Abstract
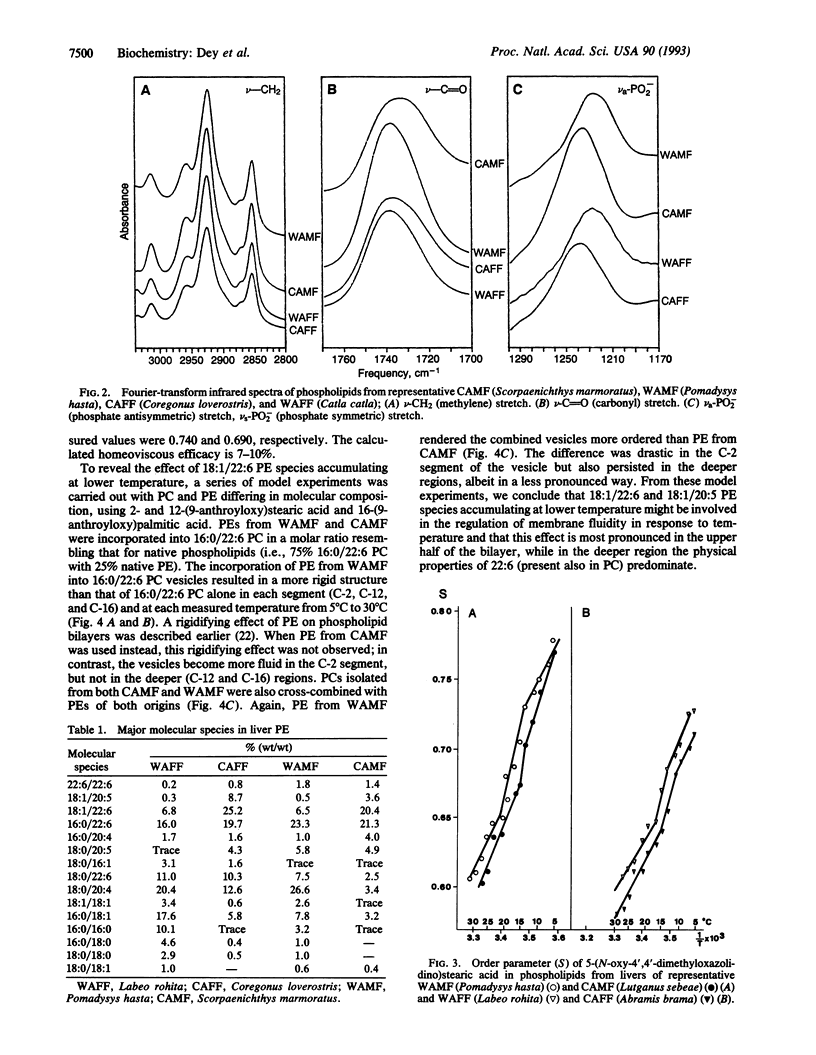
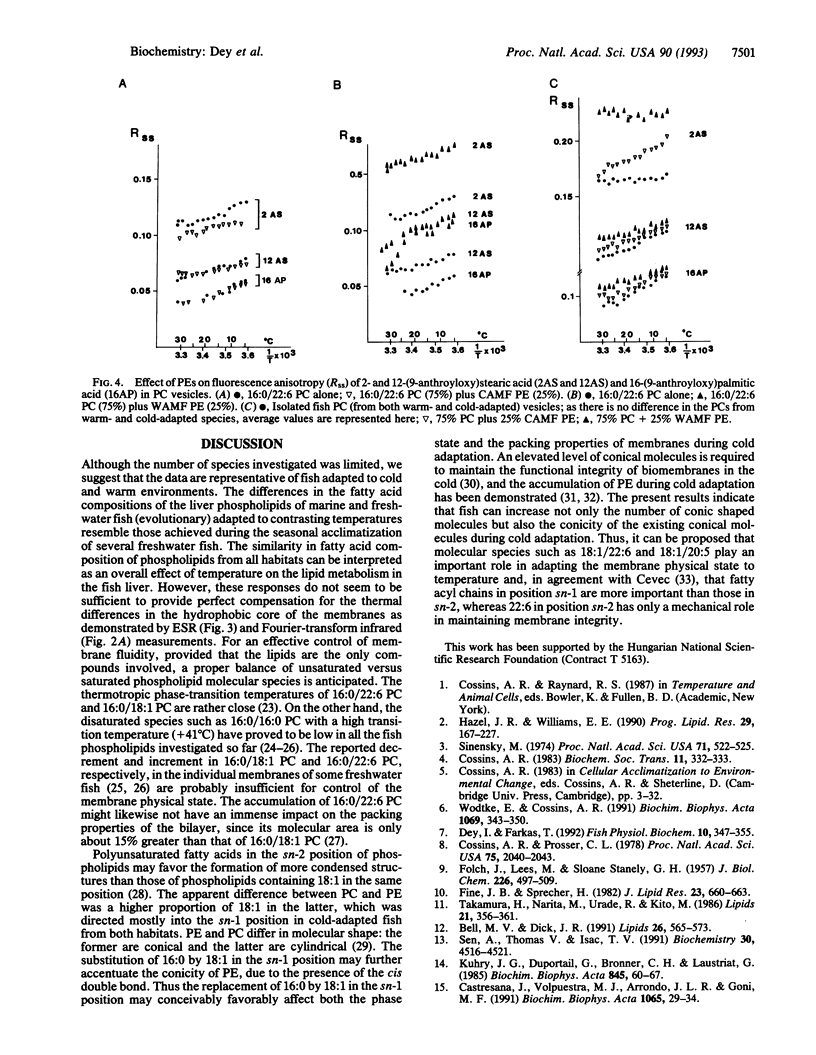
The compositions and physical states of the liver phospholipids of marine and freshwater fish adapted to relatively constant but radically different temperatures were investigated. Fish adapted to low temperature (5-10 degrees C) accumulated more unsaturated fatty acids than those in a warm (25-27 degrees C) environment. There were no measurable differences in the gross fatty acid compositions of the total liver phospholipids from identical thermal environments. Docosahexaenoic acid (22:6) did not seem to participate in the process of adaptation. Cold adaptation was coincidental with oleic acid (18:1) accumulation, preferentially in the phosphatidylethanolamine. Determination of the molecular species composition of phosphatidylethanolamine revealed a 2- to 3-fold and 10-fold increase in the level of 18:1/22:6 and 18:1/20:5 species, respectively. ESR spectroscopy revealed a 7-10% compensation in the ordering state of native phospholipids with temperature. Combination of 16:0/22:6 phosphatidylcholine with phosphatidylethanolamines of cold-adapted marine fish showed a drastic fluidization near the C-2 segment of the bilayer, but not in the deeper regions. An appropriate combination (75:25) of phosphatidylcholines from warmth-adapted marine fish with phosphatidylethanolamines from cold-adapted marine fish mimicked a 100% adaptational efficacy in the C-2 segment as compared with the phosphatidylethanolamines of warmth-adapted marine fish. A specific role of 18:1/22:6 phosphatidylethanolamine in controlling membrane structure and physical state with thermal adaptation is proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applegate K. R., Glomset J. A. Effect of acyl chain unsaturation on the packing of model diacylglycerols in simulated monolayers. J Lipid Res. 1991 Oct;32(10):1645–1655. [PubMed] [Google Scholar]
- Castresana J., Valpuesta J. M., Arrondo J. L., Goñi F. M. An infrared spectroscopic study of specifically deuterated fatty-acyl methyl groups in phosphatidylcholine liposomes. Biochim Biophys Acta. 1991 May 31;1065(1):29–34. doi: 10.1016/0005-2736(91)90006-t. [DOI] [PubMed] [Google Scholar]
- Coolbear K. P., Berde C. B., Keough K. M. Gel to liquid-crystalline phase transitions of aqueous dispersions of polyunsaturated mixed-acid phosphatidylcholines. Biochemistry. 1983 Mar 15;22(6):1466–1473. doi: 10.1021/bi00275a022. [DOI] [PubMed] [Google Scholar]
- Cossins A. R. Adaptive responses of fish membranes to altered environmental temperature. Biochem Soc Trans. 1983 Aug;11(4):332–333. doi: 10.1042/bst0110332. [DOI] [PubMed] [Google Scholar]
- Cossins A. R., Prosser C. L. Evolutionary adaptation of membranes to temperature. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2040–2043. doi: 10.1073/pnas.75.4.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Williams M. A., Tinoco J. Surface areas of 1-palmitoyl phosphatidylcholines and their interactions with cholesterol. Biochem J. 1987 Jul 15;245(2):455–462. doi: 10.1042/bj2450455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fine J. B., Sprecher H. Unidimensional thin-layer chromatography of phospholipids on boric acid-impregnated plates. J Lipid Res. 1982 May;23(4):660–663. [PubMed] [Google Scholar]
- Hazel J. R., Landrey S. R. Time course of thermal adaptation in plasma membranes of trout kidney. II. Molecular species composition. Am J Physiol. 1988 Oct;255(4 Pt 2):R628–R634. doi: 10.1152/ajpregu.1988.255.4.R628. [DOI] [PubMed] [Google Scholar]
- Hazel J. R., Williams E. E. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res. 1990;29(3):167–227. doi: 10.1016/0163-7827(90)90002-3. [DOI] [PubMed] [Google Scholar]
- Kuhry J. G., Duportail G., Bronner C., Laustriat G. Plasma membrane fluidity measurements on whole living cells by fluorescence anisotropy of trimethylammoniumdiphenylhexatriene. Biochim Biophys Acta. 1985 Apr 22;845(1):60–67. doi: 10.1016/0167-4889(85)90055-2. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Martin A., Cameron D. G. Characterization by infrared spectroscopy of the bilayer to nonbilayer phase transition of phosphatidylethanolamines. Biochemistry. 1981 May 26;20(11):3138–3145. doi: 10.1021/bi00514a024. [DOI] [PubMed] [Google Scholar]
- Michaelson D. M., Horwitz A. F., Klein M. P. Head group modulation of membrane fluidity in sonicated phospholipid dispersions. Biochemistry. 1974 Jun 4;13(12):2605–2612. doi: 10.1021/bi00709a021. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Holmgren A., Lindblom G. Fourier-transform infrared spectroscopy study of dioleoylphosphatidylcholine and monooleoylglycerol in lamellar and cubic liquid crystals. Biochemistry. 1991 Feb 26;30(8):2126–2133. doi: 10.1021/bi00222a017. [DOI] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P., Price S. J., Levi A. J. The kinetics of transport of lactate and pyruvate into isolated cardiac myocytes from guinea pig. Kinetic evidence for the presence of a carrier distinct from that in erythrocytes and hepatocytes. Biochem J. 1989 Dec 1;264(2):409–418. doi: 10.1042/bj2640409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Isac T. V., Hui S. W. Bilayer packing stress and defects in mixed dilinoleoylphosphatidylethanolamine and palmitoyloleoylphosphatidylcholine and their susceptibility to phospholipase A2. Biochemistry. 1991 May 7;30(18):4516–4521. doi: 10.1021/bi00232a021. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura H., Narita H., Urade R., Kito M. Quantitative analysis of polyenoic phospholipid molecular species by high performance liquid chromatography. Lipids. 1986 May;21(5):356–361. doi: 10.1007/BF02535701. [DOI] [PubMed] [Google Scholar]
- Wieslander A., Christiansson A., Rilfors L., Lindblom G. Lipid bilayer stability in membranes. Regulation of lipid composition in Acholeplasma laidlawii as governed by molecular shape. Biochemistry. 1980 Aug 5;19(16):3650–3655. doi: 10.1021/bi00557a002. [DOI] [PubMed] [Google Scholar]
- Wodtke E., Cossins A. R. Rapid cold-induced changes of membrane order and delta 9-desaturase activity in endoplasmic reticulum of carp liver: a time-course study of thermal acclimation. Biochim Biophys Acta. 1991 May 7;1064(2):343–350. doi: 10.1016/0005-2736(91)90321-x. [DOI] [PubMed] [Google Scholar]
- Wodtke E. Temperature adaptation of biological membranes. The effects of acclimation temperature on the unsaturation of the main neutral and charged phospholipids in mitochondrial membranes of the carp (Cyprinus carpio L.). Biochim Biophys Acta. 1981 Feb 6;640(3):698–709. doi: 10.1016/0005-2736(81)90100-0. [DOI] [PubMed] [Google Scholar]



