Abstract
AIM: To evaluate the tumor-positive ratio and number of perigastric lymph nodes as prognostic factors of gastric carcinoma in surgically-treated patients.
METHODS: The postoperative survival of 169 patients with gastric cancer who were performed D2 curative gastrectomy was analyzed with regard to its lymph node metastasis ratio and number. Meanwhile correlation of tumor-positive ratio and number of perigastric lymph nodes with pathological parameters of these patients was studied.
RESULTS: The overall 5-year survival rate of all the patients studied was 29.6%. The 5-year cumulative survival rate in patients with 1%-20% and more than 20% of tumor-positive lymph nodes was 70.6% and 12.0% respectively, and 46.6% and 17.4% in those with 1-5 and more than 5 of tumor-positive lymph nodes respectively, which were significantly decreased with the increment of involved lymph nodes assessed by either numbers or ratio (P < 0.05). Multiple stepwise regression analysis showed that both the positive ratio and number of tumor-involved lymph nodes were sensitive prognostic factors in these surgically-treated patients, which were also significantly correlated with tumor size and depth of submucosal invasion (P < 0.05).
CONCLUSION: Tumor-positive ratio and number of perigastric lymph nodes are associated with cancer progression and five-year survival rate, and may serve as valuable prognostic factors of gastric cancer in surgically-treated patients.
INTRODUCTION
It has been well recognized that lymph node metastasis in patients with gastric cancer is one of the important prognostic factors[1-4]. In 1997, the International Union Contrele Cancer (UICC) and American Joint Commission for Cancer (AJCC) redefined metastatic status of lymph node on the basis of the involved node number rather than its location, in which pN1 was defined as 1-6 local lymph nodes being involved, pN2 as 7-15 local lymph nodes being involved and pN3 as more than 15 local lymph nodes being involved[5-7]. Some reports strongly suggested that this classification was more sensitive with a higher reproducibility in the prognostic evaluation of gastric cancer patients than that assessed by the metastatic locations of lymph node[8,9]. However, In China there are few reports concerning the correlation of local lymph node metastatic ratio and number with the prognosis of gastric cancer patients, which was the motivation for us to initiate the present study of such cases in the Chinese population.
MATERIALS AND METHODS
Patients and materials
Between January 1995 and November 1997, 304 patients with primary gastric cancer were performed D2 radical gastrectomy in the Department of General Surgery, First Affiliated Hospital of Nanjing Medical University. Of them, 121 male and 48 female cases aging from 32 to 78 years (mean, 58.4 years) were found to have lymph node metastasis, and analyzed in the present study, with a following-up time from 0 to 61 months postoperation.
Methods
The status of lymph nodes was assessed according to the staging system formulated by UICC/AJCC in 1997. Of the 169 patients with positive lymph nodes, 32 were found to have remote lymphatic metastasis such as that in the retropancreatic, mesenteric and paraortic regions. The total number of resected lymph nodes was 10 223 (mean: 34.1, range: 11-122), the median number of examined lymph nodes was 26 (mean: 31.1, range: 12 to 91) for all 304 patients, the median number of involved regional lymph nodes was 5.0 (mean: 7.1, range: 1 to 42). Lymph node metastasis ratio was defined as the number of metastatic lymph nodes to the total number of resected ones.
To elucidate the prognostic significance of metastatic lymph nodes, the clinical and histopathological records of these 169 patients were analyzed. The relationships of 5-year survival rate with sex, age, tumor location, histopathological grading, macroscopic type, lymph node resection and depth of tumor invasion were determined. Tumor location, macroscopic type, and lymph node resection were graded according to the Japanese classification of gastric carcinoma proposed by Japanese Gastric Carcinoma Association (JGCA).Histopathological grading was defined according to the fifth edition of TNM classification. Following-up information was obtained from routine clinical examinations.
Statistical analysis
Statistical analysis was carried out using SPSS 10.0 for Windows. The 5-year survival rate of those performed using D2 radical gastrectomy was analyzed by cox’s proportional hazard models. The log rank test was used to compare the survival data between groups. Comparison between qualitative results for the PN categories and clinical or histopathological parameters was performed using the χ2 test. Independent predictors of postoperation survival were identified by logistic regression analysis. Kaplan-Meier curves were used to demonstrate survival distribution.
RESULTS
Among the 304 patients, 169 (55.6%) had lymph node metastasis. The 5-year survival rate was 29.6% for the node-positive patients, and was 91.2% for the node-negative patients. The cancer -specific 5-year survival rate of patients with lymph node metastasis was significantly lower than that of those without lymph node metastasis (P < 0.05).
Correlation between lymph node metastatic ratio and 5-year survival rate
The patients were divided into two groups by the ratio of metastatic lymph node number to the total number of resected lymph nodes. The 5-year survival rate of 51 patients with metastatic lymph nodes less than 20% was 70.6%(36 cases), while that of 118 patients with 21% or more was 12% (14 cases). A significant difference was noted between the two groups (P < 0.05, Table 1). As the ratio of lymph node metastatasis increased, the 5-year survival rate decreased (Figure 1).
Table 1.
Correlation between lymph node metastatic ratio and survival years
| Ratio of positive | n |
Survival years |
P | ||||
| lymph nodes | 1 | 2 | 3 | 4 | 5 | ||
| < 20% | 51 | 51 | 49 | 45 | 39 | 36 | < 0.05 |
| > 21% | 118 | 104 | 87 | 66 | 43 | 14a | |
P<0.05 vs the group with lymph node metastatic ratio less than 20%.
Figure 1.
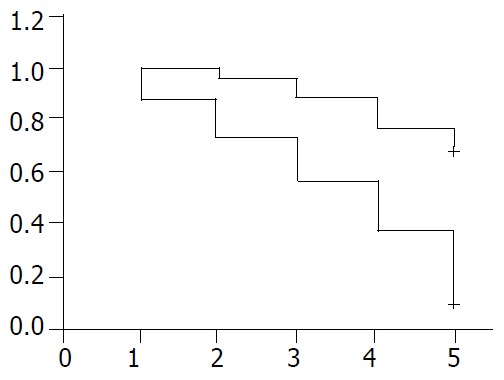
Negative correlation between survival years and ra-tio of metastatic lymph nodes in patients with gastric cancer. +: patients with metastatic ratio of lymph node less than 20%: -: patients with metastatic ratio of lymph node more than 21%.
Relation between lymph node metastasis number and 5-year survival rate
There was no significant difference in the outcome of patients with 1, 2, 3, 4 or 5 positive nodes, but there was a sharp decrease in survival rate when the sixth node was involved (Table 1), So the patients were divided into two groups according to the lymph node metastatic number. Group A having less than 5 and group B having more than 6. The 5-year survival rate of 71 patients with less than 5 metastatic lymph nodes was 46.6%(33 cases), and the 5-year survival rate of 98 patients with more than 6 metastatic lymph nodes was 17.4% (17 cases). A sharp decrease in survival was seen between two groups (P < 0.05, Table 2). The 5-year survival rate decreased as the number of lymph node metastatases increased (Figure 2).
Table 2.
Correlation between lymph node metastatic number and survival years
| Number | n |
Survival years |
P | ||||
| of positive | |||||||
| lymph node | 1 | 2 | 3 | 4 | 5 | ||
| 1 | 9 | 9 | 9 | 8 | 6 | 5 | |
| 2 | 20 | 20 | 19 | 18 | 15 | 10 | 0.908 |
| 3 | 12 | 12 | 12 | 10 | 9 | 5 | 1.0 |
| 4 | 11 | 11 | 9 | 8 | 6 | 5 | 0.79 |
| 5 | 19 | 17 | 16 | 14 | 11 | 8 | 0.704 |
| 6 | 41 | 36 | 30 | 22 | 11 | 10 | 0.009 |
| 7 | 20 | 18 | 16 | 13 | 9 | 5 | 0.555 |
| 8 | 16 | 14 | 11 | 9 | 7 | 3 | 0.698 |
| ≥ 9 | 21 | 18 | 13 | 9 | 8 | 3 | 0.688 |
| 1-5 | 71 | 69 | 66 | 58 | 47 | 33 | |
| > 5 | 98 | 86 | 70 | 53 | 35 | 17 | 0.00072a |
P < 0.05, vs the group with lymph node metastatic number more than 5.
Figure 2.
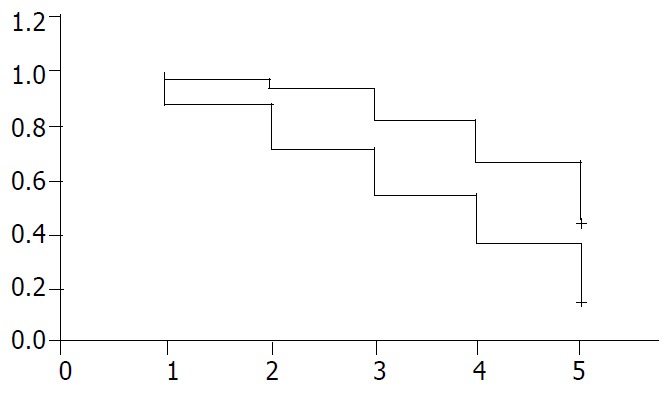
Negative correlations between survival years and num-ber of metastatic lymph nodes in patients with gastric cancer. +: patients with metastatic number of lymph nodes less than 5: -: patients with metastatic number of lymph nodes more than 6.
Correlation between positive ratio of lymph node metastasis and tumor characteristics
As shown in Table 3, lymph node metastatic ratio was positively correlated to tumor size, depth of invasion. No significant difference was found between lymph node metastatic ratio and tumor location.
Table 3.
Correlation between lymph node metastatic ratio and tumor characteristics
| Pathological | Lymph node < 20% | > 21% | P |
| characteristics | metastatic ratio | ||
| Size of tumor | |||
| < 4 cm | 42 | 43 | 0.48 |
| ≥ 4 cm | 9 | 74a | |
| location | |||
| Lower third | 5 | 8 | |
| Middle third | 22 | 47 | |
| Upper third | 24 | 63 | < 0.001 |
| Depth of invasion | |||
| Mucosa and submucosa | 12 | 9 | |
| Muscle and subserosa | 16 | 6 | |
| Serosa | 23 | 103b | < 0.001 |
P < 0.05,
P < 0.05 vs the group with lymph node metastatic ratio less than 20%.
Correlation between number of positive lymph nodes and tumor characteristics
As shown in Table 4, tumors with one to five involved lymph nodes compared with those with six or more involved lymph nodes were significantly characterized by size, depth of invasion, there was no significant difference between the number of involved lymph nodes and tumor location.
Table 4.
Correlation between number of involved lymph nodes and tumor characteristics
| Pathological | Number of positive | > 5 | P |
| characteristics | lymph nodes 1-5 | ||
| Size of tumor | |||
| < 4 cm | 51 | 34 | |
| ≥ 4 cm | 20 | 64a | 0.48 |
| location | |||
| Lower third | 7 | 6 | |
| Middle third | 31 | 38 | |
| Upper third | 33 | 54 | < 0.05 |
| Depth of invasion | |||
| Mucosa and submucosa | 20 | 1 | |
| Muscle and subserosa | 18 | 4 | |
| Serosa | 33 | 93b | < 0.05 |
P < 0.05,
P < 0.05 vs the group with lymph node metastatic num-ber more than 5.
DISCUSSION
Gastric carcinoma, one of the most common human malignant tumors, is as the first leading cause of gastrointestinal cancer-related mortality. Over 50% of patients with gastric cancer operated in China had lymph node metastasis, which resulted in poor prognosis of these patients[10-13]. As yet, lymph node status has been considered as the major determinant of gastric cancer recurrence for patients undergoing a curative gastrectomy[14-16]. However, some authors suggested that the prognostic assessment of these patients by counting the number of positive lymph nodes related to tumor locations was too complicated to be used in routine practice. A simple and easy system for lymph node staging has been considered to be urgently needed[14-16].
In the present study, we observed the effect of metastatic lymph node number on the survival years of gastric cancer patients. The result showed that the five–year survival rate in patients with 0, 1-5 and more than 5 tumor-positive lymph nodes was 91.2%, 46.6% and 17.4% respectively, indicating that considerable differences in the five-year survival rate existed between the patients. However, There was no significant difference observed between the outcomes of patients with 1, 2, 3, 4 and 5 tumor-positive lymph nodes. Thus it is more reasonable to classify gastric cancer patients with criteria of tumor-positive node numbers rather than tumor-positive nodes. Similar results have been revealed in a previous report by Wu et al[8,17,18]. They divided the gastric cancer patients into two groups according to the number of involved lymph nodes, and found that the number of positive nodes rather than lymph node involvement was suitable for the classification of nodal stages in gastric cancer.
Lymph node metastatic ratio, namely the number of metastatic lymph nodes to the total number of resected lymph nodes, has also been found to be an important prognostic factor[19-22]. We observed that the 5-year survival rate in patients with the metastatic lymph node ratio of 0%, 1%-20% and more than 21%, was 91.2%, 70.6% and 12% respectively. Similar results have been shown by Kwon and his colleagues that the metastatic ratio of lymph nodes was one of the main factors in determining the five-year survival rate of gastric cancer patients. Furthermore, the lymph node metastatic ratio has also been found to be more objective and reliable than the metastatic number in the prognosticating outcomes of these patients, because the former could effectively eliminate the influence of variance in resected lymph number on the prognosis of gastric cancer patients[23-27].
It is generally accepted the depth of cancer invasion is an other important prognostic indicator in gastric cancer. Yasuda et al[28,29] showed that the metastatic rate of lymph nodes was correlated with the depth of submucosal invasion in early stage of gastric carcinoma, which was in accordance with our present results. We found that the number and ratio of metastatic lymph nodes were positively related with the depth of cancer invasion and tumor size in gastric cancer cases.
In conclusion, gastric cancer patients with 5 or more positive lymph nodes and 20% or more of positive lymph node metastasis associated with a poor prognosis. The positive number and ratio of lymph node metastasis are simple and useful indictors in evaluating the surgical results of patients with gastric cancer[19,30,31].
Footnotes
Edited by Zhu L and Wang XL
References
- 1.Manfè AZ, Segalina P, Maffei Faccioli A. [Prognostic factors in gastric cancer. Our experience and review of the literature] Minerva Chir. 2000;55:299–305. [PubMed] [Google Scholar]
- 2.Takagane A, Terashima M, Abe K, Araya M, Irinoda T, Yonezawa H, Nakaya T, Inaba T, Oyama K, Fujiwara H, et al. Evaluation of the ratio of lymph node metastasis as a prognostic factor in patients with gastric cancer. Gastric Cancer. 1999;2:122–128. doi: 10.1007/s101200050034. [DOI] [PubMed] [Google Scholar]
- 3.Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Takahashi M, Kikuchi S, Yamauchi H. Significant prognostic factors in patients with early gastric cancer. Int Surg. 2000;85:286–290. [PubMed] [Google Scholar]
- 4.Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J Gastroenterol. 2003;9:1409–1414. doi: 10.3748/wjg.v9.i7.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jähne J. [Lymphadenectomy in gastric carcinoma] Zentralbl Chir. 2002;127:550–553; discussion 553. doi: 10.1055/s-2002-32622. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi H, Ochiai T, Suzuki T, Shimada H, Hori S, Takeda A, Miyazawa Y. Superiority of a new UICC-TNM staging system for gastric carcinoma. Surgery. 2000;127:129–135. doi: 10.1067/msy.2000.102171. [DOI] [PubMed] [Google Scholar]
- 7.Omejc M, Juvan R, Jelenc F, Repse S. Lymph node metastases in gastric cancer: correlation between new and old UICC TNM classification. Int Surg. 2001;86:14–19. [PubMed] [Google Scholar]
- 8.Wu CW, Hsieh MC, Lo SS, Shen KH, Lui WY, P'eng FK. Comparison of the UICC/AJCC 1992 and 1997 pN categories for gastric cancer patients after surgery. Hepatogastroenterology. 2001;48:279–284. [PubMed] [Google Scholar]
- 9.Katai H, Yoshimura K, Maruyama K, Sasako M, Sano T. Evaluation of the New International Union Against Cancer TNM staging for gastric carcinoma. Cancer. 2000;88:1796–1800. [PubMed] [Google Scholar]
- 10.Abe N, Watanabe T, Suzuki K, Machida H, Toda H, Nakaya Y, Masaki T, Mori T, Sugiyama M, Atomi Y. Risk factors predictive of lymph node metastasis in depressed early gastric cancer. Am J Surg. 2002;183:168–172. doi: 10.1016/s0002-9610(01)00860-1. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Sano T, Katai H, Sasako M, Maruyama K. Node-positive mucosal gastric cancer: a follow-up study. Jpn J Clin Oncol. 2001;31:153–156. doi: 10.1093/jjco/hye035. [DOI] [PubMed] [Google Scholar]
- 12.de Manzoni G, Verlato G, di Leo A, Guglielmi A, Laterza E, Ricci F, Cordiano C. Perigastric lymph node metastases in gastric cancer: comparison of different staging systems. Gastric Cancer. 1999;2:201–205. doi: 10.1007/s101200050063. [DOI] [PubMed] [Google Scholar]
- 13.Chen CY, Wu CW, Lo SS, Hsieh MC, Lui WY, Shen KH. Peritoneal carcinomatosis and lymph node metastasis are prognostic indicators in patients with Borrmann type IV gastric carcinoma. Hepatogastroenterology. 2002;49:874–877. [PubMed] [Google Scholar]
- 14.Pan W, Ishii H, Ebihara Y, Gobe G. Prognostic use of growth characteristics of early gastric cancer and expression patterns of apoptotic, cell proliferation, and cell adhesion proteins. J Surg Oncol. 2003;82:104–110. doi: 10.1002/jso.10204. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Morisaki T, Sugitani A, Ogawa T, Uchiyama A, Kinukawa N, Tanaka M. An early gastric carcinoma treatment strategy based on analysis of lymph node metastasis. Cancer. 1999;85:1500–1505. [PubMed] [Google Scholar]
- 16.Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. Lymph node status assessment for gastric carcinoma: is the number of metastatic lymph nodes really practical as a parameter for N categories in the TNM Classification Tumor Node Metastasis. J Surg Oncol. 1998;69:15–20. doi: 10.1002/(sici)1096-9098(199809)69:1<15::aid-jso4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Shimada S, Yagi Y, Honmyo U, Shiomori K, Yoshida N, Ogawa M. Involvement of three or more lymph nodes predicts poor prognosis in submucosal gastric carcinoma. Gastric Cancer. 2001;4:54–59. doi: 10.1007/pl00011724. [DOI] [PubMed] [Google Scholar]
- 18.Bouvier AM, Haas O, Piard F, Roignot P, Bonithon-Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas Results from a population based study. Cancer. 2002;94:2862–2866. doi: 10.1002/cncr.10550. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Nakane Y, Iiyama H, Sato M, Kanbara T, Nakai K, Okumura S, Yamamichi K, Hioki K. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol. 2002;9:27–34. doi: 10.1245/aso.2002.9.1.27. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda K, Shiraishi N, Suematsu T, Yamaguchi K, Adachi Y, Kitano S. Rate of detection of lymph node metastasis is correlated with the depth of submucosal invasion in early stage gastric carcinoma. Cancer. 1999;85:2119–2123. doi: 10.1002/(sici)1097-0142(19990515)85:10<2119::aid-cncr4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775–784. doi: 10.1007/BF02574500. [DOI] [PubMed] [Google Scholar]
- 22.Hyung WJ, Noh SH, Yoo CH, Huh JH, Shin DW, Lah KH, Lee JH, Choi SH, Min JS. Prognostic significance of metastatic lymph node ratio in T3 gastric cancer. World J Surg. 2002;26:323–329. doi: 10.1007/s00268-001-0227-9. [DOI] [PubMed] [Google Scholar]
- 23.Yin T, Ji XL, Shen MS. Relationship between lymph node sinuses with blood and lymphatic metastasis of gastric cancer. World J Gastroenterol. 2003;9:40–43. doi: 10.3748/wjg.v9.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee E, Chae Y, Kim I, Choi J, Yeom B, Leong AS. Prognostic relevance of immunohistochemically detected lymph node micrometastasis in patients with gastric carcinoma. Cancer. 2002;94:2867–2873. doi: 10.1002/cncr.10562. [DOI] [PubMed] [Google Scholar]
- 25.Choi HJ, Kim YK, Kim YH, Kim SS, Hong SH. Occurrence and prognostic implications of micrometastases in lymph nodes from patients with submucosal gastric carcinoma. Ann Surg Oncol. 2002;9:13–19. doi: 10.1245/aso.2002.9.1.13. [DOI] [PubMed] [Google Scholar]
- 26.Tsujitani S, Kaibara N. [Clinical significance of molecular biological detection of micrometastases in gastric carcinoma] Nihon Geka Gakkai Zasshi. 2001;102:741–744. [PubMed] [Google Scholar]
- 27.Fukagawa T, Sasako M, Mann GB, Sano T, Katai H, Maruyama K, Nakanishi Y, Shimoda T. Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer. 2001;92:753–760. doi: 10.1002/1097-0142(20010815)92:4<753::aid-cncr1379>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Mönig SP, Zirbes TK, Schröder W, Baldus SE, Lindemann DG, Dienes HP, Hölscher AH. Staging of gastric cancer: correlation of lymph node size and metastatic infiltration. AJR Am J Roentgenol. 1999;173:365–367. doi: 10.2214/ajr.173.2.10430138. [DOI] [PubMed] [Google Scholar]
- 29.Saiura A, Umekita N, Inoue S, Maeshiro T, Miyamoto S, Matsui Y, Asakage M, Kitamura M. Clinicopathological features and outcome of hepatic resection for liver metastasis from gastric cancer. Hepatogastroenterology. 2002;49:1062–1065. [PubMed] [Google Scholar]
- 30.Yoshizumi Y, Matuyama T, Koike H, Aiko S, Sugiura Y, Maehara T. Long-term survival after gastric cancer and liver and paraaortic lymph node metastases: report of a case. Surg Today. 2001;31:159–162. doi: 10.1007/s005950170202. [DOI] [PubMed] [Google Scholar]
- 31.Kologlu M, Kama NA, Reis E, Doganay M, Atli M, Dolapci M. A prognostic score for gastric cancer. Am J Surg. 2000;179:521–526. doi: 10.1016/s0002-9610(00)00385-8. [DOI] [PubMed] [Google Scholar]


