Abstract
Infection is a major complication of hematopoietic cell transplantation. Prolonged neutropenia and graft versus host disease are the two major complications with an associated risk for infection, and these complications differ according to the graft source. A phase 3, multicenter, randomized trial (BMT CTN 0201) of transplantation of bone marrow (BM) versus peripheral-blood stem cells (PBSC) from unrelated donors (URD) showed no significant differences in two-year survival between these graft sources. In an effort to provide data regarding whether bone marrow or peripheral-blood stem cells could be used as a preferential graft source for transplantation, we report a detailed analysis of the infectious complications for 2 years following transplantation from the BMT CTN 0201 trial. A total of 499 patients in this study had full audits of infection data. A total of 1347 infection episodes of moderate or greater severity were documented in 384 (77%) patients; 201/249 (81%) of the evaluable patients had received a BM graft and 183/250 (73%) had received a PBSC graft. Of 1347 infection episodes, 373 were severe and 123 were life-threatening and/or fatal; 710 (53%) of these episodes occurred on the BM arm and 637 (47%) on the PBSC arm, resulting in a two-year cumulative incidence 84.7% (95% confidence interval [CI]: 79.6–89.8) for BM vs. 79.7% (95%CI, 73.9–85.5) for PBSC, P = .013. The majority of these episodes, 810 (60%), were due to bacteria, with a two-year cumulative incidence of 72.1% and 62.9% in BM versus PBSC recipients, respectively (P = .003). The cumulative incidence of bloodstream bacterial infections during the first 100 days was 44.8% (95%CI, 38.5–51.1) for BM vs. 35.0% (95%CI, 28.9–41.1) for PBSC (P = .027). The total infection density (# infection events / 100 patient days at risk) was .67 for BM and .60 for PBSC. The overall infection density for bacterial infections was .4 in both arms; for viral infections was .2 in both arms; and for fungal/parasitic infections was .04 and .05 for BM and PBSC, respectively. The cumulative incidence of infection prior to engraftment was 47.9% (95%CI, 41.5–53.9) for BM vs. 32.8% (95%CI, 27.1–38.7) for PBSC (P = .002), possibly related to quicker neutrophil engraftment using PBSC. Infections remain frequent following URD HCT, particularly following BM grafts.
Keywords: Infection, unrelated donor transplantation, bacteremia, cytomegalovirus, aspergillosis, pre-engraftment
INTRODUCTION
Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0201 was a phase 3, multicenter, randomized trial of transplantation of bone marrow (BM) versus granulocyte-colony stimulating factor mobilized peripheral-blood stem cells (PBSC) from HLA compatible unrelated donors in patients with hematologic malignancies. There were no significant differences in 2-year survival probabilities [1]. Only 5% of patients who died had infection listed as the primary cause of death. Analysis of pre-specified secondary endpoints showed that the median time to neutrophil engraftment was 5 days shorter for those randomly assigned to receive PBSC. The total incidence of graft failure was 3% in the PBSC group, compared to 9% in the BM group. These significant findings, as well as data from other clinical trials, have led some to choose PBSC as a preferential graft source [2–6]. However, the 53% incidence of chronic graft-versus host disease at 2 years in the PBSC group was significantly higher when compared to 41% for the BM group, a finding confirmed by other studies [2–4,7–10]. These secondary analyses suggest that it may be important for patients who are immunosuppressed from prior chemotherapy, and have a lower risk of graft rejection, receive BM grafts, while patients with malignant diseases who have never undergone cytotoxic chemotherapy, who may be at increased risk of rejection of a BM graft, undergo transplant with a PBSC graft. In an effort to provide additional data regarding whether BM or PBSC could be used as a preferential graft source for transplantation, we report a detailed analysis of the infectious complications for 2 years following transplantation from the BMT CTN 0201 trial.
METHODS
Protocol Synopsis
BMT CTN Protocol 0201 opened for accrual in March 2004 and closed in September 2009 meeting the target accrual objective of 550 donor-recipient pairs. Randomization was performed 1:1 to either PBSC or BM and stratified by transplant center and disease risk. Details of the treatment schemes have been reported [1]. Key baseline characteristics were similar in the 2 treatment arms. The primary objective was to compare two-year survival probabilities in the two study arms using an intent-to-treat analysis. Secondary objectives included incidences of neutrophil and platelet engraftment, graft failure, acute graft-versus-host disease (GVHD), chronic GVHD, time off all immunosuppressive therapy, relapse, adverse events, immune reconstitution, and quality of life. Moderate or more severe infections were reported on an event-driven Case Report Form, excluding those that occurred pre-transplant, and were tabulated for two years following transplantation.
Infection Prophylaxis
Among the 40 participating transplant centers, infection prophylaxis followed local protocols that were based on national guidelines [11]. Patients underwent their transplant procedure in rooms ventilated with high efficiency particulate air filtration systems to prevent acquisition of exogenous mold infections [12]. During periods of neutropenia, broad-spectrum antibacterial agents were administered that included anti-pseudomonal antibacterial agents for febrile neutropenia [13]. During periods of fever, assessment for infection and augmentation of antimicrobial agents included empirical treatment for yeasts and molds for persistent fever [14]. Medication to prevent Pneumocystis infections generally started within one month after transplantation, following engraftment [15]. Acyclovir was used to prevent herpes simplex and varicella infections. Monitoring guidelines were followed to detect and preemptively treat cytomegalovirus (CMV) infection.[16] Diagnostic strategies for individual pathogens, particularly viral and fungal infections, varied between centers based on local laboratory practices.
Data Collection and Audit
Transplant centers prospectively reported infections for study subjects. Infection onset date, organism, body site, clinical severity, and treatment were captured. Classification of infection severity, including a fatal category, were modified from the definitions used in previous studies [17,18]: A) Life-threatening infections were those complicated by hypotension or any another event considered life-threatening; B) Severe infections often required treatment with intravenous antibiotics or involved other clinical circumstances that were considered severe.
Based on previous studies that had shown the importance of infection data auditing [17–22], the 0201 Infection Team contacted each transplant center to audit the infection data reported for local study patients. In 2012, a pilot audit of infectious disease data for 78 subjects at 15 centers involved with this study indicated value for a full data audit for all transplanted subjects. The BMT CTN Infection reporting form and Instructions for the BMT CTN 0201 Infection Data Review are included as supplementary files to this manuscript. To perform both the pilot audit and entire study audit, the Data and Coordinating Center (The EMMES Corporation, Rockville, MD) prepared an infection summary for each study patient listing the date, organism, body site, severity, and comments regarding each infection as prospectively reported. The auditor confirmed database information by reviewing electronically generated medical records of microbiology, virology, imaging, and surgical pathology reports. Study subjects who were not audited for infection data (n=27, 5%) were excluded from these analyses.
Staphylococcus epidermidis bloodstream infections were counted as an episode of infection if they were treated (even when just one positive blood culture led to treatment) because the treatment course then could have affected whether a second blood culture turned positive, altered the flora of the subject, and created the potential for drug toxicity. CMV, human herpes virus-6, and polyomavirus infections were differentiated as viremia or disease using the BMT CTN Infection reporting form which specifies a body site code; a comment box to add detail about an infection was later reviewed by the audit committee (and JHY) during data cleaning; and the specified definitions of infection severity based on body site affected. All Epstein Barr Virus (EBV) “infections” had the comments specifically reviewed to categorize infections as EBV lymphoproliferative disorder, viremia, or report of a serologic antibody status.
The BMT CTN Definitions of Infection Severity clarify that fever of undetermined origin, upper respiratory infections presumed viral, and potential infections where antibiotics were given without infectious etiology identified were not to be reported. If reported, they were removed from the dataset during final data review.
Statistical Analysis
Infectious complications were analyzed by comparison of the two treatment arms (PBSC versus BM) and adjusted by other relevant clinical risk factors. All data on time to infections or other events were calculated from the date of transplantation. Patients were censored at last follow-up, relapse, second transplant, or 2 years after transplant, whichever came first. When a single organism was recovered multiple times, we followed the algorithm for infection recurrence intervals that were specific to each microorganism, as reported previously [17].
Analysis of infection incidence (by frequency and cumulative incidence) [17] and infection density (number of infections over time for patients with repeated infections) [20] was performed for overall infections, bacterial infections, viral infections and fungal/parasitic infections, over different time periods (Day 0 to Day 100, Day 100 to 6 months, 6 months to 1 year, 1 year to 2 years, and overall within 2 years of transplantation), by treatment arm. Death was considered a competing risk in cumulative incidence analysis. Infection density was computed by infection frequencies over 100 patient days at risk. The number of patients with infections reported was summarized for each infection type and by treatment arm; specific infectious organisms of interest were explored in descriptive and cumulative incidence analyses.
Multivariate regression analyses were performed to test the contribution of the randomized stem cell source (PBSC versus BM) and other relevant covariates to the incidence of infections. Proportional rates/means models were used to model the infection density over time, with a robust variance estimator to account for within subject correlation [23]. All variables were checked for proportionality using time dependent covariates. The effect of acute and chronic GVHD on infection density was obtained using time-dependent covariates. Multivariate analyses were performed to test the impact of various types of infections on overall survival following transplantation. Cox regression models were used to model survival. The treatment arm variable was forced into all models and baseline covariates were selected using forward stepwise algorithm. Various types of infections were treated as time-dependent covariates in the model. SAS version 9.3 (SAS Institute) was used for data manipulation, infection frequency analysis, and multivariate modeling. R version 2.15.1 was used for cumulative incidence analysis.
RESULTS
Study Population and Total Infections
Among 551 patients enrolled at 48 centers between 01/2004 and 10/2009, 526 patients received a transplant. Response rate was 95% for the infection data audit (249 of the BM arm and 250 on the PBSC arm), resulting in a study population for this analysis of 499 audited patients. Table 1 lists the baseline characteristics of the patients by treatment arm. Baseline characteristics were balanced between the arms and no significant differences were noted.
Table 1.
Baseline Characteristics
| Characteristics | Treatment Arm* | |
|---|---|---|
| Bone Marrow N (%) |
Peripheral Blood Stem Cells N (%) |
|
| Total (N=499) | 249 (100.0) | 250 (100.0) |
| Age (years) | ||
| Median | 44.1 | 43.8 |
| <40 | 105 (42.2) | 103 (41.2) |
| >=40 | 144 (57.8) | 147 (58.8) |
| Recipient Gender | ||
| Male | 147 (59.0) | 135 (54.0) |
| Race | ||
| White | 226 (90.8) | 227 (90.8) |
| Others | 23 (9.2) | 23 (9.2) |
| Ethnicity | ||
| Hispanic or Latino | 9 (3.6) | 12 (4.8) |
| Others | 240 (96.4) | 238 (95.2) |
| Karnofsky Performance Score | ||
| 90–100 | 165 (66.3) | 150 (60.0) |
| <90 | 64 (25.7) | 71 (28.4) |
| Unknown | 20 (8.0) | 29 (11.6) |
| Primary Disease at Enrollment | ||
| Acute Myelogenous Leukemia | 116 (46.6) | 122 (48.8) |
| Acute Lymphoblastic Leukemia | 54 (21.7) | 52 (20.8) |
| Chronic Myelogenous Leukemia | 27 (10.8) | 32 (12.8) |
| Myelodysplastic Syndrome | 46 (18.5) | 37 (14.8) |
| Chronic Myelomonocytic Leukemia | 4 (1.6) | 3 (1.2) |
| Disease risk | ||
| Early | 182 (73.1) | 181 (72.4) |
| Advanced | 67 (26.9) | 69 (27.6) |
| Donor/Recipient Gender | ||
| Female/Female | 33 (13.3) | 50 (20.0) |
| Male/Male | 115 (46.2) | 102 (40.8) |
| Female/Male | 32 (12.8) | 33 (13.2) |
| Male/Female | 69 (27.7) | 65 (26.0) |
| Donor/Recipient CMV Status | ||
| Positive/Positive | 42 (16.9) | 52 (20.8) |
| Negative/Negative | 87 (34.9) | 102 (40.8) |
| Positive/Negative | 30 (12.0) | 30 (12.0) |
| Negative/Positive | 89 (35.7) | 65 (26.0) |
| Unknown/Negative | 1 (0.4) | 1 (0.4) |
| Number of allele level HLA mismatches at HLA-A,-B,-C,-DRB1 | ||
| 0 | 189 (76.2) | 202 (80.8) |
| 1 | 52 (21.0) | 46 (18.4) |
| >=2 | 7 (2.8) | 2 (0.8) |
| Unknown | 1 (0.4) | 0 (0.0) |
| Conditioning regimen | ||
| Cyclophosphamide and Total Body Irradiation (Cy-TBI) | 115 (46.2) | 121 (48.4) |
| Busulfan and Cyclophosphamide (Bu-Cy) | 80 (32.1) | 65 (26.0) |
| Fludarabine and Melphalan (Flu-Mel) | 16 (6.4) | 24 (9.6) |
| Fludarabine, Busulfan, and ATG (Flu-Bu-ATG) | 38 (15.3) | 40 (16.0) |
| Graft versus host disease Prophylaxis | ||
| Cyclosporine and Methotrexate | 59 (23.7) | 47 (18.8) |
| Tacrolimus and Methotrexate | 163 (65.5) | 187 (74.8) |
| Other | 27 (10.8) | 16 (6.4) |
Note:
no significant p-values for baseline characteristics between the two treatment arms
Overall, 384 patients developed infections of moderate or greater severity: 201 on the BM arm and 183 on the PBSC arm. Of 1347 infection episodes (373 severe and 123 life-threatening and/or fatal), 710 (53%) occurred on the BM arm and 637 (47%) on the PBSC arm (Figure 1). The majority of infection episodes on each treatment arm were of moderate severity (Figure 1a). However, when patients were categorized by infection of worst severity, 84 patients on the BM arm had a severe infection as their most clinically serious infection, compared to 68 patients on the PBSC arm (Figure 1b, p = 0.6).
Figure 1. Descriptive Analysis of Infection Severity.
Infection Severity by Treatment Arm. (A) All infection events. (B) Maximum severity of infections (p = 0.6).
Cumulative Incidence
Cumulative incidence described the proportion of patients developing at least one infection, with an estimated 2-year incidence of 84.7% (95% confidence interval [CI], 79.6–89.8) for the BM arm, vs. 79.7% (95%CI, 73.9–85.5) for the PBSC arm, Gray’s test P = .013 (Figure 2). In a subset analysis of the 465 patients who engrafted (excluding 34 patients with primary or secondary graft failure), the 2-year cumulative incidence rate was 85.3% (95% CI: 79.2–89.7) for the BM arm, vs. 79.8% (95%CI, 73.2–85.0) for the PBSC arm (P = .014).
Figure 2.
Cumulative Incidence of All Infections
Among 810 bacterial infection episodes, 431 occurred in patients on the BM arm and 379 in patients on the PBSC arm, with a 2-year cumulative incidence of 72.1% and 62.9%, respectively (Figure 3a, P = .003). Among Gram-positive organisms, coagulase negative Staphylococcus accounted for 224 infection episodes in 149 patients, Staphylococcus aureus for 40 infections in 29 patients, and Enterococcus (all species) for 103 infections in 82 patients (Table 2). Among Gram-negative infections, Escherichia coli accounted for 39 infection episodes in 34 patients, Klebsiella species for 36 infections in 29 patients, Pseudomonas species for 32 infections in 25 patients, Enterobacter for 14 infections in 12 patients, and Stenotrophomonas for 13 infections in 12 patients. There were 123 Clostridium difficile, 5 Nocardia, and 2 non-tuberculous mycobacterial infections. No cases of tuberculosis were reported. Of the bacterial infections, 413 were recovered from the bloodstream. The cumulative incidence of bloodstream bacterial infections during the first 100 days following transplantation was 44.8% (95%CI, 38.5–51.1) for the BM arm vs. 35.0% (95%CI, 28.9–41.1) for the PBSC arm (Figure 3b, P = .092), possibly related to quicker neutrophil engraftment using PBSC.
Figure 3.
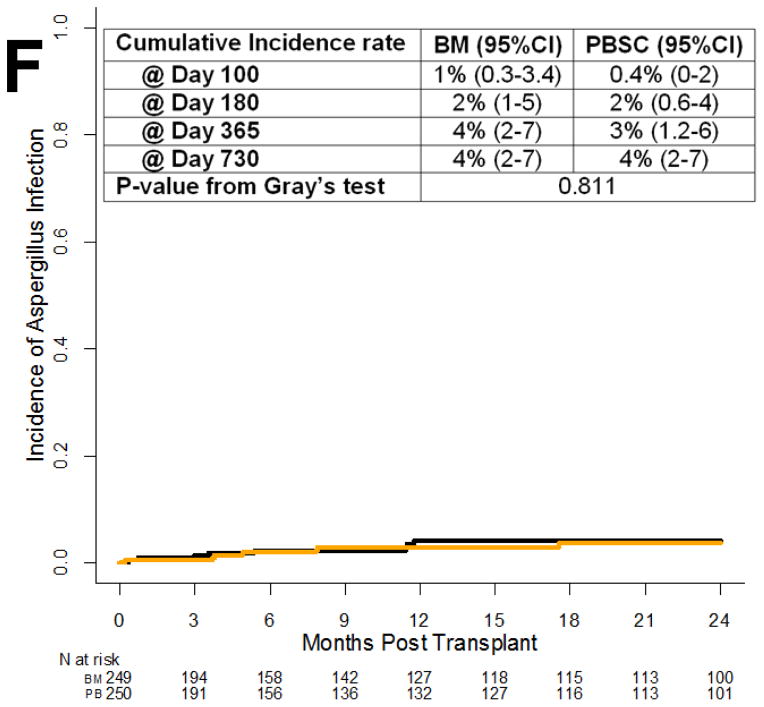
Cumulative Incidence of Specific Infections. (A) Bacteria from all sites. (B) Bloodstream bacteria. (C) All viruses. (D) Cytomegalovirus. (E) All Fungal/Parasitic Infections. (F) Aspergillus infections.
Table 2.
Pathogen Frequency by Treatment Arm (only the top 5 organisms for each infection type are listed)
| Organism | Bone Marrow N (%) |
Peripheral Blood Stem Cells N (%) |
|---|---|---|
| Bacterial infections | ||
| Staphylococcus (coagulase negative) | 123 (82) | 101 (67) |
| Enterococcus (all species) | 54 (42) | 49 (40) |
| Clostridium difficile | 69 (52) | 54 (41) |
| Staphylococcus (coagulase positive) | 10 (9) | 30 (20) |
| Escherichia (also E. coli) | 16 (15) | 23 (19) |
| Viral infections | ||
| Cytomegalovirus (CMV) | 78 (61) | 81 (57) |
| Polyomavirus | 27 (25) | 27 (24) |
| Herpes Simplex (HSV1, HSV2) | 16 (14) | 22 (17) |
| Epstein-Barr Virus (EBV) | 15 (12) | 21 (15) |
| Influenza | 22 (19) | 13 (13) |
| Fungal/Parasitic infections | ||
| Other (suspected) Fungus | 12 (11) | 13 (12) |
| Yeast other than Candida albicans | 5 (4) | 12 (10) |
| Candida albicans | 6 (6) | 10 (8) |
| Aspergillus fumigatus | 5 (5) | 6 (5) |
| Mucormycosis (Zygomycetes, Rhizopus) | 5 (4) | 2 (2) |
| Pneumocystis | 1 (1) | 2 (2) |
| Toxoplasma | 1 (1) | 1 (1) |
Among 438 viral infection episodes (136 severe and 48 life-threatening and/or fatal), 234 were on the BM arm and 204 on the PBSC arm (Figure 3c). Herpesvirus infections accounted for the majority of infections. CMV accounted for 159 infection episodes in 118 patients, herpes simplex for 38 infections in 31 patients, Epstein-Barr virus for 36 infections in 27 patients (none of these 27 patients had anti-thymocyte globulin use reported in the graft versus host disease treatment form), and varicella for 22 infections in 20 patients. Respiratory viruses accounted for 93 infection episodes including 35 for influenza, 23 respiratory syncytial virus, 17 parainfluenza virus, 14 adenovirus, and 4 rhinovirus. Additional clinically significant viral infections included a total of 54 infection episodes among 49 patients due to polyomavirus (BK virus); 10 due to human herpes virus-6; 2 due to enterovirus; and 2 due to rotavirus. The 2-year cumulative incidence of CMV infection was 25.9% (95%CI, 20.2–31.6) for the BM arm, vs. 24.4% (95%CI, 18.8–30.0) for the PBSC arm (Figure 3d, P = .62). Among 248 CMV-seropositive recipients, the cumulative incidence of CMV infection was 40.6% (95%CI, 31.8–49.2) in the BM arm compared with 36.9% (95%CI, 27.9–46.0) in the PBSC arm (P = .47). CMV infection episodes occurred in the bloodstream only in 78.6% of CMV-infected patients, versus progression to non-bloodstream sites in 21.4%. Among the CMV-infected patients, a later episode of infection occurred beyond two months from the first infection in 20.3%, although further detail regarding whether these were recurrent, refractory, or resistant infections is not within the scope of data collected by this trial. CMV seropositivity did not impact survival in the models.
Among 89 fungal infections (Figure 3e), there were 37 due to yeast (including 1 case of Saccharomyces cerevisiae in the bloodstream), 31 due to mold (20 cases of aspergillosis, 7 of mucormycosis, 2 cases of penicilliosis, 1 case of cavitary pulmonary Microascus, and 1 of fusariosis), and 1 case of coccidioidomycosis. Twenty infection episodes classified as fungal were cases of infection that were assessed to be responsive to antifungal therapy. Thirty-one of the fungal infection episodes were severe and 25 were life-threatening and/or fatal, distributed among 34 patients (13 severe and 9 life-threatening and/or fatal) on the BM arm and 37 (13 and 9) on the PBSC arm. There were 3 Pneumocystis infections and 1 case of toxoplasmosis. The Aspergillus species included 11 A. fumigatus, 2 A. niger, and 1 A. flavus. Six cases of aspergillosis were not speciated. The 2-year cumulative incidence of invasive aspergillosis was 3.9% (95%CI, 1.2–6.6) for the BM arm, vs. 3.6% (95%CI, 1.0–6.2) for the PBSC arm (Figure 3f, P = .81).
Infection Density
The overall infection density for the study was 0.63 events per 100 patient days at risk; 0.67 for BM and 0.60 for PBSC (Figure 4a). The infection density of bacterial infections was 0.4 for both arms (Figure 4b). The infection density for viral infections was 0.2 in both arms (Figure 4c). The infection density for fungal/parasitic infections was 0.04 and 0.05 for the BM and the PBSC arm, respectively (Figure 4d). Infection density for each assessment period was computed by looking into the infections reported during this period over the risk days during this period. For example, bacterial bloodstream and non-bloodstream infections had their highest density during the Day 0 to Day 100 interval (Figure 4e and 4f), and decreased over time (Table 3). The infection density for infections of moderate, severe, and life-threatening and/or fatal severity followed a similar trend with regard to infection density over time (Figures 4g, 4h, and 4i).
Figure 4.
Infection Density for each time period. (A) Total infection density. (B) Bacterial infection. (C) Viral infection. (D) Fungal infection. (E) Bloodstream bacterial infection. (F) Non-bloodstream bacterial infection. (G) Moderate infection. (H) Severe infection. (I) Life-threatening infection.
Table 3.
Overall Summary of infection frequencies
| Overall Infections * | Bone Marrow | Peripheral Blood Stem Cells | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day0-ENGR§ | ENGR-100§ | 101–180 | 181–365 | 366–730 | Overall | Day0-ENGR§ | ENGR-100§ | 101–180 | 181–365 | 366–730 | Overall | |
| Total Infection Events | 179 | 283 | 102 | 78 | 68 | 710 | 121 | 266 | 82 | 81 | 87 | 637 |
| By severity | ||||||||||||
| Moderate | 124 | 176 | 58 | 44 | 33 | 435 | 74 | 179 | 50 | 58 | 55 | 416 |
| Severe | 43 | 88 | 32 | 29 | 24 | 216 | 39 | 60 | 22 | 15 | 21 | 157 |
| Life threatening/fatal | 12 | 19 | 12 | 5 | 11 | 59 | 8 | 27 | 10 | 8 | 11 | 64 |
| By type | ||||||||||||
| Bacterial infection | 130 | 164 | 58 | 44 | 35 | 431 | 98 | 147 | 46 | 43 | 45 | 379 |
| Viral infection | 37 | 110 | 35 | 25 | 27 | 234 | 13 | 104 | 29 | 27 | 31 | 204 |
| Fungal infection | 12 | 7 | 8 | 8 | 5 | 40 | 8 | 15 | 6 | 10 | 10 | 49 |
| Parasitic infection | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 2 |
| Other types | 0 | 1 | 1 | 1 | 0 | 3 | 2 | 0 | 1 | 0 | 0 | 3 |
| Bacterial subgroups * | ||||||||||||
| Bloodstream infection | 85 | 87 | 28 | 13 | 6 | 219 | 57 | 83 | 23 | 15 | 16 | 194 |
| Nonbloodstream infection | 45 | 77 | 30 | 31 | 29 | 212 | 41 | 64 | 23 | 28 | 29 | 185 |
| Clostridium difficile infection | 23 | 29 | 8 | 7 | 2 | 69 | 22 | 14 | 5 | 10 | 3 | 54 |
| Viral subgroups * | ||||||||||||
| CMV infections | 12 | 45 | 9 | 9 | 3 | 78 | 5 | 49 | 14 | 12 | 1 | 81 |
| Respiratory infections | 12 | 10 | 10 | 11 | 15 | 58 | 2 | 5 | 5 | 6 | 13 | 31 |
| Non-CMV non-respiratory | 15 | 56 | 17 | 6 | 10 | 104 | 6 | 51 | 10 | 10 | 18 | 95 |
| EBV infections | 1 | 8 | 4 | 1 | 1 | 15 | 0 | 14 | 3 | 1 | 3 | 21 |
| Varicella | 0 | 5 | 5 | 1 | 5 | 16 | 0 | 0 | 1 | 2 | 3 | 6 |
| Fungal subgroups * | ||||||||||||
| Yeast infections | 7 | 1 | 4 | 1 | 0 | 13 | 3 | 11 | 2 | 4 | 3 | 23 |
| Mold infections | 2 | 4 | 3 | 5 | 1 | 15 | 1 | 1 | 4 | 4 | 3 | 13 |
| Aspergillus | 2 | 1 | 2 | 4 | 1 | 10 | 1 | 0 | 3 | 4 | 2 | 10 |
| Other (suspected) fungus | 3 | 2 | 1 | 2 | 4 | 12 | 4 | 3 | 0 | 2 | 4 | 13 |
Note:
Categories are not mutually exclusive. e.g. A CMV infection or an EBV infection may also fall into the category of respiratory infection, and vice versa. Categories are listed based on the study interest.
The day of engraftment (ENGR) was considered for each individual, for classification into pre- versus post-engraftment. Median engraftment was 20 days for Bone Marrow and 15 days for Peripheral Blood.
Relationship of Infection to GVHD: Multivariate Analyses
The baseline covariates in Table 1 were considered in the multivariate analyses. In multivariate analyses of infection density, there were no significant differences between PBSC and BM for density of bacterial or fungal infections (Table 4). For viral infections, a significant interaction was detected between graft type and disease risk (P < 0.001), so that for early disease the risk of viral infections was lower with PBSC compared to BM, while for advanced / late disease, risks of viral infection were higher with PBSC. However, there was no overall significant effect of graft type on viral infections if this interaction was ignored.
Table 4.
Multivariate Analysis Model of Infection Density
| Variable | Bacterial infections | Viral infections | Fungal infections | |||
|---|---|---|---|---|---|---|
| Relative density (95%CI) | P-value | Relative intensity (95%CI) | P-value | Relative intensity (95%CI) | P-value | |
| Graft type | Interacted with disease risk | <0.001 | ||||
| Bone Marrow | 1.00 | 1.00 | ||||
| Peripheral Blood | 0.88 (0.71–1.10) | 0.262 | 1.38 (0.84–2.28) | 0.207 | ||
| Disease risk | ||||||
| Early | 1.00 | |||||
| BM | 1.00 | |||||
| PBSC | 0.68 (0.49–0.93) | 0.016 | ||||
| Advanced | 1.39 (1.10–1.75) | 0.006 | ||||
| BM | 1.00 | |||||
| PBSC | 1.68 (1.1–2.57) | 0.017 | ||||
| Recipient CMV status | ||||||
| Negative | 1.00 | 1.00 | 1.00 | |||
| Positive | 1.24 (1.00–1.53) | 0.046 | 1.49 (1.15–1.94) | 0.003 | 1.66 (1.03–2.68) | 0.038 |
| HLA Match Status | 0.010 | <0.001 | ||||
| 8/8 | 1.00 | 1.00 | 1.00 | <0.001 | ||
| 7/8 | 1.41 (1.07–1.86) | 0.015 | 1.70 (1.28–2.26) | <0.001 | 1.40 (0.76–2.59) | 0.283 |
| <=6/8 | 1.90 (1.00–3.63) | 0.050 | 0.76 (0.47–1.23) | 0.265 | 7.05 (3.28–15.15) | <0.001 |
| Gender | ||||||
| Male | 1.00 | |||||
| Female | 1.30 (1.05–1.61) | 0.014 | ||||
| Karnofsky Performance Score | ||||||
| >=90 | 1.00 | |||||
| <90 | 2.23 (1.33–3.74) | 0.002 | ||||
| Unknown | 1.50 (0.61–3.66) | 0.377 | ||||
| Conditioning regimen | ||||||
| Cyclophosphamide and Total Body Irradiation (C-TBI) | 1.00 | |||||
| Busulfan and Cyclophosphamide (Bu-Cy) | 1.30 (0.95–1.77) | 0.095 | ||||
| Fludarabine and Melphalan (Flu-Mel) | 1.76 (1.16–2.68) | 0.008 | ||||
| Fludarabine, Busulfan, and ATG (Flu-Bu-ATG) | 1.77 (1.31–2.39) | <0.001 | ||||
Both acute and chronic GVHD were associated with increased rates of bacterial and fungal infections in both the BM and PBSC arms (Table 5). Only acute (but not chronic) GVHD was associated with increased rates of viral infections.
Table 5.
Multivariate Model of Infection Density: Impact of Graft Versus Host Disease
| Variable | Bacterial infection | Viral infection | Fungal infection | |||
|---|---|---|---|---|---|---|
| Relative intensity (95%CI) | P-value | Relative intensity (95%CI) | P-value | Relative intensity (95%CI) | P-value | |
| None | 1.00 | 1.00 | 1.00 | |||
| Acute GVHD only | 2.24 (1.71–2.92) | <0.001 | 1.68 (1.30–2.17) | <0.001 | 3.30 (1.56–7.01) | <0.001 |
| Chronic GVHD only | 2.39 (1.47–3.88) | <0.001 | 1.16 (0.69–1.96) | 0.571 | 7.47 (2.81–19.91) | <0.001 |
| Both | 4.16 (2.60–6.64) | <0.001 | 1.40 (0.85–2.32) | 0.183 | 5.52 (1.90–16.07) | <0.001 |
Note: other covariates in the model included those listed in Table 4.
After adjustment for significant covariates including age, conditioning regimen, HLA matching, and disease risk, bacterial and fungal, but not viral or even specifically CMV, infections were associated with worse survival in both BM and PBSC recipient groups (Table 6).
Table 6.
Multivariate Analysis of Overall Survival with Infection as time-dependent covariate
| Variable | Level | Hazard Ratio (95%CI) | P-value |
|---|---|---|---|
| Graft type | |||
| Bone Marrow | 1.00 | ||
| Peripheral Blood Stem Cells | 1.09 (0.85–1.40) | 0.487 | |
| Age | <0.001 | ||
| 0–20 | 1.00 | ||
| 21–30 | 2.06 (1.12–3.79) | 0.021 | |
| 31–40 | 1.82 (0.97–3.40) | 0.060 | |
| 41–50 | 2.22 (1.22–4.05) | 0.009 | |
| 51–60 | 3.30 (1.85–5.90) | <0.001 | |
| >=60 (combine with 51–60?) | 3.92 (2.04–7.52) | <0.001 | |
| Disease risk | |||
| Low | 1.00 | ||
| High | 1.45 (1.12–1.89) | 0.005 | |
| HLA Match Status | <0.001 | ||
| 8/8 | 1.00 | ||
| 7/8 | 1.72 (1.29–2.30) | <0.001 | |
| <=6/8 | 3.77 (1.68–8.44) | 0.001 | |
| Conditioning regimen | 0.081 | ||
| Cyclophosphamide and Total Body Irradiation (Cy-TBI) | 1.00 | ||
| Busulfan and Cyclophosphamide (Bu-Cy) | 0.67 (0.49–0.92) | 0.012 | |
| Fludarabine and Melphalan (Flu-Mel) | 0.74 (0.45–1.21) | 0.230 | |
| Fludarabine, Busulfan, and ATG (Flu-Bu-ATG) | 0.88 (0.59–1.29) | 0.503 | |
| Any bacterial infection after transplant (time-dependent covariate) | |||
| No | 1.00 | ||
| Yes | 1.49 (1.14–1.96) | 0.004 | |
| Any viral infection after transplant (time-dependent covariate) | |||
| No | 1.00 | ||
| Yes | 1.15 (0.88–1.50) | 0.317 | |
| Any fungal infection after transplant (time-dependent covariate) | |||
| No | 1.00 | ||
| Yes | 2.40 (1.71–3.35) | <0.001 | |
Infections within 30 days prior to Death
Infection was reported as a primary cause of death for 11 of 133 deceased patients on the BM arm and for 9 of 139 on the PBSC arm. Patients where infection was a de novo cause of death included 2 viral infections (2/11 = 18% of BM, and 22% of PBSC, or ~ 20%) and 1 fungal infection (9% of BM, and 11% of PBSC, or ~ 10%) on each arm, with the remainder being bacterial infections generally associated with multiorgan failure (73% of BM, and 67% of PBSC, or ~ 70%). We reviewed infections within 30 days of death to examine the proximate relationship of any infections to death. On the BM arm, 111 infections were reported on 50 patients within 30 days prior to death; on the PBSC arm, 96 infections were reported on 39 patients. Of these 207 infections reported within 30 days of death, bacteria accounted for 138, viruses for 38, and fungi/parasites for 30. Patients with acute or chronic GVHD reported as the cause of death, and an infection noted as being the most severe within 30 days of death, had a high percentage of fungal infections (20/52 or ~ 38%), with fewer viral (~ 14%), bacterial (~44%), or protozoal infections (~ 4%).
Relationship of Infection to Engraftment
The cumulative incidence of infections that occurred among engrafted patients, 1 year following engraftment, was 71.1% (95%CI, 64.4–76.9) for the BM arm vs. 67.2% (95%CI, 60.1–73.2) for the PBSC arm (P = .23) (Figure 5a). The cumulative incidence of pre-engraftment infections (occurring before Day 30) was 47.9% (95%CI, 41.5–53.9) for the BM arm vs. 32.8% (95%CI, 27.1–38.7) for the PBSC arm (P = .002) (Figure 5b).
Figure 5.
Relationship of Infection to Engraftment. (A) Cumulative incidence of infections that occurred among engrafted patients. (B) Cumulative incidence of pre-engraftment infections.
DISCUSSION
We analyzed all infection events during two years of follow-up for two randomly assigned graft source treatment arms for unrelated donor myeloablative hematopoietic cell transplantation patients who participated in BMT CTN 0201 (a minority were not myeloablative). When the trial was designed, we hypothesized that infection may play a major role in augmenting morbidity and mortality in this randomized trial, so infection events were collected prospectively as a prespecified secondary outcome. This multicenter, randomized phase 3 trial demonstrated that infections remain common and occurred for 85% of BM recipients and 80% of PBSC recipients. Strengths of this prospective study are the large sample size, as well as the detailed and audited data capture on all infections during extended follow-up. No previous comparison of these 2 graft sources for use in transplantation has such a rich and detailed infection database with which to examine infectious complications for this important question of PBSC or BM as the graft source for allogeneic hematopoietic cell transplantation.
In this large randomized study of allogeneic hematopoietic cell transplantation, we observed a cumulative incidence of bacterial infections that is higher among patients who received BM rather than PBSC grafts for transplantation. The cumulative incidence of infections was driven by bacterial infections occurring before Day 100. Since the median time to neutrophil engraftment was 5 days longer for BM recipients than for those randomly assigned to receive PBSC, it is not surprising to see many bacterial infections early after transplant. These bacterial infections were more common prior to engraftment and over the two years following transplantation, but speed of engraftment or graft failure alone did not wholly account for this finding. Bacterial bloodstream infections accounted for 30% of all infections, a finding similar to an earlier prospective study of T cell depletion to limit GVHD after unrelated donor hematopoietic cell transplantation [17].
There are no data regarding pre- or peri-transplant prophylaxis agents used, especially relevant for quinolones with the 123 cases of Clostridium difficile infection during this time prior to use of toxin-based PCR assays. As a result, antibacterial prophylaxis is a baseline characteristic that probably modulates the events seen but cannot otherwise be assessed.
During the analysis of transplant-related endpoints for this study, recipients of PBSC grafts had a higher rate of chronic GVHD, so infections that occurred after Day 100 were of particular interest, especially viral and fungal infections. CMV infections were not more frequent using BM or PBSC, even in the setting of GVHD. This study is the first comparative, long-term follow-up trial with infection data and does not support late CMV disease as a common event. Our database was not structured to capture self-limited (i.e., untreated) CMV viremia. Our database did not capture recurrent, refractory, or resistant CMV.
The probability of developing a clinically important CMV infection was less than 30% in each arm, a rate consistent with other studies published in the last 10 years, and lower than studies published earlier [17,24–26]. In our study, there were 61 CMV infection episodes on the BM arm and 57 infections for patients transplanted using PBSC. The infection density for CMV infections was 0.25 for BM and 0.24 for PBSC during the Day 0 to Day 100 interval, declining to 0.06 for BM and to 0.10 for PBSC during the Day 100 to Day 180 interval, and to 0.03 for BM and to 0.05 for PBSC during the Day 181 to Day 365 interval. Survival among patients who developed a CMV infection was 41% for the BM arm and 49% for the PBSC arm. Regardless of study arm, this overall improved rate of any CMV infection, in comparison to studies published over the last 20 years, likely reflects changes in CMV monitoring/preemptive schemas, anti-CMV prophylaxis, the stem cell product itself, and in conditioning regimens over time. The rapid turnaround time in the newest generation of CMV diagnostic testing, and the availability of oral antiviral agents (e.g., valganciclovir), may have contributed to the low and comparable rates of CMV between the study arms.
By 2 years after transplant, the cumulative incidence of Aspergillus infection was 4% in each study arm, much lower than previously reported rates of 10–15% [17,27]. The 4% rate (20 cases) represents some form of culture and/or histopathologic documentation, so these are cases of proven or probable infection but not possible infection. This is the lowest estimate of invasive aspergillosis in any allogeneic hematopoietic stem cell transplant series. The low rates of invasive aspergillosis may be related to ascertainment bias. Centers participating in this Clinical Trials Network perform bronchoscopies with various levels of aggressiveness, and when a bronchoscopy is performed, may have variable testing for Aspergillus galactomannan using lavage fluid. Thus, there is an undercount of probable and proven invasive aspergillosis since many of these pneumonias end up being “possible” invasive fungal infections or merely are treated empirically as fungal infection; in fact, there were 20 infection episodes classified as fungal that were cases of infection that were assessed to be responsive to antifungal therapy.
Diagnostic testing for fungal infections was in a state of evolution during the years that this study was conducted. While the improvement in cumulative incidence is likely related to advances in antifungal agents that can be successfully employed with less toxicity and with prophylactic intent over the last 15 years, there was still a nontrivial rate of invasive fungal infections for the patients on this study. The 2-year cumulative incidence of invasive fungal infections was 16% for BM patients and 18% for PBSC patients. This includes 7 cases of mucormycosis, a fungal organism that is not within the spectrum of activity of either voriconazole or echinocandins, as well as 25 cases of “other fungus”, some of which were clinical cases of pulmonary nodules responsive to antifungal therapy.
This detailed review of prospectively reported infectious disease events speaks to the challenges to infection data completeness and accuracy, even in this prospective and carefully monitored randomized trial. Despite prospective reporting, the initial audit of 15% of the patients (583 infections for 78 patients) identified frequent minor errors among one or more of the different data points associated with each infection (date, organism, body site, clinical severity). While these disagreements were not entirely correct as originally entered, most of these disagreements were minor did not affect the final results.
Accuracy of infection reporting goes down markedly after Day 100, when most patients leave the transplant center. When the accumulated list of all the infections was compared to the primary source documents, 65 previously unreported infections were identified. More than half of these infections occurred after Day 100. Twenty-two infections occurred after engraftment but before Day 100, of which two thirds were bacterial often coagulase negative Staphylococcus and one third were viral often CMV. The follow-up site self-audit used for this study indicates the importance of data accuracy, and argues for this type of approach in future studies evaluating complex, multiple endpoint infectious disease data, even if prospectively collected.
The data coordinating center has submitted all study data from this clinical trial, including these infection data, to the National Heart, Lung, and Blood Institute (NHLBI) data repository, so that future users should be able to access the data from a public end.
There are several limitations to interpreting a complex infection database when collected from hospitals across the study. There are differences in diagnostic testing at the various sites, and incidence rates may be altered based on current testing strategies (particularly for respiratory viruses and fungal infections). Additionally, with the detailed and complete retrospective audit of the protocol prescribed monitoring of infections, it is unlikely that many major infections were unrecognized.
This study noted a tremendous burden of infection among transplanted patients. Fortunately, infection was an uncommon cause of death. The incidence and severity of infection is lower than historical cohorts. The greatest differences were seen in the pre-engraftment time period. We need ways to continue to drive down the burden of infection for all patients. Morbidity and mortality from infection following transplantation was frequent using either graft source, with no observed differences in fungal or viral infection rates. Among those who died, few had infection reported as the primary cause of death although many infections occurred in the final month of life. The higher observed risks of bacterial infections using BM grafts may suggest either augmented prophylaxis or heightened surveillance after these transplants.
Supplementary Material
Highlights.
Infections following unrelated donor transplantation remain frequent using either graft source, with no difference in fungal or viral rates
Bone marrow graft recipients had higher rates of bacterial and pre-engraftment infections
Acknowledgments
Supported by a grant from the National Heart, Lung, and Blood Institute and the National Cancer Institute (U10HL069294), by the Office of Naval Research, and by the National Marrow Donor Program. Disclosure forms were provided by the authors. We thank the transplantation-center teams in the United States and Canada for enrolling patients in this trial; the donor-center teams in the United States, Canada, and Germany for recruiting the donors for the trial; and the National Marrow Donor Program coordinating center for facilitating the transplantations.
We thank the infectious disease auditors at each center: Nathalie Lachapelle and Jean Roy (Hôpital Maisonneuve-Rosemont), Susan Durham (Cohen Children’s Medical Center), Karen Parrott (University of Iowa Hospitals and Clinics), Andrea Ortega (Hackensack University Medical Center), Mindy Shuster (University of Pennsylvania Cancer Center), Jessica Piggee (Vanderbilt University Medical Center), Margaret Shea and Francisco Marty (Dana-Farber Cancer Institute and Brigham and Women’s Hospital), Erik Dubberke (Washington University: Barnes-Jewish), Juliana Ongley (University of California, San Diego), Lisa Malick (University of Maryland Cancer Center), Stanley Martin (Ohio State: Arthur G. James Cancer Hospital), Valerie Dorcas (Queen Elizabeth II Health Sciences Centre), JoDell McCracken (Texas Transplant Institute), Lisa Dutton (Mayo Clinic, Rochester), Lynne Strasfeld (Oregon Health & Science University), Daniel Kaul (University of Michigan Health System), Ginger Butterworth (Baylor University Medical Center), Lisa Williams (University of Alabama at Birmingham), Michael Pulsipher (Primary Children’s Hospital/Utah BMT), David Hurd (Wake Forest University), Tia Thomas (Washington University: St. Louis Children’s Hospital), Melissa Moynihan (Vancouver General Hospital), Amy O’Sullivan, Brittni Prosdocimo, and Mounzer Agha (University of Pittsburgh Cancer Institute), Janice (Wes) Brown (Stanford Hospital and Clinics), Greg McFadden (University of Nebraska Medical Center), Lynn Savoie (Tom Baker Cancer Centre), Patti Cunningham (Oklahoma University Medical Center), Adina Londrc (City of Hope National Medical Center), John Wingard (University of Florida: College of Medicine), Rebecca Gerkin (Emory University Hospital), Tammy DeGelder (McMaster University Medical Centre: Hamilton Health Sciences), Shaun DeJarnette and Abhyankar (University of Kansas Medical Center), Carol Cutrone (Loyola University Medical Center), Sofia Qureshi (MD Anderson Cancer Center), Jueleah Expose’-Spencer (University of California, San Francisco), Isabel Belen (University of Toronto: Princess Margaret), Jessica Greene (Roswell Park Cancer Institute), Mitch Horwitz (Duke University Medical Center), and Steven Pergam (Fred Hutchinson Cancer Research Center).
Footnotes
Authorship Contribution
J.H.Y. and D.J.W. designed the infectious disease analysis for this study protocol and wrote the paper; C.A., J.R.W., M.M.H. and D.J.W. were senior advisors in the design, conduct, and analysis of the study; K.K. and C.M. organized and maintained the database, J.H.Y., J.R.W, E.R.D., S.A.P., F.M.M. L.M.S., J.M.B., A.A.L., M.G.S., D.R.K., and S.I.M. audited many infection summaries; J.W. and J.H.Y. analyzed data; B.R.L., and J.W. provided statistical analysis; all authors reviewed and provided insightful comments to better the manuscript; and J.W. and J.H.Y. drew the figures.
Disclosure of potential conflicts of interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holtick U, Albrecht M, Chemnitz JM, et al. Comparison of bone marrow versus peripheral blood allogeneic hematopoietic stem cell transplantation for hematological malignancies in adults-a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2014 doi: 10.1016/j.critrevonc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Holtick U, Albrecht M, Chemnitz JM, et al. Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults. Cochrane Database Syst Rev. 2014;4:CD010189. doi: 10.1002/14651858.CD010189.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamatovic D, Balint B, Tukic L, et al. Impact of stem cell source on allogeneic stem cell transplantation outcome in hematological malignancies. Vojnosanit Pregl. 2011;68:1026–1032. [PubMed] [Google Scholar]
- 5.Korbling M. Peripheral Blood Stem Cells: A Novel Source for Allogeneic Transplantation. Oncologist. 1997;2:104–113. [PubMed] [Google Scholar]
- 6.Appelbaum FR. Choosing the source of stem cells for allogeneic transplantation: no longer a peripheral issue. Blood. 1999;94:381–383. [PubMed] [Google Scholar]
- 7.Campregher PV, Hamerschlak N, Colturato VA, et al. Survival and graft-versus-host disease in patients receiving peripheral stem cell compared to bone marrow transplantation from HLA-matched related donor: retrospective analysis of 334 consecutive patients. Eur J Haematol. 2015 doi: 10.1111/ejh.12508. [DOI] [PubMed] [Google Scholar]
- 8.Serody JS, Sparks SD, Lin Y, et al. Comparison of granulocyte colony-stimulating factor (G-CSF)--mobilized peripheral blood progenitor cells and G-CSF--stimulated bone marrow as a source of stem cells in HLA-matched sibling transplantation. Biol Blood Marrow Transplant. 2000;6:434–440. doi: 10.1016/s1083-8791(00)70035-8. [DOI] [PubMed] [Google Scholar]
- 9.Champlin RE, Schmitz N, Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2000;95:3702–3709. [PubMed] [Google Scholar]
- 10.Barge RM, Brouwer RE, Beersma MF, et al. Comparison of allogeneic T cell-depleted peripheral blood stem cell and bone marrow transplantation: effect of stem cell source on short- and long-term outcome. Bone Marrow Transplant. 2001;27:1053–1058. doi: 10.1038/sj.bmt.1703024. [DOI] [PubMed] [Google Scholar]
- 11.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherertz RJ, Belani A, Kramer BS, et al. Impact of air filtration on nosocomial Aspergillus infections. Unique risk of bone marrow transplant recipients. American Journal of Medicine. 1987;83:709–718. doi: 10.1016/0002-9343(87)90902-8. [DOI] [PubMed] [Google Scholar]
- 13.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52:e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 14.Pollack M, Heugel J, Xie H, et al. An international comparison of current strategies to prevent herpesvirus and fungal infections in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2011;17:664–673. doi: 10.1016/j.bbmt.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuan IZ, Dennison D, Weisdorf DJ. Pneumocystis carinii pneumonitis following bone marrow transplantation. Bone Marrow Transplant. 1992;10:267–272. [PubMed] [Google Scholar]
- 16.Boeckh M, Boivin G. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin Microbiol Rev. 1998;11:533–554. doi: 10.1128/cmr.11.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Burik J-AH, Carter SL, Freifeld AG, et al. Higher-risk of cytomegalovirus and aspergillus infections in recipients of T cell depleted unrelated bone marrow: Analysis of infectious complications in patients treated with T cell depletion versus immune suppressive therapy to prevent graft-versus-host disease. Biology of Blood and Marrow Transplant. 2007;13:1487–1498. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13:1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 19.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomblyn M, Young JA, Haagenson MD, et al. Decreased infections in recipients of unrelated donor hematopoietic cell transplantation from donors with an activating KIR genotype. Biol Blood Marrow Transplant. 2010;16:1155–1161. doi: 10.1016/j.bbmt.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker JN, Hough RE, van Burik JA, et al. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant. 2005;11:362–370. doi: 10.1016/j.bbmt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Bachanova V, Brunstein CG, Burns LJ, et al. Fewer infections and lower infection-related mortality following non-myeloablative versus myeloablative conditioning for allotransplantation of patients with lymphoma. Bone Marrow Transplant. 2009;43:237–244. doi: 10.1038/bmt.2008.313. [DOI] [PubMed] [Google Scholar]
- 23.Lin D, Wei L, Yang I, Ying Z. Semiparametric Regression for the Mean and Rate Functions of Recurrent Events. Journal of the Royal Statistical Society. 2000;62:711–730. [Google Scholar]
- 24.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–4071. [PubMed] [Google Scholar]
- 25.Qayed M, Khurana M, Hilinski J, et al. Risk for CMV reactivation in children undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2014 doi: 10.1002/pbc.25237. [DOI] [PubMed] [Google Scholar]
- 26.Walker CM, van Burik JA, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant. 2007;13:1106–1115. doi: 10.1016/j.bbmt.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.