doi: 10.1111/bjh.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]