Abstract
A complex set of inflammatory molecules and their receptors has been described in epileptogenic foci in different forms of pharmacoresistant epilepsies. By activating receptor-mediated pathways in neurons, these molecules have profound neuromodulatory effects that are distinct from their canonical activation of immune functions. Importantly, the neuromodulatory actions of some inflammatory molecules contribute to hyperexcitability in neural networks that underlie seizures. This review summarizes recent findings related to the role of cytokines (IL-1beta and TNF-alpha) and danger signals (HMGB1) in decreasing seizure threshold, thereby contributing to seizure generation and the associated neuropathology. We will discuss preclinical studies suggesting that pharmacological inhibition of specific inflammatory signals may be useful to treat drug-resistant seizures in human epilepsy, and possibly arrest epileptogenesis in individuals at risk of developing the disease.
Introduction
Cytokines have been shown to modulate neuronal activity either by promoting the release of neuroactive molecules, such as nitric oxide and prostaglandins, classical neurotransmitters and neurotrophins, from glia or brain endothelium [1,2], or by activating their receptors expressed by neurons [3–5]. Proinflammatory cytokines, such as interleukin(IL)-1beta and tumor necrosis factor (TNF)-alpha, activate receptor-mediated autocrine and paracrine cell signaling that results in different pathophysiological outcomes depending on the cell type [4]. Cytokines are endowed with a variety of physiological functions, including the modulation of ion channels and the regulation of the strength of synaptic transmission and plasticity [3,5,6]. However, pathological consequences may ensue if they are over-produced, or if tissue exposure to cytokines is too prolonged, such as in neurodegenerative diseases and in epilepsy [7–10].
In the last decade, preclinical and clinical evidence demonstrated the induction of the prototypical inflammatory cytokines IL-1beta and TNF-alpha, as well as danger signals such as High Mobility Group Box 1 (HMGB1), and their related signaling molecules, in epileptogenic brain tissue surgically resected either from animal models of symptomatic epilepsy or human drug-resistant forms of epilepsy [9,11–13]. Immunohistochemical analysis of these tissues showed increased levels of inflammatory molecules mostly in activated microglia and astrocytes as well as in neurons, as compared to control tissue where these molecules were expressed at low or barely detectable levels. This phenomenon, often defined as neuroinflammation [14], raised the key question of the pathophysiological role that these molecules may play in epilepsy. Notably, pharmacological and genetic studies performed in animal models of epilepsy unveiled a direct neuromodulatory function of proinflammatory cytokines, and related effector molecules such as cyclooxygenase (COX)-2 and prostaglandin E2 [15], and the complement system [16–18], resulting in modifications in neuronal excitability. These peculiar central nervous system (CNS)-related properties of inflammatory molecules are different from those underlying their canonical role as mediators of immunity activation in response to pathogens [4].
This review focuses on the neuromodulatory properties of IL-1beta, TNF-alpha and HMGB1, and highlights that dysregulation of their receptor-mediated intracellular pathways in target cells, leads to acute and long-term modifications in neuronal network excitability. These alterations play a significant role in the mechanisms of seizures, the hallmarks of epilepsy that originate from synchronized firing of neuronal populations due to underlying hyperexcitability phenomena. In light of this evidence, targeting these cytokines may represent a novel opportunity for the development of new therapies for epilepsies associated with a pathogenic inflammatory component [9,11,12].
Cytokines and danger signals and epilepsy
The presence of inflammatory molecules in human brain tissue capable of generating spontaneous seizures is a feature of various forms of symptomatic pharmacoresistant epilepsies [9,11]. Studies in animal models have shown that this inflammatory brain substrate is associated with the activation of innate immune responses in glial cells following epileptogenic insults (e.g. neurotrauma, stroke, CNS infections, status epilepticus, febrile seizures, etc) or during recurrent seizures. The consequent rapid release of cytokines, chemokines and danger signals activates NFkB-dependent downstream inflammatory cascades involving glia, neurons and the blood brain barrier, and may subsequently lead to brain extravasation of leukocytes [19].
Brain inflammation in epilepsy has been recognized since 1958 in Rasmussen’s encephalitis [20], a chronic inflammatory disease of still unknown etiology associated with pharmacoresistant epilepsy. However, its pathophysiological relevance in the mechanisms of seizures and the associated neuropathology has been fully recognized only in the last decade thanks to the evidence that (a) inflammation represents a common substrate of drug-resistant epilepsy of differing etiologies and (b) it can directly affect neuronal excitability [4,21,22] independently of its classical homeostatic role in the immune response to infections for promoting pathogen removal and tissue healing.
The IL-1 receptor (IL-1R1) and Toll-like receptor (TLR) signaling
The induction of this signaling in immune cells is crucial for activating inflammatory pathways in tissue. This signaling is triggered either by receptor recognition of pathogen associated molecular patterns (PAMPS) during infections, or by binding of endogenous molecules released from injured cells, e.g., danger associated molecular patterns or danger signals (DAMPS) during “sterile inflammation”, to alert the microenvironment of imminent or ongoing tissue damage [23]. Recent findings provide a pathophysiological link between the activation of these receptors and rapid changes in neuronal excitability. The IL-1R1 and TLR4, and their respective cognate endogenous ligands IL-1beta and HMGB1, are induced in glia and neurons in human epilepsy and in the related experimental models [4,12,24–26]. IL-1beta and HMGB1 are strictly interconnected, as shown by the involvement of NALP3 inflammasome/caspase-1 in their biosynthesis and release, and the common molecular pathways they activate in neurons and in glia (NFkB-dependent gene transcription) [27,28]. The contribution of this signaling to seizures was shown on the one hand by the dramatic decrease in seizure frequency provoked by pharmacological interventions which prevent or reverse signaling activation in brain, and on the other hand by the exacerbation of seizures induced by brain application of either IL-1beta or HMGB1 [4,24,29]. Accordingly, decreased intrinsic seizure susceptibility was reported in transgenic mice with impaired signaling activation [30–32]. Moreover, cortical application of lipopolysaccharide (LPS), a TLR4 activator, in rats rapidly increases the excitability of local neurons as assessed by measuring amplitudes of sensory evoked field potentials and spontaneous activity [33]. A ten-fold higher LPS concentration could even evoke epileptiform activity which involved IL-1beta release from activated microglia [33].
We recently showed that the redox state of the extracellular milieu is essential for mediating the proconvulsive activity of HMGB1 [34]. In fact, only the disulfide (oxidized) isoform of this molecule activates TLR4 and promotes seizures but not the reduced form, which has instead chemoattractive properties [35,36].
The involvement of this innate immunity signals in seizures indicates that neuronal excitability is affected by both IL-1beta and HMGB1. Looking into the molecular mechanisms underlying this effect, we found that the activation of IL-1R1 or TLR4 in neurons induces, within minutes, the Src kinase–mediated phosphorylation of the NR2B subunit of the N-methyl-D-aspartate (NMDA) receptor complex, thus leading to the increased neuronal Ca2+ influx [32,34,37,38]. This post-translational molecular event underlies the proconvulsive activity of both IL-1beta and HMGB1, as well as their excitotoxic properties. Moreover, a recent paper described that the activation of TLR4 by HMGB1 increased afferent evoked dentate gyrus excitability after concussive brain injury in mice [39], an event that increases the risk of developing epileptic seizures in animal models and in humans. Additional molecular mechanisms that might contribute to hyperexcitability phenomena with relevance for seizures include the downregulation of the hyperpolarization-activated cyclic nucleotide-gated (HCN1) channel, and the associated Ih current, on dendrites of hippocampal pyramidal neurons (unpublished data) and the reduction of GABA-A receptor mediated currents [25,40]. Finally, both IL-1beta and HMGB1 have been reported to increase the extracellular glutamate levels either by inhibiting glutamate re-uptake or promoting its release from glia, or by enhancing NMDA-mediated glutamate release from synaptic terminals, thereby increasing neuronal excitability [reviewed in [10]; 41,42]. The promoting effects of IL-1beta on glutamatergic transmission can also be mediated by PKC phosphorylation of the transient receptor potential vanilloid 1 channel (TRPV1) [43]. TRPV1 also mediates the inhibitory effects of IL-1beta on spontaneous inhibitory post-synaptic potentials [44,45], thus reinforcing the evidence that this cytokine induces defects in GABAergic neurotransmission in forebrain which may be relevant for seizures [25].
TNF-alpha, p55 and p75 receptors
Emerging evidence has demonstrated that, in addition to its effects on cell survival, TNF-alpha has neuromodulatory properties by promoting fast changes in neuronal excitability [46]. In analogy with IL-1beta and HMGB1, TNF-alpha affects seizure susceptibility in animal models as shown by pharmacological interventions that either mimic cytokine’s action or block either TNFR1 (p55) or TNFR2 (p75) receptor signaling [29,38,47]. In general, TNFR1 has been reported to mediate the ictogenic effects of TNF-alpha, whereas TNFR2 mediates the neuroprotective actions of this cytokine. Interestingly, a progressive reduction of TNFR2 with a concomitant increase of TNFR1 in forebrain neurons was reported in animal models of seizures [47], therefore shifting the balance towards the excitotoxic effects of this cytokine.
TNF-alpha can induce neuronal channelopathies since it affects both the assembly and the synaptic clustering of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors as well as the membrane expression of GABA-A receptors. In particular, TNF-alpha by activating intracellular kinases induces the expression of extrasynaptic GLUR2-lacking, thus Ca2+ permeable, AMPA receptors, a mechanism involved in excitotoxicity and synaptic scaling [48–50]. TNF-alpha promotes the induction of neuronal NMDA-NR1 receptors [51] and the endocytosis of GABA-A receptors, therefore decreasing inhibitory strength and reinforcing excitability [49]. Activation of protein kinases, such as PI3K and PKC, mediates TNF-alpha as well as IL-1beta modifications in the function of both receptor- and voltage-gated ion channels in neurons [5]. TNF-alpha can also induce glutamate release from microglia [52] and astrocytes [53]. In microglia, TNF-alpha evokes glutamate release by increasing the glutaminase convertion of glutamine to glutamate which is released via connexin 36 hemi-channels [52]. The astrocytic TNF-alpha evoked release involves COX-2/PGE2 synthesis, thereby resulting in increased intracellular Ca2+ mobilization [53].
Long term modification in neuronal excitability
In addition to the rapid effects on neuronal excitability above described, which are mediated by post-translational modifications in neuronal channels, a transient raise in IL-1beta and TNF-alpha in microglial cell resident in seizure susceptible brain areas, can induce long-lasting and profound synaptic changes in brain. This results in a chronic decrease in seizure threshold, also evoking behavioral comorbidities such as anxiety, depression, and cognitive dysfunction [21,22,54]. Neuronal cell loss is increased in seizing rats if they are pre-exposed to LPS 24 h before the convulsive challenge [55], and seizure threshold is reduced in adult rats that have been exposed to LPS during the first two post-natal weeks [22].
In the frame of long-term consequences on neuronal function, there is increasing evidence that injury-induced brain inflammation contributes to the development and extension of brain tissue that generates spontaneous seizures (i.e. epileptogenesis) in animal models of symptomatic epilepsies [56].
Conclusions
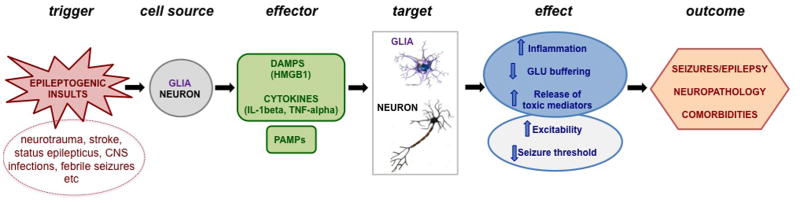
Activation of innate immunity and inflammation have been demonstrated in epilepsy also in the absence of infection or autoimmune conditions. In the context of “sterile inflammation” triggered either by recurrent seizures or epileptogenic brain injuries, neurons and glia release endogenous DAMPs such as HMGB1 and proinflammatory cytokines such as IL-1beta and TNF-alpha that, by activating their cognate receptors, trigger NFkB-dependent inflammatory gene cascades in injured tissue and exert direct neuromodulatory functions. Signaling activation in neurons increases excitability by inducing both rapid and long-term changes in receptor- and voltage-gated ion channels, and enhancing glutamate release (Table 1). Notably, these non conventional pathways activated in brain are independent on the classical immune actions mediated by the inflammatory molecules. This chain of event contributes to the generation and establishment of an hyperexcitable neuronal network which contributes to seizure mechanisms, neuropathology and comorbidities in experimental models (Figure 1).
Table 1.
Summary of cytokine and HMGB1 effects on neuronal excitability and the related molecular mechanisms in seizure and excitotoxicity models
| Cytokine/receptor | Molecular target | Cell signaling | Functional readout |
|---|---|---|---|
| IL-1β/IL-1R1 | NMDAR-NR2B | Protein kinase Src | Increased Ca2+ influx Hyperexcitability/Excitotoxicity Increased seizure susceptibility |
| GABA-A R | Protein kinase C | Decreased GABA current | |
| TRPV1 | Protein kinase C | Increased glutamate release Decreased GABA current |
|
| TNFR1(p55) | AMPAR-GLUR2 | PI3K/Akt | Increased Ca2+ influx Hyperexcitability/Excitotoxicity |
| AMPAR-GLUR1/2 | Protein phosphatase 1 | Reduction of glutamatergic drive | |
| AMPAR-GLUR1 NMDAR-NR1 |
n.d. | Increased seizure susceptibility | |
| GABA-A R β2/β3 subunits |
n.d. | Decreased inhibitory synaptic strenght | |
| CXCR4 | COX-2/PGE2 | Increased astrocytic glutamate release | |
| GLUTAMINASE | Glutamine/glutamate conversion | Increased microglial glutamate release | |
| TNFR2 (p75) | AMPAR-GLUR2/3 | Decreased response to glutamate | |
| KA-GLUR6/7 NMDAR-NR2 |
n.d. | Decreased seizure susceptibility | |
| HMGB1/TLR4 | NMDAR-NR2B | Protein kinase Src | Increased Ca2+ influx Hyperexcitability/Excitotoxicity |
| NMDAR | n.d. | Increased seizure susceptibility Increased Ca2+ influx |
|
| Glial glutamate-aspartate transporter | n.d. | Increased glutamate release from astrocytes |
Figure 1.
Schematic representation of the molecular events linking activation of innate immunity/inflammation to epilepsy. See conclusion paragraph for details.
These preclinical findings, together with the presence of inflammation in human epilepsy brain, indicates that antiinflammatory drugs might be considered to complement the symptomatic treatment provided by the available antiepileptic drugs (AEDs), particularly in epilepsies not responding to AEDs. This novel therapeutic approach by resolving the inflammatory processes in the brain would raise hyperexcitability threshold thereby decreasing the likehood of seizure recurrence, and hopefully may provide a means for disease modifications rather than a mere symptomatic control of seizures [57].
Highlights.
Inflammatory molecules have neuromodulatory effects distinct from their immune functions
Inflammatory molecules contribute to neuronal hyperexcitability underlying seizures
Inhibition of specific inflammatory signals reduces seizures and epileptogenesis
Acknowledgments
Supported by Fondazione Monzino and Epitarget (FP7/2007–2013, grant agreement n°602102) and NIH grant P20NS080185 (AV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- 1.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012;7:42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 3.Viviani B, Gardoni F, Marinovich M. Cytokines and neuronal ion channels in health and disease. Int Rev Neurobiol. 2007;82:247–263. doi: 10.1016/S0074-7742(07)82013-7. [DOI] [PubMed] [Google Scholar]
- 4*.Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25:1281–1289. doi: 10.1016/j.bbi.2011.03.018. This review describes the mechanisms by which the activation of innate immunity affects neuronal excitability and contributes to seizures. [DOI] [PubMed] [Google Scholar]
- 5*.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. doi: 10.1016/j.neuropharm.2014.10.027. This review describes the neuromodulatory properties of cytokines, that are distinct from their classical action as effector molecules of the immune system. [DOI] [PubMed] [Google Scholar]
- 6.Marin I, Kipnis J. Learning and memory..and the immune system. Learn Mem. 2013;20:601–606. doi: 10.1101/lm.028357.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 8.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. This review reports preclinical and clinical evidence of a pathogenic role of brain inflammatory processes in epilepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36:174–184. doi: 10.1016/j.tins.2012.11.008. This review describes the evidence linking astrocytes dysfunctions to epilepsy, and reports the mechanisms and signaling molecules involved in these dysfunctions. [DOI] [PubMed] [Google Scholar]
- 11.Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl 3):26–32. doi: 10.1111/j.1528-1167.2011.03033.x. [DOI] [PubMed] [Google Scholar]
- 12.Aronica E, Ravizza T, Zurolo E, Vezzani A. Astrocyte immune response in epilepsy. Glia. 2012;60:1258–1268. doi: 10.1002/glia.22312. [DOI] [PubMed] [Google Scholar]
- 13.Choi J, Koh S. Role of brain inflammation in epileptogenesis. Yonsei Med J. 2008;49:1–18. doi: 10.3349/ymj.2008.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. FEBS Lett. 2011;585:3798–3805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Rojas A, Jiang J, Ganesh T, Yang MS, Lelutiu N, Gueorguieva P, Dingledine R. Cyclooxygenase-2 in epilepsy. Epilepsia. 2014;55:17–25. doi: 10.1111/epi.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aronica E, Boer K, van Vliet EA, Redeker S, Baayen JC, Spliet WG, van Rijen PC, Troost D, da Silva FH, Wadman WJ, et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol Dis. 2007;26:497–511. doi: 10.1016/j.nbd.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Xiong ZQ, Qian W, Suzuki K, McNamara JO. Formation of complement membrane attack complex in mammalian cerebral cortex evokes seizures and neurodegeneration. J Neurosci. 2003;23:955–960. doi: 10.1523/JNEUROSCI.23-03-00955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson MJ, Thomas NK, Talwar S, Hodson MP, Lynch JW, Woodruff TM, Borges K. A novel anticonvulsant mechanism via inhibition of complement receptor C5ar1 in murine epilepsy models. Neurobiol Dis. 2015;76:87–97. doi: 10.1016/j.nbd.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 19*.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013;69:16–24. doi: 10.1016/j.neuropharm.2012.04.004. This review focuses on the involvement of IL-1R/TLR4 signaling, BBB dysfunction and COX-2 activation in epileptogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, Pardo CA, Vincent A, Mathern GW, Cross JH. Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13:195–205. doi: 10.1016/S1474-4422(13)70260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. This review reports about the long-term changes induced by cytokines in brain function during post-natal development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riazi K, Galic MA, Pittman QJ. Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res. 2010;89:34–42. doi: 10.1016/j.eplepsyres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 23*.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. This review explains what are the danger signals and when and why they are released in tissues. [DOI] [PubMed] [Google Scholar]
- 24*.Maroso M, Balosso S, Ravizza T, Liu J, Bianchi ME, Vezzani A. Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1. J Intern Med. 2011;270:319–326. doi: 10.1111/j.1365-2796.2011.02431.x. This review describes the role of IL-1R/TLR4 signaling in seizure mechanisms. [DOI] [PubMed] [Google Scholar]
- 25.Roseti C, van Vliet EA, Cifelli P, Ruffolo G, Baayen JC, Di Castro MA, Bertollini C, Limatola C, Aronica E, Vezzani A, et al. GABA currents are decreased by IL-1beta in epileptogenic tissue of patients with temporal lobe epilepsy: implications for ictogenesis. Neurobiol Dis. 2015;82:311–320. doi: 10.1016/j.nbd.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Engel T, Jimenez-Pacheco A, Miras-Portugal MT, Diaz-Hernandez M, Henshall DC. P2X7 receptor in epilepsy; role in pathophysiology and potential targeting for seizure control. Int J Physiol Pathophysiol Pharmacol. 2012;4:174–187. [PMC free article] [PubMed] [Google Scholar]
- 27.Keyel PA. How is inflammation initiated? Individual influences of IL-1, IL-18 and HMGB1. Cytokine. 2014;69:136–145. doi: 10.1016/j.cyto.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Frank MG, Weber MD, Fonken LK, Hershman SA, Watkins LR, Maier SF. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: a role for the NLRP3 inflammasome. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Balosso S, Ravizza T, Aronica E, Vezzani A. The dual role of TNF-alpha and its receptors in seizures. Exp Neurol. 2013;247C:267–271. doi: 10.1016/j.expneurol.2013.05.010. This commentary discusses the role of TNF-alpha and its receptor subtypes in neuroprotection vs seizures and excitotoxicity. [DOI] [PubMed] [Google Scholar]
- 30.Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, De Simoni MG, Sperk G, Andell-Jonsson S, Lundkvist J, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravizza T, Lucas SM, Balosso S, Bernardino L, Ku G, Noe F, Malva J, Randle JC, Allan S, Vezzani A. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia. 2006;47:1160–1168. doi: 10.1111/j.1528-1167.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 32.Iori V, Maroso M, Rizzi M, Iyer AM, Vertemara R, Carli M, Agresti A, Antonelli A, Bianchi ME, Aronica E, et al. Receptor for Advanced Glycation Endproducts is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiol Dis. 2013;58:102–114. doi: 10.1016/j.nbd.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Rodgers KM, Hutchinson MR, Northcutt A, Maier SF, Watkins LR, Barth DS. The cortical innate immune response increases local neuronal excitability leading to seizures. Brain. 2009;132:2478–2486. doi: 10.1093/brain/awp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balosso S, Liu J, Bianchi ME, Vezzani A. Disulfide-Containing High Mobility Group Box-1 Promotes N-Methyl-d-Aspartate Receptor Function and Excitotoxicity by Activating Toll-Like Receptor 4-Dependent Signaling in Hippocampal Neurons. Antioxid Redox Signal. 2014;21:1726–1740. doi: 10.1089/ars.2013.5349. [DOI] [PubMed] [Google Scholar]
- 35.Antoine DJ, Harris HE, Andersson U, Tracey KJ, Bianchi ME. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol Med. 2014;20:135–137. doi: 10.2119/molmed.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Karki R, Igwe OJ. Toll-like receptor 4 signaling: A common pathway for interactions between prooxidants and extracellular disulfide high mobility group box 1 (HMGB1) protein-coupled activation. Biochem Pharmacol. 2015;98:132–143. doi: 10.1016/j.bcp.2015.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T, Vezzani A. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain. 2008;131:3256–3265. doi: 10.1093/brain/awn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Korgaonkar AA, Swietek B, Wang J, Elgammal FS, Elkabes S, Santhakumar V. Toll-like receptor 4 enhancement of non-NMDA synaptic currents increases dentate excitability after brain injury. Neurobiol Dis. 2015;74:240–253. doi: 10.1016/j.nbd.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noam Y, Bernard C, Baram TZ. Towards an integrated view of HCN channel role in epilepsy. Curr Opin Neurobiol. 2011;21:873–879. doi: 10.1016/j.conb.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedrazzi M, Averna M, Sparatore B, Patrone M, Salamino F, Marcoli M, Maura G, Cervetto C, Frattaroli D, Pontremoli S, et al. Potentiation of NMDA receptor-dependent cell responses by extracellular high mobility group box 1 protein. PLoS One. 2012;7:e44518. doi: 10.1371/journal.pone.0044518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedrazzi M, Raiteri L, Bonanno G, Patrone M, Ledda S, Passalacqua M, Milanese M, Melloni E, Raiteri M, Pontremoli S, et al. Stimulation of excitatory amino acid release from adult mouse brain glia subcellular particles by high mobility group box 1 protein. J Neurochem. 2006;99:827–838. doi: 10.1111/j.1471-4159.2006.04120.x. [DOI] [PubMed] [Google Scholar]
- 43.Bevan S, Quallo T, Andersson DA. Trpv1. Handb Exp Pharmacol. 2014;222:207–245. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- 44.Musumeci G, Grasselli G, Rossi S, De Chiara V, Musella A, Motta C, Studer V, Bernardi G, Haji N, Sepman H, et al. Transient receptor potential vanilloid 1 channels modulate the synaptic effects of TNF-alpha and of IL-1beta in experimental autoimmune encephalomyelitis. Neurobiol Dis. 2011;43:669–677. doi: 10.1016/j.nbd.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Rossi S, Furlan R, De Chiara V, Motta C, Studer V, Mori F, Musella A, Bergami A, Muzio L, Bernardi G, et al. Interleukin-1beta causes synaptic hyperexcitability in multiple sclerosis. Ann Neurol. 2012;71:76–83. doi: 10.1002/ana.22512. [DOI] [PubMed] [Google Scholar]
- 46.Pickering M, O’Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- 47.Weinberg MS, Blake BL, McCown TJ. Opposing actions of hippocampus TNFalpha receptors on limbic seizure susceptibility. Exp Neurol. 2013;247:429–437. doi: 10.1016/j.expneurol.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 49.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 51.Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, Mattson MP, Geiger JD, Haughey NJ. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009;109:1237–1249. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 53.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 54.Riazi K, Galic MA, Kentner AC, Reid AY, Sharkey KA, Pittman QJ. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J Neurosci. 2015;35:4942–4952. doi: 10.1523/JNEUROSCI.4485-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Auvin S, Shin D, Mazarati A, Nakagawa J, Miyamoto J, Sankar R. Inflammation exacerbates seizure-induced injury in the immature brain. Epilepsia. 2007;48(Suppl 5):27–34. doi: 10.1111/j.1528-1167.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 56*.Vezzani A. Anti-inflammatory drugs in epilepsy: does it impact epileptogenesis? Expert Opin Drug Saf. 2015;14:583–592. doi: 10.1517/14740338.2015.1010508. This review discusses the clinical implications of targeting brain inflammation for therapeutic purposes. [DOI] [PubMed] [Google Scholar]
- 57.Loscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov. 2013;12:757–776. doi: 10.1038/nrd4126. [DOI] [PubMed] [Google Scholar]