Abstract
AIM: To investigate the effect of Helicobacter pylori (H pylori) infection on the expressions of Bcl-2 family members in gastric adenocarcinoma.
METHODS: Gastric adenocarcinoma and resection margin tissues of 95 patients were studied. Semi-quantitative RT-PCR was used to measure Bid, Bax and Bcl-2 mRNA expressions.
RESULTS: Expressions of Bid and Bax in gastric adenocarcinoma tissues without H pylori infection, with cagA- H pylori infection and cagA+ H pylori infection increased significantly in turn (Bid, 0.304, 0.422 and 0.855 respectively, P < 0.05; Bax, 0.309, 0.650 and 0.979 respectively, P < 0.05). Bcl-2 mRNA levels increased significantly in gastric adenocarcinoma tissues with cagA- H pylori infection and cagA+ H pylori infection, compared with those without H pylori infection (0.696 and 0.849 vs 0.411, P < 0.05). Expressions of Bid, Bax and Bcl-2 in resection margin tissues without H pylori infection, with cagA- H pylori infection and cagA+ H pylori infection increased significantly in turn (Bid, 0.377, 0.686 and 0.939 respectively, P < 0.05; Bax, 0.353, 0.645 and 1.001 respectively, P < 0.05; Bcl-2, 0.371, 0.487 and 0.619 respectively, P < 0.05). In H pylori negative specimens, expressions of Bid and Bax correlated negatively with that of Bcl-2 respectively in adenocarcinoma tissues (Bid vs Bcl-2, r = -0.409, P < 0.05; Bax vs Bcl-2, r = -0.451, P < 0.05). In H pylori positive specimens, expressions of Bid and Bax did not correlate with that of Bcl-2 in adenocarcinoma tissues (Bid vs Bcl-2, r = 0.187, P > 0.05; Bax vs Bcl-2, r = 0.201, P > 0.05), but correlated positively with that of Bcl-2 respectively in resection margin tissues (Bid vs Bcl-2, r = 0.331, P < 0.05; Bax vs Bcl-2, r = 0.295, P < 0.05).
CONCLUSION: H pylori may enhance Bid, Bax and Bcl-2 mRNA levels and cause deregulation of these apoptosis-associated genes expressions, which may play a role during development of gastric adenocarcinoma induced by H pylori.
INTRODUCTION
Helicobacter pylori (H pylori) infection is the most common chronic infection in humans and is the major cause of gastritis worldwide. This infection is also accepted as the etiological factor of the majority of peptic ulcers. It has been implicated as a significant contributing factor in the development of gastric malignancy, both gastric MALT lymphoma and gastric adenocarcinoma[1-14], and H pylori was classified as a group 1 carcinogen for gastric cancer in 1994 by the WHO and International Agency for Research on Cancer (IARC)[15]. The role of H pylori infection in the gastric carcinogenesis is not clear. It might be involved in imbalance between apoptosis and proliferation[16-33]. Bcl-2 family members have been closely related to apoptosis, which could either promote cell survival (Bcl-2, Bcl-xL, A1, Mcl-1, and Bcl-w) or promote cell death (Bax, Bak, Bcl-xS, Bad, Bid, Bik, Bim, Hrk, Bok)[34-36]. In the present study, we investigated the effect of H pylori infection on the expressions of Bcl-2 family members in gastric adenocarcinoma and resection margin tissues.
MATERIALS AND METHODS
Tissue specimens
Specimens of gastric adenocarcinoma of 95 patients (72 males and 23 females, age range 31 to 84 years, mean 56 years), who had undergone resection surgical at the Southwest Hospital in Chongqing and had not taken anti-H pylori drugs before operation, were collected from 2001 to 2002. Gastric adenocarcinoma tissues were examined microscopically. Resection margin tissues were also examined to verify that they did not contain malignant cells. The histological diagnosis was confirmed by a professional pathologist. The remaining specimens were snap-frozen and stored at -80 °C until assayed. Warthin-Starry silver staining and polymerase chain reaction (PCR) analysis for H pylori urease gene A (ureA) were performed to detect H pylori infection. PCR analysis for H pylori cagA gene was performed to verify cagA+ H pylori infection. Fifty-eight patients whose both Warthin-Starry staining and PCR for ureA showed positive results were diagnosed as suffering from H pylori infection and 37 were cagA+ H pylori.
RT-PCR analysis of Bid, Bax and Bcl-2 mRNA
According to references[37,38], primers were designed for β-actin (GenBank accession No.BC013380), 5’-GTG GGG CGC CCC AGG CAC CA-3’ (sense) and 5’-CTC CTT AAT GTC ACG CAC GAT TTC-3’ (antisense), 540 bp product; for Bid (GenBank accession No.AF087891), 5’-ATG GAC TGT TGA GGT CAA CAA C-3’ (sense) and 5’-TCA GTC CAT CCC ATT TCT GGC T-3’ (antisense), 588 bp product; for Bax (GenBank accession No.AY217036), 5’-ACC AAG AAG CTG AGC GAG TGT C-3’ (sense) and 5’-ACA AAG ATG GTC ACG GTC TGC C-3’ (antisense), 332 bp product; and for Bcl-2 (GenBank accession No.M13994), 5’-TGC ACC TGA CGC CCT TCA C-3’ (sense), 5’-AGA CAG CCA GGA GAA ATC AAA CAG-3’ (antisense), 293 bp product.
Total RNA was prepared from gastric adenocarcinoma tissues and resection margin tissues by using TriPure isolation reagent (Roche) according to the manufacturer’s protocol. Reverse transcription (RT) was performed for first-strand cDNA by using 2 μg of total RNA and 1 μl of oligo(dT)18 primer in the presence of 5 unit AMV reverse transcriptase (Promega), 20 unit RNase inhibitor, 0.5 mmol/L of each dNTP and 1×buffer in 20 μl for 60 min at 42 °C. Then 2 μl reverse transcription products were used for PCR. In a total of 20 μl reactive mixture, 5 pmol/L sense primer and 5 pmol/L antisense primer, 0.25 mmol/L of each dNTP, 1×reaction buffer and 1.5 unit Taq polymerase were mixed. The reaction was run for 33 cycles, and each consisted of denaturation at 94 °C for 60 s, annealing at 58 °C for 60 s, extension at 72 °C for 60 s and final extension prolonged for 7 min at 72°C. PCR-amplified products (8 µl each) were analyzed on 1.5% agarose gels after ethidium bromide staining. Expression levels of Bid, Bax and Bcl-2 were quantitated using Quantity One quantitation software (Bio-Rad Laboratories) and were reported to be normalized to β-actin levels.
Statistical analysis
All data were presented as means ± standard error. Differences in means were examined by ANOVA, and correlations were analyzed by using Spearman’s rank correlation coefficient (SPSS 10.0 for Windows). A P value < 0.05 was considered significant.
RESULTS
Effect of H pylori on expressions of Bid, Bax and Bcl-2 mRNA
In gastric adenocarcinoma tissues, expressions of Bid and Bax mRNA in H pylori negative group, cagA- H pylori infection group and cagA+ H pylori infection group increased in an ascending pattern, respectively (P < 0.05). Expression of Bcl-2 in H pylori negative group was significantly lower than that in H pylori infection group (P < 0.05). Levels of Bcl-2 mRNA between cagA- H pylori infection group and cagA+ H pylori infection group did not show any significant difference.
In resection margin tissues, expressions of Bid, Bax and Bcl-2 mRNA in H pylori negative group, cagA- H pylori infection group and cagA+ H pylori infection group increased in turn (P < 0.05) (Figure 1, Table 1, Table 2 and Table 3).
Figure 1.
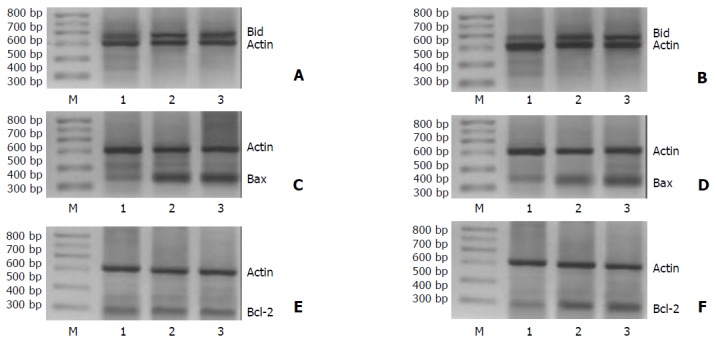
Effect of H pylori infection on mRNA expressions of Bcl-2 family members in gastric adenocarcinoma and resection margin tissues. A: Expression of Bid in gastric adenocarcinoma, B: Expression of Bid in resection margin, C: Expression of Bax in gastric adenocarcinoma, D: Expression of Bax in resection margin, E: Expression of Bcl-2 in gastric adenocarcinoma, F: Expression of Bcl-2 in resection margin, 1: H pylori negative group, 2: cagA- H pylori group, 3: cagA+ H pylori group.
Table 1.
Effect of H pylori infection on expression of Bid
| Adenocarcinoma | Resection margin | ||
| H pylori (-) | 0.304 ± 0.113 | 0.377 ± 0.119 | |
| H pylori (+) | cagA (-) | 0.422 ± 0.149a | 0.686 ± 0.285a |
| cagA (+) | 0.855 ± 0.305ac | 0.939 ± 0.383ac |
P < 0.05, vs H pylori negative group,
P < 0.05, vs cagA- H pylori infection group.
Table 2.
Effect of H pylori infection on expression of Bax
| Adenocarcinoma | Resection margin | ||
| H pylori (-) | 0.309 ± 0.123 | 0.353 ± 0.139 | |
| H pylori (+) | cagA (-) | 0.650 ± 0.393a | 0.645 ± 0.327a |
| cagA (+) | 0.979 ± 0.375ac | 1.001 ± 0.361ac |
P < 0.05, vs H pylori negative group,
P < 0.05, vs cagA- H pylori infection group.
Table 3.
Effect of H pylori infection on expression of Bcl-2
| Adenocarcinoma | Resection margin | ||
| H pylori (-) | 0.411 ± 0132 | 0.371 ± 0.153 | |
| H pylori (+) | cagA (-) | 0.696 ± 0.318a | 0.487 ± 0.241a |
| cagA (+) | 0.849 ± 0.352a | 0.619 ± 0.243ac |
P < 0.05, vs H pylori negative group,
P < 0.05, vs cagA- H pylori infection group.
Correlation among levels of Bid, Bax and Bcl-2 mRNA
In H pylori negative group, levels of Bid and Bax mRNA correlated negatively with that of Bcl-2 in gastric adenocarcinoma tissues (Bid vs Bcl-2,r = -0.409, P < 0.05; Bax vs Bcl-2, r = -0.451, P < 0.05). In H pylori positive group, expressions of Bid and Bax did not correlate with that of Bcl-2 in adenocarcinoma tissues (Bid vs Bcl-2, r = 0.187, P > 0.05; Bax vs Bcl-2, r = 0.201, P > 0.05), but correlated positively with that of Bcl-2 respectively in resection margin tissues (Bid vs Bcl-2, r = 0.331, P < 0.05; Bax vs Bcl-2, r = 0.295, P < 0.05).
DISCUSSION
Bid, Bax and Bcl-2 are representative members of Bcl-2 family. Bid and Bax are pro-apoptosis members. Bcl-2 is an anti-apoptosis member. In the mechanism of regulating apoptosis, Bid as a BH3 domain protein, is one of the initiators to apoptosis and Bax is the key member. Either Bid or Bcl-2 must rely on Bax to induce or inhibit apoptosis[39-43].
Studies have shown that H pylori infection could induce Fas antigen (Fas Ag) expression in gastric epithelial cells[44]. In addition, H pylori infection was also associated with increased mucosal inflammatory cytokines, including TNF-α[45] and IFN-γ[46]. The cytokines generated during the immune response to H pylori also increased expression of Fas Ag in gastric cell lines[47]. Fas Ag, after binding specifically to its ligand (Fas L), trimerizes and activates Caspase-8. Activation of Caspase-8 could result in the cleavage of cytosolic Bid to truncate tBID, which could translocate to mitochondria and initiate apoptosis[48]. Shibayama et al[31] found that H pylori infection induced the activation of Caspase-8 and the expression of Bid in human gastric epithelial cells, and inhibition of Caspase-8 suppressed the expression of Bid. In the present study, we found that H pylori infection upregulated expression of Bid mRNA in both gastric adenocarcinoma and resection margin tissues. That might be due to the upregulation of Fas and the activation of Caspase-8.
Bax is a cytosolic protein and translocates from the cytosol to the mitochondria for integration into the membrane following a proapoptotic stimulus. This action then results in cytochrome C release and initiates apoptosis. We have previously demonstrated that H pylori infection could promote Bax protein expression in chronic gastritis and premalignant lesions. Expression of Bax correlated positively with apoptotic index. The apoptotic index in Bax expression positive group in intestinal metaplasia, gastric dysplasia and gastric carcinoma was significantly higher than that in Bax negative group. In the present study, we found H pylori infection also increased levels of Bax mRNA in both gastric adenocarcinoma and resection margin tissues, and the effect was stronger in CagA+ H pylori group than cagA- H pylori group. These results showed H pylori infection might promote apoptosis in gastric adenocarcinoma and its resection margin tissues.
Bc1-2 is an important anti-apoptosis protein. We found that Bcl-2 mRNA levels in H pylori negative group in gastric adenocarcinoma tissues were lower than in H pylori positive group. In resection margin tissues, Bcl-2 mRNA levels in H pylori negative group, cagA- H pylori infection group and cagA+ H pylori infection group respectively increased in turn, suggesting that H pylori might promote expression of Bcl-2 in both gastric adenocarcinoma and resection margin tissues.
Although H pylori could promote expressions of Bid, Bax and Bcl-2, the correlations among them are still unclear. In the present study, it showed that levels of Bid and Bax mRNA were correlated negatively with that of Bcl-2 in gastric adenocarcinoma tissues without H pylori infection. This result is correspondent with the findings that apoptosis decreases in tumor tissues. In H pylori positive group, levels of Bid, Bax and Bcl-2 mRNA in gastric adenocarcinoma all increased. Besides, levels of Bid, Bax, and Bcl-2 did not correlate with each other. In the resection margin tissues, levels of Bid and Bax mRNA were correlated positively with that of Bcl-2. These results indicated that although H pylori could promote expressions of Bid, Bax and Bcl-2, it might play a different role in the development of gastric adenocarcinoma. In benign gastric lesions, H pylori infection might mainly upregulate expressions of pro-apoptotic genes such as Bid and Bax, and this effect might be stronger than its upregulatory effect on Bcl-2, which is consistent with the phenomena that H pylori infection increases apoptosis in atrophic gastritis and gastric ulcer. During development of gastric adenocarcinoma, upregulatory effect of H pylori on anti-apoptotic genes, for example Bcl-2, might increase gradually and counteract pro-apoptosis effect of Bid and Bax, which may induce or worsen deregulation of apoptosis-associated genes expressions during the course of the formation of gastric adenocarcinoma.
Recently, it was found that not only excessive proliferation played an important role in gastric adenocarcinoma, but also reduction of apoptosis contributed to the carcinogenesis of gastric mucosa. The abnormal expressions of Bid, Bax and Bcl-2 induced by H pylori might result in inhibition of apoptosis, which may play an important role during development of gastric adenocarcinoma induced by H pylori[49]. The detailed mechanism still remains to be studied.
Footnotes
Supported by the National Natural Science Foundation of China, No.30070043, and the Key Programs of the Military Medical and Health Foundation during the 10th Five-Year Plan Period, No.01Z075
Edited by Wang XL Proofread by Zhu LH
References
- 1.Jones RG, Trowbridge DB, Go MF. Helicobacter pylori infection in peptic ulcer disease and gastric malignancy. Front Biosci. 2001;6:E213–E226. doi: 10.2741/jones1. [DOI] [PubMed] [Google Scholar]
- 2.Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 3.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 4.Matsukura N. [Relation between Helicobacter pylori and diseases: knowledge for clinician] J Nippon Med Sch. 2002;69:200–204. doi: 10.1272/jnms.69.200. [DOI] [PubMed] [Google Scholar]
- 5.Miwa H, Go MF, Sato N. H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol. 2002;97:1106–1112. doi: 10.1111/j.1572-0241.2002.05663.x. [DOI] [PubMed] [Google Scholar]
- 6.Lan J, Xiong YY, Lin YX, Wang BC, Gong LL, Xu HS, Guo GS. Helicobacter pylori infection generated gastric cancer through p53-Rb tumor-suppressor system mutation and telomerase reactivation. World J Gastroenterol. 2003;9:54–58. doi: 10.3748/wjg.v9.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848–854. doi: 10.3748/wjg.v6.i6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR, Zhu YM, Zhang WM, Cao YX, Zhu CW, et al. Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case-control study in China. World J Gastroenterol. 2002;8:1103–1107. doi: 10.3748/wjg.v8.i6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801–804. doi: 10.3748/wjg.v7.i6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County,Fujian Province,China. World J Gastroenterol. 2000;6:374–376. doi: 10.3748/wjg.v6.i3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang ZW, Farthing MJ. Molecular mechanisms of H. pylori associated gastric carcinogenesis. World J Gastroenterol. 1999;5:369–374. doi: 10.3748/wjg.v5.i5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu GY, Yu BP, Dong WG, Li MQ, Yu JP, Luo HS, Rang ZX. Expression of TFF2 and Helicobacter pylori infection in carcinogenesis of gastric mucosa. World J Gastroenterol. 2003;9:910–914. doi: 10.3748/wjg.v9.i5.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YL, Xu B, Song YG, Zhang WD. Overexpression of c-fos in Helicobacter pylori-induced gastric precancerosis of Mongolian gerbil. World J Gastroenterol. 2003;9:521–524. doi: 10.3748/wjg.v9.i3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo XL, Wang LE, Du SY, Fan CL, Li L, Wang P, Yuan Y. Association of cyclooxygenase-2 expression with Hp-cagA infection in gastric cancer. World J Gastroenterol. 2003;9:246–249. doi: 10.3748/wjg.v9.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [Google Scholar]
- 16.Jang TJ, Kim JR. Proliferation and apoptosis in gastric antral epithelial cells of patients infected with Helicobacter pylori. J Gastroenterol. 2000;35:265–271. doi: 10.1007/s005350050344. [DOI] [PubMed] [Google Scholar]
- 17.Chan AO, Wong BC, Lam SK. Gastric cancer: past, present and future. Can J Gastroenterol. 2001;15:469–474. doi: 10.1155/2001/850308. [DOI] [PubMed] [Google Scholar]
- 18.Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15–17. doi: 10.3748/wjg.v5.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu HF, Liu WW, Fang DC, Liu FX, He GY. Clinical significance of Fas antigen expression in gastric carcinoma. World J Gastroenterol. 1999;5:90–91. doi: 10.3748/wjg.v5.i1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda S, Yoshida H, Mitsuno Hirata Y, Ogura K, Shiratori Y, Omata M. Analysis of apoptotic and antiapoptotic signalling pathways induced by Helicobacter pylori. Gut. 2002;50:771–778. doi: 10.1136/gut.50.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamajima N, Matuo K, Watanabe Y, Suzuki T, Nakamura T, Matsuura A, Yamao K, Ohashi K, Tominaga S. A pilot study to evaluate stomach cancer risk reduction by Helicobacter pylori eradication. Am J Gastroenterol. 2002;97:764–765. doi: 10.1111/j.1572-0241.2002.05571.x. [DOI] [PubMed] [Google Scholar]
- 23.Kodama M, Fujioka T, Kodama R, Takahashi K, Kubota T, Murakami K, Nasu M. p53 expression in gastric mucosa with Helicobacter pylori infection. J Gastroenterol Hepatol. 1998;13:215–219. doi: 10.1111/j.1440-1746.1998.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhu GH, Yang XL, Lai KC, Ching CK, Wong BC, Yuen ST, Ho J, Lam SK. Nonsteroidal antiinflammatory drugs could reverse Helicobacter pylori-induced apoptosis and proliferation in gastric epithelial cells. Dig Dis Sci. 1998;43:1957–1963. doi: 10.1023/a:1018830408397. [DOI] [PubMed] [Google Scholar]
- 25.Kohda K, Tanaka K, Aiba Y, Yasuda M, Miwa T, Koga Y. Role of apoptosis induced by Helicobacter pylori infection in the development of duodenal ulcer. Gut. 1999;44:456–462. doi: 10.1136/gut.44.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konturek PC, Pierzchalski P, Konturek SJ, Meixner H, Faller G, Kirchner T, Hahn EG. Helicobacter pylori induces apoptosis in gastric mucosa through an upregulation of Bax expression in humans. Scand J Gastroenterol. 1999;34:375–383. doi: 10.1080/003655299750026380. [DOI] [PubMed] [Google Scholar]
- 27.Fan X, Crowe SE, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley WK, Ernst PB, Reyes VE. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659–1669. doi: 10.1084/jem.187.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anti M, Armuzzi A, Iascone E, Valenti A, Lippi ME, Covino M, Vecchio FM, Pierconti F, Buzzi A, Pignataro G, et al. Epithelial-cell apoptosis and proliferation in Helicobacter pylori-related chronic gastritis. Ital J Gastroenterol Hepatol. 1998;30:153–159. [PubMed] [Google Scholar]
- 29.Watanabe S, Takagi A, Koga Y, Kamiya S, Miwa T. Helicobacter pylori induces apoptosis in gastric epithelial cells through inducible nitric oxide. J Gastroenterol Hepatol. 2000;15:168–174. doi: 10.1046/j.1440-1746.2000.02062.x. [DOI] [PubMed] [Google Scholar]
- 30.Takagi A, Watanabe S, Igarashi M, Koike J, Hasumi K, Deguchi R, Koga Y, Miwa T. The effect of Helicobacter pylori on cell proliferation and apoptosis in gastric epithelial cell lines. Aliment Pharmacol Ther. 2000;14 Suppl 1:188–192. doi: 10.1046/j.1365-2036.2000.014s1188.x. [DOI] [PubMed] [Google Scholar]
- 31.Shibayama K, Doi Y, Shibata N, Yagi T, Nada T, Iinuma Y, Arakawa Y. Apoptotic signaling pathway activated by Helicobacter pylori infection and increase of apoptosis-inducing activity under serum-starved conditions. Infect Immun. 2001;69:3181–3189. doi: 10.1128/IAI.69.5.3181-3189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igarashi M, Kitada Y, Yoshiyama H, Takagi A, Miwa T, Koga Y. Ammonia as an accelerator of tumor necrosis factor alpha-induced apoptosis of gastric epithelial cells in Helicobacter pylori infection. Infect Immun. 2001;69:816–821. doi: 10.1128/IAI.69.2.816-821.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol. 2001;96:16–26. doi: 10.1111/j.1572-0241.2001.03447.x. [DOI] [PubMed] [Google Scholar]
- 34.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 35.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 36.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 37.Lee EH, Wan XH, Song J, Kang JJ, Cho JW, Seo KY, Lee JH. Lens epithelial cell death and reduction of anti-apoptotic protein Bcl-2 in human anterior polar cataracts. Mol Vis. 2002;8:235–240. [PubMed] [Google Scholar]
- 38.Tafani M, Karpinich NO, Hurster KA, Pastorino JG, Schneider T, Russo MA, Farber JL. Cytochrome c release upon Fas receptor activation depends on translocation of full-length bid and the induction of the mitochondrial permeability transition. J Biol Chem. 2002;277:10073–10082. doi: 10.1074/jbc.M111350200. [DOI] [PubMed] [Google Scholar]
- 39.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 41.Theodorakis P, Lomonosova E, Chinnadurai G. Critical requirement of BAX for manifestation of apoptosis induced by multiple stimuli in human epithelial cancer cells. Cancer Res. 2002;62:3373–3376. [PubMed] [Google Scholar]
- 42.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 43.Shangary S, Johnson DE. Peptides derived from BH3 domains of Bcl-2 family members: a comparative analysis of inhibition of Bcl-2, Bcl-x(L) and Bax oligomerization, induction of cytochrome c release, and activation of cell death. Biochemistry. 2002;41:9485–9495. doi: 10.1021/bi025605h. [DOI] [PubMed] [Google Scholar]
- 44.Houghton J, Macera-Bloch LS, Harrison L, Kim KH, Korah RM. Tumor necrosis factor alpha and interleukin 1beta up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection. Infect Immun. 2000;68:1189–1195. doi: 10.1128/iai.68.3.1189-1195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, Telford JL, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 46.Harris PR, Mobley HL, Perez-Perez GI, Blaser MJ, Smith PD. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology. 1996;111:419–425. doi: 10.1053/gast.1996.v111.pm8690207. [DOI] [PubMed] [Google Scholar]
- 47.Houghton J, Korah RM, Condon MR, Kim KH. Apoptosis in Helicobacter pylori-associated gastric and duodenal ulcer disease is mediated via the Fas antigen pathway. Dig Dis Sci. 1999;44:465–478. doi: 10.1023/a:1026628601284. [DOI] [PubMed] [Google Scholar]
- 48.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 49.Konturek PC, Konturek SJ, Pierzchalski P, Bielański W, Duda A, Marlicz K, Starzyńska T, Hahn EG. Cancerogenesis in Helicobacter pylori infected stomach--role of growth factors, apoptosis and cyclooxygenases. Med Sci Monit. 2001;7:1092–1107. [PubMed] [Google Scholar]


