Abstract
Astrocytes orchestrate arrangement and functions of neuronal circuits and of the blood–brain barrier. Dysfunctional astrocytes characterize mood disorders, here showcased by deregulation of the astrocyte end-feet protein Aquaporin-4 around blood vessels and, hypothetically, of the astrocyte-specific phagocytic protein MEGF10 to shape synapses. Development of mood disorders is often a result of ‘gene × environment’ interactions, regulated among others by histone modifications and related modulator enzymes, which rapidly promote adaptive responses. Thus, they represent ideal targets of drugs aimed at inducing stable effects with quick onsets. One of the prevalent features of histone modifications and their modulators is their cell-type specificity. Investigating cell type-specific epigenetic modulations upon drug administration might therefore help to implement therapeutic treatments.
Introduction
For many years the common belief has been that brain functions would be exclusively regulated by networks of neurons. This ‘neurocentric view’ has long predominated, thereby hindering the deepening of our knowledge about the importance of other cell types for brain processes. However, the last twenty years have witnessed an increased awareness about the significance of glia cells not only as mechanically supportive, but also as regulatory elements of neuronal networks (for review, see [1]). Thus, the view of the brain has moved from an exclusively ‘neurocentric’ to an additional ‘gliocentric’. Such a change has surely highly improved our knowledge about brain processes that could not be explained by the sole activity of neurons. However, we are still far from a comprehensive understanding of how mental processes may work in healthy brains and which cell type-specific events might be dysfunctional in neurological and mood disorders. Moreover, investigating cell type-specific differences that might be responsible for the pathogenesis of brain disorders may open the avenue for a deeper examination of cellular responses to pharmacological treatments, thereby helping to optimize current treatment strategies or identify alternative targets for drug discovery. In a previous work, we already provided a detailed description of the main subtypes of glia cells, their functions and how we think that they might be involved in the pathogenesis of several neuropsychiatric disorders and in response to pharmacologic treatments [2]. Therefore, here we provide a more focused description on the functional role of astrocytes at the synaptic and vascular compartments. Furthermore, we summarize latest findings about dysfunctional astrocytes in mood disorders such as major depressive disorder (MDD) and bipolar disorder (BD). Moreover, since both disorders have a strong environmental component which can strongly impact epigenetic modifications, we compare astrocytic and neuronal epigenetic landscapes. Finally, we look into cell type-specific responses to pharmacotherapies and conceptualize a differential approach to investigate brain disorders with the perspective of boosting drug discoveries.
(Dys)functional astrocytes as drug targets in mood disorders
Astrocytes and the synaptic compartment: the ‘tripartite synapse’
Among glia cells, astrocytes actively regulate the shaping and functions of the ‘tripartite synapse’ and may therefore play major roles in the pathogenesis of brain disorders [3,4]. Recent work has in fact demonstrated that a reduction in numbers of synapses can be observed in MDD [5], thus suggesting a putative involvement of astrocytes in the aetiology of MDD. However, it is still unclear whether astrocytes become dysfunctional and thus represent a primary cause of synapses’s reduction or whether they might be secondarily affected by a synaptopathology in mood disorders. More work is needed to clarify this issue. Additionally, astrocytes regulate synaptic activity through morphological changes in ezrin-positive perisynaptic astrocyte processes (PAP) [6,7•] and through a tight modulation of metabotropic glutamate receptors [8]. Reductions in expression and functionality of metabotropic glutamate receptors have been recently described in postmortem brains of MDD, thus suggesting a putative link between these dysfunctional receptors in astrocytes and MDD [9]. Although a cell type-specific localization of deregulated metabotropic glutamate receptors has not been characterized yet, one may hypothesize that receptors localized on astrocytic PAPs are responsible for some alterations in glutamate transmission in brains of depressive patients. Furthermore, astrocytes induce synaptic changes via the regulation of the expression/release of neurotrophic factors that influence synaptic stabilization, such as glial cell derived neurotrophic factor (GDNF) [10], a factor that is reduced in the serum of depressive patients and gets restored upon successful antidepressant (AD) treatment [11]. Recently, we showed that an extracellular signal-regulated kinase1/2 (ERK1/2)-dependent GDNF release occurred in C6 glioma cells, used as model for astrocytes, only after AD treatment, and not with the antipsychotic quetiapine, suggesting a specific activation of the ERK→GDNF signalling cascade upon AD administration, in contrast to other psychotropic drugs, in astrocytes [12]. It has also been shown that astrocytes regulate synaptic densities through phagocytic mechanisms correlated with the activity of the multiple EGF-like-domain 10 (MEGF10) protein [13••]. Changes in synaptic contacts might further influence learning and memory processes. Deficits in these functions are among the hallmarks of MDD. In line with this, we have shown that several ADs may induce an astrocyte-dependent turnover of synaptic contacts in primary astrocytes-neurons co-cultures and in the prefrontal cortex of adult rat brains and this event correlates with an increased expression of astrocytic MEGF10 (Di Benedetto et al., personal communication). Interestingly, a report from Zschocke and colleagues revealed how ADs may induce the activation of autophagic mechanisms differently in astrocytes and neurons [14]. Thus, astrocytes might be central to mediate the effects of ADs on the reorganization of neuronal networks affected in MDD through both non-cell autonomous (phagocytotic) and cell-autonomous (autophagocytotic) mechanisms [15].
Astrocytes and blood vessels: the ‘neurovascular unit’
In addition to their relation to synapses, astrocytes regulate molecular transport in/out of the brain through their polarized end-feet which contact blood vessels, essential for regular brain function, since dysfunctional astrocytes at the blood–brain barrier (BBB) characterize neurological disorders [16]. Recent work by Allaman and colleagues has shown how the AD fluoxetine impacts the regulation of astrocytic glucose metabolism, with consequent effects on the availability of glucose for neuronal activity [17]. Since glucose is taken up from the bloodstream, it would be relevant to investigate whether fluoxetine influences the astrocytic end-feet and their functional role around blood vessels for the transport of glucose into the brain. Moreover, for this specific localization around blood vessels, astrocytes might also be of clinical importance for the transport of therapeutic drugs from the bloodstream into the brain parenchyma. In a previous review [2], we already introduced a first example of a transport glycoprotein localized on end-feet of astrocytes, P-glycoprotein (P-gp), which is predictive of a positive clinical response to ADs that are substrate of this protein [18]. Furthermore, it has been shown that another end-feet protein, Aquaporin-4 (Aqp-4), which is additionally expressed in adult stem cells, could regulate responses to fluoxetine on behavioral measures of depressive-like phenotypes in a chronic stress model of depression [19]. More recently, it has been described that the Aqp-4 knockout mouse displays cognitive deficits similar to those implicated in mood disorders [20,21•]. Furthermore, a post-mortem study has revealed a reduced expression of Aqp-4 around blood vessels in the prefrontal cortex of MDD patients [22••]. Thus, translational studies might be wished to clarify the functional role(s) of Aqp-4 at the BBB or in adult stem cells, which would drive the development of pharmacological tools to reverse disease phenotypes via Aqp-4 targeting.
Cell-type specificity, an underrepresented hallmark of epigenetics in MDD
Histone modifications and their impairment in MDD
Environmental challenges, such as stress exposure, can exaggerate or trigger most mood disorders and are considered to have a pivotal role in the pathogenesis of MDD. The impact of stressors is mediated via changes to the epigenome which functions as an interface between the environment and the organism with its unique genetic and epigenetic history and makeup [23]. Epigenetic mechanisms comprise a rich array of ‘tools’ to modify gene expression without changing the genetic code. Among them are methylation of DNA and RNA, changes to the chromosomal conformation and histone modifications. The most prominent and best studied histone modification processes related to depression-like symptoms are histone methylation and acetylation [24]. For instance, enzymes important for the regulation of histone acetylation are found to be dysregulated in post mortem tissue of MDD or bipolar disorder (BD) patients [25,26]. In turn, broad spectrum histone deacetylase inhibitors (HDACi) can improve depression-related symptoms in humans and in corresponding mouse models [27], while stress exposure can alter bulk histone acetylation per se [28]. Likewise, histone methylation such as changes in H3K4me3 have been linked to adaption/ maladaption to chronic stress in the mouse model [29] and were found to be dysregulated in human post mortem brains of subjects with MDD and BD [30••].
Histone modifications and astrocytes
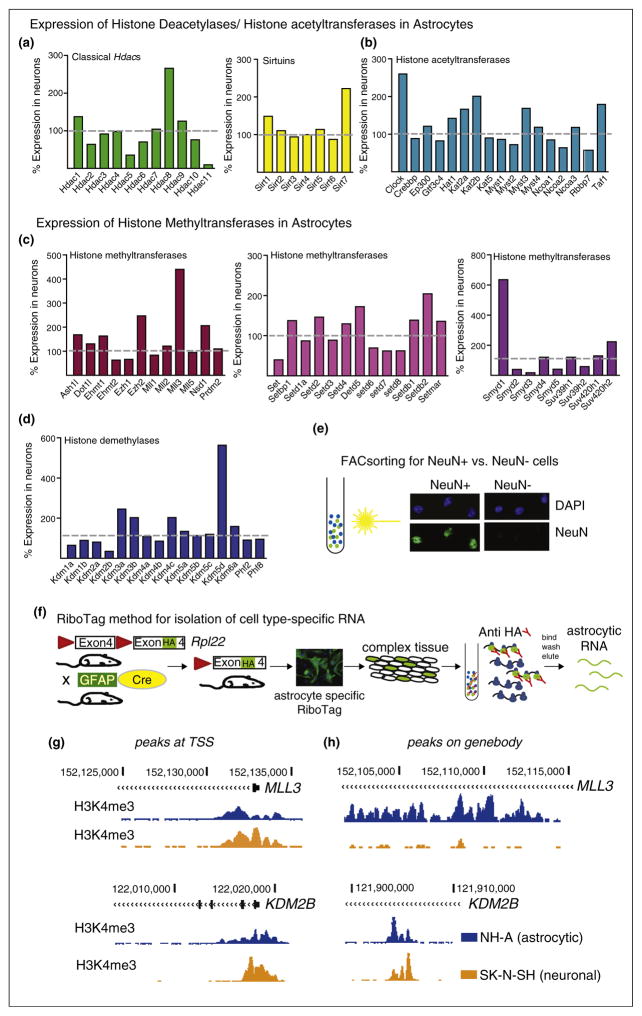
As we discussed in the previous chapter, it has become quite clear that, besides neurons, astrocytes play a major role in stress-related mood disorders [31] and in the response to ADs [32••,33,34]. However, epigenetic signatures differ between neurons and astrocytes [35], and, for example, histone deacetylases have been shown to be readily expressed in both cell types [36]. For this review we utilized a genome wide RNAseq dataset on different neural cell types derived from mouse brain published by Zhang and colleagues [37••]. The dataset includes neurons and astrocytes, allowing us to determine the relative expression levels of all to date identified histone deacetylases, histone acetyltransferases, and histone methyl-transferases and histone demethylases in these two cell types (Figure 1a–d). Interestingly, all RNAs encoding for these enzymes were expressed in astrocytes and most of them at comparable levels as in neurons, indicative of a significant biological role in this cell type. Notably, some RNAs, for example, those encoding for the histone deacetylases Hdac5 or Hdac11 (Figure 1a) and the histone demethylase Kdm2b (Figure 1d) were expressed at lower levels in astrocytes than in neurons. Other RNA transcripts, encoding for the histone deacetylase Hdac8, the Sirtuin Sirt8 (Figure 1a,b), the histone methyltransferease Mll3 (Figure 1c,d) or the histone demethylase Kdm5d were expressed 2–6 fold higher in astrocytes than in neurons. All three expression ratios call for in depth investigation of roles of these enzymes in neurons versus astrocytes in mood disorders and in response to drug treatments. We might overlook opposite changes in expression profiles of histone modifying enzymes in neurons versus astrocytes in the case of equal ratios under healthy/non-treated conditions or miss downregulation of initially higher expression levels and neglect the effects of fine tuning for enzymes that have low expression levels in astrocytes or neurons. Likewise, when we looked into published histone ChIPseq data for H3K4me3 (histone 3 lysine 4 trimethylation) in human astrocytes (NH-A) versus differentiated human neuroblastoma cells we found that at the transcription start site, where this histone mark often resides, signals in astrocytes were lower (Figure 1g). On the gene body we found as well peaks that were present in both cell lines, but with higher or lower levels in the respective other cell type. Interestingly, we found many peaks that were only present in astrocytes, but not in neurons (Figure 1h, upper panel).
Figure 1.
Epigenetics and astrocytes, rationale and methodological approach. (a)–(d) Expression levels of histone-modifying enzymes in astrocytes presented relative to neuronal expression levels. Data is derived from a RNAseq dataset using isolated neuronal and astrocytic RNA from mouse forebrain. Different cell types were isolated either by using EGFP reporter mice in conjunction with flow cytometry or by binding to panning plates [37]. Similarly, purified nuclei from specific cell types can be obtained from isolation of nuclei (transgenically) tagged in specific cell types (INTACT) using antibody coated magnetic beads [39]. These sorting methods can be used for cell type specific RNAseq and ChIPseq approaches. (a) Expression levels of deacetylases (HDACs and Sirtuins) and (b) histone acetyltransferases (HATs) and (c) histone methyl-transferases and (d) histone demethylases. (e,f) Alternative methods well suited for cell type specific ChIPseq [38,39] and RNAseq [40], respectively, are (e) fluorescence activated cellsorting (FACsorting) or (f) the use of RiboTag mice. (e) Photomicrographs show positive selection of neuronal (NeuN+) versus non-neuronal (NeuN−) cells from mouse brain after FACS. Nuclei were counterstained with DAPI. Note absence of NeuN staining in NeuN− fraction. (f) Cartoon-like representation of the RiboTag model/technology for isolation of cell type-specific RNA from mouse brain. This approach is utilizing the Cre/loxP system to tag ribosomes (ribosomal protein RPL22) in specific cell populations with an HA, for example, by using the astrocyte specific GFAP promoter, expression of Cre and therefore activation of the HA tag will occur in astrocytes only. Subsequently, HA tagged ribosomes can be immunoprecipitated and the enclosed RNA purified. (g,h) Cell type specific gene expression profiles and histone landscapes in human neural cells can be studied in post mortem brain using FACS [38,39] or using differentiated iPSCs or neuronal or astrocytic cell lines. UCSC genome browser tracks to visualize sequencing tracks from ChIPseq for the active histone mark H3K4me3 (Histone 3 lysine 4 trimethylation) on NH-A (astrocytic, blue) and SK-N-SH (neuronal, orange) cells. (g) Peaks at the transcription start site (TSS, left panels) and on the (h) gene body (right panels) on the MLL3 (higher expression levels in astrocytes) and the KDM2B (lower expression levels in astrocytes) genes.
Thus, it will be important to understand functions of histone modifications in astrocytes as well. We need to be able to compare neuron-related versus astrocyte-related epigenetic data and to understand how both cell types contribute to stress vulnerability and to the development of MDD. This is necessary, since we need to know whether observed changes (at behavioral, gene expression and epigenetic landscape levels) are affected in the same or opposite manner or not at all, to develop more specific treatment options with less side effects. This is now possible, since advancement of cellular and molecular techniques of isolation and analysis has greatly refined research into subtypes of specific neural population such as astrocytes.
Conclusions
Research to improve our knowledge about cell type-specific differences typical of different mood disorders would be essential to possibly categorize disease subtypes, otherwise hidden in studies that analyze the whole cellular content of brain tissues. Although it is clear that such an aim is not readily achievable using human samples, the availability of several animal models of mood disorders may help to screen cell type-specific distributions of epigenetic modifications and their modulators and identify their molecular targets. Getting a clearer picture on the actual disease mechanism in the brain might be bliss in identifying blood biomarkers in the clinical context and may help to evaluate whether a disease related blood profile might correlate with a cell type-specific molecular signature. Further following such translational approaches, one may suggest to evaluate whether currently available pharmacological compounds used in the clinic do preferentially target such modifications in one or the other cell type. Such studies may indicate which compounds show a higher affinity for ‘diseased’ cells, thereby helping to administer drugs which might directly and faster trigger an amelioration of disease symptoms. Taken into account that cell type ratios are often shifted in subsets of mood disease types only, these studies might actually help to develop tailored treatments for the need of individual patients. Such studies will be necessary not only to understand mechanisms of antidepressant drugs, but also to promote development of cell type-specific drugs.
Acknowledgments
The work of MJ is supported by a Marie Curie Intra European Fellowship within the 7th European Community Framework Programme and by the NARSAD Young investigator grant from the Brain and Behavior Research Foundation (in cooperation with AS). The work of BDB is supported by intramural funding from the University of Regensburg and by the German Federal Ministry of Education and Research (BMBF). The sponsors did not have any role in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the article for publication.
Authors thank Dr. Jan Deussing for fruitful scientific discussions and Dr. Yan Jiang for her extremely valuable collaborative efforts to obtain FAC sorted mouse brain nuclei.
Sequencing experiments from the ENCODE projects were originally generated at the Broad Institute, Cambridge, MA, USA and the Bernstein lab at Massachusetts General Hospital, Boston, MA, USA (NH-A cell data), and the SK-N-SH data by the Stamatoyannopoulos lab at the University of Washington, Seattle, WA, USA).
Footnotes
Conflict of interest statement
Nothing declared.
MJ and BDB contributed to literature screening and to drafting of the manuscript. All authors read and approved the final version of the manuscript.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Haydon PG, Nedergaard M. How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol. 2015;7:a020438. doi: 10.1101/cshperspect.a020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Benedetto B, Rupprecht R. Targeting glia cells: novel perspectives for the treatment of neuropsychiatric diseases. Curr Neuropharmacol. 2013;11:171–185. doi: 10.2174/1570159X11311020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF, Jr, Spray DC, Reichenbach A, Pannicke T, et al. Glial cells in (patho)physiology. J Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavialle M, Aumann G, Anlauf E, Prols F, Arpin M, Derouiche A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2011;108:12915–12919. doi: 10.1073/pnas.1100957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Bernardinelli Y, Randall J, Janett E, Nikonenko I, Konig S, Jones EV, Flores CE, Murai KK, Bochet CG, Holtmaat A, et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol. 2014;24:1679–1688. doi: 10.1016/j.cub.2014.06.025. This study shows that perisynaptic astrocyte processes (PAPs) become highly motile following synaptic activity and their degree of motility correlates with synaptic coverage and stabilization to induce long-term potentiation. [DOI] [PubMed] [Google Scholar]
- 8.Panatier A, Robitaille R. Astrocytic mGluR5 and the tripartite synapse. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.03.063. http://dx.doi.org/10.1016/j.neuroscience.2015.03.063Epub ahead of print. [DOI] [PubMed]
- 9.Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, Burger C, Auberson YP, Sovago J, Stockmeier CA, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry. 2011;168:727–734. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledda F, Paratcha G, Sandoval-Guzman T, Ibanez CF. GDNF and GFRalpha1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat Neurosci. 2007;10:293–300. doi: 10.1038/nn1855. [DOI] [PubMed] [Google Scholar]
- 11.Otsuki K, Uchida S, Watanuki T, Wakabayashi Y, Fujimoto M, Matsubara T, Funato H, Watanabe Y. Altered expression of neurotrophic factors in patients with major depression. J Psychiatr Res. 2008;42:1145–1153. doi: 10.1016/j.jpsychires.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Di Benedetto B, Kuhn R, Nothdurfter C, Rein T, Wurst W, Rupprecht R. N-desalkylquetiapine activates ERK1/2 to induce GDNF release in C6 glioma cells: a putative cellular mechanism for quetiapine as antidepressant. Neuropharmacology. 2012;62:209–216. doi: 10.1016/j.neuropharm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 13••.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. This study demonstrates for the first time that astrocytes, besides microglia cells, possess phagocytic properties which are crucial to refine synaptic networks during early postnatal development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zschocke J, Zimmermann N, Berning B, Ganal V, Holsboer F, Rein T. Antidepressant drugs diversely affect autophagy pathways in astrocytes and neurons — dissociation from cholesterol homeostasis. Neuropsychopharmacology. 2011;36:1754–1768. doi: 10.1038/npp.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Benedetto B, Rupprecht R, Czeh B. Talking to the synapse: how antidepressants can target glial cells to reshape brain circuits. Curr Drug Targets. 2013;14:1329–1335. doi: 10.2174/1389450111314110011. [DOI] [PubMed] [Google Scholar]
- 16.Cabezas R, Avila M, Gonzalez J, El-Bacha RS, Baez E, Garcia-Segura LM, Jurado Coronel JC, Capani F, Cardona-Gomez GP, Barreto GE. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front Cell Neurosci. 2014;8:211. doi: 10.3389/fncel.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allaman I, Fiumelli H, Magistretti PJ, Martin JL. Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacology (Berl) 2011;216:75–84. doi: 10.1007/s00213-011-2190-y. [DOI] [PubMed] [Google Scholar]
- 18.Uhr M, Tontsch A, Namendorf C, Ripke S, Lucae S, Ising M, Dose T, Ebinger M, Rosenhagen M, Kohli M, et al. Polymorphisms in the drug transporter gene ABCB1 predict antidepressant treatment response in depression. Neuron. 2008;57:203–209. doi: 10.1016/j.neuron.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Kong H, Sha LL, Fan Y, Xiao M, Ding JH, Wu J, Hu G. Requirement of AQP4 for antidepressive efficiency of fluoxetine: implication in adult hippocampal neurogenesis. Neuropsychopharmacology. 2009;34:1263–1276. doi: 10.1038/npp.2008.185. [DOI] [PubMed] [Google Scholar]
- 20.Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, Duffy AM, Verkman AS, Hempstead BL, Wood MA, Binder DK, et al. Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci. 2011;31:6392–6397. doi: 10.1523/JNEUROSCI.6249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Kong H, Zeng XN, Fan Y, Yuan ST, Ge S, Xie WP, Wang H, Hu G. Aquaporin-4 knockout exacerbates corticosterone-induced depression by inhibiting astrocyte function and hippocampal neurogenesis. CNS Neurosci Ther. 2014;20:391–402. doi: 10.1111/cns.12222. In this paper the authors indicate Aquaporin-4 as a novel target of AD treatment, showing how the knockout mouse for Aquaporin-4 is more sensitive to a stress-induced inhibition of astrocyte function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Rajkowska G, Hughes J, Stockmeier CA, Javier Miguel-Hidalgo J, Maciag D. Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol Psychiatry. 2013;73:613–621. doi: 10.1016/j.biopsych.2012.09.024. This paper shows the relevance of Aquaporin-4 for depressive disorder using an immunohistochemical staining on postmortem brain tissue from depressive patients. In their brains the lack of blood vessel coverage with Aquaporin-4-positive astrocyte end-feet is evident. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopizzo N, Bocchio Chiavetto L, Cattane N, Plazzotta G, Tarazi FI, Pariante CM, Riva MA, Cattaneo A. Gene-environment interaction in major depression: focus on experience-dependent biological systems. Front Psychiatry. 2015;6:68. doi: 10.3389/fpsyt.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun H, Kennedy PJ, Nestler EJ. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology. 2013;38:124–137. doi: 10.1038/npp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma RP, Grayson DR, Gavin DP. Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: analysis of theNational BrainDatabank microarray collection. Schizophr Res. 2008;98:111–117. doi: 10.1016/j.schres.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hobara T, Uchida S, Otsuki K, Matsubara T, Funato H, Matsuo K, Suetsugi M, Watanabe Y. Altered gene expression of histone deacetylases in mood disorder patients. J Psychiatr Res. 2010;44:263–270. doi: 10.1016/j.jpsychires.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, Suzuki T, Miyata N, Watanabe Y. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 30••.Cruceanu C, Alda M, Nagy C, Freemantle E, Rouleau GA, Turecki G. H3K4 tri-methylation in synapsin genes leads to different expression patterns in bipolar disorder and major depression. Int J Neuropsychopharmacol. 2013;16:289–299. doi: 10.1017/S1461145712000363. The authors demonstrate the specific association of the H3K4me3 with changes in synaptic proteins, thereby suggesting an association between observed synaptic changes in BD and MDD with this epigenetic mark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayhew J, Beart PM, Walker FR. Astrocyte and microglial control of glutamatergic signalling: a primer on understanding the disruptive role of chronic stress. J Neuroendocrinol. 2015;27:498–506. doi: 10.1111/jne.12273. [DOI] [PubMed] [Google Scholar]
- 32••.Lima A, Sardinha VM, Oliveira AF, Reis M, Mota C, Silva MA, Marques F, Cerqueira JJ, Pinto L, Sousa N, et al. Astrocyte pathology in the prefrontal cortex impairs the cognitive function of rats. Mol Psychiatry. 2014;19:834–841. doi: 10.1038/mp.2013.182. This article offers an important proof of concept for the involvement of dysfunctional astrocytes in the pathology of depressive disorder in an animal model of depression and suggests that targeting astrocytes with pharmacological compounds might be sufficient to rescue the disease phenotype. [DOI] [PubMed] [Google Scholar]
- 33.Sanacora G, Banasr M. From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry. 2013;73:1172–1179. doi: 10.1016/j.biopsych.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–773. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 35.Shulha HP, Cheung I, Whittle C, Wang J, Virgil D, Lin CL, Guo Y, Lessard A, Akbarian S, Weng Z. Epigenetic signatures of autism: trimethylated H3K4 landscapes in prefrontal neurons. Arch Gen Psychiatry. 2012;69:314–324. doi: 10.1001/archgenpsychiatry.2011.151. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald JL, Roskams AJ. Histone deacetylases 1 and 2 are expressed at distinct stages of neuroglial development. Dev Dyn. 2008;237:2256–2267. doi: 10.1002/dvdy.21626. [DOI] [PubMed] [Google Scholar]
- 37••.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. This work offers important insights into methodological issues to identify cell type specific changes which might help to generate tools for a cell type restricted analysis of disease-related gene expression changes. We used their sequencing data to generate the relative expression levels in astrocytes versus neurons for histone modifying enzymes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung I, Shulha HP, Jiang Y, Matevossian A, Wang J, Weng Z, Akbarian S. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proc Natl Acad Sci U S A. 2010;107:8824–8829. doi: 10.1073/pnas.1001702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jakovcevski M, Ruan H, Shen EY, Dincer A, Javidfar B, Ma Q, Peter CJ, Cheung I, Mitchell AC, Jiang Y, et al. Neuronal Kmt2a/ Mll1 histone methyltransferase is essential for prefrontal synaptic plasticity and working memory. J Neurosci. 2015;35:5097–5108. doi: 10.1523/JNEUROSCI.3004-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]