Abstract
AIM: To compare the therapeutic effect of transcatheter arterial chemoembolization (TACE), laparoscopic radiofrequency ablation (LRFA), and conservative treatment for the therapy of decompensated liver cirrhosis patients with hepatocellular carcinomas (HCC).
METHODS: Between October 2000 and July 2003, one hundred patients with histologically proven primary HCC and clinical decompensated liver cirrhosis (Child classification B or C) were included in this study. Forty patients received LRFA (LRFA group), twenty received TACE (TACE group), and forty received conservative treatment (control group). We compared the survival, recurrence, and complication rates in these three groups, making adjustment using the tumor metastastic node staging system.
RESULTS: The major complication rate in the TACE group (9/20) was significantly higher than that in the LRFA group (7/40). For patients with TMN stage II HCC, the survival rate of the LRFA group was better than that of the TACE and control groups (P = 0.003) but the recurrence rates befween the LRFA and TACE groups did not differ.
CONCLUSION: The LRFA group of patients had better clinical outcomes in terms of survival and complication rates in comparison with the TACE group or conservative treatment in patients with decompensated liver cirrhosis, especially in TMN patients with stage II HCC. LRFA is thus an appropriate alternative treatment for poor liver function among patients with HCC.
INTRODUCTION
Surgical resection is the preferred treatment for patients with hepatocellular carcinomas (HCC), as it offers the potential for cure of primary hepatic malignancies[1,2]. Unfortunately, only 10% to 20% of patients with HCC are suitable candidates for resection because of constraints of size, location, extent of the tumors[3] or poor liver function. The impaired liver function of HCC patients is thus a major limitation for surgical resection.
Over the last decade, other treatment modalities have been used in the management of these patients with unresectable HCC, such as cryoablation[4], microwave coagulation therapy[5,6], alcohol ablation, laser photocoagulation, high-intensity ultrasound, regional chemotherapy infusion[7], transcatheter arterial chemoembolization (TACE)[8], and radiofrequency ablation (RFA)[9].
Recently both TACE and RFA have received increasing attention as promising treatments for patients with unresectable HCC[4,8]. TACE is a liver-directed therapy that takes advantage of the relatively selective vascularization of hepatic arterial tumors. HCC derives approximately 80% to 85% of their blood supply from the hepatic artery, whereas the portal vein as well as the hepatic artery supply the normal hepatic parenchyma. Chemotherapeutic agents can thus be delivered angiographically with concomitant embolization to increase local chemotherapeutic dwell time and induce tumor ischemia[10].
Investigation and use of thermal ablation have increased with advances in radiofrequency ablation (RFA) technology. This approach has been used to treat small lesions measuring 5 cm or less in diameter, and complete necrosis was achieved in 76% - 100% of lesions[4]. It has few complications while achieving safe[11] and excellent local control[12].
The aim of this study was to compare the therapeutic effect of TACE, laparoscopic RFA (LRFA), and conservative treatment for patients with decompensated liver cirrhosis (Child classification B or C) with HCC.
MATERIALS AND METHODS
Between October 2000 and July 2003, one hundred patients with histologically proven primary hepatocellular carcinoma were included in this study. All patients were Child classification B or C[12] and not suitable to receive surgical resection. Patients with tumor size larger than 5 cm or with more than three tumors were considered suitable for repeated TACE or conservative treatment. Patients with serum total bilirubin concentrations of more than 2 mg/dL were considered for LRFA or conservative treatment. Patients with fewer than three tumors smaller than 5 cm were considered for TACE, LRFA or conservative treatment, according to their own preferences or those of their families (Figure 1). Forty patients received LRFA (LRFA group), twenty received TACE (TACE group), and forty received conservative treatment (control group). We compared the survival, recurrence, and complication rates in these three groups. The comparison was based on the AJCC TMN staging system modified in 1998[13].
Figure 1.
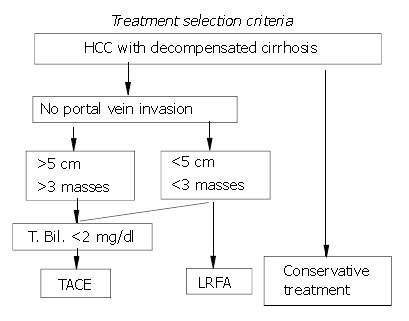
Criteria of treatment selection.
LRFA technique
Patients were considered for LRFA if they had less than three tumors smaller than 5 cm, regardless of the proximity of the lesions to major portal or hepatic vein branches. The LRFA needle was passed transcutaneously under laparoscopic ultrasound guidance in the operation room with the patient under general anesthesia. The Radio Therapeutics RF3000 system (Radio Therapeutics Corp, Mountain View, CA) was used in this study. It uses an insulated monopole LeVeen needle electrode consisting of ten hook-like projections that were deployed after the cannula was inserted into the target tissue. Once in place, power was applied by the RF3000 generator, which can deliver up to 100 W. Power was increased in a stepwise fashion beginning at 50 W until the maximum power was reached. Tumor ablation was continued at maximum power until tissue impedance increased to the point when power output fell rapidly (termed ‘roll-off’). If roll-off was not achieved, ablation was continued at maximum power for 15 min. This procedure was repeated until roll-off, or for 10 min if roll-off could not be achieved.
Small tumors (less than 3 cm) were ablated after a single passage of the electrode array into the center of the lesions. For larger tumors (more than 3 cm), the electrode array was repositioned at 2 cm intervals and ablation was carried out as above to allow complete destruction of the tumor with a 1 cm margin. For tumors located in the posterior segment or lobes of the liver where trans-abdominal needle insertion was impossible, trans-thoracic needle insertion was performed using a chest tube.
TACE technique
Vascular access was obtained via the right common femoral artery and a guide wire was advanced under fluoroscopic guidance. A 5-Freches sheath was then inserted over the guide wire. The superior mesenteric artery was selected and an angiogram was completed to identify any aberrant arterial anatomy and verify portal vein patency. The celiac axis was then selected and an angiogram was completed. The catheter and guide wire were used to select the proper hepatic artery and a limited angiogram was completed to identify the branches of the hepatic artery. The right or left hepatic artery was selected for lesions in the right or left lobe, respectively, and an angiogram was completed. Any tumors were identified using a rapid contrast blush method.
Once the vascular supply of the tumor had been identified, chemoembolization of the supplying artery was started.
Doxorubicin (50 mg: NovaPlus, Novation, Irving, TX) with one-third of a vial of 250-355 µm diameter polyvinyl alcohol particles (Boston Scientific, Natick, MA) was used as the chemoembolic agent. Successful embolization of the feeding vessel was confirmed by angiogram. The catheter and wire were then removed and direct pressure was applied for 20 minutes.
Follow-up
Computed tomographic (CT) scans were obtained from all patients one week postoperatively to document ablation. Follow-up CT scans were obtained every three months for one year and every six months thereafter. Serum Alpha-fetoprotein (AFP) concentrations were also monitored postoperatively. Elevated AFP concentrations, increases in size, or changes in the computer tomography (CT) contrast-enhanced appearance of the original tumors were used to diagnose any tumor recurrence.
Any mortality within one month after surgery (30 days) was recorded. Any complications were registered on a computer database for each patient. Major complications were regarded as any prolongation of stay in hospital caused by hepatic failure, pulmonary embolism, stroke, pneumonia, upper GI bleeding, or refractory ascites. Hepatic failure was defined based on symptoms such as hepatoencephalopathy, varices bleeding and the need for readmission and further treatment. Minor complications were regarded as those that did not cause any extension of hospital stay, such as pneumothorax, wound infections, burns and post embolization fever.
Statistical analysis
Analyses were performed using S-Plus®2000 for Windows statistical software (CANdiensten, Amsterdam, The Netherlands). The level of significance was set at P < 0.05 for all tests. Continuous variables were expressed as mean ± SD, and tested using Student’s t test and categorical variables were tested using Fisher’s exact test. Survival rates were calculated using the Kaplan-Meier method and compared using the log-rank test.
RESULTS
The demographic data of these three groups of patients are summarized in Table 1. The mean ages and follow-up times of the three groups were not different. In this population, males predominated, the main etiology of HCC was hepatitis B virus (HBV) infection and the second was hepatitis C virus infection. This situation is common in Asia. All patients had decompensated liver cirrhosis and most were classified as Child class B, 33/100 were Child class C. Portal vein thromboses only appeared in the control group (n = 12). The mean tumor diameter of the LRFA group was significantly smaller than that of the TACE and control groups (3.2±1.0 cm vs 6.8±3.7 cm and 6.5±3.1 cm, respectively; P < 0.05). All three groups had patients with TNM stage II tumors (Table 2). We chose these patients to compare the survival and recurrence rate among the three different groups. The mean tumor diameter did not differ significantly. It was 3.4±0.8 cm (n = 37) for LRFA patients, 3.7±1.0 cm (n = 9) for TACE patients and 3.6±0.9 (n = 10) for the controls. There were 11/37, 1/9 and 4/10 TMN stage II patients with Child class C, respectively, among these groups (too few for statistical significance).
Table 1.
Demographic data
| LRFA | TACE | Conservative | |
| treatment | |||
| No. of patients | 40 | 20 | 40 |
| Age (years) | 66.5 ± 9.5 | 65.0 ± 7.9 | 69.0 ± 5.7 |
| Sex (male/female) | 35/5 | 17/3 | 32/8 |
| Etiology (HBV/HCV) | 29/11 | 11/9 | 26/14 |
| Child-Pugh class B/C | 28/12 | 17/3 | 22/18 |
| AFP > 400 ng/ml | 22 | 13 | 25 |
| Portal vein thrombosis | 0 | 0 | 12 |
| Tumor diameter (cm): | 3.2 ± 1.0a | 6.8 ± 3.7a | 6.5 ± 3.1 |
| —in TNM stage | 3.4 ± 0.8 (37) | 3.7 ± 1.0 (9) | 3.6 ± 0.9 (10) |
| II tumorsb | |||
| TNM stage I tumors | 3 | 0 | 0 |
| TNM stage II tumors | 37 | 9 | 10 |
| Child grade C in | 11/37 | 1/9 | 4/10 |
| TMN stage II tumors | |||
| —in stage III tumors | 0 | 11 | 14 |
| —in stage IV tumors | 0 | 0 | 16 |
| Mean follow up (months and ranges) | 12.5 (3–30) | 11.3 (2.5–29) | 10.5 (3.1–30) |
Mean tumor diameter in the LRFA group was significantly smaller than that in the TACE group (P < 0.05).
TNM stage was allocated according to the 1998 modified edition[13].
Table 2.
Comparison between LRFA and TACE treatment groups in mortality, complication, and recurrence rates
| LRFA | TACE | P value | |||
| (n = 40) | (n = 20) | ||||
| One-month mortalitya | 1 | (2.5%) | 1 | (5%) | |
| Major complications: total | 7 | (17.5%) | 9 | (45%) | < 0.05 |
| Hepatic failureb | 3 | 3 | |||
| Pulmonary embolism | 0 | 1 | |||
| Stroke | 0 | 1 | |||
| UGIc bleeding | 2 | 2 | |||
| Pneumonia | 1 | 0 | |||
| Refractory ascites | 1 | 2 | |||
| Minor complications: total | 7 | (17.5%) | 7 | (35%) | |
| Pneumothorax | 3 | 0 | |||
| Wound infection | 2 | 0 | |||
| Burns | 2 | 0 | |||
| Post embolization syndromed | 0 | 7 | |||
| Local recurrence rate | |||||
| One year | 12 | 7 | |||
| Two years | 19 | 11 | |||
One-month mortality,
Hepatic failure: readmission due to chronic hepatic failure within three months after procedure,
UGI: upper gastrointestinal tract.
Post embolization syn-dromes included fever, pain, nausea, vomiting, leukocytosis and adynamic ileus.
Two patients died of hepatic failure within one month, one in each experimental group. We excluded these from the survival analysis. The complication and recurrence rates are summarized in Table 2. The major complication rate of the LRFA group was significantly lower than that of the TACE group (P < 0.05). Three patients developed hepatic failure and two developed upper gastrointestinal tract (UGI) bleeding in both treatment groups. One patient in the LRFA group and two in the TACE group developed refractory ascites after treatment. In the TACE group, one patient developed a pulmonary embolism and another had a stroke within three months. In the LRFA group, one patient developed pneumonia within three months. The minor complication and recurrence rates were not different after one and two years.
Patients classified as Child class B or C were also graded with TMN stage II tumors, the survival rate of the LRFA group was better than both the TACE and control groups (Figure 2) (P = 0.003), whereas the recurrence rates for the LRFA and TACE groups were not significantly different (Figure 3).
Figure 2.
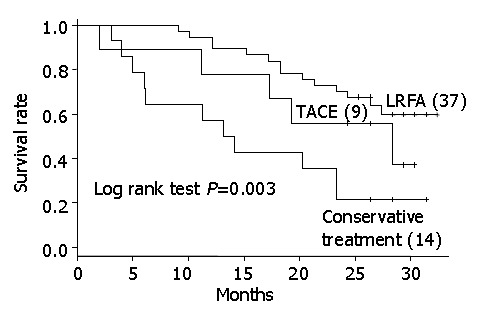
Kaplan-Meier curves of survival rates of TACE-, LRFA- and conservatively-treated groups of patients with TMN stage II hepatocarcinomas.
Figure 3.
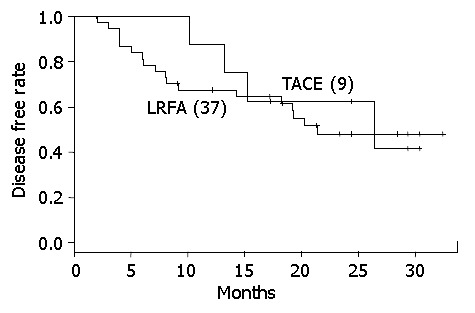
Kaplan-Meier curve of recurrence rate of TACE and LRFA in TMN stage II.
DISCUSSION
It is very difficult to perform randomized clinical trials among patients with HCC, as each treatment modality has its own specific indications and contraindications. In this study, there were some internal biases, such as tumor size and location, occurrence of portal vein thrombosis, treatment modalities, and liver functions. For example, if the tumor was larger than 5 cm or very near the artery, the patient could only choose TACE or conservative treatment. This made it difficult to compare these three different treatment modalities.
Systems for staging and classifying cancers are attracting interest worldwide. Such systems allow a selection between primary and adjuvant therapy, estimation of prognosis, assistance in evaluating the results of treatment, facilitation of the exchange of information among treatment centers, and contribution to the continuing investigation of human cancers[13]. Among these scoring systems, Child classification[14], Okuda, Cancer of the Liver Italian Program (CLIP), Barcelona Clinic Liver Cancer (BCLC), TMN[15], and Child-Pugh staging are the most widely used for classifying patients with HCC. In this study, we needed a staging system that included both liver function parameters and tumor parameters and allowed the patient groups to be statistically comparable. We combined the Child classification (classes B and C patients) and the TMN staging system (less than three tumors with their diameter less than 5 cm) to select a comparable set of patients. Based on this analysis, the survival rate of the LRFA group was better than that of the TACE and control groups. Thus, LRFA is a good adjuvant therapy for decompensated cirrhotic patients with TMN stage II HCC tumors, especially if the tumors are smaller than 5 cm. The mechanism is unknown.
RFA had an advantage over surgical resection or other palliative treatments in that it could spare more normal liver tissue and pose less risk than surgery[16]. It was more effective and required less sessions than percutaneous ethanol injection (PEI), and had fewer complications than cryosurgery[17]. As with resection of liver tumors, the goal of the RFA methods was to destroy the tumor and a small margin of the adjacent normal liver[18]. RFA offered some significant advantages over other palliative techniques, such as brief treatment time, precise production of necrotic lesions, and minimal morbidity. RFA is thus a potentially valuable treatment for patients with unresectable liver tumors. It is safe, effective, and repeatable, and local control of hepatic tumors using RFA has been shown to be effective and to prolong the survival of patients with unresectable or advanced liver tumors[11,19]. We found similar results here.
RFA can be performed by percutaneous, laparoscopic or exploratory surgical means. The laparoscopic approach could offer a minimally invasive procedure with the ability to perform intra-operative laparoscopic ultrasound guidance for better tumor detection and more accurate targeting[20]. The decompensated liver was relatively small and lay low in the hepatic fossa, making the percutaneous RFA needle approach difficult. During laparoscopic RFA, achieving a pneumoperitoneum would cause elevation of the diaphragm, which increased the operative space to avoid adjacent organ injury and facilitated needle placement.
The goal of chemoembolization therapy is to prolong tumor exposure to the chemotherapeutic agent and to add an ischemic component (i.e., particles) to enhance tumor necrosis. This treatment is based on the hypothesis that increased exposure time leads to improved response. The localized nature of this treatment could reduce many adverse side effects compared with systemic chemotherapy agents, which have been proven ineffective[19]. By contrast, TACE has the disadvantage in that chemoembolization damages more normal liver tissue than RFA. This causes more postoperative complications, such as liver failure. We think that is why the survival rate of the TACE group of patients was lower than that of the LRFA group in this series.
The major complication rate among the LRFA-treated patients in this study (17.5%) was significantly higher than that reported by Iannitti et al[21] (7.1%) and Curley et al[22]. The complication rate was 8% in Child class A patients, 6.5% in Child class B patients and 27.6% in Child class C patients. All the patients in our study had significantly impaired liver function (Child class B or C) and a high major complication rate was expected. These major complications in the LRFA group arose from the decompensated cirrhotic liver except for the development of pneumonia.
The major complication rate seen in the TACE group in this study (45%) was significantly higher than that in other studies (20%)[23]. As in the LRFA group, the major complications were caused by severely impaired liver function. TACE treatment could damage normal liver tissue and induce postembolization hepatic failure. We believe that this caused the high complication rate in our study.
In conclusion, decompensated cirrhotic liver patients with TMN stage II HCC treated with LRFA had better clinical outcomes, such as survival and lower complication rates than those treated with TACE. We suggest that LRFA may be a better choice for treating Child class B or C patients with TMN stage I or II HCC.
Footnotes
Edited by Wang XL Proofread by Zhu LH
References
- 1.Bloomston M, Binitie O, Fraiji E, Murr M, Zervos E, Goldin S, Kudryk B, Zwiebel B, Black T, Fargher S, et al. Transcatheter arterial chemoembolization with or without radiofrequency ablation in the management of patients with advanced hepatic malignancy. Am Surg. 2002;68:827–831. [PubMed] [Google Scholar]
- 2.Bowles BJ, Machi J, Limm WM, Severino R, Oishi AJ, Furumoto NL, Wong LL, Oishi RH. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg. 2001;136:864–869. doi: 10.1001/archsurg.136.8.864. [DOI] [PubMed] [Google Scholar]
- 3.Holt DR, Thiel DV, Edelstein S, Brems JJ. Hepatic resections. Arch Surg. 2000;135:1353–1358. doi: 10.1001/archsurg.135.11.1353. [DOI] [PubMed] [Google Scholar]
- 4.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radiofrequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 5.Matsukawa T, Yamashita Y, Arakawa A, Nishiharu T, Urata J, Murakami R, Takahashi M, Yoshimatsu S. Percutaneous microwave coagulation therapy in liver tumors. A 3-year experience. Acta Radiol. 1997;38:410–415. doi: 10.1080/02841859709172092. [DOI] [PubMed] [Google Scholar]
- 6.Seki T, Tamai T, Nakagawa T, Imamura M, Nishimura A, Yamashiki N, Ikeda K, Inoue K. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. 2000;89:1245–1251. [PubMed] [Google Scholar]
- 7.Aguayo A, Patt YZ. Nonsurgical treatment of hepatocellular carcinoma. Semin Oncol. 2001;28:503–513. doi: 10.1016/s0093-7754(01)90143-5. [DOI] [PubMed] [Google Scholar]
- 8.Rose DM, Chapman WC, Brockenbrough AT, Wright JK, Rose AT, Meranze S, Mazer M, Blair T, Blanke CD, Debelak JP, et al. Transcatheter arterial chemoembolization as primary treatment for hepatocellular carcinoma. Am J Surg. 1999;177:405–410. doi: 10.1016/s0002-9610(99)00069-0. [DOI] [PubMed] [Google Scholar]
- 9.Kato T, Reddy KR. Radiofrequency ablation for hepatocellular carcinoma: help or hazard. Hepatology. 2001;33:1336–1337. doi: 10.1053/jhep.2001.24738. [DOI] [PubMed] [Google Scholar]
- 10.Liu CL, Fan ST. Nonresectional therapies for hepatocellular carcinoma. Am J Surg. 1997;173:358–365. doi: 10.1016/S0002-9610(96)00384-4. [DOI] [PubMed] [Google Scholar]
- 11.Jiang HC, Liu LX, Piao DX, Xu J, Zheng M, Zhu AL, Qi SY, Zhang WH, Wu LF. Clinical short-term results of radiofrequency ablation in liver cancers. World J Gastroenterol. 2002;8:624–630. doi: 10.3748/wjg.v8.i4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuschieri A, Bracken J, Boni L. Initial experience with laparoscopic ultrasound-guided radiofrequency thermal ablation of hepatic tumours. Endoscopy. 1999;31:318–321. doi: 10.1055/s-1999-16. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual. 6th Ed. Springer-Verlag. 2002:3–8. [Google Scholar]
- 14.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 15.American Liver Tumor Study Group. A randomized prospective multi-institutional trial of orthotopic liver transplantation or partial hepatic resection with or without adjuvant chemo-therapy for hepatocellular carcinoma. Investigator booklet and protocol. 1998 [Google Scholar]
- 16.Onik GM, Atkinson D, Zemel R, Weaver ML. Cryosurgery of liver cancer. Semin Surg Oncol. 1993;9:309–317. doi: 10.1002/ssu.2980090406. [DOI] [PubMed] [Google Scholar]
- 17.Caridi J. Radiofrequenct ablation. Available from: http://www.miit.com/2002/abst/caridi_rfa.pdf [Google Scholar]
- 18.D'Agostino HB, Solinas A. Percutaneous ablation therapy for hepatocellular carcinomas. AJR Am J Roentgenol. 1995;164:1165–1167. doi: 10.2214/ajr.164.5.7717225. [DOI] [PubMed] [Google Scholar]
- 19.Scudamore CH, Lee SI, Patterson EJ, Buczkowski AK, July LV, Chung SW, Buckley AR, Ho SG, Owen DA. Radiofrequency ablation followed by resection of malignant liver tumors. Am J Surg. 1999;177:411–417. doi: 10.1016/s0002-9610(99)00068-9. [DOI] [PubMed] [Google Scholar]
- 20.Rospond RM, Mills W. Hepatic artery chemoembolization therapy for hepatic tumors. AORNJ. 1995;61:573–576. doi: 10.1016/s0001-2092(06)63746-0. [DOI] [PubMed] [Google Scholar]
- 21.Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg. 2002;137:422–446; discussion 427. doi: 10.1001/archsurg.137.4.422. [DOI] [PubMed] [Google Scholar]
- 22.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747–1752. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]


