Abstract
AIM: To investigate the dynamic changes of capillarization and peri-sinusoid fibrosis in an alcoholic liver disease model induced by a new method.
METHODS: Male SD rats were randomly divided into 6 groups, namely normal, 4 d, 2 w, 4 w, 9 w and 11 w groups. The animals were fed with a mixture of alcohol for designated days and then decollated, and their livers were harvested to examine the pathological changes of hepatocytes, hepatic stellate cells, sinusoidal endothelial cells, sinusoid, peri-sinusoid. The generation of three kinds of extra cellular matrix was also observed.
RESULTS: The injury of hepatocytes became severer as modeling going on. Under electronic microscope, fatty vesicles and swollen mitochondria in hepatocytes, activated hepatic stellate cells with fibrils could been seen near or around it. Fenestrae of sinusoidal endothelial cells were decreased or disappeared, sinusoidal basement was formed. Under light microscopy typical peri-sinusoid fibrosis, gridding-like fibrosis, broaden portal areas, hepatocyte’s fatty and balloon denaturation, iron sediment, dot necrosis, congregated lymphatic cells and leukocytes were observed. Type I collagen showed an increasing trend as modeling going on, slightly recovered when modeling stopped for 2 weeks. Meanwhile, type IV collagen decreased rapidly when modeling began and recovered after modeling stopped for 2 weeks. Laminin increased as soon as modeling began and did not recover when modeling stopped for 2 weeks.
CONCLUSION: The pathological changes of the model were similar to that of human ALD, but mild in degree. It had typical peri-sinusoid fibrosis, however, capillarization seemed to be instable. It may be related with the reduction of type IV collagen in the basement of sinusoid during modeling.
INTRODUCTION
In China alcoholic liver disease patients have been on the rise. Acetaldehyde and hydroxy free radicals oxidized from alcohol can injure hepatocytes and activate lipid peroxidation. Hydroxy free radicals are able to activate phagocytes to secrete cytokines and activate hepatic stellate cells (HSC). The activation of HSC leads to the production of various components of extracelluar matrix (ECM). Researchers have reported that peri-sinusoid fibrosis, capilllarization, gridding-like fibrosis, bridging fibrosis and even cirrhosis are most typical morphological changes in alcoholic consumers for more than 10 or even 20 years. But mild and/or moderate drinkers may not experience such severe damages of the liver. Among the ECM produced during fibrosis, types I and IV collagen and laminin are closely related to the formation of capillarization and peri-sinusoid fibrosis. The Tsukamoto-French model has been used to investigate ALD, but it is expensive and complicated. Many researchers like to make use of the gavage model because of its simplicity and convenience, but there are many inconsistent reports about the content of alcohol ingested, the time of modeling, etc. In order to probe into the mechanism of ALD and find a more suitable model, we investigated the dynamic changes of capillarization and peri-sinusoid fibrosis, the contents of types I and IV collagen and laminin, etc. in male SD rats during modeling so as to explore whether it was acceptable.
MATERIALS AND METHODS
Materials
Male SD rats, weighing 150 ± 5g, were purchased from Beijing Vital River Company. Corn oil was from Carafour Supermarket. Xanthan gum and maltose were from Beijing Chemical Agent Company. Edible alcohol was from Beijing General Alcohol Brewing Company. Carbonyl iron and pirazole were from Sigma, USA. First antibody to type I collagen, type IV collagen and laminin were from Antibody Diagnostic Inc, ADI, USA. PV-6001 Kits were from Power Vision, USA. ZLI-9030 and ZLI9001 were from Beijing Zhongshan Company.
Methods
Fifty-six rats were divided into normal(6), 4 d(8), 2 w(8), 4 w(10), 9 w(12), 11 w(12) groups.
ALD model was induced by intragastric infusion of a mixture made of alcohol (5 g/d·kg), pirazole (30 mg/d·kg), corn oil (3 ml/d·kg), carbonyl iron (35 mg/d·kg, which was decreased to 15 mg after 4 w), a little xanthan gum and maltose once a day for 5 days consecutively, with 2 days off per week, until 9 w. The rats were fed with normal diet and water ad libitum.
The rats were executed at the end of 4 d, 2 w, 4 w, 9 w and 11 w, respectively. Harvested livers were split and fixed for electron microscopy, hematoxylin and eosin, and Masson complex staining. A portion was snap frozen for biochemical and molecular analysis. Histological analysis of each liver was undertaken. Further sections were cut from each liver, deparaffinized and subjected to amylopsin antigen retrieval before being immunostained with primary antigen and second envision agents for types I and IV collagen and laminin. Semi-quantitive computation of types I and IV collagen and laminin was done by the image analyzing system MIS-2000, which was from 3Y Company, USA. The slides for electron microscopic examination were made according to routine protocol, sub-cellular morphology was investigated and photographed under JEM-1200EX (80KV).
Statistics
SPSS Version 10.0 was used. All values were expressed as mean ± SD. One-way ANOVA was used to determine the significance of differences among the six groups. P < 0.05 was considered statistically significant.
RESULTS
HE staining
Normal hepatocytes in neat plates, had big and round nuclei, with a clear profile. Hepatocytes in Four d group became swollen and turbid, or balloon denatured. Sinus stricture was evident. Hepatocytes in 2 w group showed bridging balloon denaturation at around-lobules and portal area, dot necrosis and congregating inflammatory cells could be seen. Four w group exhibited evident hepatocytes coagulation necrosis, mesenchymal cells hyperplasia, broadened portal area with fibrosis. In the Nine w group Mallory body and widespread fatty vesicle denaturation in hepatocytes, piecemeal necrosis, congregating inflammatory cells and fibrosis could be seen. Eleven w group had denatured hepatocytes and a little necrosis, hepatic stellate cells showed dark nuclei (Figure 1. A, B, C)
Figure 1.
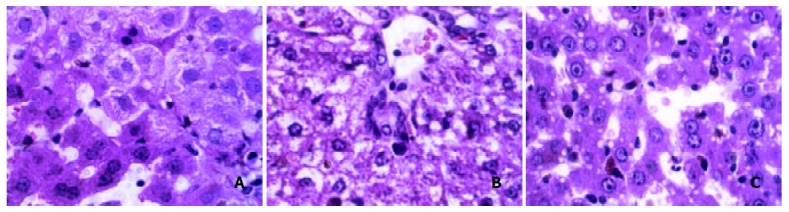
Changes of hepatocytes after HE staining. A: normal HE, B: 4 d HE, C: 4 w HE.
HE-masson staining
Normal group showed very light stain of the basement of central veins of lobules, Which was mildly evident in blood vessels at the portal area. Four d group showed more evident stain of central vein basement, parts of the hepatocytes were surrounded by gridding-like stains. Two w group showed more gridding-like stains. In 4 w group almost all hepatocytes were surrounded by gridding-like fibrosis and thicker basements of central veins were seen. Thick fibers in portal area bridged with those in hepatic lobules. The fibrotic changes in 9 w group deteriorated. In 11 w group the gridding-like fibrosis slightly ameliorated, but no evident changes were seen in central veins and portal areas (Figure 2. A, B, C).
Figure 2.
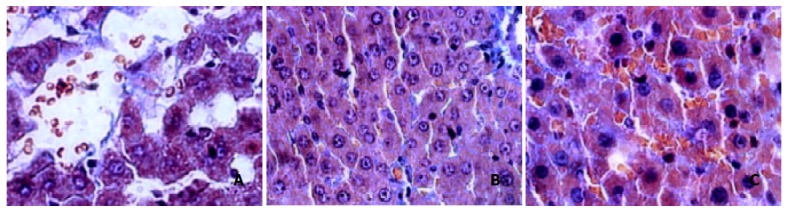
Changes of hepatocytes after Masson staining. A: normal Masson, B: 4 w Masson, C: 11 w Masson
Electron microscopic examination
Normal hepatocytes had round and clear nuclei, many microvilli extended to touch sinusoidal endothelial cells, or encircle hepatic stellate cells. Its mitochondria presented oval profiles and clear crests. Sinusoidal endothelial cells had many fenestrae and flat nuclei, without obvious basement and fibril underneath. HSC showed tight nuclear chromatin, few in number, and without fibril surrounded by. As modeling going on, the microvilli of hepatocytes became swollen and broken, rough endoplasmic reticulum showed turbulence, mitochondria became swollen and crest broken. The nuclei of sinusoidal endothelial cells became darker and thicker, nuclei chromatin grew crassitude. Fewer fenestrae and basement could be seen under which a large amount of fibrils was found. HSC proliferated actively, with nuclear chromatin turned into crassitude, and lots of fibril surrounded lay (Figure 3. A, B, C, D).
Figure 3.
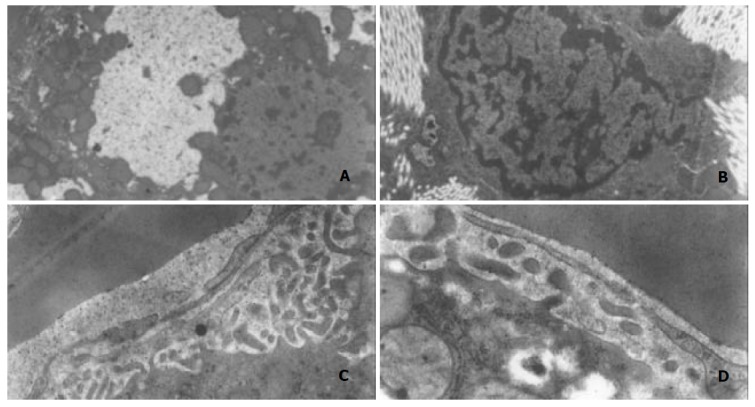
Changes of hepatocytes observed by electron microscopy. A: fatty vesicle in hepatocytes, B: activated HSC and fibril, C: sinusoidal endothelium and basement, D: endothelium,less fenestrae.
Immunohistochemical staining
The content of type I collagen grew gradually from Two w and reached the peak in Nine w. After 2 week recovery, it dropped a little in 11 week but was still higher than that of normal rats (Figure 4. A, B, C).
Figure 4.
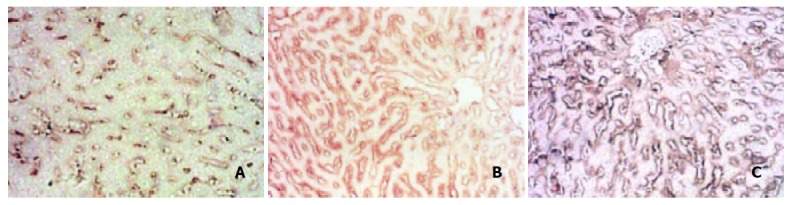
Changes of type I collagen after immunohistochemical staining. A: col-I Normal group, B: Col-I in 4 w group, C: Col-I in 9 w group.
The content of type IV collagen in normal rats was moderate, but it rapidly decreased after the infusion of alcohol mixture, and was sustained at a low level during the course of modeling. However, it recovered in Eleven w (Figure 5. A, B, C, D, E, F).
Figure 5.
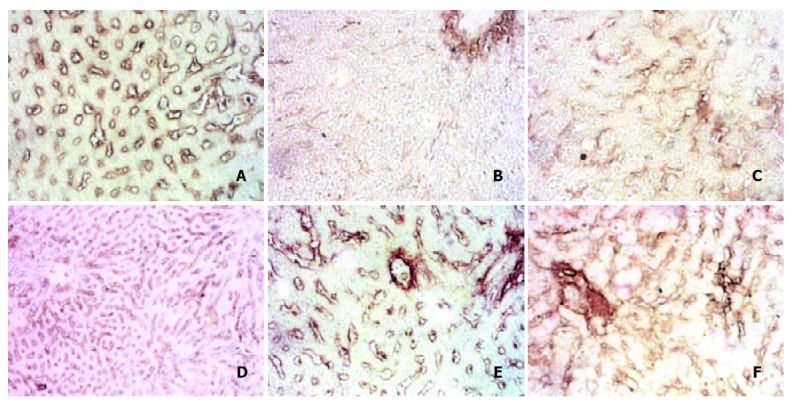
Changes of type IV collagen after immunohistochemical staining. A: col-IV Normal group, B: Col-IV in 4 d group, C: Col-IV in 2 w group, D: Col-IV in 4 w group, E: Col-IV in 9 w group, F: Col-IV in 11 w group.
There were few positive stains of laminin in normal rats, but as soon as the modeling began, its content increased rapidly, maintaining at high levels during the course, and did not return to normal in 11 w group, but much higher than that of normal rats (Figure 6. A, B, C, D, E, Table 1).
Figure 6.
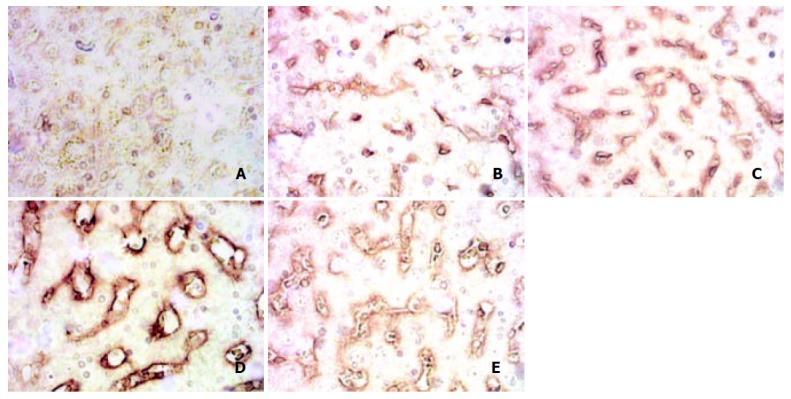
Changes of laminin after immunohistochemical staining. A: laminin Normal group, B: Laminin in 4 d group, C: Laminin in 4 w group, D: Laminin in 9 w group, E: Laminin in 11 w group.
Table 1.
Dynamic changes of types I and IV collagen and laminin (-x ± s)
| Group | Type I Collagen | Type IV Collagen | Laminin |
| Normal | 0.2874 ± 0.0224 | 0.3421 ± .0217 | 0.2263 ± 0.0010 |
| 4 d | 0.2811 ± 0.0151 | 0.2907 ± 0.0109a | 0.2975 ± 0.0034a |
| 2 w | 0.2912 ± 0.0122 | 0.2463 ± 0.0062a | 0.2962 ± 0.0048a |
| 4 w | 0.3853 ± 0.0401a | 0.3165 ± 0.0049a | 0.3336 ± 0.0091a |
| 9 w | 0.4262 ± 0.0992a | 0.3202 ± 0.0039a | 0.3523 ± 0.0108a |
| 11 w | 0.3734 ± 0.1239a | 0.3249 ± 0.0119 | 0.3549 ± 0.0120a |
P < 0.05 vs normal
DISCUSSION
The viewpoints[1-3] that moderate drinking might be beneficial to heart and vascular system or might improve the ability of senior citizens to cognize imply that the disadvantages of alcohol have not been sufficiently recognized. Investigations on pathological changes of mild and moderate drinkers in the liver, especially capillarization and peri-sinusoid fibrosis are not so profound.
Alcohol abuse has become a severe problem in China. The modeling method for the purpose of ALD research is one of the key factors. ALD may be formed in more than 10 or even 20 years, meanwhile have been found many differences due to their various genotypes[4,5]. As to modeling, pure alcohol intake was difficult in inducing a successful model in a relative short period of time[6]. Tsukamoto-French model can do so by means of consecutive infusion of alcohol into stomach through a gastric fistula and does not need quite a long time, but it is complicated and expensive. At present the most widely used method of modeling is “gavage” of alcohol[7,8]. In order to make a model in a relative short period of time, other assisting materials besides alcohol must be added to promote the pathological progress. But the materials added should be of or about the same mechanism as that of alcohol, and the contents of them have to be restrained in order not to overcome alcohol. The core is whether the model has similar pathological changes to ALD and suitable for drug researches, whether it can illustrate the mechanism of treatment so to serve clinic investigation reliably.
The mechanism of ALD has been thought to have a close relation to many factors such as lipid peroxidation[9], endotoxin[10-13], acetaldehyde and immunological injuries it induced[14-16], gender[17], sediment of iron[18-25], free radicals[26], etc. Analysis[27] of alcohol-responsive genes showed that alcohol might injure the liver omnidirectionally, causing not only fatty liver, necrosis and inflammation, hepatocyte apoptosis and hyperhomocysteinemia, but also turbulence in glucose metabolism and DNA damages, etc. It has been recognized that the occurrence of fibrosis induced by alcohol or other factors is due to the activation of hepatic stellate cells[18,28-40]. All materials added besides alcohol such as iron, polyunsaturated fatty acid and endotoxin during modeling should be designed to strengthen the common mechanism. Our model accepted iron as an additive to promote the course of lipid peroxidation. Corn oil had similar functions. Pirazole could delay the course of alcohol to be cleared from plasmid. Meanwhile we used pirazole to cause or promote blood stasis and flatulence of the intestinal tract, this might be due to its stimulating effect on local mucosa. It needs more evidences to say that pirazole can promote endotoxin absorption. But whether the fibrosis model induced by alcohol and CCI4 is suitable for ALD research should be under further observation[41].
Some researchers reported[42] that capillarization, peri-sinusoid fibrosis and gridding-like fibrosis were typical morphological changes in alcoholic cirrhosis of human beings which are rather different from that of fibrosis or cirrhosis induced by virus infection, yet we do not know whether it would happen in average moderate drinkers, and how the extracellular matrix was involved in changes of types I and IV collagen and laminin. Since there was no unification about the species of rats, the dosage of alcohol and additives used during modeling, we introduced a model established by intragastric infusion of a new mixture of alcohol for 9 weeks consecutively and studied the dynamic changes of types I and IV collagen as well as laminin, in order to find out whether this model was suitable or not for ALD research[43,44].
The end products of alcohol in the liver include acetaldehyde and hydroxy free radicals which can injure hepatocytes and activate lipid peroxidation. Phagocytes are susceptible to the course, and would excrete many cytokines such as TNF-α, TGF-β1, which in turn activate hepatic stellate cells. Activation of HSC is the key event of various kinds of fibrosis. We found in this model, however, as modeling going on, the pathological changes of hepatocytes and fibrosis were gradually deteriorated, The rats in 2 w group were low in spirit with hair standing and diarrhea, the rats in 4 w group became worse and even died. However, because of sustained alcohol intake, after 4 w the rats seemed to get better in spirits and activities, implying that they might develop some mechanisms to adapt to alcohol intake.
In this model we found that the pathological changes of hepatocytes, HSC, endothelia under electronic microscopy, HE stain, HE-masson complex stain were consistent with that of ALD in human beings. Histological analysis showed that the degree of fibrosis was stages I-II[45,46]. This model may be a good representative of average moderate drinkers who have extensive mild pathological injuries of the liver. Our study may provide some clues for investigation about mild and moderate drinkers in clinic.
Capillarization was a process in which the liver sinusoid became consecutive capillaries with evident basement around. The morphological changes mainly included decreased fenestrae in number or even disappearance in endothelia, and the formation of evident basement[47-50]. The progression of fibrosis was directly linked to the activation of HSC, and always companied by the activation of matrix metalloprotenase2 (MMP-2), without strong expression of the family of tissue inhibitor of matrix metalloprotinases(TIMPS) which was usually observed at the primary stages of fibrosis. Capillarization might not be always stably formed. Our observation demonstrated this might be true, because the content of type IV collagen was low and changed during the modeling, the basement might be mainly composed of laminin. Some of the basements of the liver sinusoid in the rats of 4 w and 9 w groups were not so typical but indeed existed.
The laminin content showed a consecutive high level during the modeling, and did not return to normal even after 2 week recovery. It implied that decomposition of laminin in this model was slightly tough. It deserves further investigation in human ALD. Thus MMP-2’s activity alone may not be able to promote the reverse of capillarization.
The content of type I collagen is less than that of type III collagen in normal rats’ liver, but it is not so when fibrosis occurs. In normal rats we saw few fibrils near the sinusoid, as modeling going on, many fibrils appeared around sinusoidal endothelial cells. We postulate that type I collagen may take part in this course. Because most researchers believed that type I collagen was not involved in the formation of basement during capillarization. We conclude that the peri-sinusoid fibrosis may be mainly composed of fibrils consisting of type I collagen. Electron microscopic observation showed that peri-sinusoid fibrils were not prone to decomposition after modeling stopped. Immunohistochemical staining observation was consistent with this.
This model had stable alcohol intake during modeling, this ensured less deaths but resulted in moderate pathological changes. If we increase the content of alcohol intake after 4 w and last it for a longer time, or do not reduce the content of iron intake, there may be more severe injuries of the liver such as cirrhosis, but also more dead animals would come forth which may lead to modeling ending in failure. However, the model still needs further improvements.
Footnotes
Edited by Wang XL and Zhu LH
References
- 1.Laatikainen T, Manninen L, Poikolainen K, Vartiainen E. Increased mortality related to heavy alcohol intake pattern. J Epidemiol Community Health. 2003;57:379–384. doi: 10.1136/jech.57.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambler G, Royston P, Head J. Non-linear models for the relation between cardiovascular risk factors and intake of wine, beer and spirits. Stat Med. 2003;22:363–383. doi: 10.1002/sim.1359. [DOI] [PubMed] [Google Scholar]
- 3.Zuccalà G, Onder G, Pedone C, Cesari M, Landi F, Bernabei R, Cocchi A. Dose-related impact of alcohol consumption on cognitive function in advanced age: results of a multicenter survey. Alcohol Clin Exp Res. 2001;25:1743–1748. [PubMed] [Google Scholar]
- 4.Tadic SD, Elm MS, Li HS, Van Londen GJ, Subbotin VM, Whitcomb DC, Eagon PK. Sex differences in hepatic gene expression in a rat model of ethanol-induced liver injury. J Appl Physiol (1985) 2002;93:1057–1068. doi: 10.1152/japplphysiol.00568.2001. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Wang B, Liu C. [Relation between activities of hepatic and gastric alcohol dehydrogenases and formation of chronic alcoholic liver diseases] Zhonghua Gan Zang Bing Za Zhi. 2001;9:265–267. [PubMed] [Google Scholar]
- 6.Ren C, Paronetto F, Mak KM, Leo MA, Lieber CS. Cytokeratin 7 staining of hepatocytes predicts progression to more severe fibrosis in alcohol-fed baboons. J Hepatol. 2003;38:770–775. doi: 10.1016/s0168-8278(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 7.Li J, French BA, Fu P, Bardag-Gorce F, French SW. Mechanism of the alcohol cyclic pattern: role of catecholamines. Am J Physiol Gastrointest Liver Physiol. 2003;285:G442–G448. doi: 10.1152/ajpgi.00093.2003. [DOI] [PubMed] [Google Scholar]
- 8.French SW. Intragastric ethanol infusion model for cellular and molecular studies of alcoholic liver disease. J Biomed Sci. 2001;8:20–27. doi: 10.1007/BF02255967. [DOI] [PubMed] [Google Scholar]
- 9.Chen WH, Liu P, Xu GF, Lu X, Xiong WG, Li FH, Liu CH. Role of lipid peroxidation in liver fibrogenesis induced by dimethylnit-rosamine in rats. Shijie Huaren Xiaohua Zazhi. 2001;9:645–648. [Google Scholar]
- 10.Murohisa G, Kobayashi Y, Kawasaki T, Nakamura S, Nakamura H. Involvement of platelet-activating factor in hepatic apoptosis and necrosis in chronic ethanol-fed rats given endotoxin. Liver. 2002;22:394–403. doi: 10.1034/j.1600-0676.2002.01552.x. [DOI] [PubMed] [Google Scholar]
- 11.Tamai H, Horie Y, Kato S, Yokoyama H, Ishii H. Long-term ethanol feeding enhances susceptibility of the liver to orally administered lipopolysaccharides in rats. Alcohol Clin Exp Res. 2002;26(8 supply):75S–80S. doi: 10.1097/01.ALC.0000026981.32386.FD. [DOI] [PubMed] [Google Scholar]
- 12.Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- 13.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 14.Järveläinen HA, Väkevä A, Lindros KO, Meri S. Activation of complement components and reduced regulator expression in alcohol-induced liver injury in the rat. Clin Immunol. 2002;105:57–63. doi: 10.1006/clim.2002.5267. [DOI] [PubMed] [Google Scholar]
- 15.Shimada S, Yamauchi M, Takamatsu M, Uetake S, Ohata M, Saito S. Experimental studies on the relationship between immune responses and liver damage induced by ethanol after immunization with homologous acetaldehyde adducts. Alcohol Clin Exp Res. 2002;26(8 supply):86S–90S. doi: 10.1097/01.ALC.0000026983.26366.D0. [DOI] [PubMed] [Google Scholar]
- 16.Searashi Y, Yamauchi M, Sakamoto K, Ohata M, Asakura T, Ohkawa K. Acetaldehyde-induced growth retardation and micro-heterogeneity of the sugar chain in transferrin synthesized by HepG2 cells. Alcohol Clin Exp Res. 2002;26(8 supply):32S–37S. doi: 10.1097/01.ALC.0000027179.33964.E0. [DOI] [PubMed] [Google Scholar]
- 17.Colantoni A, Emanuele MA, Kovacs EJ, Villa E, Van Thiel DH. Hepatic estrogen receptors and alcohol intake. Mol Cell Endocrinol. 2002;193:101–104. doi: 10.1016/s0303-7207(02)00102-8. [DOI] [PubMed] [Google Scholar]
- 18.Boireau A, Maréchal PM, Meunier M, Dubédat P, Moussaoui S. The anti-oxidant ebselen antagonizes the release of the apoptogenic factor cytochrome c induced by Fe2+/citrate in rat liver mitochondria. Neurosci Lett. 2000;289:95–98. doi: 10.1016/s0304-3940(00)01267-2. [DOI] [PubMed] [Google Scholar]
- 19.Karbownik M, Reiter RJ, Garcia JJ, Cabrera J, Burkhardt S, Osuna C, Lewinski A. Indole-3-propionic acid, a melatonin-related molecule, protects hepatic microsomal membranes from iron-induced oxidative damage: relevance to cancer reduction. J Cell Biochem. 2001;81:507–513. [PubMed] [Google Scholar]
- 20.Schümann K. Safety aspects of iron in food. Ann Nutr Metab. 2001;45:91–101. doi: 10.1159/000046713. [DOI] [PubMed] [Google Scholar]
- 21.Valerio LG, Petersen DR. Characterization of hepatic iron overload following dietary administration of dicyclopentadienyl iron (Ferrocene) to mice: cellular, biochemical, and molecular aspects. Exp Mol Pathol. 2000;68:1–12. doi: 10.1006/exmp.1999.2278. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald GA, Bridle KR, Ward PJ, Walker NI, Houglum K, George DK, Smith JL, Powell LW, Crawford DH, Ramm GA. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J Gastroenterol Hepatol. 2001;16:599–606. doi: 10.1046/j.1440-1746.2001.02445.x. [DOI] [PubMed] [Google Scholar]
- 23.Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 24.De Feo TM, Fargion S, Duca L, Cesana BM, Boncinelli L, Lozza P, Cappellini MD, Fiorelli G. Non-transferrin-bound iron in alcohol abusers. Alcohol Clin Exp Res. 2001;25:1494–1499. doi: 10.1097/00000374-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Tsukamoto H. Iron regulation of hepatic macrophage TNFalpha expression. Free Radic Biol Med. 2002;32:309–313. doi: 10.1016/s0891-5849(01)00772-9. [DOI] [PubMed] [Google Scholar]
- 26.Kono H, Bradford BU, Rusyn I, Fujii H, Matsumoto Y, Yin M, Thurman RG. Development of an intragastric enteral model in the mouse: studies of alcohol-induced liver disease using knockout technology. J Hepatobiliary Pancreat Surg. 2000;7:395–400. doi: 10.1007/s005340070034. [DOI] [PubMed] [Google Scholar]
- 27.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 28.Friedman SL. Stellate cell activation in alcoholic fibrosis--an overview. Alcohol Clin Exp Res. 1999;23:904–910. [PubMed] [Google Scholar]
- 29.Okuno M, Sato T, Kitamoto T, Imai S, Kawada N, Suzuki Y, Yoshimura H, Moriwaki H, Onuki K, Masushige S, et al. Increased 9,13-di-cis-retinoic acid in rat hepatic fibrosis: implication for a potential link between retinoid loss and TGF-beta mediated fibrogenesis in vivo. J Hepatol. 1999;30:1073–1080. doi: 10.1016/s0168-8278(99)80262-1. [DOI] [PubMed] [Google Scholar]
- 30.Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129–140. doi: 10.1055/s-2007-1007105. [DOI] [PubMed] [Google Scholar]
- 31.Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J Gastroenterol Hepatol. 1999;14:618–633. doi: 10.1046/j.1440-1746.1999.01928.x. [DOI] [PubMed] [Google Scholar]
- 32.Whalen R, Rockey DC, Friedman SL, Boyer TD. Activation of rat hepatic stellate cells leads to loss of glutathione S-transferases and their enzymatic activity against products of oxidative stress. Hepatology. 1999;30:927–933. doi: 10.1002/hep.510300404. [DOI] [PubMed] [Google Scholar]
- 33.Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol. 2000;279:G7–G11. doi: 10.1152/ajpgi.2000.279.1.G7. [DOI] [PubMed] [Google Scholar]
- 34.Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis. 2001;5:315–334, v-vi. doi: 10.1016/s1089-3261(05)70168-9. [DOI] [PubMed] [Google Scholar]
- 35.Eng FJ, Friedman SL. Transcriptional regulation in hepatic stellate cells. Semin Liver Dis. 2001;21:385–395. doi: 10.1055/s-2001-17553. [DOI] [PubMed] [Google Scholar]
- 36.Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62–73. doi: 10.1053/jhep.2002.30362. [DOI] [PubMed] [Google Scholar]
- 37.Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808–d826. doi: 10.2741/reeves. [DOI] [PubMed] [Google Scholar]
- 38.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Safadi R, Friedman SL. Hepatic fibrosis--role of hepatic stellate cell activation. MedGenMed. 2002;4:27. [PubMed] [Google Scholar]
- 40.Albanis E, Safadi R, Friedman SL. Treatment of hepatic fibrosis: almost there. Curr Gastroenterol Rep. 2003;5:48–56. doi: 10.1007/s11894-003-0009-7. [DOI] [PubMed] [Google Scholar]
- 41.Chae HB, Jang LC, Park SM, Son BR, Sung R, Choi JW. [An experimental model of hepatic fibrosis induced by alcohol and CCl4: can the lipopolysaccharide prevent liver injury induced by alcohol and CCl4] Taehan Kan Hakhoe Chi. 2002;8:173–178. [PubMed] [Google Scholar]
- 42.Zhao JB, Wang TL, Zhang DM. [Morphological study on 40 cases of alcoholic liver disease] Zhonghua Bing Li Xue Za Zhi. 1994;23:14–16. [PubMed] [Google Scholar]
- 43.Bo AH, Tian CS, Xue GP, Du JH, Xu YL. Morphology of immune and alcoholic liver diseases in rats. Shijie Huaren Xiaohua Zazhi. 2001;9:157–160. [Google Scholar]
- 44.Lin H, Lü M, Zhang YX, Wang BY, Fu BY. Induction of a rat model of alcoholic liver diseases. Shijie Huaren Xiaohua Zazhi. 2001;9:24–28. [Google Scholar]
- 45.[Consensus on evaluation of the diagnosis and efficacy of hepatic fibrosis] Zhonghua Gan Zang Bing Za Zhi. 2002;10:327–328. [PubMed] [Google Scholar]
- 46.Infectious, parasitic and hepatic diseases branch of Chinese Medical Association. Prevention and treatment program for Vi-rus Hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324–329. [Google Scholar]
- 47.Xu GF, Tian DL. Progress in capillarization research of hepatic sinusoid. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2002;10:314–316. [Google Scholar]
- 48.Lu X, Xu GF, Chen WH, Liu CH, Liu P. The dynamic changes of capillarization during hepatic fibrosis induced by DMN in rats. S. hijie Huaren Xiaohua Zazhi. 2000;8:1415–1416. [Google Scholar]
- 49.Lu X, Liu CH, Xu GF, Chen WH, Liu P. Successive observation of laminin and collagen IV on heptatic sinusoid during the formation of the liver fibrosis in rats. Shijie Huaren Xiaohua Zazhi. 2001;9:260–262. [Google Scholar]
- 50.Cheng J. The biology of molecules of extracellular matrix and the relation with clinical diseases. 1st ed, Beijing: The Publishing Company of Beijing University of Medical Sciences; 1999. [Google Scholar]


