Abstract
Thrombotic microangiopathy (TMA) after hematopoietic stem cell transplant (HSCT) associated with terminal complement activation, as measured by elevated plasma terminal complement (sC5b-9) concentrations, has a very high mortality. The complement inhibitor eculizumab may be a therapeutic option for HSCT-associated TMA. We examined the pharmacokinetics and pharmacodynamics (PK/PD) of eculizumab in children and young adult HSCT recipients with TMA and activated complement to determine drug dosing requirements for future efficacy trials. We analyzed prospectively collected laboratory samples and clinical data from 18 HSCT recipients with high-risk TMA presenting with complement activation who were treated with eculizumab. We measured eculizumab serum concentrations, total hemolytic complement activity (CH50), and plasma sC5b-9 concentrations. Population PK/PD analyses correlated eculizumab concentrations with complement blockade and clinical response and determined inter-individual differences in PK parameters. We also compared transplant survival in patients treated with eculizumab (n=18) to patients with the same high-risk TMA features who did not receive any targeted therapy during a separate prospective observational study (n=11). In the PK analysis, we found significant inter-patient variability in eculizumab clearance, ranging from 16 to 237 mL/hr/70kg in the induction phase. The degree of complement activation measured by sC5b-9 concentrations at the start of therapy, in addition to actual body weight, were significant determinants of eculizumab clearance and disease response. Sixty one percent of treated patients had complete resolution of TMA and were able to safely discontinue eculizumab without disease recurrence. Overall survival was significantly higher in treated subjects compared to untreated patients (56% versus 9%, p=0.003). Complement blocking therapy is associated with improved survival in HSCT patients with high-risk TMA who historically have dismal outcomes, but eculizumab pharmacokinetics in HSCT recipients differ significantly from reports in other diseases like atypical hemolytic uremic syndrome and paroxysmal nocturnal hemoglobinurina. Our eculizumab dosing algorithm, including pre-treatment plasma sC5b-9 concentrations, patient’s actual body weight, and the first eculizumab dose (mg), accurately determined eculizumab concentration-time profiles for HSCT recipients with high-risk TMA. This algorithm may guide eculizumab treatment and ensure that future efficacy studies use the most clinically appropriate and cost-efficient dosing schedules.
Keywords: thrombotic microangiopathy, eculizumab, CH50, membrane attack complex, complement, sC5b-9
Introduction
Thrombotic microangiopathy (TMA) is a common complication after hematopoietic stem cell transplantation (HSCT).1–4 The reported incidence of TMA after HSCT varies from 0–74% in retrospective studies.5 Our recent prospective observational study, using rigorous monitoring for microangiopathy, identified TMA in 39% of patients.6 Consistent with the literature, the clinical presentation of TMA ranged from mild (laboratory test changes only) to severe life-threatening disease.7–11 Half of the patients with TMA in our study had severe multi-visceral disease contributing to dismal transplant outcomes in untreated patients.
Traditional risk factors for TMA include endothelial injury from conditioning chemotherapy, radiation, calcineurin inhibitors, or infections.12–15 However, there is increasing evidence that complement is involved in the pathophysiology of HSCT-associated TMA and ensuing renal injury, similar to what occurs in atypical hemolytic uremic syndrome (aHUS). In our recently published prospective study HSCT recipients with proteinuria and terminal complement activation, defined as elevated plasma concentrations of the soluble terminal complement complex (sC5b-9), in addition to hematologic markers of TMA, had very poor survival (<20%) and were classified as having high-risk TMA16. In contrast, patients with hematologic TMA markers but without evidence of complement activation or proteinuria all survived despite not receiving any targeted interventions, and were classified as having low-risk TMA. Following this prospective study, all HSCT recipients at our center with high-risk TMA were offered eculizumab therapy in light of the known poor prognosis. Eculizumab is a humanized monoclonal antibody against the complement component C5 that prevents endothelial damage by blocking formation of the membrane attack complex (Supplemental Figure S1). In our initial report of the first six treated patients we noted a lag in clinical response, despite achieving an eculizumab serum concentration expected to be therapeutic of >99 µg/mL.17 In the current prospective study we performed population based pharmacokinetic and pharmacodynamic (PK/PD) analyses in an extended cohort of children and young adult receiving HSCT who were treated with eculizumab, using plasma sC5b-9 concentrations as a marker of TMA disease activity to establish dosing and monitoring regimens for future prospective efficacy studies.
Methods
Study subjects
All consecutive HSCT recipients who received eculizumab18 for high-risk TMA at our center from January 2012 to June 2014 were included in the PK/PD analyses. All study subjects had high-risk TMA features including plasma sC5b-9 concentrations above normal (>244 ng/mL) and nephrotic range proteinuria (random urine protein/creatinine ratio >2 mg/mg) present at the time of TMA diagnosis, in addition to hematologic TMA markers (schistocytes, elevated lactate dehydrogenase (LDH), reduced haptoglobin, de novo anemia and thrombocytopenia) as previously determined in our prospective observational study.6 Clinical and laboratory data were prospectively captured from the electronic medical record into HSCT databases. The institutional review board at our center approved the study. Informed consent was obtained from all study subjects.
Response assessment
A hematologic response to eculizumab was defined as normalization of LDH, resolution of the need for red blood cell (RBC) and platelet transfusions, and disappearance of schistocytes. A complete clinical response was defined as resolution of organ failure, normalization of the hematologic parameters noted above combined with a doubling of the cystatin C-estimated glomerular filtration rate (eGFR) and improvement of proteinuria to values below the nephrotic range, as defined by a random spot urine protein-to-creatinine ratio <2 mg/mg and normalization of plasma sC5b-9.17 Discontinuation of therapy was considered successful if there was no TMA recurrence eight weeks after the last eculizumab dose with normal sC5b-9 and CH50 values.
Eculizumab blood concentration and complement testing
Soluble terminal complement complex activity (sC5b-9) was measured in plasma by enzyme-linked immunosorbent assay (normal plasma concentration is below 244ng/mL). CH50 was measured in serum using a hemolytic assay (normal 101–300 units). ADAMTS13 activity (normal >67%) was measured at the time of TMA diagnosis to rule out thrombotic thrombocytopenic purpura.19 All assays used for this study are validated and available for clinical use at our institution. Eculizumab serum concentrations were measured at Cambridge Biomedical, Inc. (Boston, MA).20 Recommended therapeutic eculizumab concentrations during eculizumab induction therapy was >99 µg/mL based on recent publications in patients with aHUS21 and by Cambridge Biomedical laboratory recommendations for clinical testing.
Eculizumab treatment protocol
Eculizumab dosing was performed using CH50 monitoring as previously published by our group.1 CH50 was measured prior to starting eculizumab to assure that the patient did not have underlying hypocomplementemia that would preclude the use of CH50 for complement blockade monitoring and was then measured daily during therapy. The first eculizumab dose was based on weight as recommended for children with aHUS.20 In brief, patients weighing <40 kg started with 600 mg intravenously and patients weighing ≥40 kg started with 900 mg intravenously. Subsequent dose adjustments were as follows: if CH50 after the first eculizumab dose remained suppressed (<10% of normal) for at least six days, the subsequent dose was administered on the seventh day and then weekly while maintaining CH50 <10%.22,23 If CH50 increased above 10% of normal sooner than six days, the next dose was given when CH50 elevation above 10% normal was documented. If there was no adequate CH50 suppression after intensifying dosing interval or there was no hematologic response for longer than ten days, the eculizumab dose was increased by 300 mg/dose. After establishing the required dosing schedule to maintain adequate CH50 suppression, induction therapy was continued until patients achieved a hematologic TMA response and had a documented eculizumab serum concentration >99 µg/mL, at which point a maintenance schedule was started.17 Complete blood counts (including schistocytes) and LDH were monitored daily. Haptoglobin, urinalyses, random urine protein/creatinine ratio, and cystatin C-eGFR were monitored weekly. Eculizumab serum concentrations were measured daily during induction therapy, and plasma sC5b-9 was monitored at least three times per week during therapy. In addition, we measured plasma sC5b-9 weekly starting prior to HSCT therapy until clinical TMA diagnosis to evaluate the relationship between the first plasma sC5b-9 elevation and appearance of hematologic signs of TMA. Eculizumab serum concentration results were not available in real-time for drug dose adjustments, but were used later for PK/PD analysis to correlate eculizumab serum concentrations with sC5b-9 and CH50 values and clinical response.
Eculizumab induction dosing was continued until hematologic TMA response was achieved and CH50 remained suppressed below 10% of normal for four weeks, at which point a maintenance schedule was started by giving the same dose every two weeks while maintaining CH50<10%.1 When CH50 remained suppressed for longer than two weeks during the maintenance therapy without drug re-dosing and without active TMA signs eculizumab therapy was stopped. TMA laboratory markers, serum CH50 and plasma sC5b-9 continued to be monitored 2–3 times per week for at least eight weeks. All patients received antibacterial prophylaxis against Neisseria meningitidis until at least eight weeks after discontinuation of eculizumab or until normalization of CH50, since meningococcal vaccination does not provide protection in severely immunocompromised HSCT patients.24
Eculizumab pharmacokinetic and pharmacodynamics analysis
Standard PK analyses were performed using a one compartment model to obtain eculizumab PK parameters such as systemic clearance (CL) and volume of distribution (Vd), as previously described.17 Population PK modeling was performed using NONMEM version 7.2 (ICON Development Solutions, Ellicott City, MD, USA) to characterize population PK parameters, focusing on the induction phase (1st dose), and to identify significant covariates for eculizumab PK parameters (Supplement). A one compartment PK model was used as the structural base model. Total body weight (BW) and plasma sC5b-9 concentration at initiation of the therapy were tested as potential covariates for each PK parameter in the covariate analysis. Selection of covariates was based on a significant reduction of the objective function value (OFV) by stepwise forward inclusion (p<0.05), backward elimination (p<0.01), and by graphical evaluation of goodness-of-fit plots. The eculizumab serum concentration required to suppress CH50 to <10% of normal (complete blockade) was determined based on a receiver operating characteristic (ROC) curve to maximize the Youden’s Index which is defined as specificity+sensitivity-1 (Supplemental Figure S1).25
Post-transplant survival
Since high-risk TMA has very high mortality and all patients presenting with high-risk features during the study period received eculizumab therapy, we were not able to perform a direct comparison of treated and untreated patients with the same disease risk. Instead, to preliminarily assess outcomes among HSCT recipients treated with eculizumab for high-risk TMA (n=18), we performed a survival analysis using untreated subjects as a comparator group who were consecutive (unselected) cases with the same high-risk TMA features (n=11) from a separate prospective observational study aiming to determine TMA risk stratification performed at our institution September 2010–January 2012.6
Simulation of eculizumab serum concentrations-time profiles
Eculizumab concentration-time profiles were simulated in this patient population using various pre-treatment plasma sC5b-9 concentrations (ng/mL) using Berkley Madonna software (http://www.berkeleymadonna.com/) based on our developed PK model and PK parameter estimates.
Statistical analysis
Median (interquartile range) and frequencies (percent) were used to describe continuous and categorical variables, respectively. Differences by group for continuous and categorical variables were determined using Fisher exact and Wilcoxon tests, respectively. Survival curves were estimated using the Kaplan-Meier method. Log-rank tests were used to assess the difference in overall survival by group. Analyses were performed using R version 3.1.3. All statistical tests were two-sided and significance was assessed at p<0.05.
Results
Study subjects
Demographic and disease characteristics are shown in Table 1. Most subjects were children younger than 18 years of age, but the eculizumab treatment group included three young adults and the untreated group had two young adults 19–29 years of age. The degree of terminal complement activation, organ injury, and incidence of acute stage 3–4 graft versus host disease (GVHD) was similar in treated patients (n=18) and untreated patients (n=11). All 29 subjects had a normal plasma sC5b-9 concentration (<244ng/mL) prior to starting transplant chemotherapy. Plasma sC5b-9 became elevated above normal a median of three days after HSCT (range day −9 to day +13, where day 0 is day of stem cell infusion) in the 18 subjects treated with eculizumab, and continued to rise until eculizumab therapy was initiated. Hematologic TMA signs in these patients appeared at median day +30 after HSCT (range day +18 to day +56). ADAMTS13 activity was >10% in all 29 study subjects (median of 53%, range 29–95%) at TMA diagnosis. All patients had a normal INR (International normalized ratio of prothrombin time of blood coagulation) and negative direct antibody test (DAT).
Table 1.
Study demographics and disease characteristics
| Treated with eculizumab n=18 |
Untreated subjects n=11 |
p-value | |
|---|---|---|---|
| Transplant characteristics | |||
| Age (years)# | 4.6 (2 – 15.2) | 8.2 (1.8 – 11.8) | 0.9 |
| Actual weight (kg) | 17 (13.5 – 44.5) | 15.4 (12.6 – 44) | 0.77 |
| Male gender | 11 (61.1%) | 7 (63.6%) | 1 |
| Race | 0.4 | ||
| Caucasian | 13 (72.2%) | 6 (54.5%) | |
| Non-Caucasian | 5 (27.8%) | 5 (45.5%) | |
| Underlying diagnosis | 0.03 | ||
| Bone marrow failure | 0 (0%) | 3 (27.3%) | |
| Immune Deficiency | 9 (50%) | 6 (54.5%) | |
| Malignancy | 8 (44.4%) | 1 (9.1%) | |
| Other | 1 (5.6%) | 1 (9.1%) | |
| Donor type | 0.04 | ||
| Related | 1 (5.6%) | 4 (36.4%) | |
| Unrelated | 13 (72.2%) | 7 (63.6%) | |
| Autologous | 4 (22.2%) | 0 (0%) | |
| Stem cell source | 1 | ||
| Bone Marrow | 13 (72.2%) | 7 (63.6%) | |
| PBSCs | 4 (22.2%) | 3 (27.3%) | |
| Cord Blood | 1 (5.6%) | 1 (9.1%) | |
| HLA Match | 0.11 | ||
| Matched | 4 /14 (22.2%) | 7/11 (63.6%) | |
| Mismatched | 10 /14 (55.6%) | 4/11 (36.4%) | |
| Conditioning Regimen | 0.44 | ||
| Myeloablative | 12 (66.7%) | 5 (45.5%) | |
| Reduced intensity | 6 (33.3%) | 6 (54.5%) | |
| Cyclosporine GVHD prophylaxis | 14/14 (100%) | 11/11(100%) | 1 |
| GVHD (grade 3–4) | 9 (50%) | 6 (54.5%) | 0.70 |
| Disease features at TMA diagnosis | |||
| TMA diagnosis (day after HSCT) | 30 (18 – 55.5) | 26 (17 – 38.5) | 0.79 |
| Blood sC5b-9 (normal <244ng/ml) | 373.5 (301 – 744) | 458.5 (324.2 – 708.4) | 0.24 |
| Cystatin C estimated GFR (mg/ml) | 29.5 (17.8 – 46) | 45 (34.5 – 56) | 0.77 |
| Urine random protein/creatinine ratio | 10.3 (6.5 – 20.8) | 2.6 (2.5 – 3.3) | <0.0001 |
| Renal replacement therapy | 7 (38.9%) | 4 (36.4%) | 1 |
| Hypertension | 18 (100%) | 11(100%) | 1 |
| Pericardial effusion | 15 (83.3%)* | 9 (81.8%)** | 1 |
| PRES | 2 (11%) | 1(9.1%) | 1 |
| CNS bleed | 1(5.6%) | 0(0%) | 1 |
| Pulmonary hypertension | 3 (16.7%) | 2 (18%) | 1 |
| Gastrointestinal bleeding | 11(61%) | 7(63.6%) | 1 |
| Respiratory failure | 2 (11%) | 1(9.1%) | 1 |
Data shown as median (interquartile range) or n(%).
three subjects in treated group and two in untreated group were young adults 19–29 years of age.
* 4/15 (26.7%) and **1/9 (11%) had cardiac tamponade.
GVHD, graft versus host disease; TMA, thrombotic microangiopathy; GFR, glomerular filtration rate; PRES: posterior reversible encephalopathy syndrome; CNS, central nervous system.
Pharmacokinetic and Pharmacodynamic analyses
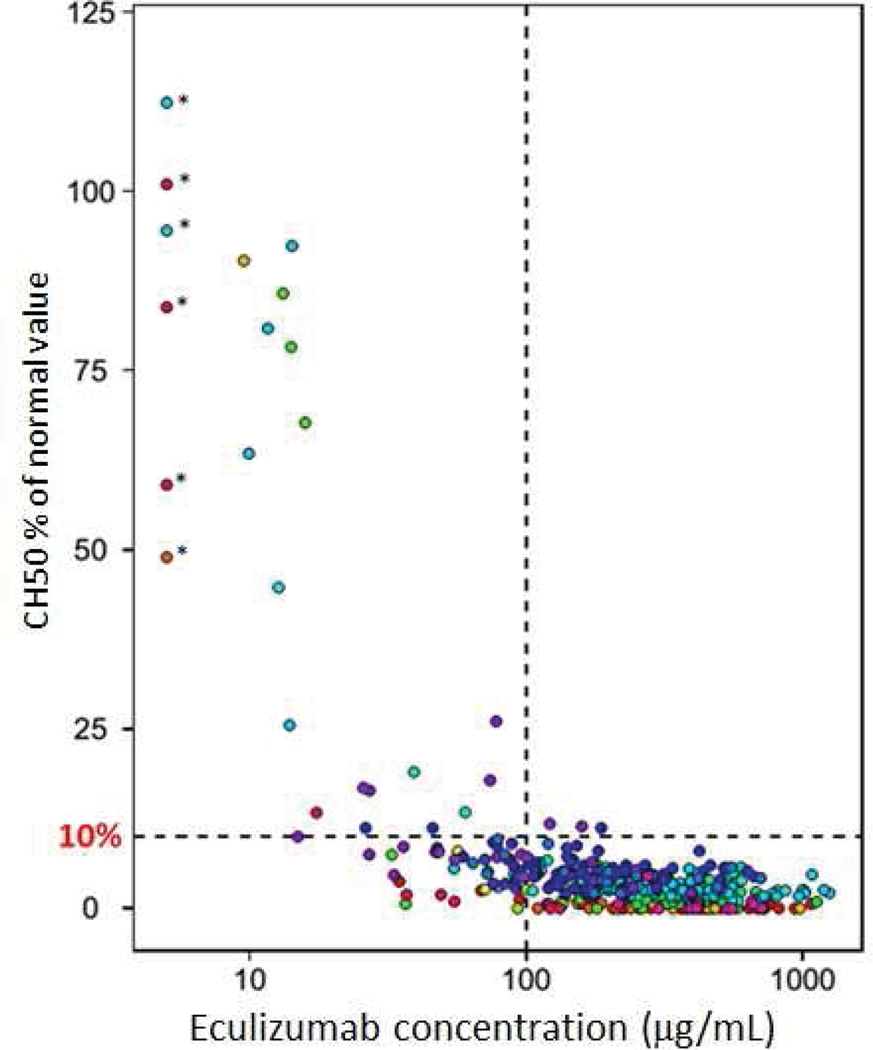
A total of 824, 316 and 401 observations were available for CH50, sC5b9 and eculizumab concentrations, respectively. First, we determined that a serum eculizumab concentration of >99 µg/mL was sufficient to suppress total hemolytic activity (CH50) to <10% of the normal value during induction therapy (Figure 1), but the time that complement activity remained blocked after each drug dose was quite variable between subjects, and in the same subject over time, indicating variable eculizumab clearance. A decline of eculizumab serum concentration to <99 µg/mL as drug was cleared from the circulation was correlated with a rising CH50, as expected, indicating incomplete complement blockade (Figure 1). Based on this observation, we further examined serum eculizumab concentration-time profiles with the goal of identifying co-variates that contributed to the inter-individual variability in eculizumab clearance seen during the course of the therapy in order to propose dosing strategies that would allow sustained complement blockade (CH50<10%).
Figure 1. Relationship between serum eculizumab serum concentration and CH50 suppression.
A total of 365 pairs of serum eculizumab concentrations and CH50 levels from 18 patients are displayed on this plot. Each individual subject is marked in different color. The x-axis shows eculizumab concentrations in log-scale. The y-axis shows the percent of pre-treatment (normal) CH50 serum concentration determined by the assay, where CH50 values in each patient were normalized by their individual pre-treatment CH50 value. The six observations with asterisks were CH50 levels when the eculizumab concentration was below the limit of detection (<10 µg/mL) during therapy. Dashed horizontal line marks 10% of CH50 normal value. Dashed vertical line represents the suggested therapeutic serum eculizumab concentration of >99 µg/mL.
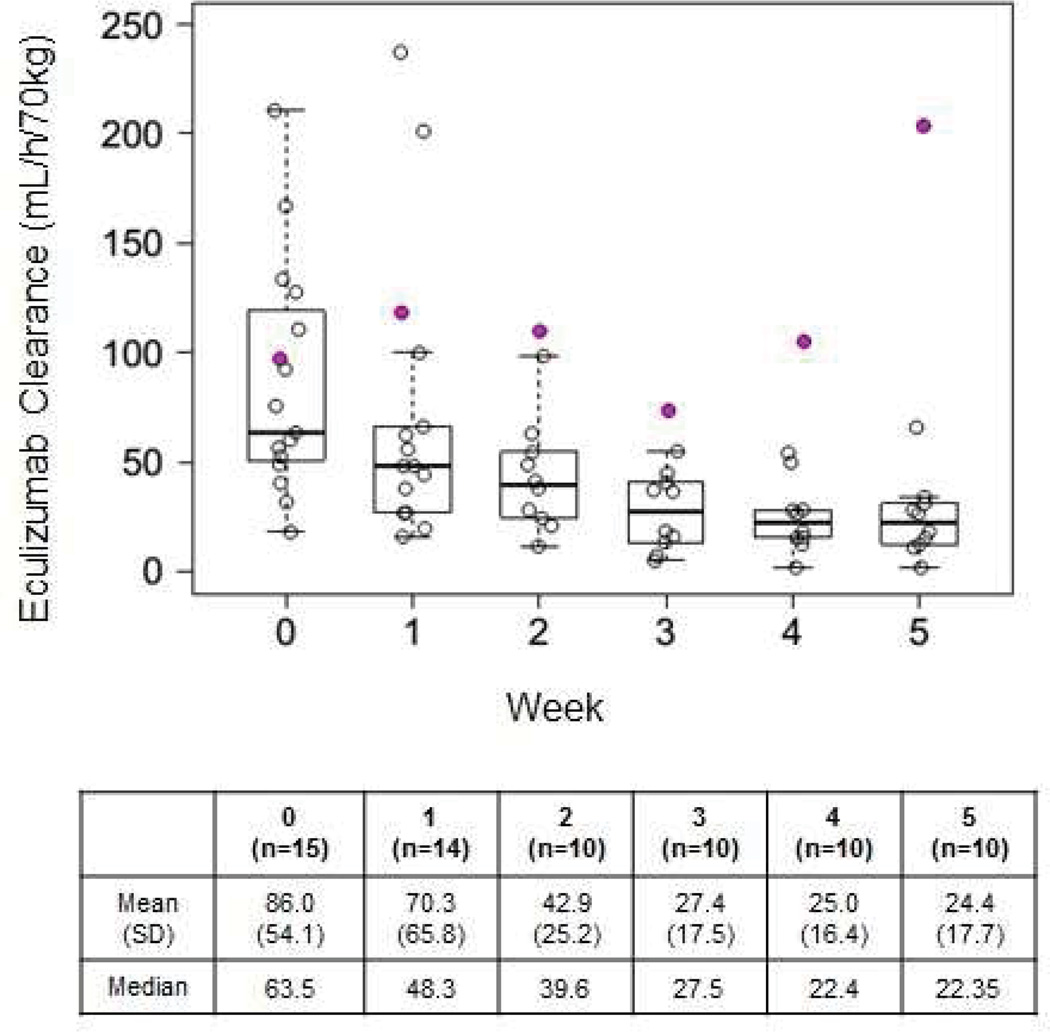
We analyzed changes in systemic eculizumab clearance during the first five weeks of therapy by performing PK analyses (Figure 2).26 Apparent systemic clearances were normalized by allometrically scaled body weight to account for body size differences and to allow comparison across the range of patients’ age.27 We found significant inter-patient variability in eculizumab clearance, ranging from 16 to 237 mL/hr/70kg during the induction phase. Mean drug clearance normalized for weight during the first week of therapy was 3.5-fold higher than during the fifth week (86.0 mL/hr/70kg-patient versus 24.4mL/hr/70kg-patient). In a previous clinical trial for adults with aHUS, population pharmacokinetic analysis found that eculizumab had a volume of distribution of 6.14 L and a clearance of 14.6 mL/h in a patient with aHUS who weighed 70 kg. In contrast,, modeling in a PNH patient weighing 70 kg found the mean clearance of eculizumab was 22 mL/h and the mean volume of distribution was 7.7 L..18,28,29 Interestingly, mean drug clearance during the fifth week of therapy in our HSCT recipients was still higher (24.4 ml/h/70kg) than mean clearance reported in patients with aHUS (13.9 mL/hr/70kg) or PNH (21.7 mL/hr/70kg) receiving maintenance therapy. It is important to note that HSCT recipients with severe and persistent gastrointestinal bleeding requiring >20 ml/kg/day of red cell transfusions and >10 ml/kg/day of apheresis platelets had high drug clearance during all five weeks of therapy, suggesting that specific clinical events such as severe blood loss can further accelerate drug clearance, emphasizing the need for continuous PD monitoring to optimize complement blockade.
Figure 2. Eculizumab clearance during the first five weeks of therapy.
This figure illustrates eculizumab clearance during first five weeks of therapy. Open circles show individual clearance values standardized by body actual body weight using allometric scaling. Individual clearance estimates were plotted weekly using the pharmacokinetic analysis. Box plot shows 25th, 50th and 75th percentiles with range. Eculizumab clearance is highest at the start of the therapy and declines by week five to become nearly equal to the eculizumab clearance reported during maintenance therapy in patients in aHUS or PHN. Shaded red circles represent the clearance trajectory in a patient with severe gastrointestinal bleeding. These clearances were excluded from summary statistics and are displayed here to illustrate high drug clearance in a patient with clinically significant blood loss and high blood product support.
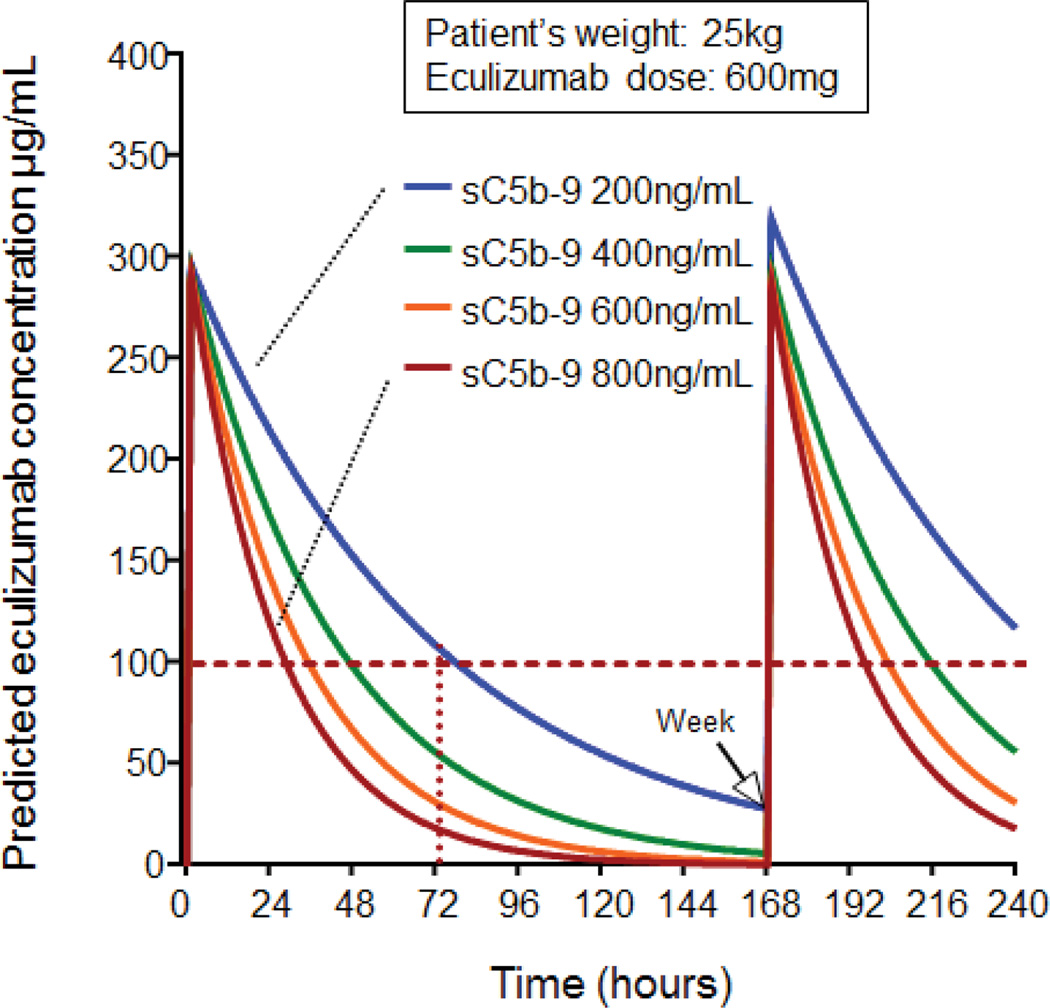
Our analysis identified that pre-treatment plasma sC5b-9 concentration was a significant covariate for initial eculizumab clearance in addition to the patient’s actual body weight. The mean pre-treatment plasma sC5b-9 concentration in our study cohort was 422 ng/mL (normal <244 ng/ml). PK modeling showed that 70 kg subject with sC5b-9 of 422 ng/mL will have a mean eculizumab clearance of 98.6 mL/hr with a mean volume of distribution was 5.72 L (Table 2, Supplemental Tables S1 and S2 and supplemental figures S2 and S3). In Figure 3, we provide an example of changes in eculizumab clearance (PK profiles) for a 25 kg child receiving 600mg eculizumab based on different pre-treatment plasma sC5b-9 concentrations using our pharmacokinetic model. This simulation uses patient’s actual weight (kg), eculizumab dose (mg) and pre-treatment plasma sC5b-9 concentration (ng/mL) and allows us to define the exact time (in hours) when the eculizumab serum concentration would decline below our targeted therapeutic level of 99 µg/mL required to suppress CH50 <10%, indicating the time when a subsequent eculizumab dose is needed. For example, in a 25 kg HSCT recipient with TMA with a pre-treatment plasma sC5b-9 concentration of 422 ng/mL, the eculizumab serum concentration is predicted to decline below 99 µg/mL around 48 hours after a first drug dose of 600 mg, while current recommendations in a 25 kg child with aHUS suggest weekly administration of 600 mg doses.20 Simulations using different body weights and eculizumab doses predicted that eculizumab serum concentration will decline below 99µg/mL in less than 72 hours in any HSCT recipient who has elevated plasma sC5b-9 concentration above 244 ng/mL.
Table 2.
Population pharmacokinetic parameter estimates in the induction phase (after first eculizumab dose)
| Parameter | Population estimates Mean (%RSE) |
Inter-individual variability (CV%) Mean (%RSE) |
|---|---|---|
| CLpop (mL/h/70kg) | 98.6 (9%) | 20.3% (26%) |
| Vdpop(L/70kg) | 5.72 (21%) | 63.1% (21%) |
| Exponent for pre-sC5b-9 | 0.73 (14%) | 0% (fix) |
Final model: CL= CLpop × (WT/70)0.75 × (preC5b9/422)0.73; Vd=Vdpop × (WT/70)1.0 CLpop: mean population clearance; Vdpop: mean population volume of distribution; WT: Actual body weight (kg); pre-sC5b-9: soluble C5b-9 plasma concentration at initiation of treatment; Actual body weight (WT) was included using allometric scaling and was identified as a significant covariate for CL and Vd (reduction of objective function value (OFV) by 17.3 (p<0.01)). Inclusion of pre-sC5b-9 onto CL led to a reduction of OFV by 9.55 (p<0.01) with a power exponent estimate of 0.73. See supplemental tables 1–2 and figures 2–4 for more details.
Figure 3. Pre-treatment plasma sC5b-9 concentration predicts eculizumab clearance.
This figure displays representative case of eculizumab serum concentrations-time profiles simulated with mean population estimates (Table 2) for 25 kg HSCT recipient receiving the eculizumab induction dose of 600 mg every 7 days as currently recommended for the same weight patient with aHUS. The y-axis shows predicted eculizumab serum concentration. The x-axis shows time in hours from the first eculizumab dose administered (hour 0) to the second dose given in one week (hour 168). The horizontal dotted red line marks the suggested therapeutic eculizumab target above >99 µg/mL required for complement blockade. Eculizumab serum concentration-time clearance curves are marked in different colors representing different plasma sC5b-9 concentration (from 200 to 800 ng/mL) at the start of eculizumab therapy (normal plasma sC5b-9 concentration is 74–244 ng/mL). Eculizumab elimination rate increases along with increasing plasma sC5b-9 concentration. This algorithm using patient’s actual weight, initial eculizumab dose (mg) and plasma sC5b-9 concentration value at the start of therapy allows prediction of the time when eculizumab serum concentration declines below required therapeutic level and when next medication dose should be given to sustain complement blockade.
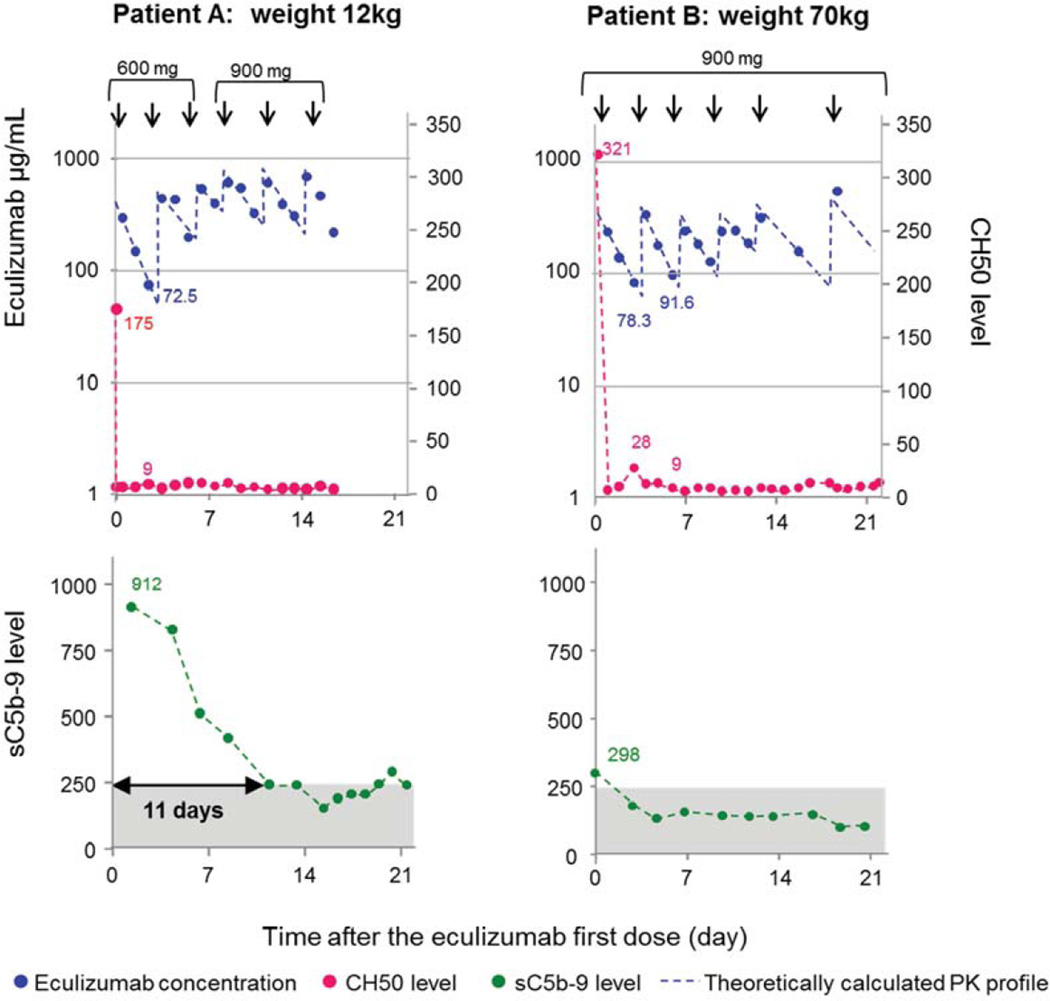
In agreement with the PK/PD modeling, all treated patients in our cohort required eculizumab re-dosing in fewer than 7 days, at least for the first 1–2 weeks of induction therapy, to achieve and sustain CH50 <10% and to maintain an eculizumab serum concentration of ≥99µg/ml. Patients with plasma sC5b-9 ≥488ng/mL (> twice normal value) at TMA diagnosis required 11–13 days of therapy with adequately suppressed CH50 to normalize sC5b-9, while patients with plasma sC5b-9 <488 ng/mL required 2–5 days to normalize sC5b-9 (Figure 4). Normalization of plasma sC5b-9 correlated with the beginning of clinical response reflected in hematologic TMA markers and organ function. Based on these data we updated our previously proposed dosing algorithm to include pre-treatment plasma sC5b-9 concentration to determine the need for subsequent dosing during the induction phase of therapy (Figure 5). A decline in plasma sC5b-9 concentration during eculizumab therapy also correlated with longer intervals of sustained CH50 suppression, indicating slower drug elimination. This was also reflected in advancement of eculizumab dosing intervals to once a week, then once every two weeks and eventually discontinuation of therapy when CH50 remained suppressed <10% for longer than 2 weeks without receiving drug. In responding patients who discontinued therapy, CH50 remained <10% of normal for a median of a 48 days (range 21–81) after the last eculizumab dose, indicating that drug clearance became very slow after TMA was controlled.
Figure 4. Correlation of eculizumab serum concentration with blood CH50 and sC5b-9.
Eculizumab pharmacokinetic and pharmacodynamic markers are illustrated over time in two representative cases during induction therapy. The left and right upper y-axes show eculizumab concentrations and total complement activity (CH50), respectively. The lower y-axis shows plasma sC5b-9 concentration. The x-axis shows time as days from the start of eculizumab therapy. Dosage (mg) and the timing of administration are indicated with arrows on the top of each figure. Blue circles represent observed eculizumab serum concentrations. Actual measured values are noted beside the circles only when eculizumab serum concentrations were below 99 µg/mL. Blue dashed lines represent predicted eculizumab pharmacokinetic profiles based on one compartmental analysis (see Methods for detail). Red circles represent the CH50. The actual values are listed on the graph only when the CH50 is above 10% of normal value. Red circles are connected with red solid lines when CH50 measurement are continuous (daily) or dashed lines when intermittent, respectively. Green circles show sC5b-9. The actual numbers mark plasma sC5b-9 concentration at initiation of the therapy. Grey box marks normal sC5b-9 value of <244 ng/mL. This figure shows that patients (representative case A) with pre-treatment sC5b-9 value at least double of normal (> 488 ng/mL) require 11–13 days of steady eculizumab serum concentration of ≥99 µg/mL with adequately suppressed CH50 (<10% lower limit of normal) to normalize sC5b-9, while patients with pre-treatment sC5b-9 value less than double normal <488 ng/mL (representative case B) take 2–5 days to normalize sC5b-9. Clinical response corresponds with normalization of sC5b-9.
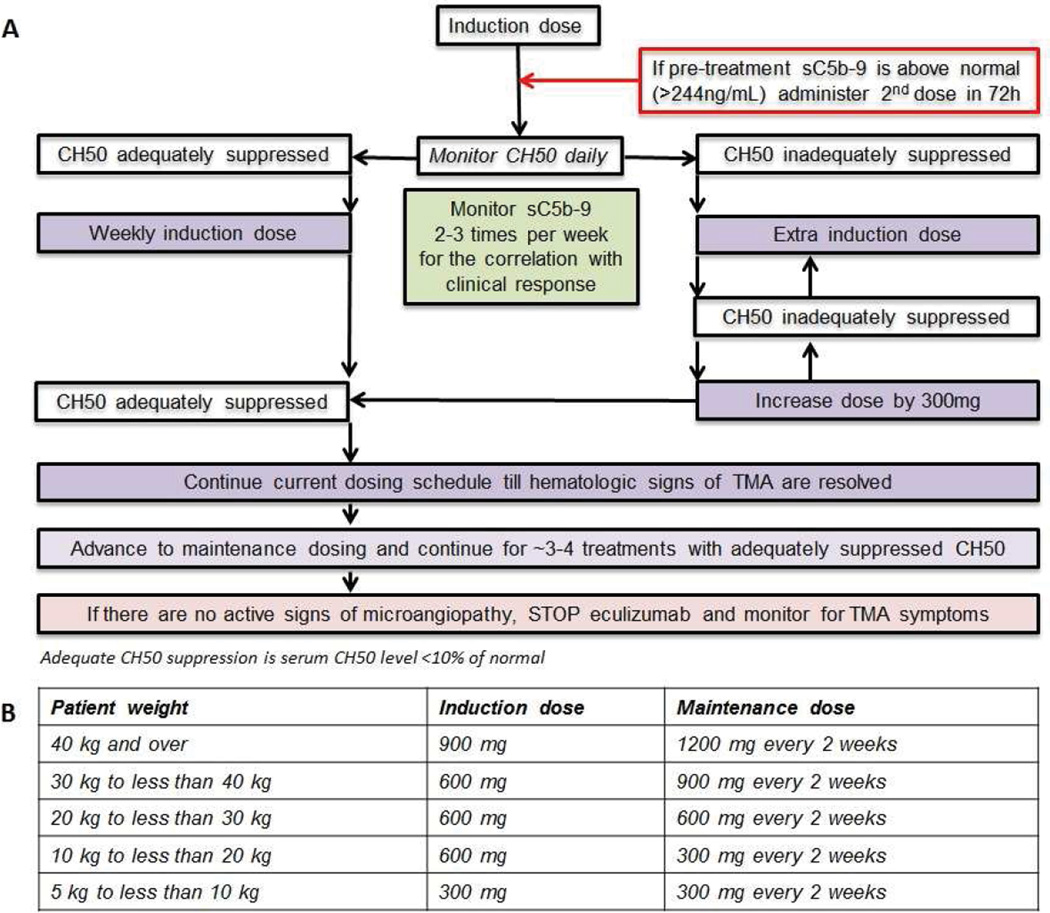
Figure 5. Eculizumab administration and monitoring schema for HSCT patients with TMA.
The first eculizumab dose for HSCT-associated TMA should be given based on patient’s weight, as listed in the table (B) according to the eculizumab package insert. If pre-treatment plasma sC5b-9 concentration is above normal >244 ng/mL, the second dose should be administered at no later than 72 hours. CH50 should be monitored each day during eculizumab induction therapy to determine the subsequent dosing schedule, since patients with TMA often require eculizumab dosing more often than weekly in the beginning of the induction therapy to sustain a therapeutic eculizumab serum concentration. To achieve complement blockade in the blood and to maintain therapeutic eculizumab serum concentration of ≥99 µg/mL, CH50 needs to remain adequately suppressed (less than 10% of the lower limit of normal). Subsequent eculizumab doses need to be given when CH50 becomes inadequately suppressed (rises above 10% of normal laboratory value), but no longer than every 7 day intervals. If CH50 remains inadequately suppressed by dosing at less than 7 day intervals and sC5b-9 remains elevated, dose should be increased by 300 mg/dose to the maximum of 1200 mg/dose and daily CH50 monitoring should continue. If CH50 is adequately suppressed for at least 6 days, then eculizumab induction doses should be given weekly. When steady CH50 suppression is achieved and hematologic TMA parameters and plasma sC5b-9 normalize, eculizumab should be advanced to a maintenance schedule as listed in table (B) based on patient’s weight, as recommended in eculizumab package insert. CH50 should be checked at least prior to each eculizumab dose to assure adequate complement blokade. If TMA remains controlled after 3–4 maintenance doses, eculizumab may be discontinued. Patients should be carefully monitored with twice a week LDH, CBC and differential, weekly urinalysis, and twice a week sC5b-9 for 4 weeks after eculizumab therapy is discontinued. Weekly CH50 should be checked until it returns to normal. Anti-meningococcal prophylaxis should be provided from the start of the therapy until about 8 weeks after stopping eculizumab and CH50 has returned to normal.
Clinical response to eculizumab
Eleven of 18 treated patients (61%) achieved complete resolution of TMA with eculizumab therapy. Responders received a median of 16 doses of eculizumab (range 4–38 doses). An HSCT recipient with persistent gastrointestinal bleeding and high drug clearance received the most doses and had the slowest disease response. Median day to hematologic TMA response was 36 days (range 14–90 days), and complete recovery of organ injury occurred in 90 days (range 24–270 days). Proteinuria was the last clinical feature of TMA to resolve. None of the patients received therapeutic plasma exchange (TPE) or fresh frozen plasma (FFP) during eculizumab therapy. TPE was attempted in the first five subjects without adequate response and was stopped prior to starting eculizumab. In subsequent cases, we proceeded directly to eculizumab if supportive measures (withdrawal of calcineurin inhibitors, treatment of GVHD and infections) were not effective.
Eculizumab therapy was discontinued when the following criteria were met: there were no active hematologic TMA symptoms with improvement in overall clinical condition and renal function, sustained normal plasma sC5b-9 concentration <244ng/ml and CH50 suppression <10% of normal value for longer than two weeks without drug re-dosing. All eleven responders successfully discontinued therapy without TMA recurrence with median follow up of 37 weeks (range 8–128 weeks). One responder died from acute GVHD three months after resolution of TMA. Seven patients (39%) who did not respond to therapy died with active TMA symptoms after receiving a median of four doses of eculizumab (range 2–24 doses). These patients were not able to sustain suppressed CH50 <10% and continued to have elevated plasma sC5b-9 concentrations. Five of these seven patients had grade 2–4 acute GVHD at the time of death.
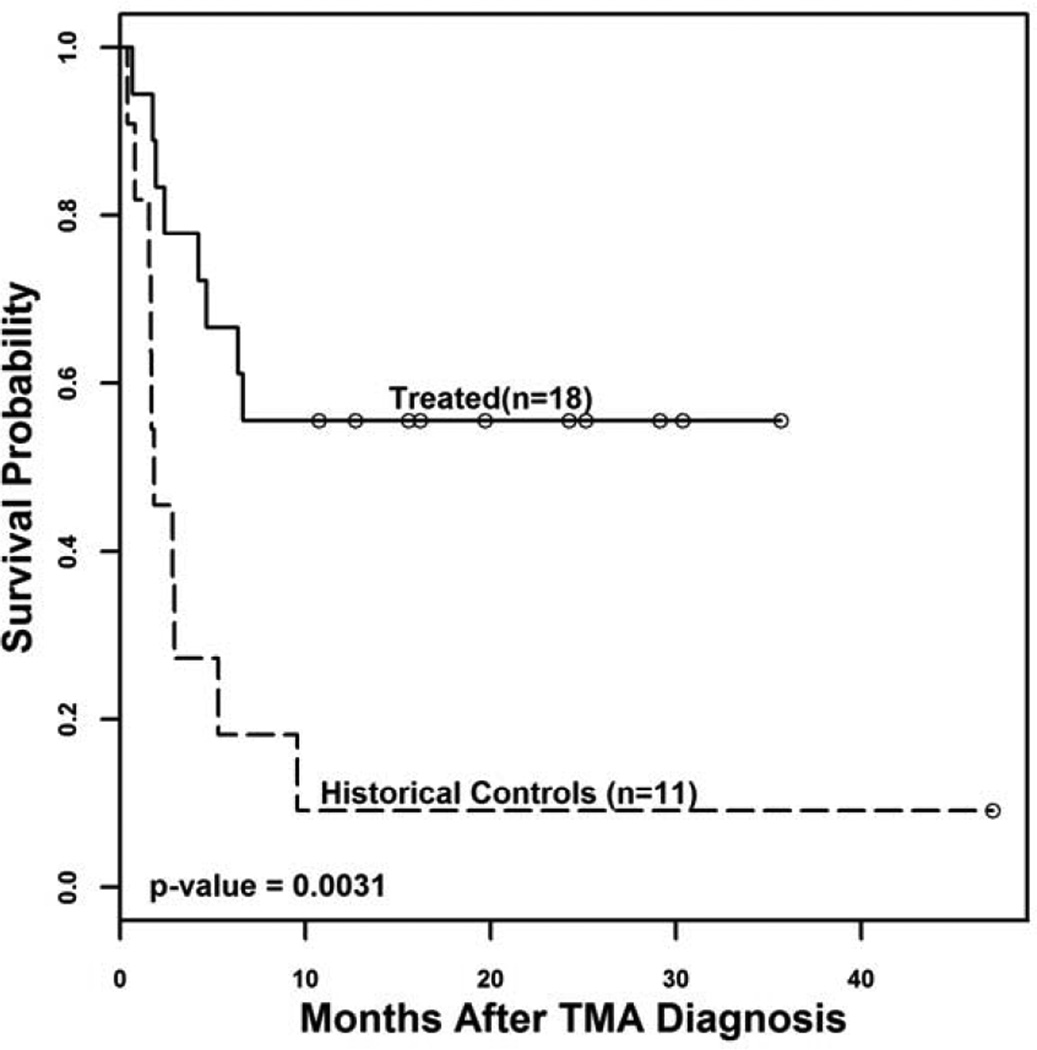
Overall survival one year after TMA diagnosis was 56% in subjects treated with eculizumab while untreated HSCT recipients with the same high-risk TMA features from our separate prospective observational study had 9% survival (p=0.003) (Figure 6). The primary causes of death in patients treated with eculizumab were GVHD (n=3), pulmonary hemorrhage (n=1), TMA/multi-organ failure (n=2), and fungal infection (n=2). The primary causes of death for the untreated patients from our prospective observational study were TMA/multi-organ failure (n=4), GVHD (n=4), and viral infection (n=2). There were no primary disease relapses and all deaths occurred due to transplant-related mortality. We did not observe any toxicities directly attributed to eculizumab. Infection rates were similar in treated and untreated patients.
Figure 6. Survival in HSCT recipients with high-risk TMA.
Survival curves for patients with high risk TMA who were treated with the terminal complement blocker eculizumab (“Treated”, n=18) and historical controls from prospective observational TMA study with the same high risk TMA features who did not receive eculizumab (“Historical Controls”, n=11) were calculated using Kaplan-Meier and the Log Rank test starting at TMA diagnosis to assess statistical significance. Patients with high risk TMA who received eculizumab therapy had better survival than untreated patients who historically are known to have nearly dismal outcome (56% versus 9% at 1 year from TMA diagnosis, p=0.003).
Discussion
Our PK/PD data indicate that there are significant differences in eculizumab clearance based on TMA activity after HSCT, and personalized pharmacodynamically-directed drug dosing is needed for the best clinical response and most economical use of this expensive drug. While eculizumab clearance in HSCT recipients was much higher at TMA diagnosis than reported in patients with aHUS or PNH, drug clearance became progressively slower as TMA responded to therapy, allowing safe tapering and discontinuation of therapy in all responding patients after TMA resolved. Our PK/PD model using the pre-treatment plasma sC5b-9 concentration, actual patient’s weight, and the first eculizumab dose (mg) was accurate in predicting eculizumab serum concentration-time profiles and the required dosing schedule for each individual patient in induction therapy when prompt disease control is essential to avoid multi-organ injury. Such a tool, when validated, can be used to predict when the eculizumab serum concentration will decline below levels required to block complement activity and allow accurate determination of the need for subsequent dosing required to sustain complement blockade and to control TMA.
Our data show that eculizumab suppresses hemolytic complement activity in the blood, measured as CH50, very quickly, but this suppression is short-lived in HSCT recipients with plasma sC5b-9 concentrations above normal. In addition, the higher sC5b-9 is at the start of therapy, the longer it takes for it to normalize and to achieve a clinical response. Higher pre-treatment sC5b-9 indicates greater tissue injury and inflammation, takes longer to resolve, and requires a more intense dosing regimen, in agreement with our finding of more severe TMA phenotype and higher mortality in transplant recipients with higher plasma sC5b-9 values.
All subjects with sC5b-9 above normal (>244 ng/mL) cleared eculizumab to <99 µg/ml in less than 72 hours, indicating the need for more frequent dosing than the weekly induction therapy currently recommended for other microangiopathies. Based on these observations, we suggest that HSCT recipients with high-risk TMA who have elevated pre-treatment plasma sC5b-9 concentrations should receive initial eculizumab dosing at least every 72 hours for the first one to two weeks of therapy or until the plasma sC5b-9 concentration normalizes to <244 ng/mL. Notably, in our cohort, plasma sC5b-9 elevation occurred during conditioning chemotherapy in some cases, while hematologic TMA markers did not become apparent until about 20 days later (range 10–28 days), perhaps indicating early vascular injury from chemotherapy or radiation contributing to TMA etiology. These observations suggest that prospective TMA monitoring and early prompt initiation of therapy may prevent TMA-mediated vascular injury and progression to multi-organ failure. Furthermore, early initiation of therapy when the pre-treatment plasma sC5b-9 concentration is lower, may allow for faster complement blockade and fewer doses of eculizumab to abort TMA.
Our PK/PD data showed that eculizumab clearance became progressively slower after normalization of plasma sC5b-9 concentrations, indicating that disease activity was controlled and drug could be dosed at longer intervals. In fact, after five weeks of therapy, initially high drug clearance in HSCT patients became nearly comparable to the drug clearance reported in the maintenance phase for patients with aHUS or PNH. Interestingly, in HSCT recipients who resolved TMA, CH50 remained suppressed after the last eculizumab dose for longer than three weeks, suggesting that drug dosing in the maintenance phase can perhaps be extended over a longer interval than currently recommended for aHUS or PNH and CH50 monitoring could serve as a simple and widely available test for safe tapering of eculizumab therapy.
Notably, drug clearance remained high in a patient with biopsy-proven gastrointestinal TMA associated with severe intestinal bleeding requiring significant blood product support, indicating that personalized eculizumab dosing is needed in HSCT recipients, especially those with other transplant complications. Additionally, the use of plasma containing products (FFP, or high volumes of platelet products) will increase eculizumab clearance by providing exogenous complement factors, and eculizumab will need to be given more frequently in this setting to maintain complement blockade. ADAMTS13 activity is often decreased in patients with acute hemolysis and plasma therapy (FFP or TPE) should not be administered in subjects receiving eculizumab who do not meet criteria for a diagnosis of TTP (ADAMTS13 <10%).19,30,31 In our study, reduced ADAMTS13 levels recovered to normal after resolution of TMA with eculizumab therapy without need for plasma replacement.
Our ability to successfully discontinue eculizumab therapy in responding patients indicates a unique mechanism of complement dysregulation in TMA after HSCT occurring in response to triggers like chemotherapy, infections, or GVHD in susceptible individuals. It appears that complement blockade is only required as a temporary measure to abort the TMA process, and is not needed as life-long therapy, as currently recommended in aHUS and PNH.
Patients treated with eculizumab for high-risk TMA had much better outcomes than historically reported with this severe HSCT complication. We recognize that our comparison of cases treated with eculizumab with prior consecutive cases diagnosed with high-risk TMA using identical criteria is un-randomized. However, the striking improvement in survival that we see supports future prospective studies of efficacy, and our pharmacodynamically-guided individualized dosing strategy should be applied to future studies to ensure that optimal eculizumab dosing is tested.
We conclude that eculizumab is a potential therapeutic option for HSCT recipients with complement-mediated TMA, but personalized PD guided dosing is strongly recommended, due to variable drug clearance based on disease activity. We found marked inter-individual variability in clearance of eculizumab, and also found that clinical events such as GI bleeding importantly changed clearance, indicating need for real-time dose adjustment to optimize complement blockade. Moreover, clearance of eculizumab slows as TMA resolves and real-time PD monitoring can reduce the amount of therapy needed. We hypothesize that initiation of therapy early in the disease course will likely be cost-effective, as these patients should need less frequent dosing and shorter therapy with careful real-time PD monitoring, and will likely avoid severe organ damage. Patients should be carefully selected for therapy, with early treatment of those with a severe phenotype, as defined in this study, and observation only for those with mild phenotypes. Plasma sC5b-9 concentrations measured prior to therapy can guide eculizumab dosing in induction when prompt disease control is essential to avoid multi-organ injury. Even though HSCT recipients with TMA require frequent dosing initially, eculizumab dosing can be tapered using routine CH50 monitoring and discontinued safely after TMA is controlled resulting in improved long term outcomes.
Our study was performed in children and young adults and is limited by small sample size and the inclusion of patients with a wide range of ages and body sizes. Our data and our PK/PD model needs to be validated in larger cohorts of pediatric and adult HSCT recipients. In addition, despite significant improvement in outcomes with the complement blocking therapy, we still need better understanding of TMA pathogenesis in patients with concomitant transplant complications such as acute GVHD or viral infections, in order to develop comprehensive therapeutic strategies targeting diverse pathways of endothelial injury.4,32–34
Supplementary Material
Highlights.
Eculizumab is a promising therapeutic option in HSCT recipients with high-risk TMA.
Eculizumab clearance in HSCT recipients depends on TMA disease activity.
Plasma sC5b-9 concentration can be used to determine eculizumab dosing regimen.
CH50 monitoring aids decision to discontinue therapy when TMA is controlled.
PK/PD guided eculizumab therapy improves survival in HSCT recipients with TMA.
Acknowledgements
We thank Ms. Thelma Kathman and the staff of the Nephrology Clinical Laboratory at CCHMC for their assistance with CH50 testing and Dr. Ralph Gruppo and Ms. Mary Block and the staff of Hematology Clinical laboratory at CCHMC for their assistance with sC5b-9 testing.
SJ, BL, KJ, FZ, SD have a US Provisional patent application for methods and compositions related to transplant-associated thrombotic microangiopathy. SJ research is supported by CCHMC Center for Pediatric Genomics and Innovation grants. BD has served on the speaker’s bureau for Alexion Pharmaceuticals. BL is supported by an American Society for Blood and Marrow Transplantation New Investigator Award. None of the listed organizations had any role in the study design, data analysis, manuscript preparation, decision to submit for publication, or study funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement
Other authors have no disclosures to report.
References
- 1.Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood reviews. 2014 May;29(3):191–204. doi: 10.1016/j.blre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Fontbrune FS, Galambrun C, Sirvent A, et al. Use of Eculizumab in Patients With Allogeneic Stem Cell Transplant-Associated Thrombotic Microangiopathy: A Study From the SFGM-TC. Transplantation. 2015 Sep;99(9):1953–1959. doi: 10.1097/TP.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 3.Kim SS, Patel M, Yum K, Keyzner A. Hematopoietic stem cell transplant-associated thrombotic microangiopathy: review of pharmacologic treatment options. Transfusion. 2015;55:452–458. doi: 10.1111/trf.12859. [DOI] [PubMed] [Google Scholar]
- 4.Labrador J, Lopez-Corral L, Lopez-Godino O, et al. Risk factors for thrombotic microangiopathy in allogeneic hematopoietic stem cell recipients receiving GVHD prophylaxis with tacrolimus plus MTX or sirolimus. Bone Marrow Transplant. 2014 May;49(5):684–690. doi: 10.1038/bmt.2014.17. [DOI] [PubMed] [Google Scholar]
- 5.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90:918–926. doi: 10.1097/TP.0b013e3181f24e8d. [DOI] [PubMed] [Google Scholar]
- 6.Jodele S, Davies SM, Lane A, et al. Refined diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a prospective study in children and young adults. Blood. 2014 doi: 10.1182/blood-2014-03-564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laskin BL, Maisel J, Goebel J, et al. Renal Arteriolar C4d Deposition: A Novel Characteristic of Hematopoietic Stem Cell Transplantation-Associated Thrombotic Microangiopathy. Transplantation. 2013 Jul 27;96(2):217–223. doi: 10.1097/TP.0b013e31829807aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jodele S, Licht C, Goebel J, et al. Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood. 2013;122:2003–2007. doi: 10.1182/blood-2013-05-501445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricklin D, Cines DB. TMA: beware of complements. Blood. 2013;122:1997–1999. doi: 10.1182/blood-2013-07-512707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190:3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meri S. Complement activation in diseases presenting with thrombotic microangiopathy. European journal of internal medicine. 2013;24:496–502. doi: 10.1016/j.ejim.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Kojouri K, George JN. Thrombotic microangiopathy following allogeneic hematopoietic stem cell transplantation. Curr Opin Oncol. 2007;19:148–154. doi: 10.1097/CCO.0b013e3280148a2f. [DOI] [PubMed] [Google Scholar]
- 13.Nakamae H, Yamane T, Hasegawa T, et al. Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. American journal of hematology. 2006;81:525–531. doi: 10.1002/ajh.20648. [DOI] [PubMed] [Google Scholar]
- 14.Willems E, Baron F, Seidel L, Frere P, Fillet G, Beguin Y. Comparison of thrombotic microangiopathy after allogeneic hematopoietic cell transplantation with high-dose or nonmyeloablative conditioning. Bone Marrow Transplant. 2010;45:689–693. doi: 10.1038/bmt.2009.230. [DOI] [PubMed] [Google Scholar]
- 15.Uderzo C, Bonanomi S, Busca A, et al. Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82:638–644. doi: 10.1097/01.tp.0000230373.82376.46. [DOI] [PubMed] [Google Scholar]
- 16.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–653. doi: 10.1182/blood-2014-03-564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jodele S, Fukuda T, Vinks A, et al. Eculizumab Therapy in Children with Severe Hematopoietic Stem Cell Transplantation-Associated Thrombotic Microangiopathy. Biol Blood Marrow Transplant. 2013 Apr;20(4):518–525. doi: 10.1016/j.bbmt.2013.12.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidtko J, Peine S, El-Housseini Y, Pascual M, Meier P. Treatment of atypical hemolytic uremic syndrome and thrombotic microangiopathies: a focus on eculizumab. Am J Kidney Dis. 2013;61:289–299. doi: 10.1053/j.ajkd.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Shah N, Rutherford C, Matevosyan K, Shen YM, Sarode R. Role of ADAMTS13 in the management of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP) Br J Haematol. 2013;163:514–519. doi: 10.1111/bjh.12569. [DOI] [PubMed] [Google Scholar]
- 20.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 21.Legendre CM, Licht C, Loirat C. Eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;369:1379–1380. doi: 10.1056/NEJMc1308826. [DOI] [PubMed] [Google Scholar]
- 22.Jodele S, Fukuda T, Vinks A, et al. Eculizumab Therapy in Children with Severe Hematopoietic Stem Cell Transplantation-Associated Thrombotic Microangiopathy. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014 Apr;20(4):518–525. doi: 10.1016/j.bbmt.2013.12.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peffault de Latour R, Fremeaux-Bacchi V, Porcher R, et al. Assessing complement blockade in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Blood. 2015;125:775–783. doi: 10.1182/blood-2014-03-560540. [DOI] [PubMed] [Google Scholar]
- 24.Prasad K, Karlupia N. Prevention of bacterial meningitis: an overview of Cochrane systematic reviews. Respiratory medicine. 2007;101:2037–2043. doi: 10.1016/j.rmed.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Zheng S, Gaitonde P, Andrew MA, Gibbs MA, Lesko LJ, Schmidt S. Model-based assessment of dosing strategies in children for monoclonal antibodies exhibiting target-mediated drug disposition. CPT: pharmacometrics & systems pharmacology. 2014;3:e138. doi: 10.1038/psp.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson BJ, Holford NH. Tips and traps analyzing pediatric PK data. Paediatric anaesthesia. 2011;21:222–237. doi: 10.1111/j.1460-9592.2011.03536.x. [DOI] [PubMed] [Google Scholar]
- 28.Keating GM. Eculizumab: a review of its use in atypical haemolytic uraemic syndrome. Drugs. 2013;73:2053–2066. doi: 10.1007/s40265-013-0147-7. [DOI] [PubMed] [Google Scholar]
- 29.McKeage K. Eculizumab: a review of its use in paroxysmal nocturnal haemoglobinuria. Drugs. 2011;71:2327–2345. doi: 10.2165/11208300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.van den Born BJ, van der Hoeven NV, Groot E, et al. Association between thrombotic microangiopathy and reduced ADAMTS13 activity in malignant hypertension. Hypertension. 2008;51:862–866. doi: 10.1161/HYPERTENSIONAHA.107.103127. [DOI] [PubMed] [Google Scholar]
- 31.Feng S, Eyler SJ, Zhang Y, et al. Partial ADAMTS13 deficiency in atypical hemolytic uremic syndrome. Blood. 2013;122:1487–1493. doi: 10.1182/blood-2013-03-492421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shayani S, Palmer J, Stiller T, et al. Thrombotic microangiopathy associated with sirolimus level after allogeneic hematopoietic cell transplantation with tacrolimus/sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2013;19:298–304. doi: 10.1016/j.bbmt.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rachakonda SP, Penack O, Dietrich S, et al. Single-Nucleotide Polymorphisms Within the Thrombomodulin Gene (THBD) Predict Mortality in Patients With Graft-Versus-Host Disease. J Clin Oncol. 2014;32:3421–3427. doi: 10.1200/JCO.2013.54.4056. [DOI] [PubMed] [Google Scholar]
- 34.Dietrich S, Falk CS, Benner A, et al. Endothelial vulnerability and endothelial damage are associated with risk of graft-versus-host disease and response to steroid treatment. Biol Blood Marrow Transplant. 2013;19:22–27. doi: 10.1016/j.bbmt.2012.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.