Abstract
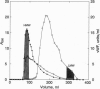
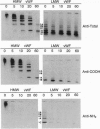
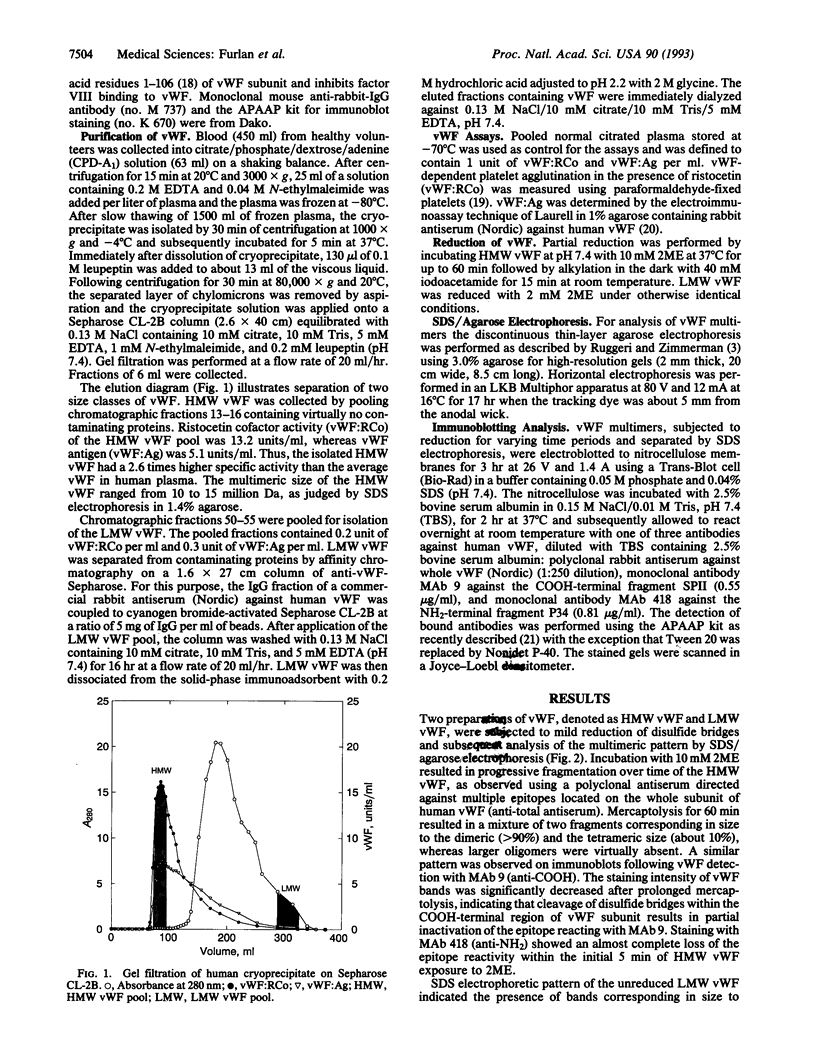
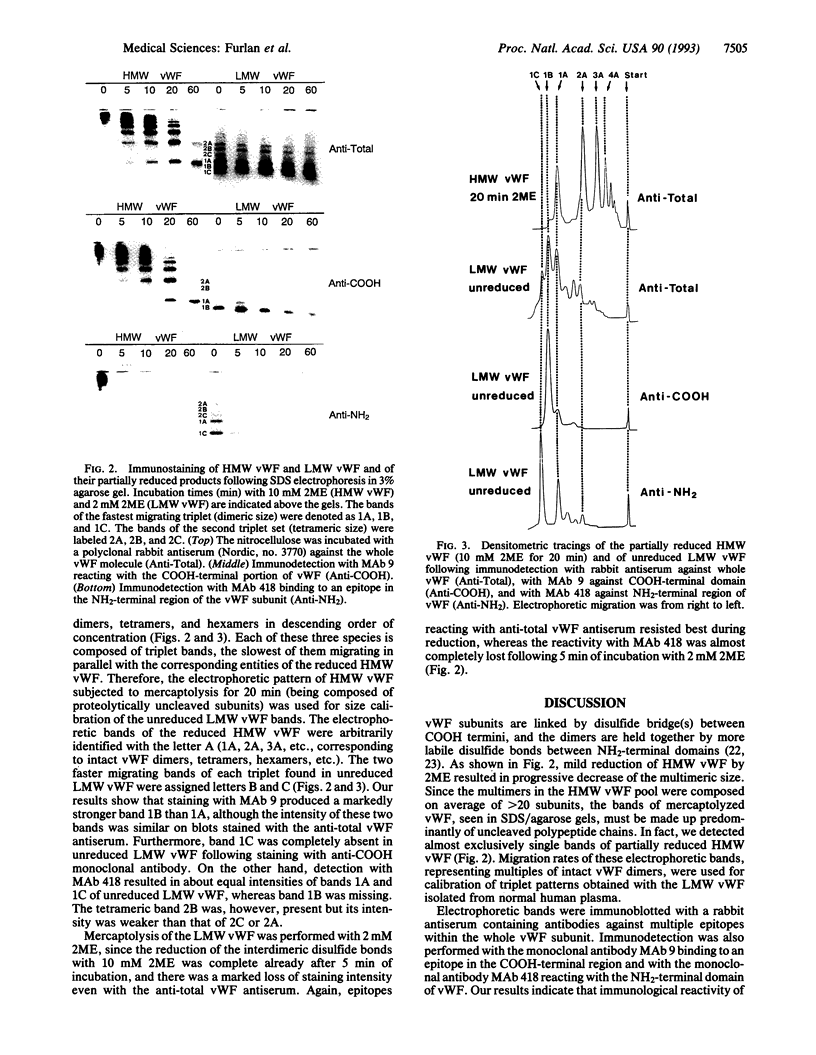
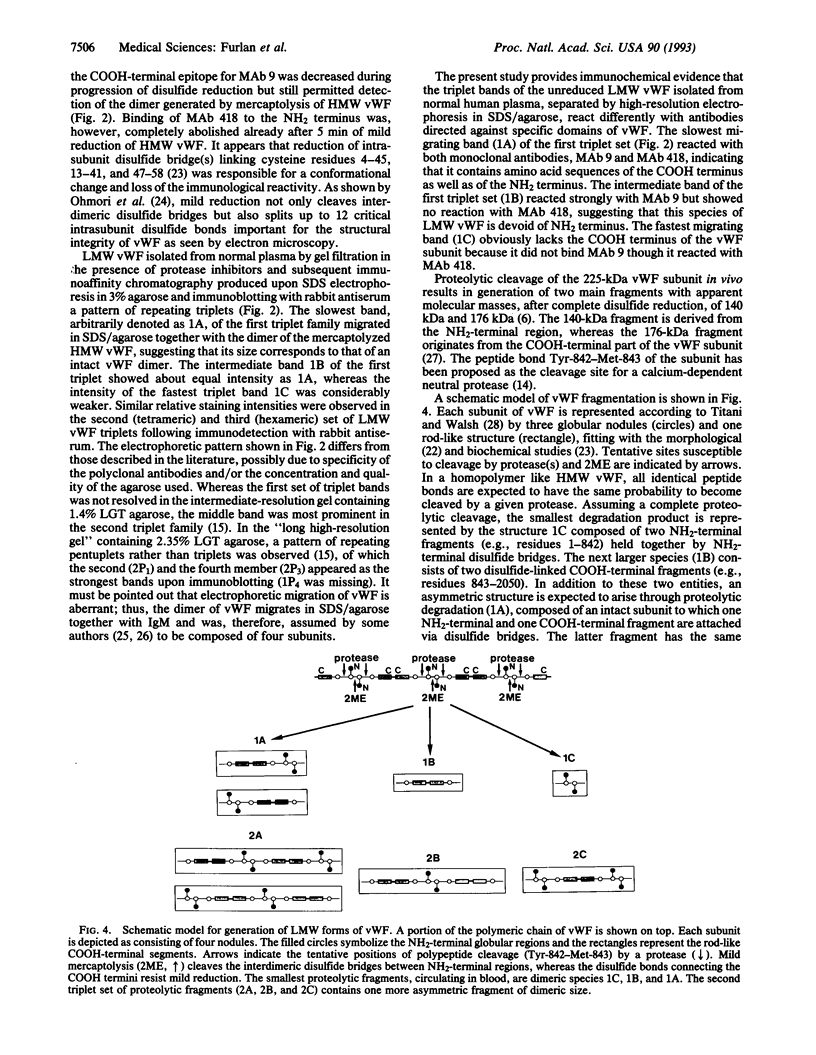
High molecular weight (HMW) and low molecular weight (LMW) forms of von Willebrand factor (vWF) were isolated from normal human plasma in the presence of protease inhibitors. HMW and LMW vWF preparations were subjected to reduction of interdimeric disulfide bridges under mild reducing conditions. Following sodium dodecyl sulfate electrophoresis in 3% agarose, the vWF bands were detected by immunoblotting with a polyclonal rabbit anti-vWF antiserum as well as with two monoclonal antibodies directed against epitopes located in the NH2-terminal (MAb 418) or in the COOH-terminal (MAb 9) region of the vWF subunit. Our results suggest that the slowest migrating band of the dimeric triplet set of LMW vWF represents an asymmetric structure composed of an intact subunit to which one NH2-terminal and one COOH-terminal fragment are linked by disulfide bridges. The intermediate band of the first triplet of LMW vWF strongly reacted with MAb 9 but not with MAb 418, indicating that it represents a dimer of COOH-terminal fragments. The fastest migrating band of the same triplet is apparently a dimer of the NH2-terminal fragments because it reacted with MAb 418 but not with MAb 9. Each next higher family of triplets seems to contain one more asymmetric fragment of dimeric size. These results are compatible with a model according to which LMW forms of vWF are derived from HMW vWF by proteolytic cleavage in the circulating blood.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baillod P., Affolter B., Kurt G. H., Pflugshaupt R. Multimeric analysis of von Willebrand factor by vertical sodium dodecyl sulphate agarose gel electrophoresis, vacuum blotting technology and sensitive visualization by alkaline phosphatase anti-alkaline phosphatase complex. Thromb Res. 1992 Jun 15;66(6):745–755. doi: 10.1016/0049-3848(92)90050-k. [DOI] [PubMed] [Google Scholar]
- Berkowitz S. D., Dent J., Roberts J., Fujimura Y., Plow E. F., Titani K., Ruggeri Z. M., Zimmerman T. S. Epitope mapping of the von Willebrand factor subunit distinguishes fragments present in normal and type IIA von Willebrand disease from those generated by plasmin. J Clin Invest. 1987 Feb;79(2):524–531. doi: 10.1172/JCI112843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde U., Dent J. A., Berkowitz S. D., Ruggeri Z. M., Zimmerman T. S. Subunit composition of plasma von Willebrand factor in patients with the myeloproliferative syndrome. Blood. 1986 Dec;68(6):1213–1217. [PubMed] [Google Scholar]
- Castaman G., Rodeghiero F., Lattuada A., Mannucci P. M. A new variant of von Willebrand disease (type II I) with a normal degree of proteolytic cleavage of von Willebrand factor. Thromb Res. 1992 Feb 1;65(3):343–351. doi: 10.1016/0049-3848(92)90165-7. [DOI] [PubMed] [Google Scholar]
- Chopek M. W., Girma J. P., Fujikawa K., Davie E. W., Titani K. Human von Willebrand factor: a multivalent protein composed of identical subunits. Biochemistry. 1986 Jun 3;25(11):3146–3155. doi: 10.1021/bi00359a012. [DOI] [PubMed] [Google Scholar]
- Counts R. B., Paskell S. L., Elgee S. K. Disulfide bonds and the quaternary structure of factor VIII/von Willebrand factor. J Clin Invest. 1978 Sep;62(3):702–709. doi: 10.1172/JCI109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent J. A., Berkowitz S. D., Ware J., Kasper C. K., Ruggeri Z. M. Identification of a cleavage site directing the immunochemical detection of molecular abnormalities in type IIA von Willebrand factor. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6306–6310. doi: 10.1073/pnas.87.16.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent J. A., Galbusera M., Ruggeri Z. M. Heterogeneity of plasma von Willebrand factor multimers resulting from proteolysis of the constituent subunit. J Clin Invest. 1991 Sep;88(3):774–782. doi: 10.1172/JCI115376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici A. B., Mannucci P. M., Lombardi R., Lattuada A., Colibretti M. L., Dent J. A., Zimmerman T. S. Type II H von Willebrand disease: new structural abnormality of plasma and platelet von Willebrand factor in a patient with prolonged bleeding time and borderline levels of ristocetin cofactor activity. Am J Hematol. 1989 Dec;32(4):287–293. doi: 10.1002/ajh.2830320409. [DOI] [PubMed] [Google Scholar]
- Fowler W. E., Fretto L. J., Hamilton K. K., Erickson H. P., McKee P. A. Substructure of human von Willebrand factor. J Clin Invest. 1985 Oct;76(4):1491–1500. doi: 10.1172/JCI112129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girma J. P., Kalafatis M., Piétu G., Lavergne J. M., Chopek M. W., Edgington T. S., Meyer D. Mapping of distinct von Willebrand factor domains interacting with platelet GPIb and GPIIb/IIIa and with collagen using monoclonal antibodies. Blood. 1986 May;67(5):1356–1366. [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., McKeown L. P., Maisonneuve P., Jenneau C., Sultan Y. A variant of type II von Willebrand disease with an abnormal triplet structure and discordant effects of protease inhibitors on plasma and platelet von Willebrand factor structure. Am J Hematol. 1987 Mar;24(3):259–266. doi: 10.1002/ajh.2830240305. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W., Shainoff J. R. Factor VIII-related protein circulates in normal human plasma as high molecular weight multimers. Blood. 1980 Jun;55(6):1056–1059. [PubMed] [Google Scholar]
- Kinoshita S., Harrison J., Lazerson J., Abildgaard C. F. A new variant of dominant type II von Willebrand's disease with aberrant multimeric pattern of factor VIII-related antigen (type IID). Blood. 1984 Jun;63(6):1369–1371. [PubMed] [Google Scholar]
- Macfarlane D. E., Stibbe J., Kirby E. P., Zucker M. B., Grant R. A., McPherson J. Letter: A method for assaying von Willebrand factor (ristocetin cofactor). Thromb Diath Haemorrh. 1975 Sep 30;34(1):306–308. [PubMed] [Google Scholar]
- Mannucci P. M., Lombardi R., Federici A. B., Dent J. A., Zimmerman T. S., Ruggeri Z. M. A new variant of type II von Willebrand disease with aberrant multimeric structure of plasma but not platelet von Willebrand factor (type IIF). Blood. 1986 Jul;68(1):269–274. [PubMed] [Google Scholar]
- Marti T., Rösselet S. J., Titani K., Walsh K. A. Identification of disulfide-bridged substructures within human von Willebrand factor. Biochemistry. 1987 Dec 15;26(25):8099–8109. doi: 10.1021/bi00399a013. [DOI] [PubMed] [Google Scholar]
- Meyer D., Dreyfus M. D., Larrieu M. J. Willebrand factor: immunological and biological study. Pathol Biol (Paris) 1973 Nov;21(Suppl):66–71. [PubMed] [Google Scholar]
- Ohmori K., Fretto L. J., Harrison R. L., Switzer M. E., Erickson H. P., McKee P. A. Electron microscopy of human factor VIII/Von Willebrand glycoprotein: effect of reducing reagents on structure and function. J Cell Biol. 1982 Nov;95(2 Pt 1):632–640. doi: 10.1083/jcb.95.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret B. A., Furlan M., Beck E. A. Studies on factor VIII-related protein. II. Estimation of molecular size differences between factor VIII oligomers. Biochim Biophys Acta. 1979 May 23;578(1):164–174. doi: 10.1016/0005-2795(79)90124-7. [DOI] [PubMed] [Google Scholar]
- Piétu G., Ribba A. S., Chérel G., Meyer D. Epitope mapping by cDNA expression of a monoclonal antibody which inhibits the binding of von Willebrand factor to platelet glycoprotein IIb/IIIa. Biochem J. 1992 Jun 15;284(Pt 3):711–715. doi: 10.1042/bj2840711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piétu G., Ribba A. S., Meulien P., Meyer D. Localization within the 106 N-terminal amino acids of von Willebrand factor (vWF) of the epitope corresponding to a monoclonal antibody which inhibits vWF binding to factor VIII. Biochem Biophys Res Commun. 1989 Aug 30;163(1):618–626. doi: 10.1016/0006-291x(89)92182-7. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Nilsson I. M., Lombardi R., Holmberg L., Zimmerman T. S. Aberrant multimeric structure of von Willebrand factor in a new variant of von Willebrand's disease (type IIC). J Clin Invest. 1982 Nov;70(5):1124–1127. doi: 10.1172/JCI110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest. 1980 Jun;65(6):1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Walsh K. A. Human von Willebrand factor: the molecular glue of platelet plugs. Trends Biochem Sci. 1988 Mar;13(3):94–97. doi: 10.1016/0968-0004(88)90048-5. [DOI] [PubMed] [Google Scholar]
- Tsai H. M., Nagel R. L., Hatcher V. B., Sussman I. I. Multimeric composition of endothelial cell-derived von Willebrand factor. Blood. 1989 Jun;73(8):2074–2076. [PubMed] [Google Scholar]
- Tsai H. M., Nagel R. L., Sussman I. I. Subunit composition of plasma von Willebrand factor multimers: evidence for a non-proteolytic mechanism resulting in apparent increase in proteolytic fragments. Thromb Res. 1991 Jul 1;63(1):179–188. doi: 10.1016/0049-3848(91)90280-a. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. S., Dent J. A., Ruggeri Z. M., Nannini L. H. Subunit composition of plasma von Willebrand factor. Cleavage is present in normal individuals, increased in IIA and IIB von Willebrand disease, but minimal in variants with aberrant structure of individual oligomers (types IIC, IID, and IIE). J Clin Invest. 1986 Mar;77(3):947–951. doi: 10.1172/JCI112394. [DOI] [PMC free article] [PubMed] [Google Scholar]