Abstract
AIM: To investigate the effect of acupuncture and moxibustion on epithelial cell apoptosis and expression of Bcl-2, Bax, fas and FasL proteins in rat ulcerative colitis.
METHODS: A rat model of ulcerative colitis was estabelished by immunological methods and local stimulation. All rats were randomly divided into model control group (MC), electro-acupuncture group (EA), herbs-partition moxibustion group (HPM). Normal rats were used as normal control group (NC). Epithelial cell apoptosis and expression of Bcl-2, Bax, fas and FasL proteins were detected by TUNEL and immunohistochemiscal method respectively.
RESULTS: The number of epithelial cell apoptosis in MC was significantly higher than that in NC, and was markedly decreased after the treatment with herbs-partition moxibustion or electro-acupuncture. The expression of Bcl-2, Bax, fas and FasL in colonic epithelial cells in MC was higher than that in NC, and was markedly down- regulated by herbs-partition moxibustion or electro-acupuncture treatment.
CONCLUSION: The pathogenesis of ulcerative colitis in rats involves abnormality of apoptosis. Acupuncture and moxibustion can regulate the expression of Bcl-2, Bax, fas and FasL proteins and inhibit the apoptosis of epithelial cells of ulcerative colitis in rats by Bcl-2/Bax, fas/FasL pathways.
INTRODUCTION
Ulcerative colitis (UC) is a non-specific inflammatory intestinal disease. The pathogenesis of ulcerative colitis involves abnormality of apoptosis which is affected by a variety of factors[1-3]. At present, increasing evidence suggests that acceleration of apoptosis of epithelial cells and inhibition of apoptosis of inflammatory cells (such as neutrophil) are closely associated with colonic tissue injury and immunological abnormality in ulcerative colitis.
Apoptosis is determined by the relative expression of serial genes involved in the regulation of apoptosis. Fas/FasL is one of the important pathways of epithelial cell apoptosis in UC. In tissues of UC, the number of FasL positive cells is significantly increased, resulting in apoptosis. FasL expression increases in the focal region of active UC, which directly promotes apoptosis of Fas expressing colonic epithelium. The apoptosis promoting gene bax also plays an important role in apoptosis. The ratio of bax/bcl-2 determines whether apoptosis occurs or not. Excessive expression of bax promotes apoptosis.
In the present study, a rat model of UC was established by immunological method and local stimulation. After the treatment with electro-acupuncture and herbs-partition moxibustion, the number of colonic epithelial cell apoptosis and the expression of Bcl-2/Bax and Fas/FasL proteins were detected by TUNEL and immunohistochemistry respectively for elucidating the mechanism of acupuncture and moxibustion underlying colonic epithelial cell apoptosis in rat UC.
MATERIALS AND METHODS
Experimental animals and materials
Two hundred male SD rats (weighting 200 ± 20 g) were provided by Experimental Animal Center of Shanghai University of TCM. TUNEL kits was purchased from Boehringer Mannheim (Germany). Bax, Bcl-2 and FasL kits were from Dako (Denmark). Fas was from Santa-cruz (USA).
Methods
Animal model and therapeutic methods Establishment of animal model: According to Experimental Methodology of Pharmacology[4], UC rat model was established by immunological method and local stimulation. Colonic mucosa was prepared from human fresh surgical colonic specimens, homogenized by adding appropriate amount of normal saline and centrifuged for 30 min at 3000 r/min. The supernatant was removed for the measurement of protein concentration and then mixed with Freund adjuvant. The antigen fluid was first injected into the plantar pedis of the model group rats, then into the plantar pedis, dorsum, inguen and abdominal cavity (no Freund adjuvant in the last injection) on the tenth, seventeenth, twenty-fourth and thirty-first day respectively. When a certain titer of serum anti-colonic antibody was reached, 3 mL 3% formalin and 2 mL antigen fluid (no Freund adjuvant) were administered by enema successively. The rats in NC were administrated with normal saline as the same procedure of MC.
Treatment: After the ulcerative colitis rat model was built, the animals were randomly divided into model control group (MC 8), electro-acupuncture group (EA 8), herbs-partition moxibustion group (HPM 8) and normal control group (NC 6). HPM: Moxa cones made of refined mugwort floss were placed on the medicinal formula (medicinal formula dispensing: Radix Aconiti praeparata, cortex Cinnamomi, et al) for Qihai (RN6) and Tianshu (ST 25, bilateral) and ignited. Two moxa cones were used for each treatment once a day and 14 times as a course. EA: Tianshu (bilateral) and Qihai were acupunctured and then stimulated by intermittent pulse with 2 HZ frequency, 4 mA intensity for 20 minutes once a day and 14 times as a course.
After treatment, four gronp rats were killed simultaneously. The distal 6 cm long colons were dissected and reserved in formaldehyde solution.
TUNEL analysis Formalin fixed specimens were embedded in paraffin using standard procedures. Deparaffinised and rehydrated sections were immersed in 3 mL/L H2O2 for 30 min at room temperature and digested with proteinase K for 20 min at 37 °C. The sections were immersed in 1 g/L Triton-100 and then incubated with TUNEL mixture for 1 hour at room temperature, with streptavidin-HRP (1:400) for 30 min at 37 °C. The sections were stained with 0.4 g/L DAB and treated with 3 mL/L H2O2 for 10 min and with hematoxylin for 1 min. The results were observed with light microscopy. Positive reaction was shown by brown color. The apoptotic cells were counted as the mean of cells in 3 visual fields of one section. The data were analysed by q test, using statistical package SPSS.
Immunohistochemistry Formalin fixed specimens were embedded in paraffin using standard procedures. Sections attached on carry sheet glass were autoclaved at 58 °C for 24 h. Deparaffinised and rehydrated sections were immersed in 10 mL/L H2O2 for 20 min and washed three times, each time for 3 min with PBS. Sections were preincubated with 10 mL/L normal goat anti rabbit serum for 20 min at room temperature and then incubated with the first antibodies diluted for 18 h at 4 °C and Envision reagent for 30 min at 37 °C. Sections were stained by 0.4 g/L DAB with 0.3 mL/L H2O2 for 8 min and hematoxylin for 30 s. The results were observed under light microscope.
Positive specimens were used as positive controls. The result of PBS instead of the first antibody was used as negative control. The positive reactions showed brown particles. The positive cells expressing Bcl-2, Bax, fas and FasL were counted as the mean of cells in 3 visual fields of one section. The data were analysed by q test, using statistical package SPSS.
RESULTS
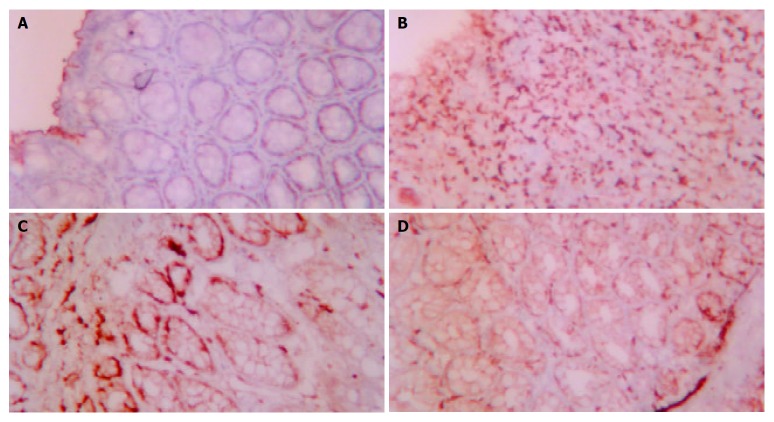
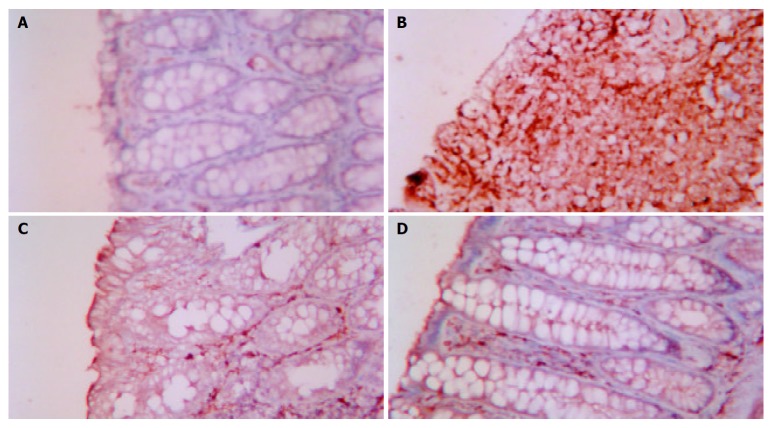
The effect of acupuncture and moxibustion on epithelial cell apoptosis in UC rats is shown in Table 1 and Figure 1 (A-D).
Table 1.
Results of epithelial cell apoptosis in different groups
| Group | n | Number of apoptotic cells (mean ± SD) |
| NC | 6 | 20.61 ± 1.99 |
| MC | 8 | 66.21 ± 8.51b |
| HPM | 8 | 33.58 ± 3.59bd |
| EA | 8 | 34.29 ± 2.70bd |
P < 0.01 vs NC;
P < 0.01 vs MC.
Figure 1.
A: Epithelial cell apoptosis in NC ×200, B: Epithelial cell apoptosis in MC ×200, C: Epithelial cell apoptosis in EA ×200, D: Epithelial cell apoptosis in HPM ×200.
Table 1 shows that the number of epithelial cell apoptosis in MC was significantly increased compared with that of NC (P < 0.01). The number of epithelial cell apoptosis in EA and HPM was remarkedly decreased compared with that of MC (P < 0.01), but was not as low as that of NC (P < 0.01).
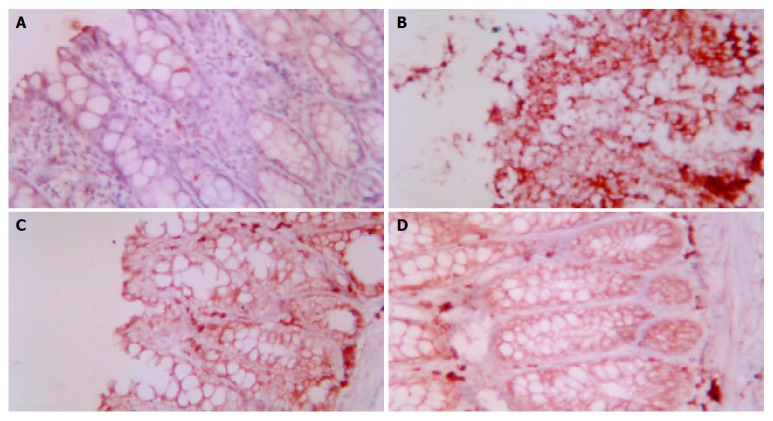
The effect of acupuncture and moxibustion on Bax expression in colonic epithelia of UC rats is shown in Table 2 and Figure 2 (A-D)
Table 2.
Bax expression in colonic epithelia of different groups (mean ± SD)
| Group | n | Area of expression | Intensity of expression |
| NC | 6 | 35905.06 ± 2987.97 | 0.1683 ± 0.0105 |
| MC | 8 | 52451.13 ± 3174.10b | 0.2558 ± 0.0142b |
| HPM | 8 | 39561.50 ± 1382.94d | 0.1900 ± 0.0047d |
| EA | 8 | 38477.79 ± 3309.19d | 0.1796 ± 0.0117d |
P < 0.01 vs NC;
P < 0.01 vs MC.
Figure 2.
A: Bax expression in colonic epithelia of NC ×200, B: Bax expression in colonic epithelia of MC ×200, C: Bax expression in colonic epithelia of EA ×200, D: Bax expression in colonic epithelia of HPM ×200.
Table 2 shows that the area and intensity of Bax expression in the colonic epithelia of MC were significantly increased compared with that of NC (P < 0.01). The area and intensity of Bax expression in the colonic epithelia of PHM and EA were markedly decreased compared with that of MC, but showed no significant difference when compared with that of NC.
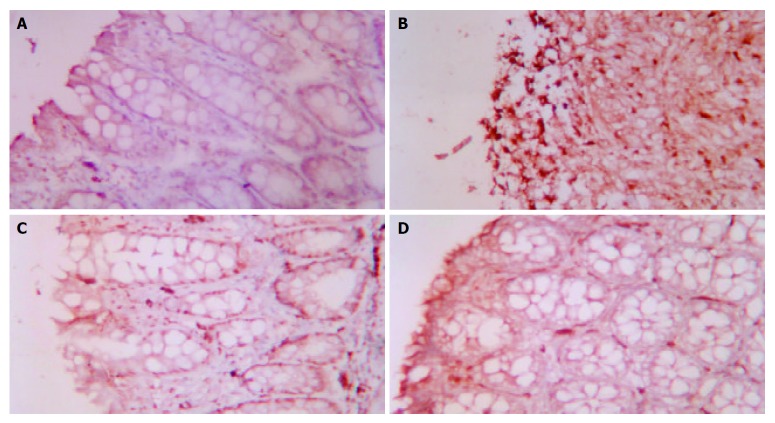
The effect of acupuncture and moxibustion on Bcl-2 expression in the colonic epithelium of UC rats is shown in Table 3 and Figure 3 (A-D)
Table 3.
Bcl-2 expression in colonic epithelia of different groups (mean ± SD)
| Group | n | Area of expression (µm2) | Intensity of expression |
| NC | 6 | 30863.61 ± 2273.44 | 0.1539 ± 0.0114 |
| MC | 8 | 44757.67 ± 28.1.53b | 0.2242 ± 0.0196b |
| HPM | 8 | 40061.63 ± 4937.84be | 0.1979 ± 0.0177bd |
| EA | 8 | 39219.04 ± 3449.84db | 0.1875 ± 0.0133bd |
P < 0.01 vs NC;
P < 0.01 vs MC;
P < 0.05 vs MC.
Figure 3.
A: Bcl-2 expression in colonic epithelia of NC ×200, B: Bcl-2 expression in colonic epithelia of MC ×200, C: Bcl-2 expres-sion in colonic epithelia of EA ×200, D: Bcl-2 expression in colonic epithelia of HPM ×200.
Table 3 shows that the area and intensity of Bcl-2 expression in the colonic epithelia of MC were significantly increased compared with that of NC (P < 0.01). The area and intensity of Bcl-2 expression in the colonic epithelia of PHM and EA were markedly decreased compared with that of MC, but which were not as low as that of NC.
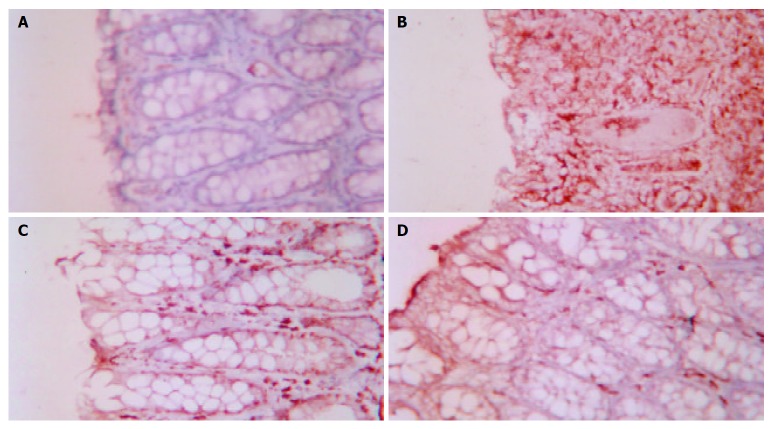
The effect of acupuncture and moxibustion on Fas expression in the colonic epithelium of UC rats is shown in Table 4 and Figure 4 (A-D)
Table 4.
Fas expression in colonic epithelia of different groups (mean ± SD)
| Group | n | Area of expression (µm2) | Intensity of expression |
| NC | 6 | 33764.67 ± 4422.37 | 0.1722 ± 0.0153 |
| MC | 8 | 50262.08 ± 4780.34b | 0.2500 ± 0.0212b |
| HPM | 8 | 37992.29 ± 3239.23de | 0.1825 ± 0.0200d |
| EA | 8 | 38913.21 ± 4669.80de | 0.1850 ± 0.0138d |
P < 0.01 vs NC;
P < 0.01 vs MC;
P < 0.05 vs NC.
Figure 4.
A: Fas expression in colonic epithelia of NC ×200, B: Fas expression in colonic epithelia of MC ×200, C: Fas expression in colonic epithelia of EA ×200, D: Fas expression in colonic epithelia of HPM ×200.
Table 4 shows that the area and intensity of Fas expression in the colonic epithelia of MC were significantly increased compared with that of NC (P < 0.01). The area and intensity of Fas expression in the colonic epithelia of PHM and EA were markedly decreased compared with that of MC(P < 0.01), but still had a significant difference compared with that of NC (P < 0.05).
The effect of acupuncture and moxibustion on FasL expression in the colonic epithelium of UC rats is shown in Table 5 and Figure 5 (A-D)
Table 5.
FasL expression in colonic epithelia of different groups (mean ± SD)
| Group | n | Area of expression (µm2) | Intensity of expression |
| NC | 6 | 33063.56 ± 3347.24 | 0.1561 ± 0.0080 |
| MC | 8 | 44566.58 ± 4637.23b | 0.2600 ± 0.0105b |
| HPM | 8 | 38825.58 ± 2495.51bd | 0.1838 ± 0.0156bd |
| EA | 8 | 38553.29 ± 3489.38bd | 0.1850 ± 0.0108bd |
P < 0.01 vs NC;
P < 0.01 vs MC.
Figure 5.
A: FasL expression in colonic epithelia of NC ×200, B: FasL expression in colonic epithelia of MC ×200, 5: FasL expression in colonic epithelia of EA ×200, D: FasL expression in colonic epithelia of HPM ×200.
Table 5 shows that the area and intensity of FasL expression in the colonic epithelia of MC were significantly increased compared with that of NC(P < 0.01). The area and intensity of FasL expression in the colonic epithelia of PHM and EA were markedly decreased compared with that of MC(P < 0.01), which had a significant difference compared with that of NC(P < 0.01).
DISCUSSION
UC is a non-specific inflammatory intestinal disease. The incidence of UC in our country has an increasing trend yearly. The pathogenesis of ulcerative colitis in rats involved in the abnormality of apoptosis[5-8]. Increasing evidence showed that acceleration of epithelial cell apoptosis and inhibition of inflammatory cell apoptosis were closely associated with colonic tissue injury and immunological abnormality in ucerative colitis[9-11].
Studies showed that cell proliferation, differentiation and apoptosis of epithelial cells in intestines mucosa were a dynamic equilibrium process, and neonate epithelial cells migration along crypt villi from pit cells became mature villous epithelial cells. In physiological condition, apoptosis only occurred on superficial epithelial cells of intestine. In pathologic status, this sequence was destructive. For example, at the area of active inflammation, the apoptotie rate of neonate epithelial cells is accelerated and pit cells were superproliferative[12-14]. This alteration would lead to destruction of epithelial barrier of colon and imbalance of intestinal function of absorption and excretion.
Apoptosis is a procedure of death adjusted by a flock of apoptotic genes, the cell apoptosis was determined by the relative gene expression level of a series of apoptosis genes[15-17]. The bcl-2 gene kindred is an important apoptosis adjusting gene, the position of Bcl-2 protein is at mitochondrial membrane, endoplasmic reticulum and nuclear membrane. As it could prolong the life of cells, it has been generally accepted as an antiapoptosis gene[18-23]. Bax is a new member of bcl-2 gene kindred, it could form a dimer with bcl-2 to inhibit its function[24-27]. The relative expression ratio of bcl-2 and bax determines whether apoptosis happens in cells or not. When expression of bax gained advantage, apoptosis would occur and when the expression of bcl-2 gained advantage, the cells would continue to exist[28-32]. Many studies have shown that abnormal apoptosis in ulcerative colitis could be affected by many agents[33-36].
This study showed that persistent inflammation resulted in the abnormal increase of epithelial cell apoptosis in UC rats. Meanwhile, the area and intensity of Bcl-2 and Bax expression in colonic epithelia of MC were significantly increased compared with that of NC, suggesting that epithelial cell apoptosis is abnormally active. The upregulation of Bcl-2 and Bax expression in colonic epithelia increased the number of apoptotic cells, which might be one of the important mechanisms of colonic pathological changes in UC. After the treatment with electro-acupuncture and herbs-partition moxibustion, the ulceration of colonic tissues in both groups was markedly improved and the tissue structure was well restored. The number of apoptotic cells in colonic tissues in EA and HPM was significantly decreased compared with that of NC. The area and intensity of Bcl-2 and Bax expression in colonic epithelia of PHM and EA were markedly decreased compared with that of MC. Especially, Bax expression was much downregulated. The above results showed that electro-acupuncture and herbs-partition moxibustion could inhibit colonic epithelial cell apoptosis of UC rats by decreasing Bcl-2 and Bax expression. The extent of Bcl-2 and Bax expression in colonic epithelia of UC rats downregulated by acupuncture and herbs-partition moxibustion was different, thus the raletive ratio of Bcl-2 and Bax expression in colonic epithelia was changed due to the inhibition of the abnormal increase of epithelial cell apoptosis in UC. The above results showed that down-regulation of the inflammatory reaction of colonic epthelia in UC rats, inhibition of the injurious effect of a variety of proinflammatory cytokines on colonic tissues, decrease of Bcl-2 and Bax expression of epithelial cells and regulation of the relative ratio of Bcl-2 and Bax expression could change the active state of colonic epithelial cell apoptosis due to its decrease. This is one of the important mechanisms of acupuncture and moxibustion in regulating apoptosis and treating UC.
Many studies[37-39] have shown Fas/FasL is an important pathway of epithelial cell apoptosis in UC. Fas is also named Apo-1 or CD95, belongs to tumour necrosis factor receptor (TNFR) kindred, it has comprehensive expressions in various histocytes and can bind anti-Fas antibody or FasL to change the constitution of cell surface. So it can transmit signals in cells to switch on apoptosis mechanism, leading to apoptosis of cells that express Fas. The function of Fas/FasL is to maintain immune stablity of body and balance of body’s apoptosis[40-44]. Normally colonic epithelium could express Fas, and a small quantity of cells could express FasL in the site where the number of apoptosis cells was markedly increased. When UC occurred, because of stimulation by inflammation, the increase of FasL expression would cause apoptosis[45,46]. The high expression of FasL in active UC could cause apoptosis of cells expressing Fas[47-49], this would accelerate migration and activity of neutrophils and lymphocytes, causing progressive mucosal lesion of UC [50-52]. Therefore, the study of epithelial cell apoptosis may develop a new effective therapeutic approach to UC.
The study showed that the area and intensity of Fas and FasL expression in colonic epithelia of MC were significantly increased compared with that of NC as apoptosis increased, suggesting that the high expression of Fas and FasL plays an important role in epithelial cell apoptosis in UC. After the treatment with electro-acupuncture and herbs-partition moxibustion, Fas and FasL expressions in colonic epithilia of both groups were markedly downregulated compared with MC, and the number of apoptosis cells was also decreased. The above results showed that regulating the abnormal expression of Fas and FasL in colonic tissues of UC rats and decreasing its epithilial cell apoptosis might be one of the important mechanisms of acupuncture and moxibustion in treating UC. Our previous study showed that acupuncture and moxibustion could markedlly inhibit the expression of proinflammatory cytokines such as IL-1β and IL-6[53-56]. It is suggested that acupuncture and moxibustion can regulate Fas and FasL expression in colonic tissues of UC rats, and may be associated with the inhibition of the activation of macrophages in colonic epithilia and decrease of the expression of proinflammatory cytokines such as IL-1β and IL-6. Further activation of macrophages in the blocked initial cascade reaction of inflammation and immunity in colonic epithilia can be effectively controlled. Stimulation of inflammatory cytokines on colonic tissues is relieved and stability of immunological function in UC rats is restored.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30200368 and Shanghai Commission of Science and Technology, No. 02DZ19150-3 and key program of Shanghai and State Administration of Traditional Chinese Medicine of China
Edited by Wang XL
References
- 1.Xia B, Shivananda S, Zhang GS, Yi JY, Crusius JBA, Pe-a AS. Inflammatory bowel disease in Hubei Province of China. China Natl J New Gastroenterol. 1997;3:119–120. doi: 10.3748/wjg.v3.i2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xia B, Guo HJ, Crusius J, Deng CS, Meuwissen S, Pena A. In vitro production of TNF-alpha,IL-6 and sIL-2R in Chinese patients with ulcerative colitis. World J Gastroenterol. 1998;4:252–255. doi: 10.3748/wjg.v4.i3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu QY, Hu XY, Jiang Y. Clinical investigation of ulcerative coli-tis patients treated by integrated traditional Chinese and West-ern medicine. World J Gastroenterol. 1998;4(Suppl 2):93–94. [Google Scholar]
- 4.Xu SY, Bian RL, Chen X. Experimental methodology of pharmacology. Beijing: People's Health Publishing House; 1982. p. 892. [Google Scholar]
- 5.Seidelin JB, Nielsen OH. [Apoptosis in chronic inflammatory bowel disease. The importance for pathogenesis and treatment] Ugeskr Laeger. 2003;165:790–792. [PubMed] [Google Scholar]
- 6.Sasaki S, Yoneyama H, Suzuki K, Suriki H, Aiba T, Watanabe S, Kawauchi Y, Kawachi H, Shimizu F, Matsushima K, et al. Blockade of CXCL10 protects mice from acute colitis and enhances crypt cell survival. Eur J Immunol. 2002;32:3197–3205. doi: 10.1002/1521-4141(200211)32:11<3197::AID-IMMU3197>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Vetuschi A, Latella G, Sferra R, Caprilli R, Gaudio E. Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2002;47:1447–1457. doi: 10.1023/a:1015931128583. [DOI] [PubMed] [Google Scholar]
- 8.Sipos F, Molnár B, Zágoni T, Tulassay Z. [Changes in cell kinetics and clinical course in inflammatory bowel diseases] Orv Hetil. 2002;143:1175–1181. [PubMed] [Google Scholar]
- 9.Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 10.Arai N, Mitomi H, Ohtani Y, Igarashi M, Kakita A, Okayasu I. Enhanced epithelial cell turnover associated with p53 accumulation and high p21WAF1/CIP1 expression in ulcerative colitis. Mod Pathol. 1999;12:604–611. [PubMed] [Google Scholar]
- 11.Bu P, Keshavarzian A, Stone DD, Liu J, Le PT, Fisher S, Qiao L. Apoptosis: one of the mechanisms that maintains unresponsiveness of the intestinal mucosal immune system. J Immunol. 2001;166:6399–6403. doi: 10.4049/jimmunol.166.10.6399. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki S, Yoneyama H, Suzuki K, Suriki H, Aiba T, Watanabe S, Kawauchi Y, Kawachi H, Shimizu F, Matsushima K, et al. Blockade of CXCL10 protects mice from acute colitis and enhances crypt cell survival. Eur J Immunol. 2002;32:3197–3205. doi: 10.1002/1521-4141(200211)32:11<3197::AID-IMMU3197>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Dieckgraefe BK, Crimmins DL, Landt V, Houchen C, Anant S, Porche-Sorbet R, Ladenson JH. Expression of the regenerating gene family in inflammatory bowel disease mucosa: Reg Ialpha upregulation, processing, and antiapoptotic activity. J Investig Med. 2002;50:421–434. doi: 10.1136/jim-50-06-02. [DOI] [PubMed] [Google Scholar]
- 14.Ruemmele FM, Seidman EG. Cytokine--intestinal epithelial cell interactions: implications for immune mediated bowel disorders. Zhonghua Minguo Xiaoerke Yixuehui Zazhi. 1998;39:1–8. [PubMed] [Google Scholar]
- 15.Huang XM. Bcl-2 with its protein and regulation of apoptosis. Foreign Med Sci Sec Mol Biol. 1997;19:16–19. [Google Scholar]
- 16.Wu K, Zhao Y. Investigation progres of apoptosis. Foreign Med Sci Sec Mol Biol. 2001;24:134–136. [Google Scholar]
- 17.Mercer WE, Shields MT, Lin D, Appella E, Ullrich SJ. Growth suppression induced by wild-type p53 protein is accompanied by selective down-regulation of proliferating-cell nuclear antigen expression. Proc Natl Acad Sci USA. 1991;88:1958–1962. doi: 10.1073/pnas.88.5.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohman L, Franzén L, Rudolph U, Birnbaumer L, Hörnquist EH. Regression of Peyer's patches in G alpha i2 deficient mice prior to colitis is associated with reduced expression of Bcl-2 and increased apoptosis. Gut. 2002;51:392–397. doi: 10.1136/gut.51.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan J, Ouyang Q, Liu WP, Li GD, Li FY. Apoptosis and prolif-eration of epithelial cells in ulcerative colitis. Zhonghua Xiaohua Neijing Zazhi. 2001;18:161–163. [Google Scholar]
- 20.Jiang XL, Quan QZ, Sun ZQ, Wang YJ, Qi F, Wang D, Zhang XL. The expression of apoptosis adjust protein of ulcerative colitis patien's lymphocyte. Shijie Huaren Xiaohua Zazhi. 1999;7:903–904. [Google Scholar]
- 21.Mayer B, Oberbauer R. Mitochondrial regulation of apoptosis. News Physiol Sci. 2003;18:89–94. doi: 10.1152/nips.01433.2002. [DOI] [PubMed] [Google Scholar]
- 22.Buduneli E, Genel F, Atilla G, Kütükçüler N. Evaluation of p53, bcl-2, and interleukin-15 levels in gingival crevicular fluid of cyclosporin A-treated patients. J Periodontol. 2003;74:506–511. doi: 10.1902/jop.2003.74.4.506. [DOI] [PubMed] [Google Scholar]
- 23.Sohn SK, Jung JT, Kim DH, Kim JG, Kwak EK, Park Ti, Shin DG, Sohn KR, Lee KB. Prognostic significance of bcl-2, bax, and p53 expression in diffuse large B-cell lymphoma. Am J Hematol. 2003;73:101–107. doi: 10.1002/ajh.10333. [DOI] [PubMed] [Google Scholar]
- 24.Chiu CT, Yeh TS, Hsu JC, Chen MF. Expression of Bcl-2 family modulated through p53-dependent pathway in human hepatocellular carcinoma. Dig Dis Sci. 2003;48:670–676. doi: 10.1023/a:1022816204831. [DOI] [PubMed] [Google Scholar]
- 25.Korkolopoulou P, Lazaris ACh, Konstantinidou AE, Kavantzas N, Patsouris E, Christodoulou P, Thomas-Tsagli E, Davaris P. Differential expression of bcl-2 family proteins in bladder carcinomas. Relationship with apoptotic rate and survival. Eur Urol. 2002;41:274–283. doi: 10.1016/s0302-2838(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 26.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 27.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 28.Korkolopoulou P, Oates J, Kittas C, Crocker J. p53, c-myc p62 and proliferating cell nuclear antigen (PCNA) expression in non-Hodgkin's lymphomas. J Clin Pathol. 1994;47:9–14. doi: 10.1136/jcp.47.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iimura M, Nakamura T, Shinozaki S, Iizuka B, Inoue Y, Suzuki S, Hayashi N. Bax is downregulated in inflamed colonic mucosa of ulcerative colitis. Gut. 2000;47:228–235. doi: 10.1136/gut.47.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller E, Vieth M, Stolte M, Mueller J. The differentiation of true adenomas from colitis-associated dysplasia in ulcerative colitis: a comparative immunohistochemical study. Hum Pathol. 1999;30:898–905. doi: 10.1016/s0046-8177(99)90242-3. [DOI] [PubMed] [Google Scholar]
- 31.Ina K, Itoh J, Fukushima K, Kusugami K, Yamaguchi T, Kyokane K, Imada A, Binion DG, Musso A, West GA, et al. Resistance of Crohn's disease T cells to multiple apoptotic signals is associated with a Bcl-2/Bax mucosal imbalance. J Immunol. 1999;163:1081–1090. [PubMed] [Google Scholar]
- 32.Kraus MD, Shahsafaei A, Antin J, Odze RD. Relationship of Bcl-2 expression with apoptosis and proliferation in colonic graft versus host disease. Hum Pathol. 1998;29:869–875. doi: 10.1016/s0046-8177(98)90459-2. [DOI] [PubMed] [Google Scholar]
- 33.Ilyas M, Tomlinson IP, Hanby AM, Yao T, Bodmer WF, Talbot IC. Bcl-2 expression in colorectal tumors: evidence of different pathways in sporadic and ulcerative-colitis-associated carcinomas. Am J Pathol. 1996;149:1719–1726. [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh J, de La Motte C, Strong SA, Levine AD, Fiocchi C. Decreased Bax expression by mucosal T cells favours resistance to apoptosis in Crohn's disease. Gut. 2001;49:35–41. doi: 10.1136/gut.49.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui YF, Xia GW, Fu XB, Yang H, Peng RY, Zhang Y, Gu QY, Gao YB, Cui XM, Hu WH. Relationship between expression of Bax and Bcl-2 proteins and apoptosis in radiation compound wound healing of rats. Chin J Traumatol. 2003;6:135–138. [PubMed] [Google Scholar]
- 36.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 37.Sträter J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Möller P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology. 1997;113:160–167. doi: 10.1016/s0016-5085(97)70091-x. [DOI] [PubMed] [Google Scholar]
- 38.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–159. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 39.Iwamoto M, Makiyama K, Koji T, Kohno S, Nakane PK. [Expression of Fas and Fas-ligand in epithelium of ulcerative colitis] Nihon Rinsho. 1996;54:1970–1974. [PubMed] [Google Scholar]
- 40.Mountz JD, Zhou T, Su X, Wu J, Cheng J. The role of programmed cell death as an emerging new concept for the pathogenesis of autoimmune diseases. Clin Immunol Immunopathol. 1996;80:S2–14. doi: 10.1006/clin.1996.0136. [DOI] [PubMed] [Google Scholar]
- 41.Nagata S. Fas and Fas ligand: a death factor and its receptor. Adv Immunol. 1994;57:129–144. doi: 10.1016/s0065-2776(08)60672-0. [DOI] [PubMed] [Google Scholar]
- 42.Yukawa M, Iizuka M, Horie Y, Yoneyama K, Shirasaka T, Itou H, Komatsu M, Fukushima T, Watanabe S. Systemic and local evidence of increased Fas-mediated apoptosis in ulcerative colitis. Int J Colorectal Dis. 2002;17:70–76. doi: 10.1007/s003840100340. [DOI] [PubMed] [Google Scholar]
- 43.Coffey JC, Bennett MW, Wang JH, O'Connell J, Neary P, Shanahan F, Redmond HP, Kirwan WO. Upregulation of Fas-Fas-L (CD95/CD95L)-mediated epithelial apoptosis--a putative role in pouchitis. J Surg Res. 2001;98:27–32. doi: 10.1006/jsre.2001.6129. [DOI] [PubMed] [Google Scholar]
- 44.Iwamoto M, Makiyama K, Koji T, Kohno S, Nakane PK. [Expression of Fas and Fas-ligand in epithelium of ulcerative colitis] Nihon Rinsho. 1996;54:1970–1974. [PubMed] [Google Scholar]
- 45.Möller P, von Reyher U, Leithäuser F, Sträter J. [CD95 (APO-1/Fas) and CD95-ligand (CD95L). Implications of these apoptosis mediating receptor/ligand systems in the pathogenesis of autoimmune diseases] Verh Dtsch Ges Pathol. 1996;80:12–20. [PubMed] [Google Scholar]
- 46.Wu HG, Zhou LB, Shi DR, Liu SM, Liu HR, Zhang BM, Chen HP, Zhang LS. Morphological study on colonic pathology in ulcerative colitis treated by moxibustion. World J Gastroenterol. 2000;6:861–865. doi: 10.3748/wjg.v6.i6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97:2820–2828. doi: 10.1111/j.1572-0241.2002.07029.x. [DOI] [PubMed] [Google Scholar]
- 48.Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J Pathol. 2003;199:28–35. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- 49.Brown KA, Back SJ, Ruchelli ED, Markowitz J, Mascarenhas M, Verma R, Piccoli DA, Baldassano RN. Lamina propria and circulating interleukin-6 in newly diagnosed pediatric inflammatory bowel disease patients. Am J Gastroenterol. 2002;97:2603–2608. doi: 10.1111/j.1572-0241.2002.06030.x. [DOI] [PubMed] [Google Scholar]
- 50.Ueyama H, Kiyohara T, Sawada N, Isozaki K, Kitamura S, Kondo S, Miyagawa J, Kanayama S, Shinomura Y, Ishikawa H, et al. High Fas ligand expression on lymphocytes in lesions of ulcerative colitis. Gut. 1998;43:48–55. doi: 10.1136/gut.43.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sträter J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Möller P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology. 1997;113:160–167. doi: 10.1016/s0016-5085(97)70091-x. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki A, Sugimura K, Ohtsuka K, Hasegawa K, Suzuki K, Ishizuka K, Mochizuki T, Honma T, Narisawa R, Asakura H. Fas/Fas ligand expression and characteristics of primed CD45RO+ T cells in the inflamed mucosa of ulcerative colitis. Scand J Gastroenterol. 2000;35:1278–1283. doi: 10.1080/003655200453629. [DOI] [PubMed] [Google Scholar]
- 53.Wu HG, Chen HP, Shi Z, Hua XG, Zhao C. Clinical study of the treatment of chronic ulcerative colitis with moxibustion. Int J Clin Acupunct. 1999;1:26–28. [Google Scholar]
- 54.Wu HG, Shi Z, Zhou LB, Pan YY, Tan WL. Acupuncture and moxibustion's effect on cytokine of ulcerative colitis rat. Int J Clin Acupunct. 2000;3:43–48. [Google Scholar]
- 55.Wu HG, Zhou LB, Pan YY, Huang C, Chen HP, Shi Z, Hua XG. Study of the mechanisms of acupuncture and moxibustion treatment for ulcerative colitis rats in view of the gene expression of cytokines. World J Gastroenterol. 1999;5:515–517. doi: 10.3748/wjg.v5.i6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu HG, Chen HP, Zhou LB, Pan YY, Huang C, Shi Z. molecu mechanism of acupuncture and moxibustion's therapy effect on ulcerative colitis rat. Shanghai Zhenjiu Zazhi. 1998;17:30. [Google Scholar]