Abstract
AIM: To investigate maspin expression in tumorigenesis and progression of gastric cancer and to explore its relevant molecular mechanisms.
METHODS: Formalin-fixed and paraffin-embedded tissues from normal mucosa (n = 182), dysplasia (n = 69), cancer (n = 113) of the stomach were studied for maspin expression by immunohistochemistry. Microvessel density (MVD) in gastric cancer was labeled using anti-CD34 antibody. Maspin expression was compared with clinical parameters and MVD of tumors. Caspase-3 expression was also detected in gastric carcinoma by immunohistochemistry. The relationship between Caspase-3 and maspin expression was concerned as well.
RESULTS: The positive rates of maspin expression were 79.8% (145/182), 75.4% (52/69) and 50.4% (57/113) in normal mucosa, dysplasia and cancer of the stomach, respectively. Cancer less frequently expressed maspin than normal mucosa and dysplasia (P < 0.05). Maspin expression showed a significantly negative association with invasive depth, metastasis, Lauren’s and Nakamura’s classification (P < 0.05), but not with tumor size, Borrmann’s classification, growth pattern or TNM staging (P > 0.05). The positive rate of Caspase-3 was significantly lower in gastric cancer than in normal gastric mucosa (P < 0.05,32.7% vs 50.4%). It was noteworthy that maspin expression was negatively correlated with MVD, but positively correlated with expression of Caspase-3 in gastric cancer (P < 0.05).
CONCLUSION: Down-regulated maspin expression is a late molecular event in gastric carcinogenesis. Reduced expression of maspin contributes to progression of gastric cancer probably by inhibiting cell adhesion, enhancing cell mobility, decreasing cell apoptosis and facilitating angiogenesis. Additionally altered expression of maspin underlies the molecular mechanism of differentiation of gastric cancer and supports the different histogenetic pathways of intestinal and diffuse gastric cancers. Maspin expression can be considered as an effective and objective marker to reveal biological behaviors of gastric cancer.
INTRODUCTION
Mammary serine protease inhibitor (maspin) was identified by subtractive hybridization as a candidate tumor suppressor protein in normal mammary epithelial cells[1]. Maspin gene maps to human chromosome 18q21.3-q23, whose cDNA consists of 2584 nucleotides encoding for a 42 ku peptide[2]. A number of findings support its inhibitory effects on tumors. Levels of maspin expression showed an inverse correlation with progression of malignancies. Mammary carcinoma cells transfected with maspin showed a reduction of tumor growth and metastasis in nude mice, the addition of recombinant maspin decreased the migration potential of breast and prostate cancer cells across a reconstituted basement membrane. More recently, maspin has been shown to inhibit angiogenesis by blocking in vitro migration of vascular endothelial cells and by in vivo inhibition of rat cornea neovascularization[1-8]. It has been documented that maspin transgene expression in mouse mammary gland inhibited Simian virus 40 (SV40) large T-antigen induced breast carcinogenesis and was correlated with increased apoptosis of mammary gland cells[9].
Gastric cancer is one of the commonest malignancies in China, and even in the world. However, the molecular aspects of carcinogenesis and progression of gastric cancer remain elusive[10-19]. The purposes of this study were to examine maspin expression in normal gastric mucosa, gastric dysplasia, gastric cancer, and to compare its expression with clinicopathological features of gastric cancer, and to analyze the correlation of maspin expression with Caspase-3 expression and MVD in gastric cancer.
MATERIALS AND METHODS
Patients and samples
Normal mucosa (n = 182), dysplasia (n = 69), cancer (n = 113) of the stomach were collected from the Second Affiliated Hospital of China Medical University between Sept. 1996 and Feb. 2002. All tissues were fixed in 40 g/L formaldehyde, embedded in paraffin and incised into 4 μm sections. These sections were stained by hematoxylin-and-eosin method to confirm their histological diagnosis and other microscopic characteristics. All patients did not undergo chemotherapy and radiotherapy before operation.
Evaluation of clinicopathological parameters of gastric cancer
Clinicopathological staging for each gastric carcinoma was evaluated according to the TNM system. Gross appearance of the tumors was described according to Borrmann’s classification. Histomorphological architecture of the tumor samples was expressed according to Lauren’s and Nakamura’s classifications. Growth patterns of gastric cancer were classified into mass-,nest-, or diffuse- type. Furthermore, tumor diameter, invasive depth and metastasis were determined.
Immunohistochemistry
Representative and consecutive sections were studied with streptavidin-biotin-peroxidase immunohistochemistry (SABC kit from Boster Biotech.). Anti-maspin, anti-Caspase-3 and anti-CD34 antibodies were purchased from Novocastra, Zhongshan (China) and DAKO respectively. All procedures were implemented according to the instructions of the product. For negative controls, sections were processed as above but treated with PBS (0.01 mol/L, pH7.4) instead of primary antibodies.
Evaluation of maspin and Caspase-3 immunostaining
The immunoreactivity to maspin and Caspase-3 was localized in cytoplasms. One hundred cells were selected and counted from 5 representative fields of each section by two independent observers. The positive percentage of counted cells was graded semi-quantitatively into one of the four-tier scoring system: negative (-), 5%; weakly positive (+), 5% - 25%; moderately positive (++), 25% - 50%; and strongly positive (+++), 50%.
Microvessel density counting
CD34 antibodies were distributed in cell membranes and cytoplasms of vascular endothelial cells. Modified Weidner’s method was used to calculate the microvessel density, which was described as follows. Microvessels in tumor were considered as hot points in vessel counts. Any brown staining endothelial cell or endothelial cell cluster was regarded as a single, countable microvessel. Observers selected such five areas and counted individual microvessels at 400× magnification (i.e. 40× objective lens and 10× ocular lens, 0.1885 mm2 per field).
Statistical analysis
Statistical evaluation was performed using chi-square test to differentiate the rates between different groups, using Spearman correlation test to analyze the rank data, and using one-way ANOVA to differentiate means of different groups. P < 0.05 was considered as statistically significant. SPSS 10.0 software was employed to analyze all data.
RESULTS
Maspin expression in normal mucosa, dysplasia, and cancer of the stomach
Figures 1 A-D show the positive immunoreactivity to maspin in cytoplasms of epithelial, dysplasia and cancer cells of the stomach. As summarized in Table 1, the positive rates of maspin expression were 79.8% (145/182), 75.4% (52/69), and 50.4% (57/113) in normal mucosa, dysplasia and cancer of gastric cancer, respectively. Normal mucosa and dysplasia more frequently expressed maspin than cancer (P < 0.05).
Figure 1.
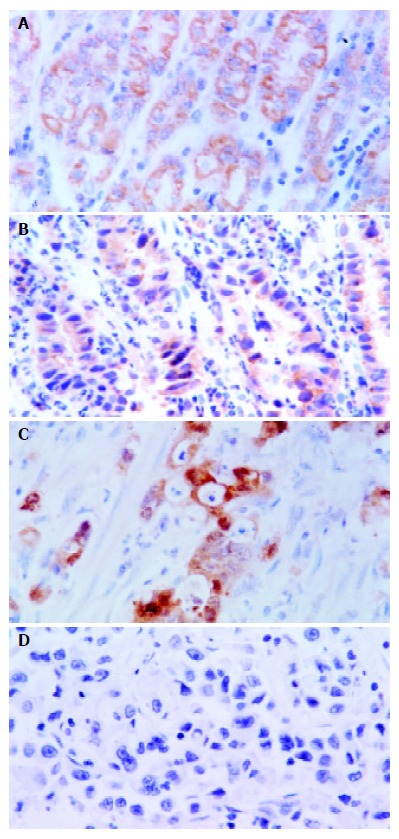
A: Localization of maspin in cytoplasms, and strong expression in gastric epithelial cells (SABC, ×400), B: Strong immunoreactivity of gastric dysplastic cells to maspin (SABC, ×400), C: Strong immunostaining of maspin in gastric papil-lary adenocarcinoma cells (SABC, ×400), D: Negative expres-sion of maspin in poorly-differentiated gastric adenocarcinoma cells (SABC, ×400).
Table 1.
Maspin expression in normal mucosa, dysplasia, and primary cancer of the stomach
| Groups | n |
Maspin expression |
||
| - | +~+++ | % | ||
| Normal mucosa | 182 | 37 | 145 | 79.8b |
| Dysplasia | 69 | 17 | 52 | 75.4d |
| Primary cancer | 113 | 56 | 57 | 50.4 |
Compared with primary cancer
P = 0.000 (Χ2 = 27.589, Pearson’ r = 0.306),
P = 0.000 (Χ2 = 11.075, Pearson’ r = 0.247).
Relationship between maspin expression and clinicopathological features of gastric cancer
Table 2 shows that maspin expression had a significantly negative association with invasive depth, metastasis, Lauren’s and histological classifications (P < 0.05), but not with tumor size, Borrmann’s classification, growth pattern or TNM staging (P > 0.05).
Table 2.
Relationship between maspin expression and clinico-pathological features of gastric cancer
| Clinicopathological features | n |
Maspin expression |
||||||
| - | + | ++ | +++ | % | rs | P value | ||
| Tumor size | 0.000 | 0.999 | ||||||
| < 4 cm | 47 | 22 | 13 | 7 | 5 | 53.2 | ||
| ≥ 4 cm | 66 | 34 | 11 | 15 | 6 | 48.5 | ||
| Borrmann’s classification | 0.014 | 0.896 | ||||||
| I, II | 28 | 12 | 9 | 5 | 2 | 57.1 | ||
| III, IV | 59 | 30 | 10 | 13 | 6 | 49.2 | ||
| Invasive depth | 0.280 | 0.03 | ||||||
| Above submucosa | 26 | 10 | 4 | 6 | 6 | 61.5 | ||
| Muscularis propria | 34 | 12 | 11 | 7 | 4 | 64.7 | ||
| Below subserosa | 53 | 34 | 9 | 9 | 1 | 35.8 | ||
| Metastasis | 0.208 | 0.027 | ||||||
| - | 75 | 31 | 18 | 19 | 7 | 58.7 | ||
| + | 38 | 25 | 6 | 3 | 4 | 34.2 | ||
| TNM staging | 0.004 | 0.967 | ||||||
| O, I | 46 | 22 | 11 | 10 | 3 | 52.2 | ||
| II, III, IV | 67 | 34 | 13 | 12 | 8 | 49.3 | ||
| Growth pattern | 0.032 | 0.767 | ||||||
| Mass | 23 | 13 | 4 | 5 | 1 | 43.5 | ||
| Nest | 30 | 11 | 6 | 8 | 5 | 63.63 | ||
| Diffuse | 34 | 18 | 9 | 5 | 2 | 47.1 | ||
| Lauren’s classification | 0.228 | 0.015 | ||||||
| Intestinal | 36 | 12 | 8 | 11 | 5 | 66.7 | ||
| Diffuse | 57 | 33 | 12 | 8 | 4 | 42.1 | ||
| Mixed | 20 | 11 | 4 | 3 | 2 | 45.0 | ||
| Nakamura’s classification | 0.212 | 0.024 | ||||||
| Differentiated | 53 | 20 | 14 | 12 | 7 | 62.3 | ||
| Undifferentiated | 60 | 30 | 10 | 1 | 4 | 10.0 | ||
| Caspase-3 expression | 0.246 | 0.09 | ||||||
| - | 78 | 44 | 17 | 12 | 5 | 56.4 | ||
| +~+++ | 35 | 12 | 7 | 10 | 6 | 65.7 | ||
Relationship between maspin and Caspsae-3 expression, MVD in gastric cancer
The positive rate of Caspase-3 expression was lower in gastric cancer than in normal gastric mucosa (P < 0.05, 32.7% vs 50.4%). It was noticeable that maspin expression was positively correlated with Caspase-3 expression, but negatively with MVD in gastric cancer (P < 0.05) (Table 2 and Table 3, Figure 2 and Figure 3).
Table 3.
Relationship between maspin expression and MVD in primary gastric cancer
| Maspin expression | n | MVD (mean ± SD) | F value | P value |
| - | 56 | 51.72 ± 26.87 | 4.911 | 0.029 |
| +~+++ | 57 | 41.70 ± 20.92 | ||
| Total | 113 | 46.67 ± 24.47 |
Figure 2.
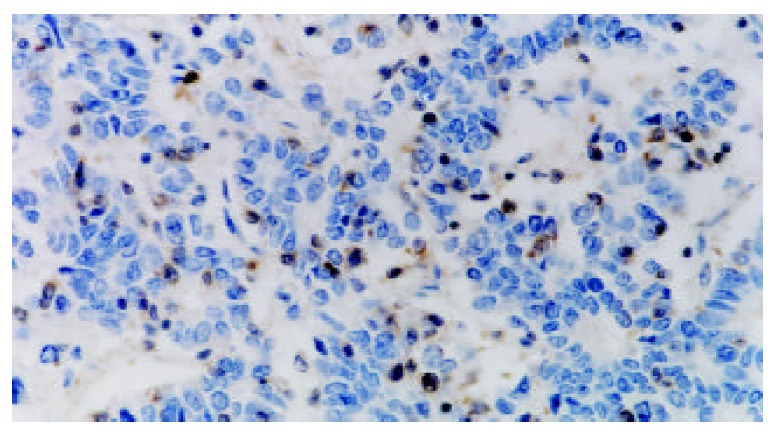
Negatively immunostaining of Caspase-3 in poorly-differentiated gastric adenocarcinoma cells and positive expres-sion in infiltrating lymphocytes (SABC, ×400).
Figure 3.
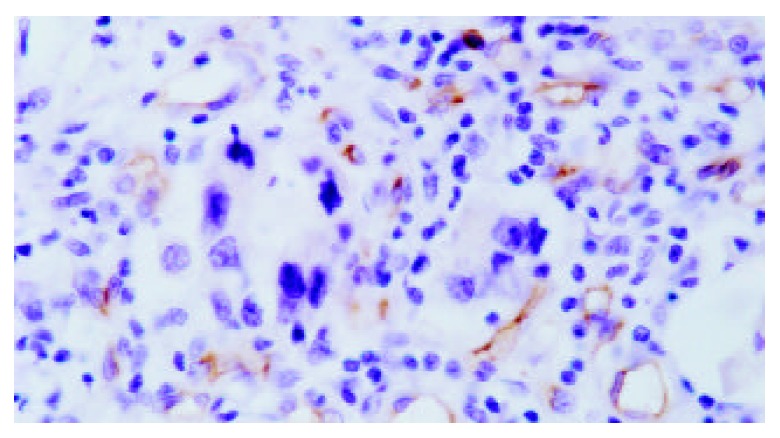
Localization of CD34 antigens in cell membranes and cytoplasms of vascular endothelial cells (SABC, ×400).
DISCUSSION
Carcinogenesis and progression of malignancies are a complicated multistage process that requires the coordination of multiple genes, including oncogenes and tumor suppressor genes. Genomic instability is one of the driving forces for tumor development. Among all genetic alterations, inactivation of metastasis suppressor genes has been found to be an important factor because of their contribution to malignant change of normal cells and metastasis of tumor cells[20,21].
It is known that maspin is highly expressed in various kinds of normal cells and lowly expressed in cancer cells. Our data showed that maspin expression was gradually reduced from normal mucosa, through dysplasia to cancer of the stomach. The positive rate of maspin expression was lower in gastric cancer than in normal gastric mucosa and gastric dysplasia with no significant difference in the latter two. These results suggested that reduced maspin expression contributed to malignant change of gastric epithelial cells. The down-regulated expression of maspin could be considered as a late molecular event of gastric carcinogenesis. Previous reports indicated that DNA methylation, histone deacetylation or LOH was partially responsible for the silencing of maspin gene expression[22-24]. Decreased expression of maspin might be attributable to these genetic alterations in gastric carcinogenesis as mentioned above.
Our study showed that maspin expression was negatively associated with invasion and metastasis of tumors, suggesting its inhibitory effects on progression of gastric cancer. Biliran et al[25] found that maspin could specifically inhibit cell surface-associated urokinase-type plasminogen activator and fibrinogen-bound tissue-type plasminogen activator, which was correlated with significantly decreased cell invasion potential and motility in vitro. Blacque et al[26] reported that interaction between recombinant maspin and some collagens might contribute to cell adhesion, cell migration and angiogenesis. Seftor et al[27] demonstrated that maspin was able to regulate integrin expression, indicating that maspin could reduce the invasive phenotype of cancer cells by altering their integrin profile. These findings suggested that maspin expression could inhibit tumor progression in vivo, likely through a combination of increased cell adhesion, decreased angiogenesis, and inhibition of tumor cell migration.
Additionally, it was found that undifferentiated gastric carcinomas had a lower expression of maspin as compared with the differentiated ones,suggesting that down-regulated expression of maspin was closely associated with the differentiation of gastric cancer. Diffuse-type gastric cancers had less expression of maspin as compared with the intestinal-type ones. It supported that there were different tumorigenetic pathways between diffuse-type and intestinal-type gastric carcinomas. Diffuse-type gastric cancer, the main part of which was undifferentiated carcinoma, displayed a diffusely invasive growth pattern. It is possible that down-regulation of maspin expression could influence mobility and adhesion of cancer cells.
Our study also showed a negative correlation between maspin expression and MVD in gastric cancer. Song et al[28] found that maspin-positive colonic adenocarcinomas showed less MVD than maspin-negative ones. Zhang et al[8] reported that maspin might act directly on vascular endothelial cells to stop their migration towards basic fibroblast growth factor and vascular endothelial growth factor and to limit mitogenesis and tube formation, which could dramatically reduce tumor-associated MVD. These in vivo and in vitro data suggest that the tumor suppressor activity of maspin may depend in large part on its ability to inhibit angiogenesis and raise the possibility that maspin and similar serpins may be excellent targets for the development of drugs that modulate angiogenesis.
Furthermore, it was found that Caspase-3 expression was increased in gastric cancer with maspin positively expressed. Jiang et al[29] reported that endogenous maspin expression could enhance staurosporine-induced apoptosis of carcinoma cells as judged by the increased fragmentation of DNA, increased proteolytic inactivation of poly-[ADP-ribose]-polymerase, as well as the increased activation of Caspase-8 and Caspase-3. Li et al[30] showed that apoptosis induced by manganese-containing superoxide dismutase was associated with elevated maspin expression level. These results indicated that it was possible that maspin expression might inhibit progression of gastric cancer by inducing apoptosis.
In summary, down-regulated maspin expression is a late molecular event in gastric carcinogenesis. Reduced expression of maspin can contribute to progression of gastric cancer by inhibiting cell adhesion, enhancing cell mobility, decreasing cell apoptosis and facilitating angiogenesis. Additionally altered expression of maspin underlies the molecular mechanism of differentiation of gastric cancer and supports the different histogenetic pathways of intestinal and diffuse gastric cancers. Maspin expression can be considered as an effective marker to reveal biological behaviors of gastric cancer.
Footnotes
Edited by Wang XL Proofread by Zhu LH
References
- 1.Maass N, Hojo T, Zhang M, Sager R, Jonat W, Nagasaki K. Maspin--a novel protease inhibitor with tumor-suppressing activity in breast cancer. Acta Oncol. 2000;39:931–934. doi: 10.1080/02841860050215909. [DOI] [PubMed] [Google Scholar]
- 2.Reis-Filho JS, Torio B, Albergaria A, Schmitt FC. Maspin expression in normal skin and usual cutaneous carcinomas. Virchows Arch. 2002;441:551–558. doi: 10.1007/s00428-002-0710-1. [DOI] [PubMed] [Google Scholar]
- 3.Kim DH, Yoon DS, Dooley WC, Nam ES, Ryu JW, Jung KC, Park HR, Sohn JH, Shin HS, Park YE. Association of maspin expression with the high histological grade and lymphocyte-rich stroma in early-stage breast cancer. Histopathology. 2003;42:37–42. doi: 10.1046/j.1365-2559.2003.01567.x. [DOI] [PubMed] [Google Scholar]
- 4.Odero-Marah VA, Khalkhali-Ellis Z, Schneider GB, Seftor EA, Seftor RE, Koland JG, Hendrix MJ. Tyrosine phosphorylation of maspin in normal mammary epithelia and breast cancer cells. Biochem Biophys Res Commun. 2002;295:800–805. doi: 10.1016/s0006-291x(02)00764-7. [DOI] [PubMed] [Google Scholar]
- 5.Zou Z, Zhang W, Young D, Gleave MG, Rennie P, Connell T, Connelly R, Moul J, Srivastava S, Sesterhenn I. Maspin expression profile in human prostate cancer (CaP) and in vitro induction of Maspin expression by androgen ablation. Clin Cancer Res. 2002;8:1172–1177. [PubMed] [Google Scholar]
- 6.Shi HY, Liang R, Templeton NS, Zhang M. Inhibition of breast tumor progression by systemic delivery of the maspin gene in a syngeneic tumor model. Mol Ther. 2002;5:755–761. doi: 10.1006/mthe.2002.0602. [DOI] [PubMed] [Google Scholar]
- 7.Streuli CH. Maspin is a tumour suppressor that inhibits breast cancer tumour metastasis in vivo. Breast Cancer Res. 2002;4:137–140. doi: 10.1186/bcr437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Shi Y, Magit D, Furth PA, Sager R. Reduced mammary tumor progression in WAP-TAg/WAP-maspin bitransgenic mice. Oncogene. 2000;19:6053–6058. doi: 10.1038/sj.onc.1204006. [DOI] [PubMed] [Google Scholar]
- 10.Yin T, Ji XL, Shen MS. Relationship between lymph node sinuses with blood and lymphatic metastasis of gastric cancer. World J Gastroenterol. 2003;9:40–43. doi: 10.3748/wjg.v9.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Kuang LG, Zheng HC, Li JY, Wu DY, Zhang SM, Xin Y. PTEN encoding product: a marker for tumorigenesis and progression of gastric carcinoma. World J Gastroenterol. 2003;9:35–39. doi: 10.3748/wjg.v9.i1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang YA, Zhang YY, Luo HS, Xing SF. Mast cell density and the context of clinicopathological parameters and expression of p185, estrogen receptor, and proliferating cell nuclear antigen in gastric carcinoma. World J Gastroenterol. 2002;8:1005–1008. doi: 10.3748/wjg.v8.i6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002;8:994–998. doi: 10.3748/wjg.v8.i6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, Yang JM. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8:987–993. doi: 10.3748/wjg.v8.i6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang DC, Luo YH, Yang SM, Li XA, Ling XL, Fang L. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J Gastroenterol. 2002;8:787–791. doi: 10.3748/wjg.v8.i5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS,VEGF,tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591–595. doi: 10.3748/wjg.v8.i4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao XX, Yin L, Sun ZC. The expression of hTERT mRNA and cellular immunity in gastric cancer and precancerosis. World J Gastroenterol. 2002;8:586–590. doi: 10.3748/wjg.v8.i4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niu WX, Qin XY, Liu H, Wang CP. Clinicopathological analysis of patients with gastric cancer in 1200 cases. World J Gastroenterol. 2001;7:281–284. doi: 10.3748/wjg.v7.i2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin Y, Li XL, Wang YP, Zhang SM, Zheng HC, Wu DY, Zhang YC. Relationship between phenotypes of cell-function differentiation and pathobiological behavior of gastric carcinomas. World J Gastroenterol. 2001;7:53–59. doi: 10.3748/wjg.v7.i1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaeger EB, Samant RS, Rinker-Schaeffer CW. Metastasis suppression in prostate cancer. Cancer Metastasis Rev. 2001;20:279–286. doi: 10.1023/a:1015587411668. [DOI] [PubMed] [Google Scholar]
- 21.Debies MT, Welch DR. Genetic basis of human breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2001;6:441–451. doi: 10.1023/a:1014739131690. [DOI] [PubMed] [Google Scholar]
- 22.Maass N, Biallek M, Rösel F, Schem C, Ohike N, Zhang M, Jonat W, Nagasaki K. Hypermethylation and histone deacetylation lead to silencing of the maspin gene in human breast cancer. Biochem Biophys Res Commun. 2002;297:125–128. doi: 10.1016/s0006-291x(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 23.Spring P, Nakashima T, Frederick M, Henderson Y, Clayman G. Identification and cDNA cloning of headpin, a novel differentially expressed serpin that maps to chromosome 18q. Biochem Biophys Res Commun. 1999;264:299–304. doi: 10.1006/bbrc.1999.1453. [DOI] [PubMed] [Google Scholar]
- 24.Domann FE, Rice JC, Hendrix MJ, Futscher BW. Epigenetic silencing of maspin gene expression in human breast cancers. Int J Cancer. 2000;85:805–810. doi: 10.1002/(sici)1097-0215(20000315)85:6<805::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Biliran H, Sheng S. Pleiotrophic inhibition of pericellular urokinase-type plasminogen activator system by endogenous tumor suppressive maspin. Cancer Res. 2001;61:8676–8682. [PubMed] [Google Scholar]
- 26.Blacque OE, Worrall DM. Evidence for a direct interaction between the tumor suppressor serpin, maspin, and types I and III collagen. J Biol Chem. 2002;277:10783–10788. doi: 10.1074/jbc.M110992200. [DOI] [PubMed] [Google Scholar]
- 27.Seftor RE, Seftor EA, Sheng S, Pemberton PA, Sager R, Hendrix MJ. maspin suppresses the invasive phenotype of human breast carcinoma. Cancer Res. 1998;58:5681–5685. [PubMed] [Google Scholar]
- 28.Song SY, Lee SK, Kim DH, Son HJ, Kim HJ, Lim YJ, Lee WY, Chun HK, Rhee JC. Expression of maspin in colon cancers: its relationship with p53 expression and microvessel density. Dig Dis Sci. 2002;47:1831–1835. doi: 10.1023/a:1016409031562. [DOI] [PubMed] [Google Scholar]
- 29.Jiang N, Meng Y, Zhang S, Mensah-Osman E, Sheng S. Maspin sensitizes breast carcinoma cells to induced apoptosis. Oncogene. 2002;21:4089–4098. doi: 10.1038/sj.onc.1205507. [DOI] [PubMed] [Google Scholar]
- 30.Li JJ, Colburn NH, Oberley LW. Maspin gene expression in tumor suppression induced by overexpressing manganese-containing superoxide dismutase cDNA in human breast cancer cells. Carcinogenesis. 1998;19:833–839. doi: 10.1093/carcin/19.5.833. [DOI] [PubMed] [Google Scholar]


