Abstract
AIM: To investigate the expression of hypoxia-inducible factor (HIF)-2α/endothelial PAS domain protein1 (EPAS1) in hepatocellular carcinoma (HCC).
METHODS: Expression of HIF-2α/EPAS1 was investigated immunohistochemically on paraffin-embedded sections from 97 patients with HCC. To further confirm that HIF-2α/EPAS1 in HCC tissues also correlated with angiogenesis, a parallel immunohistchemistry study of vascular endothelial growth factor (VEGF) was performed on these 97 cases.
RESULTS: HIF-2α/EPAS1 could be detected in 50 of 97 cases (51.6%), including 19 weakly positive (19.8%), and 31 strongly positive (31.1%), the other 47 cases were negative (48.4%). The expression of HIF-2α/EPAS1was significantly correlated with tumor size, capsule infiltration, portal vein invasion, and necrosis. A parallel immunohistochemical analysis of VEGF demonstrated its positive correlation with capsule infiltration, portal vein invasion, and HIF-2α/EPAS1 overexpression, which supported the correlation of HIF-2α/ EPAS1up-regulation with tumor angiogenesis. No apparent correlation was observed between HIF-2α/EPAS1 and capsular formation, presence of cirrhosis, and histological grade.
CONCLUSION: HIF-2α/EPAS1 is expressed in most of HCC with capsular infiltration and portal vein invasion, which indicates a possible role of HIF-2α/EPAS1 in HCC metastasis.
INTRODUCTION
Hypoxia is an essential development and physiological stimulus and plays a key role in the physiology of cancer, heart attack, stroke, and other major causes of mortality[1]. Recently, hypoxia-inducible factors HIF-1α and HIF-2α/endothelial PAS domain protein1 (EPAS1) have been identified. They are basic helix-loop-helix/Per-Arnt-Sim (PAS) transcription factors induced under hypoxia[2,3]. When dimerized with aryl hydrocarbon nuclear translocator (also known as HIF-3α, another basic helix-loop-helix family member), both HIF-1α and HIF-2α can bind to the hypoxia response element in a battery of genes and transactivate their expression, including vascular endothelial growth factor (VEGF)[3,4]. These genes are important for tumor adaptation to hypoxia, implicating the possible role of HIFs in tumor progression. Tumor can not grow larger than 1-2 mm3 in the absence of angiogenesis, because of the lack of oxygen in the center of tumors, which result in apoptosis and necrosis[5]. Many tumors contain hypoxic microenvironments, a condition associated with poor prognosis and resistant to clinical treatment. Along with HIF-1α, EPAS1 is also known as HIF-2α[6,7], HIF-like factor[8], and HIF-related factor[9]. The expression of HIF-2α/EPAS1 has been reported in many human malignancies[10,11]. We chose to study HIF-2α/ EPAS1 expression in order to evaluate its clinical significance and identify the relation with some important markers of carcinoma progression, which are also predictors of metastasis.
MATERIALS AND METHODS
Clinical material
We examined 97 surgical resection specimens from patients (76 males, 21 females) with HCC, who underwent surgery at Xiangya hospital between January 1994 and December 2001. The age of patients ranged from 34 to 78 years (61.4 ± 8.9 years). The clinical features of the patients were noted with reference to clinical reports and pathology reports, including Edmondson-Steiner classification. In principle, grades I, II and III-IV and the characteristics of these patients are summarized in Table 1.
Table 1.
Correlation between HIF-2α/EPAS1 and clinicopathological features of HCC patients
| Variant |
HIF-2α/EPAS1 expression (No of cases) |
No of cases 97 | Significance | ||
| - | + | ++ | |||
| Pathological grade | |||||
| Grade I | 9 | 4 | 3 | 16 | NS |
| Grade II | 23 | 8 | 7 | 38 | |
| Grade III-IV | 15 | 7 | 221 | 43 | |
| Tumor size | |||||
| 2 | 22 | 3 | 8 | 33 | |
| 2 - 5 | 16 | 14 | 13 | 43 | 0.001a |
| > 5 | 9 | 2 | 10 | 21 | |
| Cirrhosis | |||||
| With | 27 | 9 | 17 | 53 | NS |
| Without | 20 | 10 | 14 | 44 | |
| Capsule formation | |||||
| With | 8 | 1 | 9 | 18 | NS |
| Without | 39 | 18 | 22 | 79 | |
| Capsule infiltration | |||||
| With | 3 | 3 | 10 | 16 | 0.011a |
| Without | 44 | 16 | 21 | 81 | |
| Portal vein invasion | |||||
| With | 15 | 15 | 18 | 48 | 0.001a |
| Without | 32 | 4 | 13 | 49 | |
| Necrosis | |||||
| With | 19 | 12 | 23 | 54 | 0.010a |
| Without | 28 | 7 | 8 | 43 | |
P < 0.05 vs the expression of HIF-2α/EPAS1 was significant in HCC tissues with capsule infiltration, portal vein invasion, necrosis, and tumor size. NS, not significant.
Immunohistochemical determination
Tissues were fixed with 10% formaldehyde in phosphate-buffered saline, embedded in paraffin, and cut into 5 µm-thick tissue sections. The sections were deparaffinized in xylene and rehydrated in grade ethanol. Endogenous peroxidase was blocked by immersing the sections in 3% H2O2 in 100% methanol for 20 minutes at room temperature. Antigen retrieval was achieved by micro-waving sections at 95 °C for 10 minutes in 0.001 M citrate buffer (pH 6.7). For immunochemical detection of diluted mouse monoclonal antibodies against HIF-2α/EPAS1 (190b) (DAKO, Glostrup, Denmark) and 1:200 diluted mAb against VEGF (Ab3) (Fremont, CA). Substitution of the primary antibodies with normal mouse IgG was used as a negative control. After washing, specimens were incubated with peroxidase-conjugated goat anti-mouse IgG for 30 min at room temperature. The color reaction was developed by incubating the sections with 0.5 mg/ml 3,3’-diaminobenzidine and 3% (vol/vol) H2O2 in phosphate-buffer saline for 7 min. The sections were counterstained slightly with Meyer hematoxylin and mounted.
VEGF determination and assessment
Mouse monoclonal antibody Ab3, neomarker (Fremon, CA) for detecting a smaller isoform (VEGF121) of VEGF were used for the identification and evaluation of VEGF expression in this study. Staining with VEGF monoclonal antibody required antigen retrieval, which was best done by microwave pretreatment. In brief, paraffin-fixed slides were autoclaved for 7 min in pretreatment before deparaffinization and rehydration. Evaluation of staining was semiquantitatively graded based on score determination by intensity distribution as strong ++ (dark brown), weak + (brown), or negative + (no staining). The score was determined independently by at least three of four observers.
Statistical analysis
A computer software SPSS 10.0 program was used for statistical analysis. Spearman’s correlation coefficient test was used to assess the relationship between HIF-2α/EPAS1, VEGF expression versus histological grade and tumor size. χ2 test was used to assess the correlation between HIF-2α/EPAS1, VEGF, versus existence of necrosis, cirrhosis, capsular formation, capsular infiltration and portal vein invasion. P < 0.05 was considered to be statistically significant.
RESULTS
In the 97 cases studied, positive staining of HIF-2α/EPAS1 protein was localized mainly in the cytoplasm, and occasionally and faintly in the membrane of HCC cells (Figures 1A and 1B). In general, most positive stains were observed in the perinecrotic regions near the tumor foci (Figure 1C). There was expression of HIF-2α/EPAS1 in cancer cells, whereas no positive staining was seen in the stroma cells of HCC (Figure 1D). Immunoreactivity of HIF-2α/EPAS1 was also observed in macrophages, as previously reported[10]. In addition, we also found positive staining in the intraluminal surfaces of hepatic vessels. All the positive immunoreactivity in macrophages was in the cytoplasm. The immunoreativity was even stronger in macrophages than that in tumor cells (Figure 1E). All the tumor cluster infiltrating to the tissue showed moderate to strong positive staining of HIF-2α/EPAS1 (Figure 1F). Fifty of 97 cases (51.5%) were positive, including 31 strongly positive (31.9%), 19 weakly positive (19.8%). In the 50 HIF-2α/EPAS1-positive cases, 35 cases (64.8%) had necrosis, and 15 cases (16.27%) had no necrosis. Positive staining was seen in 33.3% of the small-sized HCC (≤ 2 cm), 62.7% of the medium-sized (2-5 cm), and 57.1% of the HCCs larger than 5 cm. HCC larger than 5 cm in diameter though not significantly different between the two groups, had a relatively lower HIF-2α/EPAS1 expression compared to medium-sized tumors. HIF-2α/EPAS1 staining was seen in 81.2% of HCC patients with capsule infiltration, and 45.6% in those without them. Positive staining was also seen in 68.7% in HCC patients with portal vein invasion, and 34.6% in those without them (Table 1). HIF-2α/ EPAS1 positivity was significantly correlated with tumor size, capsule infiltration, portal vein invasion, and existence of necrosis (P < 0.05 respectively). To further confirm whether HIF-2α/EPAS1 expression in HCC tissues also correlated with angiogenesis, a parallel immunohistochemical study of VEGF was performed on these 97 cases, in which VEGF expression was assessed as a major marker for angiogenesis (Table 2). Positive staining was found mostly in the cytoplasm of tumor cells (Figures 2A and 2B), and only weaker staining in stroma areas was detectable. VEGF expression in tumors had strong correlation with capsule infiltration, portal vein invasion, and necrosis (P < 0.05 respectively). The overexpression of VEGF in capsule infiltration and portal vein invasion was found to correlate positively with HIF-2α/EPAS1 expression (P < 0.05, Table 3), supporting the correlation of HIF-2α/EPAS1 up-regulation with tumor metastasis and angiogenesis in HCC.
Figure 1.
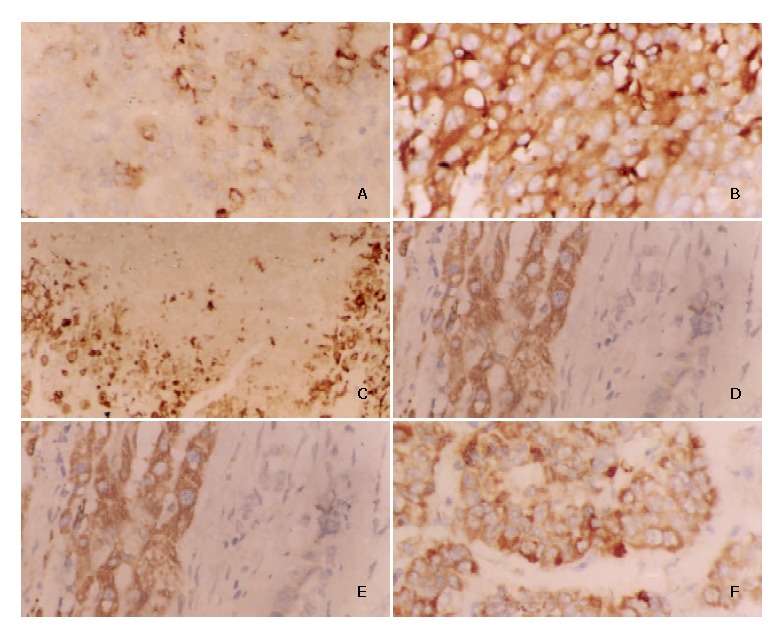
Characterization of HIF-2α/EPAS1 expression in human hepatocellular carcinoma tissues by IHC technique. A: Weak expression of HIF-2α/EPAS1 in membranes and cytoplasms of HCC cells (× 400). B: Strong cytoplasmic immunoreactivity of HIF-2α/EPAS1 in HCC cells (× 400). C: HIF-2α/EPAS1 positive staining (arrows) in perinecrotic region near tumor in a HCC sample (with capsular infiltration and portal vein invasion) (× 400). D: Strong HIF-2α/EPAS1 expression in HCC tissues whereas no staining in adjacent stroma cells (× 400). E: Strong staining in the cytoplasm of macrophages compared with cancer cells, showing weak staining for HIF-2α/EPAS1 (× 400). F: Moderate to strong positive staining of HIF-2α/EPAS1 in tumor clusters infiltrating to the tissue (× 400).
Table 2.
Correlation between VEGF protein and clinicopathological features of HCC patients
| Variants | NO of cases 97 |
VEGF expression |
Significance | ||
| - | + | ++ | |||
| Pathological grade | |||||
| Grade I | 6 | 6 | 0 | 0 | NS |
| Grade II | 76 | 28 | 22 | 26 | |
| Grade III-IV | 15 | 8 | 3 | 4 | |
| Tumor size | |||||
| 2 | 13 | 11 | 0 | 2 | NS |
| 2-5 | 49 | 16 | 14 | 19 | |
| > 5 | 35 | 15 | 11 | 9 | |
| Cirrhosis | |||||
| With | 53 | 20 | 10 | 23 | NS |
| Without | 44 | 22 | 15 | 7 | |
| Capsule formation | |||||
| With | 18 | 7 | 5 | 6 | NS |
| Without | 79 | 35 | 20 | 24 | |
| Capsule infiltration | |||||
| With | 16 | 4 | 2 | 10 | 0.011a |
| Without | 81 | 38 | 23 | 20 | |
| Portal vein infiltration | |||||
| With | 48 | 14 | 16 | 18 | 0.020a |
| Without | 49 | 20 | 9 | 12 | |
| Necrosis | |||||
| With | 54 | 15 | 17 | 22 | 0.011a |
| Without | 43 | 27 | 8 | 8 | |
P < 0.05 vs the expression of VEGF was significant in HCC with capsule infiltration, portal vein invasion, and with necrosis. NS, not significant.
Figure 2.
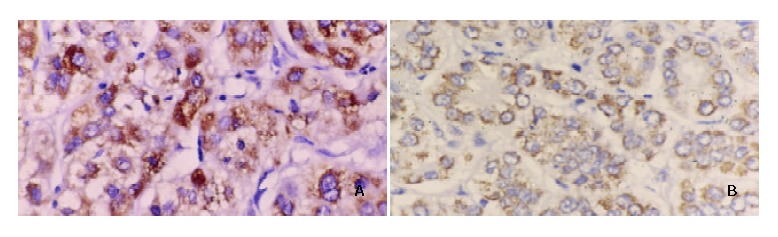
Parallel study of VEGF protein expression in HCC samples. A mAb against VEGF was used for immunostainig of slides from HCC patients. A: HCC with capsular infiltration and portal vein invasion showing strong staining (++) in the cytoplasm of tumor cells. B: HCC without capsular infiltration and portal vein invasion showing weak staining (+) (× 200).
Table 3.
Correlation between HIF-2α/EPAS1 and VEGF protein expression in HCC tissues
| Variants | NO of cases |
Staining score |
Significance | ||
| - | + | ++ | |||
| HIF-2α/EPAS1 | 97 | 47 | 19 | 31 | 0.017 |
| VEGF | 97 | 42 | 25 | 30 | |
DISCUSSION
Angiogenesis appeared to be one of the crucial steps in tumor’s transition from small, harmless cluster of mutated cells to a large, malignant growth, capable of spreading to other organs throughout the body[12]. HCC is a typical hypervascular tumor of the digestive organs. It seems likely that the formation of tumor vessels precedes tumor growth and is indispensable in maintaining tumor viability, because hepatic arterial embolization frequently causes necrosis and induces a marked reduction in tumor size. In the present study, we investigated the expression of HIF-2α/EPAS1 in HCC tissues. To further confirm whether HIF-2α/EPAS1 in HCC tissues also correlated with angiogenesis, we performed an immunohistchemistry study of VEGF protein. We also examined the correlation between HIF-2α/EPAS1, VEGF protein expression and clinicopathological features.
Our data showed that tumor size was correlated with HIF-2α/EPAS1. Small HCC had significantly lower HIF-2α/EPAS1 expression compared with medium-sized tumors. What was contrary to previous finding[13,14] was that tumors with large sizes had higher expression than smaller and moderate sizes, even these tumors were relatively less vascular compared with the large-sized ones. However, it has been reported that the intercapillary distance increased as the tumor size or weight increased, possibly because of different rates of endothelial cells and neoplastic cell turnover[15,16]. The turnover time of endothelial cells was 50 to 60 hours while that of the neoplastic cells was 22 hours, and significantly shorter[17]. On the other hand, the characteristics of tumor microcirculation could offer another explanation for the reduction of HIF-2α/EPAS1 expression as the tumor became smaller or larger. Generally, blood flow, oxygen pressure, and pH values were less in tumors than in the counterpart normal tissues[18], and because of the absence of lymphatic vessels, the interstitial pressure was often high in tumors, leading further transport problems[19]. As a result, hypoxia and necrosis were a general phenomenon of tumors, especially large ones[20]. It was assumed that rapid cell proliferation at the center of tumor could lead to increased interstitial pressure, which may lead to compression closure of capillaries and consecutive tumor necrosis[21]. The current results showed that HCCs of 2 to 5 cm in diameter had the highest HIF-2α/EPAS1 expression compared with larger and smaller tumors. This observation could be considered important for regional chemotherapy, because intuitively, tumor, tumor metastasis, and tumor death should be closely correlated with tumor-induced hypoxia and necrosis. Cells in hypoxic regions have been thought to be more resistant to the effects of radiotherapy and many conventional chemotherapeutic agents than their normoxic counterparts[22,23].
We also found strong immunoreactivity in macrophages. The significance of HIF-2α/EPAS1 in these cells warrants further study. As they have been shown to be one of the terminally differentiated cells that can produce a number of potent angiogenic cytokines such as VEGF[24,25], their chemotaxis, infiltration, degranulation may promote tumor angiogenesis and progression. A parallel IHC study of angiogenesis marker demonstrated that in HCC tissues, overexpression of both VEGF and HIF-2α/EPAS1 was coincidentally found, supporting the notion that HIF-2α/ EPAS1 expression is correlated with angiogenesis in HCC.
In the current work, significantly more HIF-2α/EPAS1 protein expression was present in perinecrotic regions. This, when taken with the fact that macrophages appeared to be more pro-angiogenic at these sites[26] may help to explain our observation. As HIF-2α/EPAS1 has been shown to be accumulated by hypoxic macrophages in human tumor[11,27], our finding may indicate that HIF-2α/EPAS1 protein may be released by macrophages and is part of the mechanism by which this protein is most expressed in perinecrotic regions. On the other hand, direct support for microenvironmental mechanisms of HIF-α activation in diverse types of human tumor could offer an alternative explanation.
Results from our analysis of HIF-2α/EPAS1 expression in perinecrotic areas were consistent with a number of reports from clinical studies on breast[28-30], ovarian[31], and lung[32,33] cancers and in hemangioblastomas[10]. These reports all demonstrated that macrophage hotpots were remotely located from the vascular hotpots of tumors, suggesting that macrophages may preferentially migrate toward areas of relative hypoxia[29]. This in turn might attract macrophages into tumor, which then contribute to angiogenic process, giving rise to association between high levels of angiogenesis and extensive necrosis[29]. Macrophages might be attracted to necrotic tumors by chemotactic factors, such as VEGF[26,34,35].
As a potent pro-angiogenic cytokine, VEGF has been reported to be overexpressed in both malignant tumors[30,36] and stroma cells[30,34] and macrophages[26,34,37]. Expression of VEGF was up-regulated in poorly vascularized areas of breast carcinomas[28,29,38]. VEGF positive macrophages were restricted to areas of VEGF production[26,28]. Evidence is accumulating that VEGF might be activated in stroma cells, especially in macrophages, with the process mediated by the VEGF receptor ftl-1[35]. Thus, the subcellular mechanisms mediating hypoxia on VEGF gene by macrophages are not known at present. This most likely involved one or more of the pathways activated by hypoxia in transformed cells[37,40], including the activation of such transcription factors as hypoxia-inducible factors (HIFs) 1, 2 (otherwise known as EPAS1). In this study, we observed the overexpression of VEGF protein in tumor and VEGF expression positively correlated with HIF-2α/EPAS1 expression. We found that the highest VEGF expression was detectable mostly in tumor areas and only weaker staining in necrotic and stroma areas was detectable.
It is widely accepted that angiogenesis necessitates the degradation of extracellular matrix, this process requires protease activation and release. Plasmin was thought to be one of the key proteases involved in this process[39]. Angiogenesis also appear to be involved in the invasion of tumors into surrounding tissues, because this invasion requires concomitant neovascularization through the sprouting of endothelial cells in the tumor stroma. It has been reported that VEGF induced both urokinase-type plasminogen activator (PA) and tissue type PA in endothelial cells[40], and hypoxia might promote cellular invasion by stimulating the expression of urokinase type plasminogen activator (uPAR)[41]. Therefore, enhanced expression of these angiogenic factors would likely indicate the ability of tumors to invade the tumor stroma as well as the ability to promote the development of new blood vessels. Based on these considerations, we examined both portal involvement and capsule infiltration. These two clinicopathological features have been thought to be the most important clinical factors in assessing liver tumor, and HCC in particular, as they were strongly correlated with the metastasis of HCC[42-44]. Our results were in agreement with this concept. We found that the portal vein involvement and capsular infiltration were correlated with the expression of both HIF-2α/EPAS1 and VEGF proteins. HCC patients with capsular infiltration and portal vein invasion had more HIF-2α/EPAS1 and VEGF expression than those without them, indicating that HIF-2α/EPAS1 and VEGF expression may be associated with a poor prognosis of patients with HCCs.
The clinical significance of HIF-2α/EPAS1 expression in tumors remains largely unexplored as monoclonal antibodies available for immunohistochemistry have been recently developed. Talks et al[11] recently reported the expression of HIF-α in a panel of normal human tissues and benign or malignant tumors and first showed the expression of the molecule in a good percentage of human carcinomas. However, studies on the HIF-2α/EPAS1 expression with angiogenic factors and receptors, with microvessel density or with other molecular markers or with prognosis of human carcinomas are few. Investigations regarding these angiogenic factors which have been partially done for endothelial carcinoma[3,33,45], and regarding the status of signal transduction via HIF-2α/EPAS1 when the receptors do and do not bind to this protein and when dimerization with aryl hydrocarbon receptor nuclear translocator occurs between HIF-2α/EPAS1 and other HIF-α protein family, should help clarify the significance of HIF-2α/EPAS1 in human cancers, including HCC.
In this study, we found cytoplasmic immunoreactivity of HIF-2α/EPAS1, but equivocal staining was sometimes observed in the nuclear which we did not regard as positive. Although it was assumed that nuclear HIF was the active form, clearly it was synthesized and also degraded in the cytoplasm[45,46]. These findings, at least in part, could explain the cytoplamic location of HIF-2α/EPAS1, which was a tumor specific finding and could better reflect the HIF up-regulation pathways in paraffin embedded material. This suggestion was in accordance with the scoring system proposed by Zhong et al[46].
In conclusion, HIF-2α/EPAS1 expression in HCC and its clinical association with necrosis seem to be a good predictive tool and possibly a target therapy for metastasis of liver cancer, especially HCC. The finding that medium-sized HCCs had the highest expression of HIF-2α/EPAS1 compared with smaller and larger HCCs can be used, after further evaluation, as a therapeutic guide during the selection of cases for chemotherapy.
Footnotes
Supported by the National Key Technologies R and D Program, No. 2001BA703B04
Edited by Gupta MK and Wang XL
References
- 1.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2α plays an important role in vascular remodeling. Proc Natl Acad Sci USA. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 5.Morrow CS, Cowan KH. Antineoplastic drug resistance and breast cancer. Ann N Y Acad Sci. 1993;698:289–312. doi: 10.1111/j.1749-6632.1993.tb17220.x. [DOI] [PubMed] [Google Scholar]
- 6.Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1α. Blood. 1998;92:2260–2268. [PubMed] [Google Scholar]
- 7.Jain S, Maltepe E, Lu MM, Simon C, Bradfield CA. Expression of ARNT, ARNT2, HIF1α, HIF2α and Ah receptor mRNAs in the developing mouse. Mech Dev. 1998;73:117–123. doi: 10.1016/s0925-4773(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 8.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flamme I, Fröhlich T, von Reutern M, Kappel A, Damert A, Risau W. HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1α and developmentally expressed in blood vessels. Mech Dev. 1997;63:51–60. doi: 10.1016/s0925-4773(97)00674-6. [DOI] [PubMed] [Google Scholar]
- 10.Flamme I, Krieg M, Plate KH. Up-regulation of vascular endothelial growth factor in stromal cells of hemangioblastomas is correlated with up-regulation of the transcription factor HRF/HIF-2α. Am J Pathol. 1998;153:25–29. doi: 10.1016/s0002-9440(10)65541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkman J. Fighting cancer by attacking its blood supply. Sci Am. 1996;275:150–154. doi: 10.1038/scientificamerican0996-150. [DOI] [PubMed] [Google Scholar]
- 13.Favier J, Plouin PF, Corvol P, Gasc JM. Angiogenesis and vascular architecture in pheochromocytomas: distinctive traits in malignant tumors. Am J Pathol. 2002;161:1235–1246. doi: 10.1016/S0002-9440(10)64400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cayre A, Rossignol F, Clottes E, Penault-Llorca F. aHIF but not HIF-1α transcript is a poor prognostic marker in human breast cancer. Breast Cancer Res. 2003;5:R223–R230. doi: 10.1186/bcr652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaupel P. Hypoxia in neoplastic tissue. Microvasc Res. 1977;13:399–408. doi: 10.1016/0026-2862(77)90106-6. [DOI] [PubMed] [Google Scholar]
- 16.Tannock IF, Steel GG. Quantitative techniques for study of the anatomy and function of small blood vessels in tumors. J Natl Cancer Inst. 1969;42:771–782. [PubMed] [Google Scholar]
- 17.Tannock IF, Hayashi S. The proliferation of capillary endothelial cells. Cancer Res. 1972;32:77–82. [PubMed] [Google Scholar]
- 18.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 19.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 20.Lyng H, Skretting A, Rofstad EK. Blood flow in six human melanoma xenograft lines with different growth characteristics. Cancer Res. 1992;52:584–592. [PubMed] [Google Scholar]
- 21.Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- 22.Blancher C, Harris AL. The molecular basis of the hypoxia response pathway: tumour hypoxia as a therapy target. Cancer Metastasis Rev. 1998;17:187–194. doi: 10.1023/a:1006002419244. [DOI] [PubMed] [Google Scholar]
- 23.Richard DE, Berra E, Pouysségur J. Angiogenesis: how a tumor adapts to hypoxia. Biochem Biophys Res Commun. 1999;266:718–722. doi: 10.1006/bbrc.1999.1889. [DOI] [PubMed] [Google Scholar]
- 24.Harmey JH, Dimitriadis E, Kay E, Redmond HP, Bouchier-Hayes D. Regulation of macrophage production of vascular endothelial growth factor (VEGF) by hypoxia and transforming growth factor beta-1. Ann Surg Oncol. 1998;5:271–278. doi: 10.1007/BF02303785. [DOI] [PubMed] [Google Scholar]
- 25.Gaudry M, Brégerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood. 1997;90:4153–4161. [PubMed] [Google Scholar]
- 26.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths L, Binley K, Iqball S, Kan O, Maxwell P, Ratcliffe P, Lewis C, Harris A, Kingsman S, Naylor S. The macrophage - a novel system to deliver gene therapy to pathological hypoxia. Gene Ther. 2000;7:255–262. doi: 10.1038/sj.gt.3301058. [DOI] [PubMed] [Google Scholar]
- 28.Leek RD, Hunt NC, Landers RJ, Lewis CE, Royds JA, Harris AL. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J Pathol. 2000;190:430–436. doi: 10.1002/(SICI)1096-9896(200003)190:4<430::AID-PATH538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79:991–995. doi: 10.1038/sj.bjc.6690158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hlatky L, Tsionou C, Hahnfeldt P, Coleman CN. Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res. 1994;54:6083–6086. [PubMed] [Google Scholar]
- 31.Negus RP, Stamp GW, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997;150:1723–1734. [PMC free article] [PubMed] [Google Scholar]
- 32.Shoji M, Hancock WW, Abe K, Micko C, Casper KA, Baine RM, Wilcox JN, Danave I, Dillehay DL, Matthews E, et al. Activation of coagulation and angiogenesis in cancer: immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am J Pathol. 1998;152:399–411. [PMC free article] [PubMed] [Google Scholar]
- 33.Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1α and 2α in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polverini PJ, Leibovich SJ. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab Invest. 1984;51:635–642. [PubMed] [Google Scholar]
- 35.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marmé D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 36.Huang GW, Yang LY, Sheng LX, Li HL, Qing JQ, Yang ZL. The relationship between VEGF and HIF-1α protein in hepatocellular carcinoma. Zhonghua Xiaohua Zazhi. 2000;10:627–628. [Google Scholar]
- 37.Xiong M, Elson G, Legarda D, Leibovich SJ. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol. 1998;153:587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.Pepper MS, Montesano R. Proteolytic balance and capillary morphogenesis. Cell Differ Dev. 1990;32:319–327. doi: 10.1016/0922-3371(90)90046-y. [DOI] [PubMed] [Google Scholar]
- 40.Pepper MS, Vassalli JD, Orci L, Montesano R. Proteolytic balance and capillary morphogenesis in vitro. EXS. 1992;61:137–145. doi: 10.1007/978-3-0348-7001-6_22. [DOI] [PubMed] [Google Scholar]
- 41.Graham CH, Fitzpatrick TE, McCrae KR. Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood. 1998;91:3300–3307. [PubMed] [Google Scholar]
- 42.Los M, Zeamari S, Foekens JA, Gebbink MF, Voest EE. Regulation of the urokinase-type plasminogen activator system by the von Hippel-Lindau tumor suppressor gene. Cancer Res. 1999;59:4440–4445. [PubMed] [Google Scholar]
- 43.Arii S, Tanaka J, Yamazoe Y, Minematsu S, Morino T, Fujita K, Maetani S, Tobe T. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer. 1992;69:913–919. doi: 10.1002/1097-0142(19920215)69:4<913::aid-cncr2820690413>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 44.Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 45.Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, Zee B, Mo F, Teo PM, Huang DP, et al. Coexpression of hypoxia-inducible factors 1α and 2α, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–2604. [PubMed] [Google Scholar]
- 46.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]


