Abstract
Hair and feathers are unique because 1) their stem cells are contained within a follicle structure, 2) they undergo cyclic regeneration repetitively throughout life, 3) regeneration occurs physiologically in healthy individuals and 4) regeneration is also induced in response to injury. Precise control of this cyclic regeneration process is essential for maintaining the homeostasis of living organisms. While stem cells are regulated by the intra-follicle adjacent micro-environmental niche, this niche is also modulated dynamically by extra-follicular macro-environmental signals, allowing stem cells to adapt to a larger changing environment and physiological needs. Here we review several examples of macro-environments that communicate with the follicles: intradermal adipose tissue, innate immune system, sex hormones, aging, circadian rhythm and seasonal rhythms. Related diseases are also discussed. Unveiling the mechanisms of how stem cell niches are modulated provides clues for regenerative medicine. Given that stem cells are hard to manipulate, focusing translational therapeutic applications at the environments appears to be a more practical approach.
Graphical abstract
Specialized stem cells residing in most tissues and organs possess the capacity for self-renewal as well as for multipotent differentiation to maintain organ function and organismal health. In some tissues, such as the skin and intestines, stem cells remain in a prolonged quiescent state. However in most tissues stem cells may be transiently activated when needed during physiological organ regeneration or in response to injury [1,2]. Therefore, it seems that our ability to overcome degenerative disorders and aging problems is not just a dream but is a reachable goal if we can identify and harvest stem cells in various tissues. However, stem cells are relatively rare and are difficult to distinguish from their neighbors with current molecular markers. Rather than isolating and transplanting stem cells, one could simply augment natural mechanisms to activate resident stem cells within the tissue of interest. To date, it has not been easy to regulate stem cell activity, even though they are controlled in part by their specialized shelter, the so called niche [3–5]. Using a variety of approaches it has become apparent that regulating stem cell activity is more complicated than previously imagined so it will take a concerted effort to resolve this puzzle.
The skin as a model organ
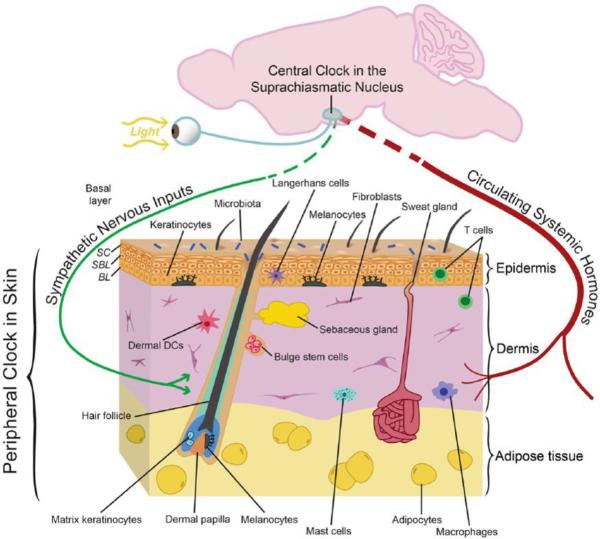
The skin is a multi-layered epidermis overlying the dermis which rests upon adipose tissue. One of the main functions of skin is to form a barrier to prevent loss of fluids. It also serves to prevent infection using an immune system composed of Langherhans cells in the epidermis and macrophages, mast cells and lymphocytes within the dermis. The skin is highly vascularized and innervated. Hair follicles and sweat glands are mini organs which reside within the skin (Fig. 1). Thus the skin is a complex organ which serves many functions that are essential to life.
Figure 1. Skin structure with many different tissue components within and input from the external environment.
The epidermis is composed of three layers. 1) Epidermis, the outermost layer, is mainly composed by keratinocytes. It prevents water loss and functions as a barrier to infection through immune cells, like Langerhans cells, residing within. 2) Dermis, the middle layer of the skin, contains three major cell types, fibroblasts, adipocytes and immune cells, including macrophages, mast cells and lymphocytes. 3) Adipocytes reside within the subcutaneous layer. Melanocytes, the pigment producing cells, are also located within epidermis and color the skin and hairs. In humans, melanocytes appear in both hair follicles and inter-follicular epidermis, however, in mouse, melanocytes can only be found within hair follicles and become activated during hair regeneration. Hair follicles, which regenerate cyclically throughout life and sweat glands, the skin cooling system are two other important mini organs inhabiting the skin. The skin is also richly vascularized and innervated; cells within these structures likely have their own circadian clock that could modify their functions including sensory responses, heat regulation, and oxygenation. There is evidence for an active circadian clock in all cell types of the skin, and it is highly likely that distinct functions are modulated in different cell types. It is also known that circadian clock activity in skin is coordinated by the suprachiasmatic nucleus, presumably through neuronal and hormonal mediators, although this remains to be defined in skin. (Adopted from Plikus et al., 2015)
The hair follicle stem cell model
The hair follicle is a great model in which to study stem cell biology because it is one of the few organs that can regenerate cyclically throughout life. The cyclic process goes through phases of anagen (growth phase), catagen (involution phase) and telogen (resting phase) (Fig. 2). This cycle allows hair stem cells to briefly exit their quiescent status to generate transient amplifying progeny and differentiate into different portions of the hair follicles. Hair stem cells located in the bulge area can be activated by physiological processes or in response to injury.
Figure 2. Regenerative cycling of hair and feather follicles, and the regulation of stem cell quiescence / activation.
(A–B) The structure and regeneration cycle of a hair follicle. (C) The status of hair stem cells, quiescent or active, is decided by the balance between Wnt or BMP signals within the hair follicle. There are multiple molecules that can modulate Wnt and BMP activity, either from intra-follicular niche, or from extra follicular environment. Stem cells basically sum up these activities to decide to remain quiescent or become activated status. (D–E) The structure and regeneration cycle of a feather follicle. (Panel A is adopted from Chen et al., 2012. Panel C is adopted from Kandyba et al., 2013. Panel D, E are adopted from Yue et al., 2012 and Chu et al., 2014)
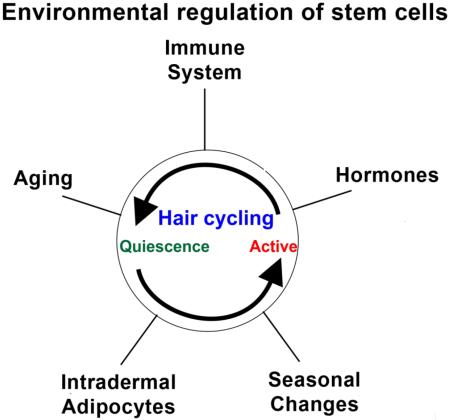
For many years, hair follicle cycling was thought to be controlled exclusively by regulators such as periodic β-catenin activity which emanate from within the follicle [6–8]. They showed that during the refractory telogen period (early telogen or more quiescent status), high levels of BMP6 and FGF-18 are secreted by K6+ inner bulge layer of hair follicles to quiescent the hair stem cells [9]. Upon transition into the competent telogen phase (late telogen or ready to regenerate status), FGF-7, FGF-10, TGF-β2 and noggin (inhibitor of BMP) are secreted from the dermal papilla, a population of specialized mesenchymal cells surrounded by hair matrix cells. These growth factors activate the hair germ [10,11] to release Wnt/β-catenin signals which stimulates anagen re-entry (Fig. 2C) [10]. We refer to these intra-follicular factors as coming from the micro-environment. However, more recently a number of other regulators from outside of the follicle were found to control their cycle. We refer to these as coming from the macro-environment. There is a vast literature discussing intra-follicular micro-environmental factors that influence follicle cycling. This review will discuss how five different macro-environmental sources of factors regulate follicular cycling by influencing molecular signaling pathways within follicles. We mainly discuss hair stem cells, but will refer to other stem cell systems as well.
1. Intradermal adipose tissue
Dermal adipose tissue is a type of white adipose tissue (WAT) that in rodents, including rats and mice, forms a continuous thin layer sandwiched between the dermis proper and the panniculus carnosus skeletal muscle [12–15]. While the primary function of dermal WAT is to provide animals with thermo-insulation, several new roles came to light in recent studies. These include the role of dermal WAT in innate immunity [16] and the ability of WAT cells to modulate regeneration dynamics of pelage hair follicles via paracrine growth factor production [17,18].
Role of intradermal adipose tissue in adult hair cycling
A close spatial-temporal relationship between pelage hair follicles and dermal WAT was noted in classic studies. Lipid-filled dermal adipocytes in rats appear just before birth and in close association with the base of newly formed anagen hair follicles [13,14]. More recently, similar developmental dynamics for dermal WAT were confirmed for mice [19–21]. Furthermore, expression studies for WAT lineage markers, C/EBPα [20] and Fabp4 [21], as well as lineage tracing studies [22] show that in mice (embryonic day 16–17) cells committed to adipose lineage appear in the developing dermis several days prior to lipogenesis. Importantly, a recent study by Donati et al. started to unravel the signaling relationship between hair follicles and dermal adipocytes during skin morphogenesis [23]. They showed that, although hair follicles can augment WAT morphogenesis, in principle, the follicles are dispensable for this process. Dermal WAT was able to form in mutant mice whose hair morphogenesis was blocked. WAT morphogenesis could be augmented by increasing canonical WNT signaling in embryonic skin epithelium (including, but not limited to the epithelial lineage of hair follicles). This appears to depend on the epithelial cells producing various pro-adipogenic growth factors (Bone Morphogenetic Protein (BMP) and Insulin Growth Factor (IGF) family members [23]). In this sense, cutaneous epithelium can be said to form an inductive signaling environment for dermal adipogenesis.
Once formed, dermal WAT periodically cycles in synchrony with the hair cycle, such that it expands in thickness during anagen to nearly twice its size at telogen [18,23–26]. This cyclic relationship between hair follicles and WAT is particularly prominent in rabbits, where the differentiated adipose layer, which is discontinuous and clusters exclusively around compound hair follicles, is only visible during anagen and all but disappears during telogen [27]. This ability of dermal WAT to undergo cycles of expansion and collapse in synchrony with hair regeneration, sets it apart from most other WAT depots in the body, which cycle mainly in response to global metabolic requirements, such as starvation.
What is the signaling connection between dermal WAT and hair follicles? While our knowledge on this topic is fragmented, a few available studies indicate that WAT signaling can modulate several hair cycle events. During early telogen WAT produces BMP ligands, in particular Bmp2 expression cycles out of phase with the intra-follicular Wnt/β-catenin cycle, thus dividing the conventional telogen into two new functional phases: refractory and competent for hair regeneration; characterized by high and low BMP signaling, respectively. The novel finding that periodic large-scale dermal bone morphogenic proteins (BMPs) 2/4 expression can manipulate hair stem cell activity brought about a new concept that stem cells can also be regulated by the extra-niche signaling macro-environment [17,28]. This BMP-driven refractory macro-environment is essential for normal patterning of hair regeneration in adult mice, where distinct domains on hair follicles demarcated by sharp anagen-telogen boundaries form. We also discovered that intra-dermal adipose tissue is also involved in modulating the regenerative wave pattern that occurs in a population of hair follicles [28]. Following this idea, we further discovered additional intra-dermal signals, including DKK-1 and SFRP-4 and demonstrated that stem cell homeostasis can be fine-tuned by activator/inhibitor signals derived from intra/extra follicular sources [27,29]. During anagen onset, proliferative expansion of adipose progenitors in dermal WAT is accompanied by the production of Platelet-derived growth factor subunit A (Pdgfa) which appears to promote anagen entry, likely via PDGF signaling to dermal papillae (Fig. 3) [18]. Importantly, paracrine signaling from WAT may provide a modulating effect on hair cycle progression, rather than being an absolute requirement. Indeed, vibrissa hair follicles are isolated from dermal WAT by a thick collagen capsule and cavernous blood sinus, yet they undergo rapid hair cycles.
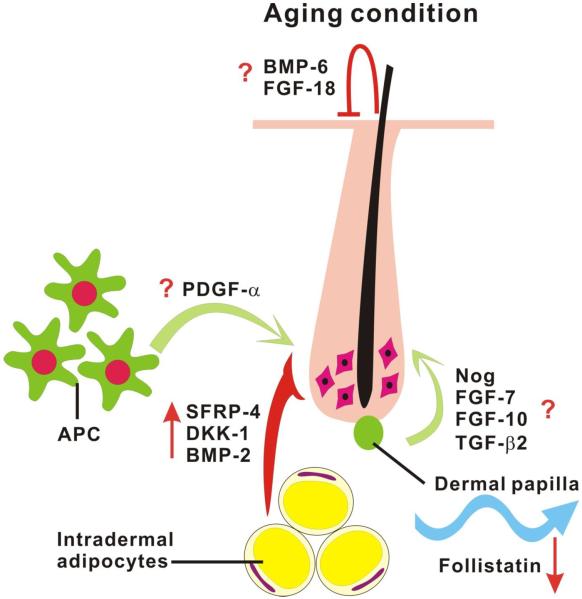
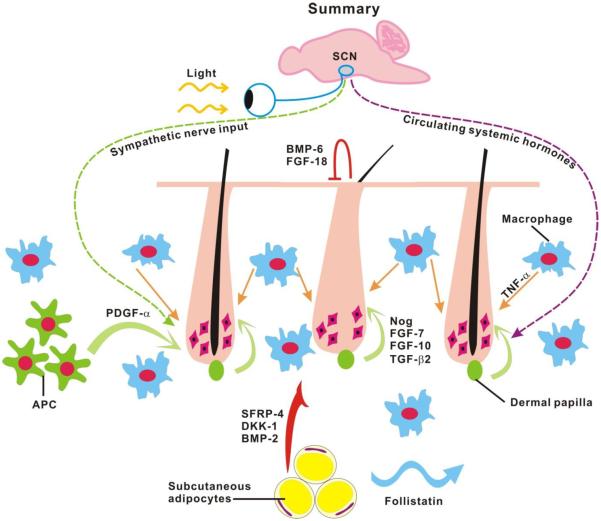
Figure 3. Alteration of skin macro-environment during aging.
During refractory telogen, BMP-6 and FGF-18 released from K6+ inner bulge layer of hair follicles suppress the activity of hair stem cells and keep them in quiescent status. As progression from refractory telogen to competent telogen, dermal papilla secretes FGF-7, FGF-10, TGF-β2 and noggin to activate the hair germ. Outside of the follicle, signals released from intra-dermal tissue or cells can also manipulate the activity of stem cells. BMP-2, DKK-1 and SFRP-4 secreted by intra-dermal adipocytes can serve as inhibitors to decrease the activation threshold of hair stem cells. In contrast, platelet-derived growth factor-α (PDGF-α) released by adipocyte precursor cells (APC) is involved in hair germ activation. Once regeneration starts, this process can then propagate to the surrounding competent telogen follicles like hair waves through follistatin emitted by intra-dermal adipocytes. In aging mice, the regeneration cycle can be delayed by over-expression of the intra-dermal inhibitors, such as BMP-2, DKK-1 and SFRP-4. In addition, the propagation signal, follistatin is also down-regulated with aging which inhibits hair wave propagation and results in a smaller regeneration area. Based on Chen et al., 2014.
Further evidence in support of signaling connecting adipose tissue and hair follicles emerges from recent studies on leptin, a WAT-derived hormone. Ablation of leptin signaling in db/db mice, mutant for leptin receptor, prominently delays anagen entry, while intradermal injection of leptin induces new anagen [30]. Moreover, at supra-physiological concentrations, leptin can inhibit anagen phase in vitro in cultured vibrissa follicles [31]. Future studies are needed to establish the contribution to hair cycle-modulating leptin from local paracrine sources (dermal WAT) vs. systemic sources that have no physical connection to hair follicles (visceral and deep subcutaneous WAT depots).
Among other specialized functions of dermal WAT, a recent study showed it to be an important component of the skin's innate immune system [16]. Dermal WAT progenitors can directly sense bacterial infection and launch a quick response, consisting of tissue expansion (via proliferative burst) and secretion of the antimicrobial peptide, Cathelicidin. Future work is likely to uncover additional specialized roles for dermal WAT and increase awareness of the regional WAT tissue heterogeneity.
2. Immune system
The immune system functions to protect the body against disease-causing microorganisms, including bacteria, fungi, virus, etc. The body defense system, which is a network coordinating the actions of cells, tissues, and organs not only protects against pathogen invasion but through inflammatory responses and cytokine production also regulates tissue regeneration.
Molecular and cellular events underlying immune response mediated hair regeneration
This idea is supported by previous research which demonstrated that skin wounds can induce hair growth through apoptotic signals regulating kinase 1 (ASK1)-dependent recruitment and activation of macrophages [32] which then increase Tnf-α expression [33]. Tnf-α was found to accelerate wound healing through paracrine mechanisms [34]. The receptor activator of NF-κB (RANK) is important in controlling the hair cycle [35]. Tnf-α has also been implicated in regeneration in other systems. For example, Tnf-α is shown to stimulate stem cell-mediated angiogenesis, protection, and survival through an NF-κB dependent mechanism [36]. In addition, RANKL-stimulated bone resorbing osteoclasts that could also affect hematopoietic progenitor mobilization by reducing the stem cell niche components SDF-1 (CXCL12), stem cell factor (SCF) and osteopontin along the endosteum [37]. Furthermore, one recent study also demonstrated that perifollicular macrophages are involved in cyclic adult hair follicle stem cell activation [38]. They found that the number of macrophages increased at middle telogen and progressively decreased at late telogen before the onset of HF-SC activation. Macrophage reduction, which resulted from apoptosis, is responsible for stimulating hair follicle stem cells and anagen re-entry through β-catenin/Wnt signaling activation.
Other inflammatory mediators, including prostaglandins (PGs), also play an important role during the wound healing process and tissue regeneration [39–41]. PGs, a group of physiologically active lipid compounds metabolized from arachidonic acid, execute their function through both autocrine and paracrine effects. Different kinds of PGs which are derived from a variety of synthase isoforms have different and even opposite functions. Hair follicles have been seen to produce and metabolize PGs [42,43]. Increasing PG levels by the ectopic expression of epidermal cyclooxygenase-2 (COX-2) leads to the formation of a sparse coat of greasy hair and to sebaceous gland hyperplasia [44]. PGE2 can protect mice from radiation induced hair loss [45] and topical application of PGF2α was found to induce anagen re-entry in the mouse model [46]. Furthermore, bimatoprost, the analog of PGF2α was approved by the FDA for use in lengthening human eyelashes [47]. However, in contrast to PGE2 and PGF2α which can stimulate hair regeneration, PGD2 produces an opposite effect that inhibits hair growth in both humans and mice through the G-protein coupled receptor Gpr44 [40,43].
Quorum sensing and tissue regeneration
Generally, the regeneration process is thought to occur via a one-to-one condition whereby stimulation of one stem cell leads to the activation of that single stem cell. However, this is an inefficient regeneration process. Can the regeneration response be thoroughly elicited by stimulating only some key cells or signals? Recently, we accidentally discovered that regeneration could occur through a collective decision making process. By plucking hairs with a proper arrangement, up to 5 times more neighboring, unplucked resting hairs were activated to regenerate. However, if the number of plucked hairs was below a threshold, no hairs including the plucked ones regenerated [48]. This type of regeneration is a threshold dependent process, which provides an organ-level example of quorum sensing. Quorum sensing is a self-driven decision-making process when certain criteria are met within the responding population. Each individual element estimates the number of other components with which they interact. Then, a response occurs when a threshold is reached. Quorum sensing has been invoked to describe bacteria cell-to-cell communication [49], affecting gene regulation in response to population density fluctuations [50]. A synthetic circuit in yeast has been used to achieve versatile social behaviors including quorum sensing [51]. Quorum sensing also has been used successfully to explain the behavior of social insects such as ants and honey bees for their collective decision-making [52,53].
In reality, how does the follicle population “cast and count its vote” in quorum sensing? The basic architectural design can be as follows. First, there is a stimulus to some, but not all, individual elements. Second, a stimulated element sends out a signal. Third, each element gauges the signal from its surroundings. Finally, a local decision is made within the population in an all-or-none fashion. The simplest models of quorum sensing are based on the idea of a signaling substance that spreads by diffusion, since the steady state concentration of such a substance will naturally reflect the spatial density of the sources that produce it. However, because the skin environment is more complex, and the mathematical model from the experimental results estimated that the quorum signal range of action was around 1 mm; greater than expected for a diffusible molecular cue. This suggested that an intermediate mobile cell vector is required for the observed response. Molecular and genetic analysis uncovered that this organ level quorum sensing is achieved by a two-step immune response cascade. First injured hair follicles will release CCL2 that recruits TNF-α secreting macrophages, which accumulate and signal to both plucked and unplucked follicles. Then TNF-α, acting through the NF-κB pathway, ultimately stimulates hair regeneration through activation of Wnt signaling (Fig. 4).
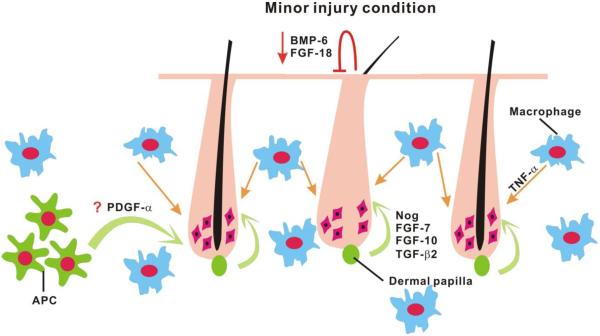
Figure 4. Quorum sensing regenerative behavior in a hair population can be induced by quantitative and topological hair plucking.
Minor injury, e.g. hair plucking would induce hair regeneration. Using a properly designed plucking procedure, one can achieve a more efficient regeneration response that induced the activation of up to 5 times more neighboring, unplucked resting hairs. This type of regeneration is a threshold dependent all-or-none process, which provides an organ-level example of quorum sensing. This process is mediated through a multi-step immune response cascade. First, hair plucking leads to hair keratinocyte apoptosis then CCL2 will be released by apoptotic keratinocytes. In the meantime, intra-follicular stem cell quiescence factors, BMP-6 and FGF-18 are down-regulated which diminishes the inhibition threshold for hair stem cell activation. Second, TNF-α secreting macrophages will be recruited by CCL2. When enough macrophages accumulate within the plucking region and reach the threshold, all the hairs, including plucked and unplucked hairs regenerate simultaneously. Based on Chen et al., 2015.
This work demonstrates that a quorum sensing cellular circuit can integrate existing cell injury, immune response and regeneration related molecular pathways from multiple organs to quantify the distress. The stem cell population then decides whether to disregard the stimulus as negligible or respond to it with a full scale cooperative regenerative response when a threshold is reached.
3. Seasons and circadian rhythm
In addition to local paracrine modulators, hair follicles are regulated by physiological changes that take place throughout the body. Perhaps one of the most prominent modulators of body physiology is the circadian clock, a signaling pathway that prominently oscillates with 24-hour periodicity due to the robust built-in negative feedback loop component. Transcriptional activity of Clock and Bmal1 regulates daily rhythmic expression of the so-called clock output genes, with some circadian targets becoming high at the nadir and others at the zenith [54]. The circadian clock phase becomes adjusted to the time of day in the central circadian clock located within the suprachiasmatic nucleus (SCN) in response to seasonal changes in daylight duration, as well as upon migration between time zones. The SCN clock then signals to adjust the phases of local clocks within various peripheral tissues (Fig. 1).
Molecular and cellular events underlying circadian rhythm effects on hair follicle regeneration
While not unique to the skin, prominent peripheral circadian clocks operate in various skin compartments, including hair follicles [55]. To-date, the clock has been implicated in modulating various aspects of hair follicle regeneration. During telogen phase and upon anagen initiation, clock genes are prominently expressed in the epithelial progenitors of secondary hair germs [56]. Functionally, Bmal1 null mice display a delay in proliferative activation of hair germ progenitors, and feature anagen initiation delay [56]. Importantly, mice with tissue specific Bmal1 deletion in skin epithelium do not have delayed anagen initiation [57], suggesting that this hair cycle defect in germline Bmal1 mutants is not cell-autonomous, but instead is dependent on either clock activity in the dermal papilla or on systemic clock-controlled humoral factors.
Clock activity is also present in bulge stem cells during both telogen and anagen phases [58,59]. Intriguingly, Janich et al. showed that circadian activity, as measured by a fluorescent clock reporter, is heterogeneous in the bulge, with reporter-high and reporter-low sub-populations [58]. Interestingly, the ratio of reporter-high stem cells undergoes prominent cyclic changes in synchrony with the hair cycle, with the highest percentage being during anagen. When compared with gene expression, the reporter-high bulge subpopulation was enriched for mediators of the Wingless-Int (WNT) and Transforming Growth Factor β (TGF-β) signaling pathways, whose activity is implicated in stem cell activation [60]. These data suggest that daily cycles of signaling responsiveness by bulge stem cells might exist, potentially making them more or less receptive for activation at different times during the day. This hypothesis remains to be comprehensively tested in in vivo assays.
During mature anagen, clock genes become prominently expressed in the hair matrix, dermal papilla and other follicular compartments [59,61]. Rather than regulating the length of anagen phase (the length of club hairs in circadian mutant mice is not significantly different from these in wild type control mice), the clock gates the progression of hair matrix cells into mitosis at the level of the G2/M cell cycle checkpoint [59]. Indeed, significantly more mitotic matrix cells are found in mouse anagen hair follicles during subjective night/early morning as compared to mid-day. This daily cycle of hair matrix proliferation has two implications. Throughout the day hair growth is uneven, but instead occurs with a subtle “bump”. More importantly, daily sensitivity of actively regenerating hair follicles to genotoxic stress, such as γ-radiation, varies significantly between night and day time. Indeed, exposure of wild type mice to the same dose of γ-radiation in the morning, during the mitotic peak, resulted in significant hair loss as compared to afternoon, when fewer mitotic cells endowed hair follicles with higher resistance. Time-of-day dependent hair loss responses to radiation were absent in Cry1/Cry2 null mice defective for the circadian clock.
Recently, an in vitro hair follicle culture system became an important tool for studying cell-autonomous effects of the circadian clock in humans. Al-Nuaimi et al. showed that siRNA mediated silencing of BMAL1 or PERIOD1 circadian genes delayed anagen-to-catagen transformation of cultured male scalp hair follicles [61]. Furthermore, Hardman et al. showed that the circadian clock also affects hair follicle melanogenesis – siRNA mediated silencing of the clock increases hair follicle pigmentation [62]. These intriguing results suggest the involvement of the circadian clock as a regulator of the hair cycle and of hair follicle pigmentation. However, this needs to be studied further to reveal the possible role of the circadian clock in these processes.
Hormones regulating seasonal molts and hair follicle cycling in mammals
Some animals are distinctly seasonal. Seasonal mammals live in colder environments with distinct seasons. Many physiological activities, such as breeding, hibernation, and fur molting are synchronized with seasonal changes due to changes in day length rather than temperature shifts [63]. For seasonal breeders who reproduce during a specific period of the year, molting occurs following the breeding season and therefore is closely related to the end of breeding activity. In mammals, studies on the weasel, mink and red deer who are short day breeders (<12 hours of light), suggest seasonal molting is mediated by melatonin, a major hormone rhythmically synthesized by the pineal gland [64–66]. Circadian rhythm regulation is achieved via melatonin's ability to manipulate expression of the clock genes [67,68]. Peripheral tissue “calendar cells” express high affinity melatonin receptors that are sensitive to the duration of melatonin exposure [69]. Upon receiving the melatonin signal, the calendar cells can mediate the secretion of prolactin, which increases during long days (summer) and decreases during short days (winter) [70,71]. Following ovulation, prolactin levels increase leading to a seasonal molt. Prolactin can induce an early molt that leads to a subsequent round of hair growth in cashmere goats [72]. It is clear that the melatonin-calendar cell-prolactin axis is one of the major pathways involved in seasonal molting. Though the precise mechanism of how melatonin regulates hair growth is still obscure, the fact that melatonin can induce prolactin secretion [70,71] which can induce hair follicle regression [73,74] provides a clue into how melatonin may work (Fig. 5).
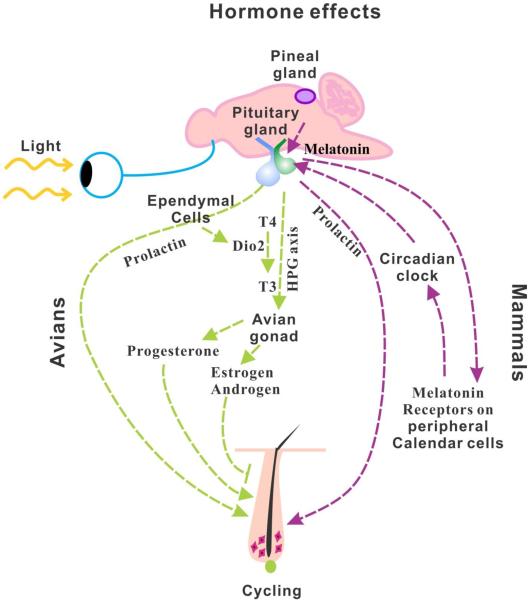
Figure 5. Hormone effects on hair and feather regeneration.
The right side of the figure depicts the role of hormones in mammalian hair cycling. The left side depicts the role of hormones in avian feather cycling. In mammals, melatonin released from the pineal gland interacts with their receptors on calendar cells of the pituitary gland and peripheral tissues to regulate the circadian clock. This then signals for the release of prolactin from the pituitary gland which stimulates hair follicle cycling. In avians, prolactin plays a similar role to stimulate feather cycling. However, layered on top of this, activation of thyroid hormone (T4 converted to T3) within the pituitary gland, controlled by DiO2 stimulates the hypothalamus pituitary gonad axis (HPG axis). The subsequent release of progesterone from the gonad induces molting and stimulates feather cycling, while the release of androgens or estrogens inhibits molting. While the influence of hormones on skin appendage regeneration and morphogenesis is well acknowledged, the mechanism remains to be investigated.
In addition to molting, melatonin was also found to be involved in the hair regeneration cycle [75]. It was reported that the melatonin receptor, MT1 exists in hair follicle keratinocytes and dermal papilla fibroblasts and MT2 is up-regulated in late-anagen through catagen, and down-regulated in telogen [76]. In addition, Kobayashi further confirms that both human and mouse hair follicles are extra-pineal sites of melatonin synthesis [77].
The feather follicle stem cell model
The convergent evolution of seasonal skin appendage molting also exists in birds where the feather follicle is another model in which to study stem cell biology (Fig. 2D–E). Feather follicles cycle through Growth, Resting and Molt phases and the whole follicle is replaced via stem cell activation. During embryonic feather development, reciprocal signals between a dermal condensation and its overlying epithelial placode determine the nature and location of the skin appendage [78]. As morphogenesis progresses, feathers evaginate from the skin surface and then grow asymmetrically, rising higher at the posterior end. Feathers then invaginate into the underlying mesenchyme and form a follicle. The mesenchymal cells at the base of the follicle are organized into a dermal papilla [79,80]. Epithelial stem cells are located in the collar above the dermal papilla adjacent to the pulp [81]. Melanocyte stem cells which give rise to the diverse feather pigmentation patterns are also localized within this region [82]. This places the proliferative region of the feather proximally while the more differentiated regions are located more distally. Since the collar is wrapped around the central pulp, the stem cells form a ring. One advantage to studying stem cells in feathers is that at different life stages, different feather types are formed which serve different needs. Newborn birds are covered in downy feathers which are replaced from the same follicles by juvenal feathers and then basic feathers [80]. Downy feathers are radially symmetric and provide warmth to the young chick. The juvenal and basic feathers enable birds to have different feather structures in different regions. This regional-specificity enabled the development of flight feathers on the wings to expand their environment to include the sky [83]. It also enabled the formation of ornate tail feathers used for display. Many adult feathers retain downy-like branches near the feather base to provide warmth and can still form vane-like branches to move the air in more distal regions.
Hormones regulating seasonal molts in birds
In birds the reproductive tract regresses in response to the increasing prolactin levels. Subsequently, the plasma sexual steroid hormones are decreased due to the low gonadal hormone secretion. It was found that early increases in prolactin are always followed by a molt [84]. Administering exogenous androgens and estrogens to domestic fowl could inhibit molting, while exogenous treatment with anti-gonadotrophin compounds, such as progesterone or prolactin could induce molting and reproductive involution (Fig. 5) [85,86]. Jungle fowl commence regression of their reproductive organs and halt egg laying just prior to shedding and replace their feathers at the onset of broodiness when prolactin levels are high [87]. A similar phenomenon also has been reported in starlings [88,89].
A great number of studies have been designed to elucidate the methods and pathways to induce and regulate molting in birds. As in mammals, seasonal photoperiod changes can induce molting. The role of thyroid hormones in the regulation of seasonal reproduction has been known for decades, but it now appears that the activation of thyroid hormone (the conversion of T4 to T3) is mainly regulated by type 2 deiodinase (DiO2) located in ependymal cells [90]. More recently, it was found that thyroid-stimulating hormone secreted from the pars tuberalis of the pituitary gland is responsible for this conversion. Thyroid hormone activation enables gonadotrophin releasing hormone (GnRH) nerve terminals to directly contact the basal lamina in the portal capillary perivascular space which then activates the HPG axis [91–95]. Nevertheless, it is still unclear whether molting is a direct effect of photoperiod duration or a secondary consequence of hormone levels influenced by the photoperiod, including the HPG axis, HPA (hypothalamus-pituitary-adrenal) axis and HPT (hypothalamus-pituitary-thyroid) axis (Fig. 5). The data does suggest that after the reproductive period, T4 becomes the dominant thyroid hormone form. Therefore, further studies are required to investigate the key regulators and modulators in seasonal molting.
4. Sex hormones
Role of sex hormones in mammals
While the hormones discussed above can influence seasonal variation in skin appendages, the sex hormones can alter skin appendage phenotypes to serve different purposes throughout the life of an organism. Sex hormones (androgens and estrogens) are mostly synthesized within the gonads. The initiation of estrogen synthesis takes place in ovarian theca cells which convert cholesterol to androstenedione. Within the ovary, androstenedione is then taken up by granulosa cells and converted to estrone or into testosterone which is then converted to estradiol [96]. Most testosterone is synthesized in the Leydig cells of the testes in response to the hypothalamic – pituitary – testicular axis. The release of GnRH from the hypothalamus stimulates pituitary release of LH and FSH which triggers the production of testosterone in the testes [97]. Aromatase converts testosterone to estradiol and 5-alpha reductase converts testosterone to dihydrotestosterone, the most active androgen form.
These hormones are also made to a lesser extent in the adrenal glands and other sites throughout the body, including the skin [98]. Skin has the ability to synthesize and convert hormone precursors into their active forms [99,100] whose actions can change the morphology of hairs and to influence skin maintenance and repair during aging. Skin appendages are cyclically replaced. This enables the formation of different shaped and pigmented skin appendages at different stages of life or in different seasons. For instance, young vertebrate hairs are fine and lack pigment. The release of sex hormones during puberty changes the nature of these skin appendages. The hair stem cells are induced to form longer and thicker hairs in different body regions. Later in life hair cycles can produce thinner hair filaments or even return to vellus hairs. The skin thickness and elasticity decreases as androgen levels decrease with advancing age [101]. Estrogen levels increase as androgen levels decrease perhaps through the action of aromatase. Hormone replacement therapy improves the condition of aging skin [102]. On the other hand, in mice castration improves the rate of wound healing [103], possibly by regulating inflammation in response to wounding [104]. Also, during pregnancy and lactation the mouse hair cycle stops [17] apparently in response to elevated prolactin levels which act through the Jak/Stat5 signaling pathway [105].
In humans, the androgen and estrogen receptors are localized to the dermal papilla, a specialized signaling center within the dermis. Human keratinocytes do not show AR signaling transactivation suggesting that they may not be the responding cells [106], however, hHa7, a type I hair keratin has an androgen response element in its promoter and was reported to be induced by androgens [107]. It has been suggested that androgen induced IGF-1 may mediate some of the paracrine signaling activities in hair follicles in response to androgen [108]. Hormone receptors belong to the family of nuclear receptors which upon binding to their respective ligands, translocate from the cytoplasm to the nucleus and induce the transcription of specific genes. Interestingly, different body regions demonstrate differential responses to circulating hormone levels. For instance, in aging animals, androgens can induce the growth of hairs on facial skin (moustache, beards) yet induce the regression of scalp hairs (see section on androgenetic alopecia below). Specificity of this response is tuned by the binding of nuclear receptors to a growing list of co-activators. Unfortunately, mouse hairs do not show sexual dimorphisms and they do not go through hair loss analogous to androgenetic alopecia, so they are not a suitable model in which to explore the etiology of this disease.
Role of sex hormones in birds
Introducing exogenous estrogens to roosters prior to gonadal development led to transient feminization [109]. Suppression of endogenous estrogen activity prior to gonadal development can lead to transient masculinization [110]. Permanent sex reversal was achieved by treating genetic hens with aromatase inhibitors prior to ovarian development [111,112]. Sex hormones can also lead to sexual dimorphisms, the appearance of specialized sex-dependent skin appendages. This suggests that skin stem cells within adult birds can respond to hormonal signals to produce different specialized skin appendage phenotypes that affect the birds adapt to different needs at different life stages.
In chickens, the estrogen and progesterone receptors have been reported to be found within feathers in the dermal papilla and epidermal cells as well as in the interfollicular epidermis [113]. While the androgen receptor has not yet been mapped in feathers, sexual dimorphisms in the size and coloring of roosters and hens suggest hormones must play a role. Sexually dimorphic characteristics in birds include the increased size of combs and wattles, the presence of spurs on the legs and the colorful ornate feather patterns present on roosters compared to hens. Interestingly, some strains of rooster have female feather shapes and colors. This so called henny feathering is due to the presence of aromatase in the skin which converts androgens to estrogens [114].
Sex hormone involvement in human diseases
Defects in hormones and downstream signaling can lead to a variety of human diseases, breast cancer, ovarian cancer, testicular feminization, hirsutism, etc.
Androgenetic alopecia
Androgenetic alopecia (AGA) is a common disease of the skin in which the anagen phase of the hair cycle is shortened in affected areas leading to the miniaturization of hairs. Although 95% of testosterone is synthesized by the testes, 5% is locally converted from precursors in peripheral tissues, including the skin [115]. The skin also expresses 5α-reductase which converts testosterone to highly active dihydrotestosterone (DHT). DHT binds with high affinity to the androgen receptor (AR), a transcription factor residing within the dermal papilla which induces the expression of a number of genes. Individuals lacking 5α-reductase do not develop AGA. Higher AR numbers are associated with the dermal papilla of bald compared to non-bald hair follicles [116]. Expression of some AR variants also correlates with AGA [117]. β-Catenin plays an early and essential role in hair cycle induction [118]. The AR has been shown to bind to β-catenin and inhibit the canonical Wnt signaling pathway by inducing Dkk [119,120] which then inhibits keratinocyte growth and leads to AGA [119,121]. The role of AR co-regulators in this process remains unclear at this time.
Possible treatments for AGA target 1) 5α-reductase or 2) the endothelia which provide nutrients to the hair follicles. 5α-reductase has been targeted by the use of inhibitors (such as Finasteride or Minoxidil) which some success. Vascularization of the skin regulates hair follicle size [122] and is reduced in the scalp of patients with AGA. Through cross-talk with the endothelial cells, human dermal papilla cells release VEGF and IGF-1 which promote angiogenesis [123,124].
In contrast to androgen, estrogen signaling through the estrogen receptor 1 (ESR1) leads to an increased telogen:anagen ratio [125,126]. During human pregnancy hair regeneration is suppressed due to increased estrogen levels [127], while hairs are held in telogen during pregnancy in mice [17]. Application of exogenous estrogen can maintain hairs in telogen while estrogen inhibitors induce proliferation and entry to anagen [128]. Estrogen induces increased TGF-β2 levels leading to early anagen-catagen transition and subsequent induction of BMP4 leads to the maintenance of telogen [129].
Hirsutism
Hirsutism is the appearance of male patterned facial and axillary hair growth in reproductive aged women. Hirsutism is often associated with polycystic ovary syndrome, in which increased circulating androgen and insulin levels accompany the formation of ovarian cysts. Research based on female lambs and rhesus monkeys found that embryonic exposure to high androgen levels led to increased circulating levels with a hypersensitivity to androgen later in life [130,131]. The syndrome seems to result from an imbalance of a number of hormones along the hypothalamic-pituitary-gonadal axis which may arise from the early exposure to androgens [132]. Hirsutism is currently treated with anti-androgens.
Hermaphroditism
People displaying both male and female gonads are referred to as Hermaphrodites. Much of our knowledge of hermaphroditism is based on animal studies. In metazoans male sex determination is regulated by the Sry gene located on the Y chromosome [133]. This leads to the expression of DMRT transcription factors [134] which may regulate testicular and ovarian determination genes. In mice, DMRT1 is required for the differentiation and maintenance of somatic and germ cells into the male gonad [135,136]. Female Dmrt1 conditional transgenic mice directing expression to the ovary suppressed Foxl2 (the gene which maintains female sex determination) and other ovary specific genes and upregulated the expression of genes involved in testis determination [137]. This led to tissue reprogramming and the conversion of the ovary into a testis.
5. Aging
Aging is a physiological process associated with a progressive deterioration in the ability of tissues to repair and regenerate. Depletion of stem cells is thought to play the major role in the aging dependent functional decline of tissue regeneration [138–140]. However, the number of tissue stem cells doesn't have to decrease during aging [141–144].
Aging effects on hair follicle regeneration
Our recent study showed that hair follicles in old skin stay in the resting phase for a much longer time than in younger skin. Moreover, the hair wave propagates at a reduced velocity and the hair domains fragment into smaller sized regions [144]. There are five possible explanations for the effects of aging on hair regeneration: 1) hair inducing signals, such as Wnt pathway proteins may become down-regulated with aging, 2) propagating signals or activators secreted from neighboring anagen follicles or macro-environments are down-regulated, 3) old HFs cannot respond effectively to propagating signals or activators, 4) the stem cells are depleted, and 5) Wnt signal inhibitors, such as DKK and SFRP or BMP, are activated in old mice and inhibit anagen re-entry and hair wave propagation. Flow Activated Cytometry Sorting (FACS) and stem cell marker analysis revealed that the hair stem cell number and the markers they express are compatible between young and old mice [144], which is consistent with the previous finding that aging associated human alopecia is not associated with stem cell loss but resulted from a deficiency of hair germ progenitors and failure to activate hair stem cells [145]. In addition, the intra-follicular hair inducing Wnt/β-catenin signaling pathway is intact in aging mice. These results imply that the extra-follicular macro-environment rather than the stem cells may be responsible for aging related hair regeneration alterations [144].
This hypothesis was confirmed by an experiment in which the regeneration defect in aging skin was rescued when it was transplanted onto a young mouse [144]. However, another recent study suggests that the systemic environment may not play a major role in delaying hair stem cell activation because delays in aging HF regeneration can't be rescued by parabiosis [146]. They found that functional declaration of HF stem cells in aging mice are resulted from enrichment of intrinsic nuclear factor of activated T-cell c1 (NFATc1) and local (or extrinsic) BMP. Through inhibition signal of NFATc1 and BMP, aged HFSCs can return into more youthful levels [146]. Similarly, our recent study demonstrated that the extra-follicular macro-environment periodically expresses both activators (follistatin, etc) and inhibitors (Bmp2, Dkk1, Sfrp4, etc) of follicle growth and coordinates with intra-follicular Wnt/β-catenin signaling to regulate the hair regeneration cycle (Fig. 3). In aging mice, the activator is down-regulated resulting in small hair cycle domains because hair wave propagation is not favored. Meanwhile, increased inhibitor expression in aging mice leads to telogen retention because anagen re-entry was blocked [144]. These two independent studies illustrated that hair stem cells are regulated by local but not systemic signals. This point of view was confirmed by another group which indicated that levels of dermal adipocyte Bmp2 is higher in aging mice which affects hair stem cell activation [146]. In addition, 1. Notch activation and Notch ligand (Delta) expression was increased while old muscle stem cells (satellite cells) were exposed to young serum [147]; 2. Aged hepatocyte proliferation was stimulated and cEBP-α complex was restored to levels observed in young animals after performing heterochronic parabiosis [148] offering further support that the function of the aging stem cells is still intact and can respond appropriately when placed in a young environment. However, though our and other studies all indicated that stem cell function can be partially rescued through correction the intrinsic and extrinsic signals, the regeneration ability is not restored totally implied that stem cell itself but not the environment may also take partial responsibility for aging process.
6. Additional tissue interactions involving hair follicles should be investigated further
The hair follicle bulge area can be divided into several small compartments, which are composed of distinct cell populations expressed different cell markers, including CK15 [149], CD34 [150], Lgr5 [151], etc. Recently Brownell et al identified a new set of cells expressing Gli1 within the bulge area which can respond to neuronal Sonic Hedgehog (Shh) signals to create a perineural stem cell niche within the telogen bulge [152]. Gli1 positive follicle cells not only function as multipotent stem cells to regenerate hair follicles cyclically but also become epidermal stem cells after wounding under the control of the perineural stem cell niche [152]. These results not only illustrate that hair follicles are maintained by adult stem cells which express the Shh response gene, Gli1 but revealed that the nervous system also plays an important role in stem cell regulation. This concept was also supported by the finding that peripheral adrenergic signals are necessary for G-CSF induced mobilization of murine progenitor cells [153].
Evidence also indicated that stem cells can be regulated by the dynamic interaction of both immune and nervous systems [154]. When facing either physiological or pathological stresses, osteoclasts will expand and activate then release various proteolytic enzymes enabling hematopoietic stems and progenitor cells (HSPCs) to leave the bone marrow (BM) and enter the bloodstream to participate in host defense and organ repair. Such a reaction is initiated by a reduction in SDF-1 levels in the BM and an increase in the peripheral blood, as well as increased CXCR4 expression in the BM, all of which will enhance the release of catecholamines and upregulate β-adrenergic receptor expression on HSPCs [154]. These observations inspire in us the concept that all the factors, activators or inhibitors derived from many sources within the macro-environment, will be integrated within a complicated network to modulate the activity of stem cells; i.e. input from different sources will all be “taken into consideration” by stem cells.
Communication between different kinds of cells and tissues are mutual and complicated. Stem cells can be regulated by the environmental signals and vice versa. For example, skin is composed of a variety of cells and tissues derived from ectoderm and mesoderm. To make these cells and tissues pattern and function smoothly and precisely, cross-talk among them is very important. It is well-known that the thickness of the intra-dermal adipocyte layer is tightly linked with the hair growth cycle. The adipose layer becomes thickened when anagen is initiated; in contrast the adipose layer starts to regress when the follicles are in telogen or resting phase. Though our and other previous work demonstrated that intra-dermal adipose tissue can regulate the cyclic regeneration of hair follicles [17,18,27], whether oscillation of the adipocyte layer thickness is regulated by epidermis or hair follicles is poorly understand. Recently, Donati et al discovered that adipocyte differentiation was elicited by activating epidermal Wnt/β-catenin signaling and this response is mediated by stimulating the secretion of adipogenic factors, including BMP and insulin signaling pathways [23]. This study points out the fact that interaction between different cells or tissues is important in organ homeostasis maintenance. Additionally, a recent study further demonstrated that different cells can cross-talk through paracrine signaling pathways [124]. By using a transwell-based co-culture system, Bassino et al., identified that human follicle dermal papilla cells (FDPC) can promote survival, proliferation and tubulogenesis of human microvascular endothelial cells (HMVEC) through the release of vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF-1), two potent proangiogenic mediators whose expression will alter the growth of hair follicles [122,123]. On the other hand, β-catenin production from FDPC, which is important for hair regeneration, can be enhanced by HMVEC [124]. This interaction between FDPC and HMVEC provides a good illustration that regeneration occurs through “team work” by collaborating among a variety of cells, tissues and even organs.
In conclusion, stem cells hold the clue for regeneration. Many people are eager to ward off aging and regenerative disorders through scientific advances unveiling the mystery of how stem cells are regulated. In the past, the stem cell niche, the micro-environment closest to or surrounding the stem cells, was thought to be the only and major “stem cell regulation center”. However, more and more evidence indicates that stem cell regulation is a complicated network through inter-actions among different layers of the micro- and macro-environment (Fig. 6) [29]. Stem cells can gather and sum up all the signals (including positive and negative modulators) from a variety of sources to decide if the regeneration response should be pursued or not. On the other hand, stem cells not only receive signals from these multi-environments, but also release signals to the surrounding cells to maintain the homeostasis. These findings demonstrate that the regeneration response relies on collaborative interactions among cells, tissues and organs. Through these interactions, mediated in part by signal transduction, an effective and collective regeneration response, perhaps in the form of tissue level “quorum sensing” will take place [48].
Figure 6. Skin appendages, stem cell niche, and the multiple extra-follicular environments, with a few known regulatory molecular circuits.
Compared to Figure 1, we can see many of the complex tissues within the skin talk to each other. Hair follicle stem cells interact with adipose layers, immune cells, blood vessels, nerves in the dermis, etc. Factors derived from the external environment of the neuroendocrine system also modulate hair follicle stem cells. The activation and quiescence of hair stem cells is regulated by the sum of activators and inhibitors. In the follicle, these factors emanate from both stem cells and their niche. In some follicles, the niche can respond to several environmental modulators, so they can assume different forms to adapt to different physiological needs. Some examples of environmental modulators discussed here include hormones, seasonal changes, the aging process, intra-dermal adipose tissue and the immune response. By summing up all these activators and inhibitors from both the intra-follicular niche and extra-follicular macro-environment, stem cells decide whether to stay in quiescence status or proceed to activation. We have just begun to know some of these molecular circuits. Continuous work in this direction will reveal more involved molecular pathways, more interactions, more on how the regulatory network is assembled, and more therapeutic possibilities to intervene on behalf of our patients' benefit.
The difficulty for scientists trying to utilize stem cells in regenerative medicine is that stem cells are hard to define and obtain. Their isolation and expansion further complicates the hurdles that face this direction of inquiry. Focusing on the environment rather than the stem cells may be a better strategy to fight against aging and degenerative disorders. By understanding the multi-layered hierarchical regulation of stem cells, we hope to open a window for therapeutic applications in the future.
Highlights.
Hair follicle stem cells are regulated by many extra-follicular sources.
Signals from the adipose tissue modulate hair stem cell behavior and aging.
Upon injury, innate immune response triggers regeneration through quorum sensing.
The effects of androgen on hair growth are different in different regions and ages.
Seasonal and circadian rhythms modulate the length of hair cycling.
Acknowledgement
CMC, RBW are supported by NIAMS RO1 AR 47364 and AR60306. CCC is supported by NSC 100-2314-B-075-044, NSC 101-2314-B-075-008 -MY3, Taipei Veterans General Hospital (VN104-12, V104C-055, VGHUST104-G1-1-1, VN103-12, V103C-010, V102B-009, R-1100404, R-1100403). MVP is supported by NIAMS R01-AR067273 and Edward Mallinckrodt Jr. Foundation grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Argyris TS. The effect of wounds on adjacent growing or resting hair follicles in mice. AMA Arch Pathol. 1956;61:31–6. [PubMed] [Google Scholar]
- [2].Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–94. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- [3].Schofield R. The relationship between the spleen colony-forming cell and the hamatopopietic stem cell. A hypothesis. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- [4].Li L, Xie T. Stem Cell Niche: Structure and Function. Annu Rev Cell Dev Biol. 2005;21:605–31. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- [5].Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- [6].Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–45. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- [7].Reddy S, 1, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- [8].Lowry WE, 1, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of b-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hsu Y-C, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–69. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oshimori N, Fuchs E. Paracrine TGF-β signaling counterbalances bmp-mediated repressionin hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fraser DA. The development of the skin of the back of the albino rat until the eruption of the first hairs. The Anatomical Record. 1928;38:203–23. [Google Scholar]
- [13].Durward A, Rudall KM. Studies on hair growth in the rat. Journal of anatomy. 1949;83:325–35. [PMC free article] [PubMed] [Google Scholar]
- [14].Hausman GJ, Campion DR, Richardson RL, Martin RJ. Adipocyte development in the rat hypodermis. The American journal of anatomy. 1981;161:85–100. doi: 10.1002/aja.1001610107. [DOI] [PubMed] [Google Scholar]
- [15].Driskell RR, Jahoda CA, Chuong CM, Watt FM, Horsley V. Defining dermal adipose tissue. Experimental dermatology. 2014;23:629–31. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Plikus MV, 1, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–4. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–71. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wolnicka-Glubisz A, King W, Noonan FP. SCA-1+ cells with an adipocyte phenotype in neonatal mouse skin. J Invest Dermatol. 2005;125:383–5. doi: 10.1111/j.0022-202X.2005.23781.x. [DOI] [PubMed] [Google Scholar]
- [20].Wojciechowicz K, Markiewicz E, Jahoda CA. C/EBPalpha identifies differentiating preadipocytes around hair follicles in foetal and neonatal rat and mouse skin. Exp Dermatol. 2008;17:675–80. doi: 10.1111/j.1600-0625.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- [21].Wojciechowicz K, Gledhill K, Ambler CA, Manning CB, Jahoda CA. Development of the mouse dermal adipose layer occurs independently of subcutaneous adipose tissue and is marked by restricted early expression of FABP4. PloS one. 2013;8:e59811. doi: 10.1371/journal.pone.0059811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–81. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Donati G, Proserpio V, Lichtenberger BM, Natsuga K, Sinclair R, Fujiwara H, et al. Epidermal Wnt/β-catenin signaling regulates adipocyte differentiation via secretion of adipogenic factors. Proc Natl Acad Sci U S A. 2014;111:E1501–9. doi: 10.1073/pnas.1312880111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Butcher EO. The hair cycles in the albino rat. The Anatomical Record. 1934;61:5–19. [Google Scholar]
- [25].Chase HB, Montagna W, Malone JD. Changes in the skin in relation to the hair growth cycle. Anat Rec. 1953;116:75–81. doi: 10.1002/ar.1091160107. [DOI] [PubMed] [Google Scholar]
- [26].Hansen LS, Coggle JE, Wells J, Charles MW. The influence of the hair cycle on the thickness of mouse skin. Anat Rec. 1984;210:569–73. doi: 10.1002/ar.1092100404. [DOI] [PubMed] [Google Scholar]
- [27].Plikus MV, Baker RE, Chen CC, Fare C, de la Cruz D, Andl T, et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–9. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Plikus MV, Widelitz RB, Maxson R, Chuong CM. Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity. Int J Dev Biol. 2009;53:857–68. doi: 10.1387/ijdb.072564mp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen CC, Chuong CM. Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. J Dermatol Sci. 2012;66:3–11. doi: 10.1016/j.jdermsci.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sumikawa Y, Inui S, Nakajima T, Itami S. Hair cycle control by leptin as a new anagen inducer. Experimental dermatology. 2014;23:27–32. doi: 10.1111/exd.12286. [DOI] [PubMed] [Google Scholar]
- [31].Yang CC, Sheu HM, Chung PL, Chang CH, Tsai YS, Hughes MW, et al. Leptin of dermal adipose tissue is differentially expressed during the hair cycle and contributes to adipocyte-mediated growth inhibition of anagen-phase vibrissa hair. Exp Dermatol. 2015;24:57–60. doi: 10.1111/exd.12566. [DOI] [PubMed] [Google Scholar]
- [32].Osaka N, Takahashi T, Murakami S, Matsuzawa A, Noguchi T, Fujiwara T, et al. ASK1-dependent recruitment and activation of macrophages induce hair growth in skin wounds. J Cell Biol. 2007;176:903–9. doi: 10.1083/jcb.200611015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jiang S, Zhao L, Teklemariam T, Hantash BM. Small cutaneous wounds induce telogen to anagen transition of murine hair follicle stem cells. J Dermatol Sci. 2010;60:143–50. doi: 10.1016/j.jdermsci.2010.10.008. [DOI] [PubMed] [Google Scholar]
- [34].Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB, Kim JH. Tumor Necrosis Factor-alpha-Activated Human Adipose Tissue-Derived Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing through Paracrine Mechanisms. J Invest Dermatol. 2011;131:1559–67. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- [35].Duheron V, Hess E, Duval M, Decossas M, Castaneda B, Klöpper JE, et al. Receptor activator of NF-{kappa}B (RANK) stimulates the proliferation of epithelial cells of the epidermo-pilosebaceous unit. Proc. Natl. Acad. Sci. U S A. 2011;108:5342–7. doi: 10.1073/pnas.1013054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–82. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- [37].Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- [38].Castellana D, Paus R, Perez-Moreno M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 2014;12:e1002002. doi: 10.1371/journal.pbio.1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kapoor M, Kojima F, Yang L, Crofford LJ. Sequential induction of pro- and anti-inflammatory prostaglandins and peroxisome proliferators-activated receptor-gamma during normal wound healing: a time course study. Prostaglandins Leukot Essent Fatty Acids. 2007;76:103–12. doi: 10.1016/j.plefa.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nelson AM, Loy DE, Lawson JA, Katseff AS, Fitzgerald GA, Garza LA. Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. J Invest Dermatol. 2013;133:881–9. doi: 10.1038/jid.2012.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nieves A, Garza LA. Does prostaglandin D2 hold the cure to male pattern baldness? Exp Dermatol. 2014;23:224–7. doi: 10.1111/exd.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Colombe L, Vindrios A, Michelet JF, Bernard BA. Prostaglandin metabolism in human hair follicle. Exp Dermatol. 2007;16:762–9. doi: 10.1111/j.1600-0625.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- [43].Garza LA, Liu Y, Yang Z, Alagesan B, Lawson JA, Norberg SM, et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012;4:126ra34. doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Neufang G, Furstenberger G, Heidt M, Marks F, Müller-Decker K. Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proc Natl Acad Sci U S A. 2001;98:7629–34. doi: 10.1073/pnas.121574098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Geng L, Hanson WR, Malkinson FD. Topical or systemic 16, 16 dm prostaglandin E2 or WR-2721 (WR-1065) protects mice from alopecia after fractionated irradiation. Int J Radiat Biol. 1992;61:533–7. doi: 10.1080/09553009214551291. [DOI] [PubMed] [Google Scholar]
- [46].Sasaki S, Hozumi Y, Kondo S. Influence of prostaglandin F2alpha and its analogues on hair regrowth and follicular melanogenesis in a murine model. Exp Dermatol. 2005;14:323–8. doi: 10.1111/j.0906-6705.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- [47].Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47:S185–S202. doi: 10.1016/s0039-6257(02)00307-7. [DOI] [PubMed] [Google Scholar]
- [48].Chen CC, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, et al. Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell. 2015;161:277–90. doi: 10.1016/j.cell.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bassler BL. Small talk. Cell-to-cell communication in bacteria. Cell. 2002;109:421–4. doi: 10.1016/s0092-8674(02)00749-3. [DOI] [PubMed] [Google Scholar]
- [50].Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–99. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- [51].Youk H, Lim WA. Secreting and Sensing the Same Molecule Allows Cells to Achieve Versatile Social Behaviors. Science. 2014;343:1242782. doi: 10.1126/science.1242782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pratt SC. Quorum sensing by encounter rates in the ant Temnothorax albipennis. Behav Ecol. 2005;16:488–96. [Google Scholar]
- [53].Visscher PK. Group decision making in nest-site selection among social insects. Annu Rev Entomol. 2007;52:255–75. doi: 10.1146/annurev.ento.51.110104.151025. [DOI] [PubMed] [Google Scholar]
- [54].Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annual review of neuroscience. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Plikus MV, Van Spyk EN, Pham K, Geyfman M, Kumar V, Takahashi JS, et al. The Circadian Clock in Skin: Implications for Adult Stem Cells, Tissue Regeneration, Cancer, Aging, and Immunity. J Biol Rhythms. 2015 doi: 10.1177/0748730414563537. pii: 0748730414563537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lin KK, Kumar V, Geyfman M, Chudova D, Ihler AT, Smyth P, et al. Circadian Clock Genes Contribute to the Regulation of Hair Follicle Cycling. PLoS Genet. 2009;5:e1000573. doi: 10.1371/journal.pgen.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11758–63. doi: 10.1073/pnas.1209592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, et al. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–14. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- [59].Plikus MV, Vollmers C, de la Cruz D, Chaix A, Ramos R, Panda S, et al. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc Natl Acad Sci U S A. 2013;110:E2106–15. doi: 10.1073/pnas.1215935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Plikus MV. New activators and inhibitors in the hair cycle clock: targeting stem cells' state of competence. J Invest Dermatol. 2012;132:1321–4. doi: 10.1038/jid.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Al-Nuaimi Y, Hardman JA, Biro T, Haslam IS, Philpott MP, Toth BI, Farjo N, et al. A meeting of two chronobiological systems: circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. J Invest Dermatol. 2014;134:610–9. doi: 10.1038/jid.2013.366. [DOI] [PubMed] [Google Scholar]
- [62].Hardman JA, Tobin DJ, Haslam IS, Farjo N, Farjo B, Al-Nuaimi Y, et al. The Peripheral Clock Regulates Human Pigmentation. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.442. doi:10.1038/jid.2014.442. [DOI] [PubMed] [Google Scholar]
- [63].Paul MJ, Galang J, Schwartz WJ, Prendergast BJ. Intermediate-duration day lengths unmask reproductive responses to nonphotic environmental cues. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1613–9. doi: 10.1152/ajpregu.91047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Allain D, Rougeot J. Induction of autumn moult in mink (Mustela vison Peale and Beauvois) with melatonin. Reprod Nutr Dev. 1980;20:197–201. doi: 10.1051/rnd:19800114. [DOI] [PubMed] [Google Scholar]
- [65].Rust CC, Meyer RK. Hair color, molt, and testis size in male, short-tailed weasels treated with melatonin. Science. 1969;165:921–2. doi: 10.1126/science.165.3896.921. [DOI] [PubMed] [Google Scholar]
- [66].Webster JR, Barrell GK. Advancement of reproductive activity, seasonal reduction in prolactin secretion and seasonal pelage changes in pubertal red deer hinds (Cervus elaphus) subjected to artificially shortened daily photoperiod or daily melatonin treatments. J Reprod Fertil. 1985;73:255–60. doi: 10.1530/jrf.0.0730255. [DOI] [PubMed] [Google Scholar]
- [67].Hiragaki S, Baba K, Coulson E, 1, Kunst S, Spessert R, Tosini G. Melatonin signaling modulates clock genes expression in the mouse retina. PLoS One. 2014;9:e106819. doi: 10.1371/journal.pone.0106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].von Gall C, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann N Y Acad Sci. 2005;1040:508–11. doi: 10.1196/annals.1327.105. [DOI] [PubMed] [Google Scholar]
- [69].Lincoln GA, Andersson H, Loudon A. Clock genes in calendar cells as the basis of annual timekeeping in mammals--a unifying hypothesis. J Endocrinol. 2003;179:1–13. doi: 10.1677/joe.0.1790001. [DOI] [PubMed] [Google Scholar]
- [70].Allain D, Ravault JP, Panaretto BA, Rougeot J. Effects of pinealectomy on photoperiodic control of hair follicle activity in the Limousine ram: possible relationship with plasma prolactin levels. J Pineal Res. 1986;3:25–32. doi: 10.1111/j.1600-079x.1986.tb00723.x. [DOI] [PubMed] [Google Scholar]
- [71].Nixon AJ, Choy VJ, Parry AL, Pearson AJ. Fiber growth initiation in hair follicles of goats treated with melatonin. J Exp Zool. 1993;267:47–56. doi: 10.1002/jez.1402670108. [DOI] [PubMed] [Google Scholar]
- [72].Dicks P, Russel AJ, Lincoln GA. The role of prolactin in the reactivation of hair follicles in relation to moulting in cashmere goats. J Endocrinol. 1994;143:441–8. doi: 10.1677/joe.0.1430441. [DOI] [PubMed] [Google Scholar]
- [73].Foitzik K, Krause K, Nixon AJ, Ford CA, Ohnemus U, Pearson AJ, et al. Prolactin and its receptor are expressed in murine hair follicle epithelium, show hair cycle-dependent expression, and induce catagen. Am J Pathol. 2003;162:1611–21. doi: 10.1016/S0002-9440(10)64295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Foitzik K, Krause K, Conrad F, Nakamura M, Funk W, Paus R. Human scalp hair follicles are both a target and a source of prolactin, which serves as an autocrine and/or paracrine promoter of apoptosis-driven hair follicle regression. Am J Pathol. 2006;168:748–56. doi: 10.2353/ajpath.2006.050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fischer TW, Slominski A, Tobin DJ, Paus R. Melatonin and the hair follicle. J Pineal Res. 2008;44:1–15. doi: 10.1111/j.1600-079X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- [76].Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol. 2003;196:144–53. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- [77].Kobayashi H, Kromminga A, Dunlop TW, Tychsen B, Conrad F, Suzuki N, et al. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005;19:1710–2. doi: 10.1096/fj.04-2293fje. [DOI] [PubMed] [Google Scholar]
- [78].Chen CF, Foley J, Tang PC, Li A, Jiang TX, Wu P, et al. Development, regeneration, and evolution of feathers. Annu Rev Anim Biosci. 2015;3:169–95. doi: 10.1146/annurev-animal-022513-114127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lillie FR, Wang H. Physiology of development of the feather V. Experimental morphogenesis. Physiol Zool. 1941;14:103–35. [Google Scholar]
- [80].Lucas AM, Stettenheim PR. Avian Anatomy Integuments Part I, II. US Gov. Print. Off; Washington, DC: 1972. [Google Scholar]
- [81].Yue Z, Jiang TX, Widelitz RB, Chuong CM. Mapping stem cell activities in the feather follicle. Nature. 2005;438:1026–9. doi: 10.1038/nature04222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lin SJ, Foley J, Jiang TX, Yeh CY, Wu P, Foley A, et al. Topology of feather melanocyte progenitor niche allows complex pigment patterns to emerge. Science. 2013;340:1442–5. doi: 10.1126/science.1230374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chuong CM, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, et al. Adaptation to the Sky: Defining the Feather With Integument Fossils From Mesozoic China and Experimental Evidence From Molecular Laboratories. J Exp. Zool. 2003;298B:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dawson A. Control of molt in birds: Association with prolactin and gonadal regression in starlings. Gen Comp Endocrinol. 2006;147:314–22. doi: 10.1016/j.ygcen.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [85].Schleussner G, Dittami JP, Gwinner E. Testosterone implants affect molt in male European starlings, Sturnus vulgaris. Physiol Zool. 1985;585:597–604. [Google Scholar]
- [86].Nolan VJ, Ketterson ED, Ziegenfus C, Cullen DP, Chandler CR. Testosterone and avian life histories: effects of experimentally elevated testosterone on prebasic molt and survival in male dark-eyed juncos. Condor. 1992;94:364–70. [Google Scholar]
- [87].Sherry DF, Mrosovsky N, Hogan JA. Weight loss and anorexia during incubation in birds. J. Comp. Physiol. Psychol. 1980;94:89–98. [Google Scholar]
- [88].Dawson A, Goldsmith AR. Prolactin and gonadotrophin secretion in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to nesting, incubation, and rearing young. Gen Comp Endocrinol. 1982;48:213–21. doi: 10.1016/0016-6480(82)90019-3. [DOI] [PubMed] [Google Scholar]
- [89].Rothery P, Wyllie I, Newton I, Dawson A, Osborn D. The timing and duration of moult in adult starlings Sturnus vulgaris in eastcentral England. Ibis. 2001;143:435–41. [Google Scholar]
- [90].Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, et al. Light-induced hormone conversion of T4 toT3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–81. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]
- [91].Yamamura T, Yasuo S, Hirunagi K, Ebihara S, Yoshimura T. T3 implantation mimics photoperiodically reduced encasement of nerve terminals by glial processes in the median eminence of Japanese quail. Cell Tissue Res. 2006;324:175–9. doi: 10.1007/s00441-005-0126-8. [DOI] [PubMed] [Google Scholar]
- [92].Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–22. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- [93].Yoshimura T. Thyroid hormone and seasonal regulation of reproduction. Front Neuroendocrinol. 2013;34:157–66. doi: 10.1016/j.yfrne.2013.04.002. [DOI] [PubMed] [Google Scholar]
- [94].Nakane Y, Yoshimura T. Universality and diversity in the signal transduction pathway that regulates seasonal reproduction in vertebrate. Frontiers Neurosci. 2014;8:1–7. doi: 10.3389/fnins.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shinomiya A, Shimmura T, Nishiwaki-Ohkawa T, Yoshimura T. Regulation of seasonal reproduction by hypothalamic activation of thyroid hormone. Front Endocrinol. 2014;5:12. doi: 10.3389/fendo.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Barbieri RL. The endocrinology of the menstrual cycle. Methods Mol Biol. 2014;1154:145–69. doi: 10.1007/978-1-4939-0659-8_7. [DOI] [PubMed] [Google Scholar]
- [97].Chen H, Ge RS, Zirkin BR. Leydig cells: from stem cells to aging. Mol Cell Endocrinol. 2009;306:9–16. doi: 10.1016/j.mce.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–23. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pomari E, Valle LD, Pertile P, Colombo L, Thornton MJ. Intracrine sex steroid synthesis and signaling in human epidermal keratinocytes and dermal fibroblasts. FASEB J. 2015;29:508–24. doi: 10.1096/fj.14-251363. [DOI] [PubMed] [Google Scholar]
- [100].Zouboulis CC. The human skin as a hormone target and an endocrine gland. Hormones (Athens) 2004;3:9–26. doi: 10.14310/horm.2002.11109. [DOI] [PubMed] [Google Scholar]
- [101].Zouboulis CC. Intrinsic skin aging. A critical appraisal of the role of hormones. Hautarzt. 2003;54:825–32. doi: 10.1007/s00105-003-0581-7. [DOI] [PubMed] [Google Scholar]
- [102].Sator PG, Schmidt JB, Sator MO, Huber JC, Hönigsmann H. The influence of hormone replacement therapy on skin ageing: a pilot study. Maturitas. 2001;39:43–55. doi: 10.1016/s0378-5122(00)00225-5. [DOI] [PubMed] [Google Scholar]
- [103].Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest. 2002;110:615–24. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119:722–32. doi: 10.1242/jcs.02786. [DOI] [PubMed] [Google Scholar]
- [105].Goldstein J, Fletcher S, Roth E, Wu C, Chun A, Horsley V. Calcineurin/Nfatc1 signaling links skin stem cell quiescence to hormonal signaling during pregnancy and lactation. Genes Dev. 2014;28(9):983–294. doi: 10.1101/gad.236554.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Inui S, Itami S, Pan HJ, Chang C. Lack of androgen receptor transcriptional activity in human keratinocytes. J Dermatol Sci. 2000;23:87–92. doi: 10.1016/s0923-1811(99)00091-2. [DOI] [PubMed] [Google Scholar]
- [107].Jave-Suarez LF, Langbein L, Winter H, Praetzel S, Rogers MA, Schweizer J. Androgen regulation of the human hair follicle: the type I hair keratin hHa7 is a direct target gene in trichocytes. J Invest Dermatol. 2004;122:555–64. doi: 10.1111/j.0022-202X.2004.22336.x. [DOI] [PubMed] [Google Scholar]
- [108].Itami S, Kurata S, Takayasu S. Androgen induction of follicular epithelial cell growth is mediated via insulin-like growth factor-I from dermal papilla cells. Biochem Biophys Res Commun. 1995;212:988–94. doi: 10.1006/bbrc.1995.2067. [DOI] [PubMed] [Google Scholar]
- [109].Scheib D. Effects and role of estrogens in avian gonadal differentiation. Differentiation. 1983;23:S87–92. doi: 10.1007/978-3-642-69150-8_15. [DOI] [PubMed] [Google Scholar]
- [110].Bruggeman V, Van As P, Decuypere E. Developmental endocrinology of the reproductive axis in the chicken embryo. Comp Biochem Physiol A Mol Integr Physiol. 2002;131:839–46. doi: 10.1016/s1095-6433(02)00022-3. [DOI] [PubMed] [Google Scholar]
- [111].Burke WH, Henry MH. Gonadal development and growth of chickens and turkeys hatched from eggs injected with an aromatase inhibitor. Poult Sci. 1999;78:1019–1033. doi: 10.1093/ps/78.7.1019. [DOI] [PubMed] [Google Scholar]
- [112].Yang X, Zheng J, Na R, Li J, Xu G, Qu L, et al. Degree of sex differentiation of genetic female chicken treated with different doses of an aromatase inhibitor. Sex Dev. 2008;2:309–15. doi: 10.1159/000195680. [DOI] [PubMed] [Google Scholar]
- [113].Herremans M, Berghman L, Decuypere E, Vandesande F. Immunocytochemical demonstration of progesterone and estrogen receptors in feathers and skin of adult hens. Acta Biol Hung. 1993;44:353–66. [PubMed] [Google Scholar]
- [114].Matsumine H, Herbst MA, Ou SH, Wilson JD, McPhaul MJ. Aromatase mRNA in the extragonadal tissues of chickens with the henny-feathering trait is derived from a distinctive promoter structure that contains a segment of a retroviral long terminal repeat. Functional organization of the Sebright, Leghorn, and Campine aromatase genes. J Biol Chem. 1991;266:19900–7. [PubMed] [Google Scholar]
- [115].Batrinos ML. The endocrinology of baldness. Hormones. 2014;13:197–212. doi: 10.1007/BF03401334. [DOI] [PubMed] [Google Scholar]
- [116].Hibberts NA, Howell AE, Randall VA. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol. 1998;156:59–65. doi: 10.1677/joe.0.1560059. [DOI] [PubMed] [Google Scholar]
- [117].Hillmer AM, Hanneken S, Ritzmann S, Becker T, Freudenberg J, Brockschmidt FF, et al. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Am J Hum Genet. 2005;77:140–8. doi: 10.1086/431425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–53. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- [119].Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008;128:262–9. doi: 10.1038/sj.jid.5700999. [DOI] [PubMed] [Google Scholar]
- [120].Chesire DR, Isaacs WB. Ligand-dependent inhibition of beta-catenin/TCF signaling by androgen receptor. Oncogene. 2002;21:8453–69. doi: 10.1038/sj.onc.1206049. [DOI] [PubMed] [Google Scholar]
- [121].Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002;16:1967–9. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- [122].Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–17. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Li W, Man XY, Li CM, Chen JQ, Zhou J, Cai SQ, et al. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp Cell Res. 2012;318:1633–40. doi: 10.1016/j.yexcr.2012.05.003. [DOI] [PubMed] [Google Scholar]
- [124].Bassino E, Gasparri F, Giannini V, Munaron L. Paracrine cross-talk between human hair follicle dermal papilla cells and microvascular endothelial cells. Exp Dermatol. 2015;24:388–90. doi: 10.1111/exd.12670. [DOI] [PubMed] [Google Scholar]
- [125].Conrad F, Ohnemus U, Bodo E, Bettermann A, Paus R. Estrogens and human scalp hair growth-still more questions than answers. J Invest Dermatol. 2004;122:840. doi: 10.1111/j.0022-202X.2004.22344.x. [DOI] [PubMed] [Google Scholar]
- [126].Moverare S, Lindberg MK, Faergemann J, Gustafsson JA, Ohlsson C. Estrogen receptor a, but not estrogen receptor b, is involved in the regulation of the hair follicle cycling as well as the thickness of epidermis in male mice. J Invest Dermatol. 2002;119:1053–8. doi: 10.1046/j.1523-1747.2002.00637.x. [DOI] [PubMed] [Google Scholar]
- [127].Montagna W. The structure and function of skin. 2nd Edit Academic Press; New York: 1962. [Google Scholar]
- [128].Oh HS, Smart RC. An estrogen receptor pathway regulates the telogen:anagen hair follicle transition and influences epidermal cell proliferation. Proc Natl Acad Sci U S A. 1996;93:12525–30. doi: 10.1073/pnas.93.22.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hu HM, Zhang SB, Lei XH, Deng ZL, Guo WX, Qiu ZF, et al. Estrogen leads to reversible hair cycle retardation through inducing premature catagen and maintaining telogen. PLoS One. 2012;7:e40124. doi: 10.1371/journal.pone.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Robinson JE, Forsdike RA, Taylor JA. Taylor. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140:5797–805. doi: 10.1210/endo.140.12.7205. [DOI] [PubMed] [Google Scholar]
- [131].Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77:167–72. doi: 10.1016/s0015-0282(01)02947-8. [DOI] [PubMed] [Google Scholar]
- [132].Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847. doi: 10.1016/j.jaad.2014.05.007. [DOI] [PubMed] [Google Scholar]
- [133].Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–21. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- [134].Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet. 2012;13:163–74. doi: 10.1038/nrg3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell. 2010;19:612–24. doi: 10.1016/j.devcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–4. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Lindeman RE, Gearhart MD, Minkina A, Krentz AD, Bardwell VJ, Zarkower D. Sexual Cell-Fate Reprogramming in the Ovary by DMRT1. Curr Biol. 2015 doi: 10.1016/j.cub.2015.01.034. pii: S0960-9822(15)00066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat Cell Biol. 2011;13:506–12. doi: 10.1038/ncb0511-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J Cell Biol. 2011;193:257–66. doi: 10.1083/jcb.201010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–4. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- [141].Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105:1493–9. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 2007;3:226–37. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]
- [143].Giangreco A, Qin M, Pintar JE, Watt FM. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell. 2008;7:250–9. doi: 10.1111/j.1474-9726.2008.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Chen CC, Murray PJ, Jiang TX, Plikus MV, Chang YT, Lee OK, et al. Regenerative hair waves in aging mice and extra-follicular modulators follistatin, dkk1, and sfrp4. J Invest Dermatol. 2014;134:2086–96. doi: 10.1038/jid.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Garza LA, Yang CC, Zhao T, Blatt HB, Lee M, He H, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121:613–22. doi: 10.1172/JCI44478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Keyes BE, Segal JP, Heller E, Lien WH, Chang CY, Guo X, et al. Nfatc1 orchestrates aging in hair follicle stem cells. Proc Natl Acad Sci U S A. 2013;110:E4950–9. doi: 10.1073/pnas.1320301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- [148].Gopinath SD, Rando TA. Stem Cell Review Series: Aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–8. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- [149].Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–37. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- [150].Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–11. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- [151].Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–9. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- [152].Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–65. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the Sympathetic Nervous System Regulate Hematopoietic Stem Cell Egress from Bone Marrow. Cell. 2006;124:407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- [154].Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem Cell Regulation via Dynamic Interactions of the Nervous and Immune Systems with the Microenvironment. Cell Stem Cell. 2008;3:484–92. doi: 10.1016/j.stem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- [155].Kandyba E, Leung Y, Chen YB, Widelitz R, Chuong CM, Kobielak K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proc Natl Acad Sci U S A. 2013;110:1351–6. doi: 10.1073/pnas.1121312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Yue Z, Jiang TX, Wu P, Widelitz RB, Chuong CM. Sprouty/FGF signaling regulates the proximal-distal feather morphology and the size of dermal papillae. Dev Biol. 2012;372:45–54. doi: 10.1016/j.ydbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Chu Q, Cai L, Fu Y, Chen X, Yan Z, Lin X, et al. Dkk2/Frzb in the dermal papillae regulates feather regeneration. Dev Biol. 2014;387:167–78. doi: 10.1016/j.ydbio.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]