Abstract
AIM: To investigate the effects of catalytically superior gene-directed enzyme prodrug therapy systems on a rat hepatoma model.
METHODS: To increase hepatoma cell chemosensitivity for the prodrug 5-fluorocytosine (5-FC), we generated a chimeric bifunctional SuperCD suicide gene, a fusion of the yeast cytosine deaminase (YCD) and the yeast uracil phosphoribosyltransferase (YUPRT) gene.
RESULTS: In vitro stably transduced Morris rat hepatoma cells (MH) expressing the bifunctional SuperCD suicide gene (MH SuperCD) showed a clearly marked enhancement in cell killing when incubated with 5-FC as compared with MH cells stably expressing YCD solely (MH YCD) or the cytosine deaminase gene of bacterial origin (MH BCD), respectively. In vivo, MH SuperCD tumors implanted both subcutaneously as well as orthotopically into the livers of syngeneic ACI rats demonstrated significant tumor regressions (P<0.01) under both high dose as well as low dose systemic 5-FC application, whereas MH tumors without transgene expression (MH naïve) showed rapid progression. For the first time, an order of in vivo suicide gene effectiveness (SuperCD>>YCD>>BCD>>>negative control) was defined as a result of a direct in vivo comparison of all three suicide genes.
CONCLUSION: Bifunctional SuperCD suicide gene expression is highly effective in a rat hepatoma model, thereby significantly improving both the therapeutic index and the efficacy of hepatocellular carcinoma killing by fluorocytosine.
Keywords: YCD/YUPRT fusion, Cytosine deaminase, GDEPT, Suicide gene therapy, Hepatoma therapy
INTRODUCTION
Suicide gene therapy involves the transfer of genes into tumor cells to render them specifically sensitive to prodrugs that are relatively nontoxic to normal tissues. Cytosine deaminase (CD) enzyme is found in many bacteria, yeast and fungi, where it deaminates cytosine to uracil[1]. It also deaminates the relatively nontoxic prodrug 5-fluorocytosine (5-FC) to the highly toxic chemotherapeutic compound 5-fluorouracil (5-FU), used in the treatment of malignant tumors (Figure 1A)[2]. Transduction of the CD gene into tumor cells combined with systemic administration of 5-FC has been shown to have anticancer effects in various animal models[3-7]. Due to their inherent bystander effect, toxic 5-FU metabolites (Figure 1A) also reach non-transduced neighboring cells[8-10]. In contrast to the herpes simplex virus type 1 thymidine kinase/ganciclovir system, this bystander effect does not depend on gap junctional intercellular communication channels[11].
Figure 1.
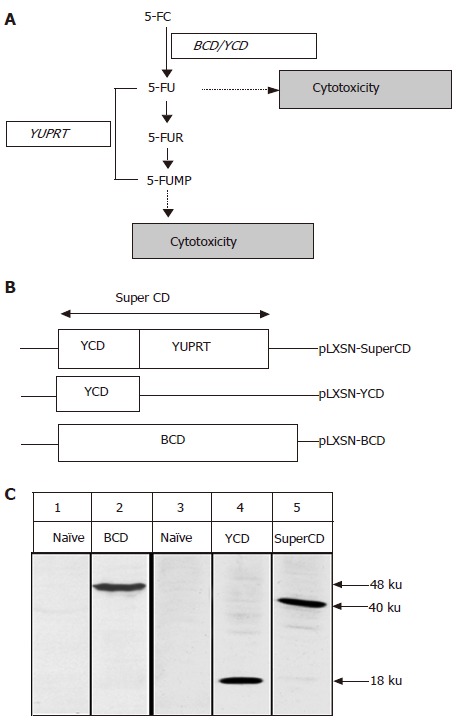
Mode of action of SuperCD (YCD::YUPRT) fusion. Panel A: Schematic representation of biological pathways resulting in cytotoxicity following cytosine deaminase/uracil phosphoribosyltransferase (UPRT)-mediated toxification of the prodrug 5-FC. 5-FC: 5-fluorocytosine; 5-FU: 5-fluorouracil; 5-FUR: 5-fluorouridine; 5-FUMP: 5-fluorouridine 5'-monophosphate; CD: cytosine deaminase; YUPRT: yeast uracil phosphoribosyltransferase. Panel B: Schematic representation of retroviral vectors used, in which suicide gene expression was placed under the retroviral 5' LTR. Panel C: Western blot detection of suicide gene expression in retrovirally generated stable SuperCD/YCD/BCD-expressing Morris hepatoma (MH) cell lines employing polyclonal rabbit antisera directed against BCD (lanes 1 and 2) and YCD (for detection of both SuperCD and YCD; lanes 3-5). Lane 1: MH naïve cells (negative control); lane 2: MH BCD cells, exhibiting a 48-ku band specific for BCD; lane 3: MH naïve cells (negative control); lane 4: MH YCD cells, exhibiting a 18-ku band specific for YCD; lane 5: MH SuperCD cells, exhibiting a 40-ku band specific for the YCD::YUPRT fusion, i.e. SuperCD. Numbers on the right hand side indicate the sizes of the expected proteins.
In this work, we focused on the optimization of the CD suicide gene system for hepatoma treatment. We compared both in vitro and in vivo therapeutic efficiency of stable expression of: (1) a new bifunctional chimeric SuperCD suicide gene, composed of the yeast cytosine deaminase (YCD) suicide gene directly fused to a 5'-terminally deleted yeast uracil phosphoribosyltransferase (YUPRT) gene[12]; (2) YCD suicide gene; and (3) bacterial cytosine deaminase (BCD) suicide gene in the MH 3924A animal model[13].
MATERIALS AND METHODS
Retroviral vector construction
Genomic DNA of Saccharomyces cerevisiae, strain KFY159 (kindly provided by K.-U. Fröhlich, Physiologisch-Chemisches Institut, Eberhard-Karls-University, Tübingen, Germany), was employed to amplify YCD and YUPRT using polymerase chain reaction technique. YCD (S. cerevisiae gene FCY-1) was amplified with primers: 5'-GGGGTACCGCCACCATGGTGACAGGGGGAATGGCAAG-3' (primer #1) and 5'-GGGCGGCCGCCTCACTCACCAATATCTTCAAA CCAATC-3' (primer #2). Alternatively, primer #3 (5'-GGGAATTCTCACCAATATCTTCAAACCAATC-3') was used as a reverse primer in combination with primer #1 to amplify the yeast FCY-1 gene. The latter YCD fragment was subsequently joined to a PCR product amplifying the yeast FUR-1 gene (YUPRT). While omitting the N-terminal domain, the remaining domains of the YUPRT open reading frame, enzymatically sufficient for efficiently transferring the phosphoribosyl residue to the deaminated 5-FC[14], were amplified with primers: 5'-CCGAATTCGGAACCATTTAAGAACGTC-3' (primer #4) and 5'-CCGCGGCCGCCTTAAACACAGTAGTATCTGTCACC-3' (primer #5). Both PCR fragments were purified for subsequent ligation as recently described elsewhere[15]. Subsequently, both YCD and YUPRT PCR amplification products were combined in a three-fragment ligation procedure employing a pUC-based cloning vehicle as a backbone. This intermediate construct was used to remove the EcoRI site between the YCD and YUPRT sequences and at the same time to generate the bifunctional SuperCD suicide fusion gene employing a site-directed mutagenesis using the Promega QuikChange method (Promega, Mannheim, Germany). With sense and anti-sense mutagenesis primers (sense sequence: 5'-ATGGTTTCCGAAGCCTCACCAATATCT-3'), the two enzymatic moieties were joined in-frame, resulting in an Ala residue linking the two yeast ORFs (now with the YUPRT gene starting at the natural S38 codon). Subsequently, the following retroviral constructs were generated (Figure 1B): (1) pLXSN-BCD contains the E. coli cytosine deaminase (BCD) suicide gene under the control of the retroviral 5´LTR in basic retroviral vector pLXSN[16] (kindly provided by R.M. Blaese, NIH, Bethesda, MD, USA; originally denominated as pCD2)[17]; (2) pLXSN-YCD harboring the YCD suicide gene was generated by the insertion of the YCD PCR amplification product into basic vector pLXSN-GreeN (derivative of vector pLXSN, in which we exchanged the neor gene by a bifunctional EGFP/neor fusion gene); (3) pLXSN-SuperCD harboring the bifunctional SuperCD suicide fusion gene was generated by insertion of the YCD/YUPRT in-frame fusion fragment into basic vector pLXSN-GreeN.
Cell culture
Morris hepatoma (MH 3924A) cells were purchased from the German Cancer Research Center (DKFZ) Tumor Collection, Heidelberg, Germany, and maintained in DMEM supplemented with 50 mL/L fetal calf serum (FCS) in a humidified incubator containing 50 mL/L CO2 at 37 °C.
Generation of stable BCD, YCD and SuperCD expressing Morris hepatoma 3924A cell lines
For the generation of suicide gene transducing retroviral particles, 5×105 PE501 ecotropic packaging cells were seeded on 60-mm diameter petri dishes. After 24 h, using lipofectAMINE reagent (Invitrogen), cells were transiently transfected with 3 µg of each retroviral vector DNA. Forty-eight hours later, the supernatant was employed for transduction of 3×105 naïve MH cells. Following G418 selection (600 µg/mL), resistant clones were analyzed for EGFP marker gene expression by fluorescence microscopy, followed by functional characterization of suicide gene expression in the sulforhodamin B (SRB) cytotoxicity assay. Cell clones exhibiting the highest cytotoxic effect were selected and named as MH BCD, MH YCD, and MH SuperCD.
Western blot analysis
Cells were lysed in SDS sample buffer and proteins were separated on 100 g/L SDS-PAGE and transferred to PVDF membranes[18]. Polyclonal rabbit antiserum directed against YCD (a generous gift from Transgene SA, Strasbourg, France), a polyclonal rabbit anti-BCD antibody (a generous gift from C. Richards, Glaxo-Wellcome, RTP, NC, USA)[19] and goat anti-rabbit IgG-horseradish peroxidase conjugates (BioRad, München, Germany) were used. Detection of reactive bands was facilitated using horseradish peroxidase-linked secondary conjugate and ECL detection reagents (Amersham Pharmacia, Freiburg, Germany).
SRB cytotoxicity assay
MH (naïve) as well as MH BCD, MH YCD, MH SuperCD cells were seeded in 24-well plates (1×104 cells/well). The next day 5-FC containing medium was added (a generous gift from Roche, Basel, Switzerland) and the cells were incubated for 4 d in order to allow untreated cells to reach confluence. Growth inhibition was evaluated by the SRB cytotoxicity assay[20] using a microtiter plate reader (Dynatech MR7000, Denkendorf, Germany) at 550 nm.
In vivo experiments
All animal experiments were performed in concordance with the laws of the German Government concerning the conduction of animal experimentation. Surgical and imaging procedures were performed under intraperitoneally applied anesthesia with ketamine hydrochloride (Ketanest, Parke-Davis, Berlin, Germany; 100 mg/kg body weight) and xylazine hydrochloride (Rompun, Bayer, Leverkusen, Germany; 10 mg/kg body weight).
The subcutaneous MH naïve and MH SuperCD tumors were established by injecting 1×107 cells (in 70 µL PBS) at the dorsum of 6-wk-old male syngeneic ACI rats (Harlan Winkelmann, Borchen, Germany). Eleven days later the rats were randomized into two groups (3 rats/group), and were treated twice daily with ip injections of 5-FC (283 mg/kg body weight) or saline (0.9% NaCl) for 7 d. Tumor sizes were measured with calipers. Tumor volumes were calculated in cubic centimeter using the formula recommended by Carlsson et al[21]: tumor volume (mm3)=largest diameter (mm)×[smallest diameter (mm)]2/2.
In order to monitor 5-FC serum levels by HPLC quantification, blood was taken via tail vein puncture on d 5 of the 5-FC application period. On d 8 of the treatment period, the animals were killed, and tumors were explanted and measured for tumor weight and volume. Tumor samples were analyzed for (1) the presence of SuperCD DNA and (2) the endurance of the SuperCD suicide gene functional activity. For PCR analysis, tumor sample DNA was extracted using DNeasy Tissue Kit (Qiagen, Hilden, Germany). MH SuperCD DNA was amplified using primers 5'-CGACCCCGCCTCGATCCTCC-3' (primer #6) and 5'-CTGCTGGGGAGCCTGGGGAC-3' (primer #7). PCR products were separated by electrophoresis on a 10 g/L agarose gel. For the functional testing of continuous suicide gene presence following the in vivo growth period of MH SuperCD tumors, tumor tissues were minced, placed into six-well plates and cultivated in DMEM medium containing 50 mL/L FCS, supplemented with 1% penicillin-streptomycin. Three days later, stably transduced MH SuperCD cells were selected by continuous addition of G418 (600 µg/mL). Two weeks later, the cells were analyzed for functional SuperCD gene activity employing the SRB cytotoxicity assay.
Orthotopic tumor implantation within the liver was performed as described elsewhere[13]. Twenty-one days later, tumor sizes were measured by magnetic resonance imaging (MRI). Subsequently, the rats were randomized into two groups (six rats/group) and were treated twice daily with ip injections of 5-FC (283 mg/kg body weight) or saline. On d 31, tumor sizes were again measured by MRI. Animals were killed, livers explanted and analyzed macroscopically for the presence of tumor tissue.
MRI measurements
Monitoring of tumor growth was performed by sequential MRI studies, using a 1.5 Tesla MR tomograph (VISION, Siemens, Erlangen, Germany). A turbospinecho sequence was applied to acquire T2 weighed images using the following parameters: 3.5 s repetition time, 96 ms echo time, field of view of 200 mm×100 mm. Fifteen slices with an in-plane resolution of 0.75 mm×0.75 mm, a slice thickness of 2 mm and a gap of 0.3 mm between the slices were acquired with six signal averages. Maximum areas of the tumors were determined by the identification of the tumor slice exhibiting largest diameters (main diameter a and minor diameter b), followed by the calculation of the respective area (a/2×b/2×π).
Statisticial analysis
Viability was explained by cell line, 5-FU concentration, and their interaction in analyses of variance. Square roots of counts were used as Box-Cox analyses and normal quantile-plots of residuals suggested so. Means and 95% confidence intervals were transformed back and divided by the fitted value of MH naïve cells. The in vivo experiment with four cell lines was analyzed by the analysis of covariance of logarithms of tumor sizes (considered censored at 30 mm² when below) with variable time factors, cell line and individual and different slopes for the cell lines. The hypotheses were: all four mean growth rates are equal, and mean growth rates of YCD and SuperCD cells are equal. As intra-individual comparison of cell lines was possible, all six possible combinations were implanted as in a balanced incomplete block plan. Sample size was planned to guarantee multiple power of more than 0.8 at effect size 30% and coefficient of variation 30%, while the multiple significance level was 0.05.
RESULTS
Through its toxic metabolites, 5-FU kills cells by binding to the thymidylate synthase, thereby causing a blockade of DNA synthesis as well as by directly interfering with the cellular RNA metabolism (Figure 1A). Because 5-FU is able to diffuse passively through cell membranes, it not only affects transduced cells, but also neighboring cells (so-called bystander cells). However, the conversion of 5-FU to 5-FUMP constitutes a rate-limiting step at least in some tumor cells[22]. In the context of suicide gene transfer, it is hypothesized that an additional encoding of the UPRT gene not only enhances tumor cell sensitivity to 5-FU, but also augments the sensitivity of tumor cells to 5-FC, when such cells are enabled to simultaneously express a CD gene (Figure 1A). Since also low tumor transduction efficiencies are recognized as a major limitation in liver tumor suicide gene therapy[23,24], 5-FC/CD/UPRT-based suicide gene systems could not only help to improve the effectiveness of 5-FC toxification, but also enhance the effectiveness of bystander killing, thereby helping to compensate for currently still low efficiencies of direct liver tumor suicide gene transfer. In this work, we therefore investigated whether hepatoma cell chemosensitivity for 5-FC could be enhanced by simultaneously addressing the two major steps of 5-FC metabolism. For this purpose, we first generated a so-called SuperCD fusion gene which combines the (1) enzymatic function of yeast CD (YCD) with (2) YUPRT-mediated phosphorylation of 5-FU to 5-FUMP (Figures 1A and B).
Construction of SuperCD/YCD/BCD suicide gene expressing retroviral vectors, and analysis of stably transduced Morris rat hepatoma cells
We employed genomic DNA of S. cerevisiae to amplify both yeast CD as well as yeast UPRT genes by PCR[22]. Subsequently, both genes were linked in-frame using a site-directed mutagenesis scheme, resulting in generation of a bifunctional suicide fusion gene which was named as SuperCD. Next, the SuperCD DNA sequence was inserted into basic retroviral vector pLXSN[16], resulting in generation of pLXSN-SuperCD (Figure 1B, upper construct), in which transgene expression is driven by the retroviral 5´LTR. For comparison of cytotoxic effectiveness, we likewise generated retroviral vectors pLXSN-YCD (Figure 1B, middle construct; solely encoding the YCD cDNA) and pLXSN-BCD (Figure 1B, lower construct; solely encoding the BCD cDNA)[25]. Employing all three constructs, PE501 ecotropic packaging cells were transiently transfected. Subsequently, respective supernatants containing recombinant retroviruses were used for transduction of MH 3924A cells. G418 selection gave rise to neor resistant clones (MH SuperCD, MH YCD, and MH BCD), which stably expressed suicide genes SuperCD, YCD, or BCD at comparable levels as detected by Western blotting (Figure 1C). Next, a SRB cytotoxicity assay was performed to compare the suicidal efficiencies of the different transgenes in the context of stable cell lines. As shown in Figure 2, a clear order of efficacy of the different suicide transgenes was found: in the SuperCD expressing cell line (MH SuperCD) even at a 5-FC concentration as low as 0.1 mmol/L, a very low cell survival of only 8% was observed. In contrast, sole expression of YCD (MH YCD) was much less efficient, resulting in a nearly 5-fold higher cell survival (38% at 0.1 mmol/L 5-FC). Finally, sole expression of BCD had only a minor cytotoxic effect (>80% cell survival at 0.1 mmol/L 5-FC). Thereby, convincing in vitro evidence was provided that the YUPRT component implemented within the SuperCD fusion gene is highly functional in the context of hepatoma cells. This corresponds to observations made by others with cell lines derived from non-liver tumor tissues (e.g. human colon cancer cells, human pancreas tumor cells, human breast cancer cells, human glioblastoma cells)[22,26]. The efficacy of much lower 5-FC doses has to be regarded as an important finding when transferring the 5-FC/SuperCD system to the preclinical/clinical situation: in the mammalian organism, 5-FC is also metabolized by the bacteria of the gut flora. An increased intestinal production of 5-FU can lead to dramatic unwanted side effects which are feared greatly in the context of 5-FU chemotherapeutic regimes[27].
Figure 2.
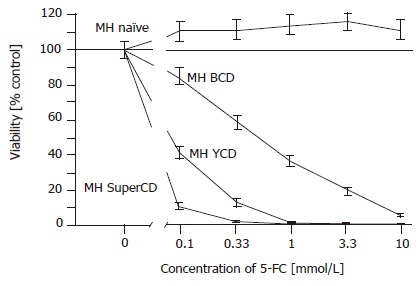
SRB cytotoxicity assay with stable BCD, YCD, SuperCD expressing Morris hepatoma (MH) cell lines generated by retroviral transduction. Untransduced MH3924A cells (MH naïve) and stably transduced Morris hepatoma cell lines (MH BCD, MH YCD, MH SuperCD) were seeded on 24-well plates. After 4 d of 5-FC treatment, the growth inhibition was determined by SRB staining and measured at 550 nm. All values were referred to that of the untransduced control cells without the addition of 5-FC and are given as percentage of surviving cells (control: 100%). All experiments were carried out at least in quadruplicate. Viability values plotted represent fitted medians and 95%CI.
Subcutaneously implanted MH SuperCD tumors demonstrate a complete tumor regression under systemic 5-FC application
A first in vivo testing of the SuperCD-enhanced suicide gene effect was performed in six animals who received two sc tumor cell injections each: (1) MH SuperCD cells into the right dorsum; and (2) as a control in the same animals MH naïve cells into the left dorsum. On d 11, when tumors had reached a measurable size, rats were randomized into two groups and treated for one week with twice daily ip injections of 5-FC or saline (control group). 5-FC serum levels of treated animals as determined by HPLC ranged from 1 to 2.6 µg/mL, whereas control animals receiving saline displayed background levels only (data not shown). During the treatment period, tumor volume increased constantly in the saline-treated negative control group, irrespectively of the tumor type implanted. In contrast, in all the three 5-FC treated animals, the MH SuperCD tumors disappeared completely, whereas the MH naïve tumors grown on the opposite dorsum further increased in volume until d 18, when the animals were killed (Table1). At post mortem examination of 5-FC-treated animals, no residual tumor tissue was found on the right dorsum in which the MH SuperCD tumors originally had grown, whereas large MH SuperCD tumors, weighing between 1.8 and 2.7 g, were found in the saline-treated animals. As expected, all animals displayed large MH naïve tumors on the opposite site, independent of 5-FC or saline treatment.
Molecular and functional analysis of subcutaneously grown control tumors explanted on d 18
In vivo selective pressure for transgene-negative tumor cells and silencing of transgenes by methylation events are well-known phenomena[28]. We, therefore, extracted DNA from subcutaneously grown xenograft tumors for PCR analysis. In all three SuperCD tumor samples obtained from saline-treated animals, a 1 143-bp SuperCD-specific PCR amplification product was detected (Figure 3A, lanes #1-#3). Thus, in vivo passage of SuperCD-expressing tumor cells does not cause a relevant selective pressure against the presence of the SuperCD transgene. From the same subcutaneously grown tumors explanted at d 18, we further performed a functional analysis on recultured tumor cells (saline-treated MH SuperCD/MH naïve tumors) by applying the SRB cytotoxicity assay (Figure 3B). A 4-d treatment with 5-FC had no toxic effect on in vivo passaged MH naïve cells (control). However, all three MH SuperCD cell cultures (#1-#3) obtained after in vivo passage demonstrated a profound 5-FC-induced cytotoxic effect (Figure 3B, lower curves). The cytotoxic effect following in vivo passage was found to be comparable with MH tumor cells that had not undergone an in vivo passage (compared with Figure 2). These results demonstrated that, irrespective of an 18-d in vivo passage, MH SuperCD tumor cells continuously exerted a strong suicide gene effect at low 5-FC prodrug levels, which is of importance for further preclinical and clinical suicide gene therapy approaches.
Figure 3.
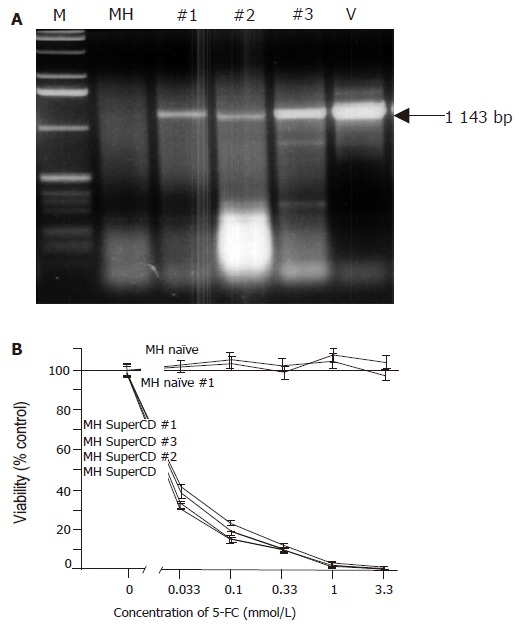
Molecular and functional analysis of subcutaneously grown control tumors explanted on d 18 (saline-treated MH SuperCD/MH naïve tumors). A: Agarose gel electrophoresis of SuperCD DNA-specific PCR products. Lane M: marker DNA ladder; lane MH: MH parental cell line (no transgene; negative control; PCR performed with 1 μg of DNA); lanes 1-3: PCR amplifications performed on MH SuperCD tumor tissues of animals 1, 2, 3 (PCR performed with 1–3 µg of DNA); lane V: vector control lane (PCR performed with 1 pg of pLXSN-SuperCD). Arrow: indicates 1 143-bp SuperCD-specific amplification product. B: SRB cytotoxicity assay with recultured tumor cells explanted after 18 d of subcutaneous growth. As in Figure 2, cytotoxicity was measured after 5-FC treatment for 4 d in vitro. MH naïve: negative control with MH parental cell line (no transgene; no animal passage); MH naïve, #1: MH naïve tumor explanted from a saline-treated animal (control); MH SuperCD, cell line: MH SuperCD parental cell line (control; no animal passage); MH SuperCD 1, 2, 3, tumor passage: MH SuperCD tumors explanted from saline-treated animals 1, 2, 3. All values were referred to that of the untransduced control cells and are given as percentage of surviving cells (control: 100%). All experiments were carried out in quadruplicate. Viability values plotted represent fitted medians and 95%CI.
Orthotopically implanted MH SuperCD tumors demonstrate a profound tumor regression under systemic 5-FC application
Based on the overwhelming efficacy of 5-FC treatment observed in subcutaneously grown MH SuperCD tumors, we next treated orthotopic liver tumors in the same rat hepatoma model in a larger number of animals, thereby investigating the statistical relevance of SuperCD suicide protein expression. As caliper measurement of small intrahepatic tumors is impossible, this study was based on non-invasive MRI imaging. Twenty-one days after the implantation of solitary MH SuperCD tumors into the livers of recipient animals, a first MRI was performed. Maximum tumor areas were analyzed. Rats with tumor areas ranging from 20 to 156 mm2 (representative day 21 MRI images shown in Figures 4B and C, respectively, column 1 “untreated”) were then randomized into two groups with six rats in each, and treated twice daily with ip injections of 5-FC or saline for 10 d.
Figure 4.
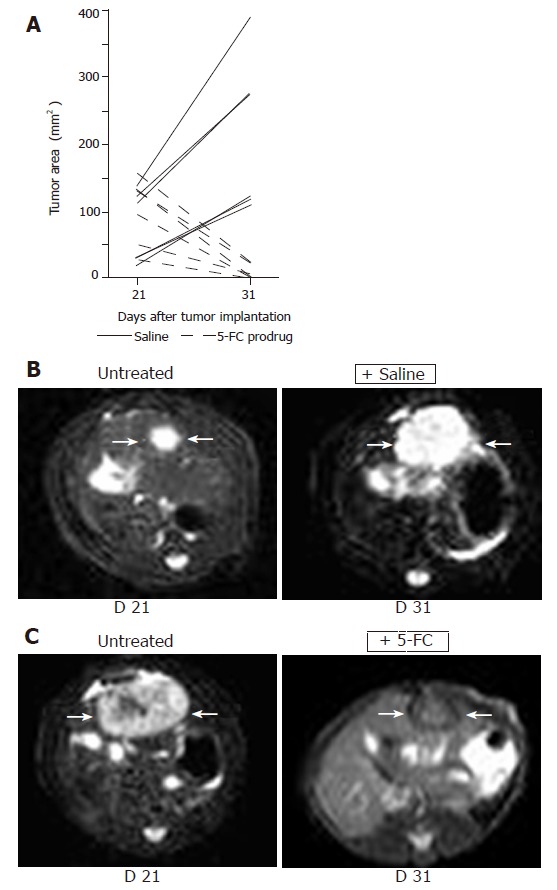
Non-invasive MRI detection of tumor growth of orthotopically implanted MH SuperCD hepatomas (saline vs 5-FC prodrug treatment). Animals with MRI detectable liver tumors in T2 weighed images were randomized into two groups (6 rats/group) and treated twice daily with ip injections of saline or 5-FC (283 mg/kg body weight) over a 10-d period. Panel A: Maximum tumor areas (mm2) at d 21 and d 31; solid lines: tumor growth of control animals (saline-treated); broken lines: tumor regression of serum animals (5-FC-treated). Panel B: Representative MRI tumor images of the saline-treated control animal exhibiting the smallest tumor area at d 21 (left); and a dramatic tumor growth was observed at d 31 (right). Panel C: Representative MRI tumor images of the serum animal (treated with 5-FC) exhibiting the largest tumor area at d 21 (left); and a dramatic tumor regression was measured at d 31 (right).
MRI-based measurement of tumor sizes at the end of the treatment (d 31) revealed a persistent orthotopic tumor growth for saline-treated control animals (Figure 4A, solid lines). In contrast, all six animals treated systemically for 10 d with 5-FC exhibited a clear decline of tumor sizes, either complete regressions or dramatic reductions (Figure 4A, broken lines). Ranking area differences revealed a significant difference between the untreated and the treated groups (U-test, P = 0.0051). Thus, bifunctional SuperCD suicide gene expression was found to be highly effective against a rat hepatoma model under both subcutaneous and orthotopic implantation conditions.
Low dose 5-FC application found to be sufficient to achieve complete tumor regressions of orthotopically implanted MH SuperCD cells
Anticipating an enhanced SuperCD suicide gene effect, we already had started to administer twice daily with 5-FC dosage of 283 mg/kg body weight, which is only half of the dosage of about 500 mg/kg body weight used by others[6,29-33]. Since this prodrug dosing regimen was found to be highly efficient for the eradication of both subcutaneous (Table 1) and orthotopic (Figure 4) MH SuperCD hepatomas, we aimed next at further decreasing the 5-FC dose by reducing the application frequency. Thereby, side effects due to 5-FU metabolites resulting from unwanted routes of “collateral” gut toxification could be even further reduced.
Table 1.
Effect of 5-FC treatment on subcutaneous MH tumor growth
|
Suicide gene |
|||
| None (control) | SuperCD | ||
| Treatment | 5-FC | 1.4 g | n.d. |
| 3.2 g | n.d. | ||
| 1.8 g | n.d. | ||
| Saline (control) | 2.5 g | 1.8 g | |
| 2.9 g | 1.9 g | ||
| 3.7 g | 2.7 g | ||
Six animals received two sc tumor cell injections (1×107 cells in 70 µL PBS/animal). On d 11, tumor-bearing rats were randomized into two groups and treatment started with twice daily ip injections of 5-FC (283 mg/kg body weight) or saline (control). At the end of 5-FC treatment (d 18), animals were killed, tumors were harvested and weighed. Complete regression of tumors was observed only in the 5-FC-treated SuperCD tumors (n.d.: no detectable tumors).
We tested this hypothesis on three animals with small MH SuperCD liver tumors (#7, #8, #9) having received saline only as control animals during the first treatment period. The other animals had to be euthanized due to their tumor burden. In these animals, low dose treatment was started on d 31 with only twice weekly ip injections of 5-FC (283 mg/kg body weight) for 28 d. When MRI was again performed at d 59, all MH SuperCD tumors had completely disappeared (Figure 5, right column). In none of these three animals, any solid tumor tissue was found at autopsy. Instead, only small yellowish fatty “scars” were visible. Thereby, bifunctional SuperCD suicide gene expression was found to be highly effective in a rat hepatoma model under both subcutaneous and orthotopic growth conditions, even when a strongly reduced 5-FC dosing regime was applied.
Figure 5.
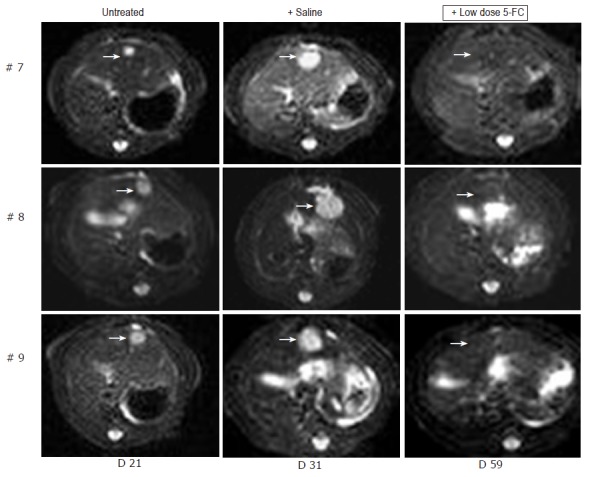
MRI detection of tumor growth of orthotopically implanted MH SuperCD hepatomas treated under a low-dose 5-FC regimen. Animals #7, #8, #9 (column 1: tumor size at d 21), which first had been treated with saline only for over a 7-d period and thereafter, uniformly exhibiting a substantial tumor growth (column 2: tumor size at d 31), were then treated by a low-dose 5-FC regimen [twice weekly ip injections of 5-FC (283 mg/kg body weight)] over a 28-d period until d 59. Analysis of subsequent MRI sections did not reveal any MH SuperCD tumors (column 3, d 59: +low dose 5-FC); T2 weighed images are depicted; arrows in column 3 point out to places of former tumor localizations before the onset of 5-FC treatment.
Determination of in vivo suicide gene effectiveness by direct in vivo comparison of suicide genes SuperCD vs YCD vs BCD
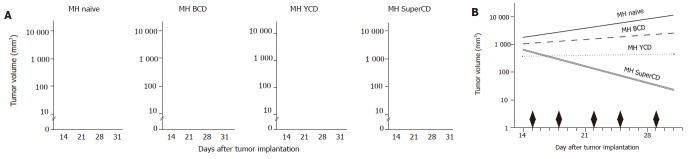
Until now, no study encompassing a direct in vivo comparison of different suicide genes (SuperCD vs YCD vs BCD) has been performed. Therefore, 1×107 cells of the four cell-lines (1) MH naїve (negative control), (2) MH BCD, (3) MH YCD, and (iv) MH SuperCD were injected subcutaneously into ACI rats. In this course, each animal received two injections with two different cell-lines, one inoculated at the left dorsum, and the other one at the right dorsum. Based on a balanced incomplete block plan, the six possible pairs of cell lines were implanted into six groups, each comprising eight animals. Tumor volumes were determined by caliper measurement. At d 15 after hepatoma cell inoculation, prodrug therapy with twice weekly ip injections of 283 mg 5-FC/kg body weight (low dose regime) was started. Interestingly, 8 out of 18 MH SuperCD tumors on d 28 and 11 out of 18 MH SuperCD-tumors on d 31 had shrunken to an immeasurable size (Figure 6A, right panel). A subsequent analysis of covariance of log tumor volumes revealed a significant reduction in median tumor volumes under our low dose 5-FC application scheme only for the MH SuperCD tumors. The reduction in median tumor volume was almost complete (95%CI for the reduction between d 14 and 31 from 98.7% to 99.6%) (Figure 6B). The maximum likelihood ratio χ² test of the global hypothesis was significant χ² = 264; 3 df; P = 6×10-57). Thus, for the first time in vivo evidence was provided demonstrating that the SuperCD in vivo suicide gene effect might be superior to the one of YCD (P <10-10), which itself was superior to BCD (Figures 6A and B).
Figure 6.
Direct in vivo comparison of SuperCD vs YCD vs BCD suicide gene effectiveness. Approximately 1×107 cells of the four cell-lines MH naїve, MH BCD, MH YCD, and MH SuperCD were injected subcutaneously into male ACI rats. After 15 d, prodrug application started with twice weekly ip injections of 283 mg of 5-FC/kg body weight as indicated by black diamonds in panel B. Panel A: Depiction of tumor volumes of all four cell lines in all animals at four time points after implantation. Panel B: Median tumor volume regression lines by analysis of covariance of log tumor volumes. As a result, significant reductions in median tumor volumes under low dose 5-FC application were observed only for the MH SuperCD tumors.
DISCUSSION
Although the study described here demonstrated that the expression of YCD and YUPRT as a fusion protein with 5-FC administration exerted a striking antitumor effect on solid tumors like hepatomas, further improvements are needed for clinical applications. Current limitations of in vivo liver tumor transduction efficiencies could be further compensated with the help of intercellular cargo proteins (e.g. VP22, our own work[18,34], and Refs. [35,36]). At the moment, we are evaluating the properties of VP22-SuperCD/SuperCD-VP22 fusion proteins to further potentiate suicide gene therapy efficiencies in our rat hepatoma model, thereby improving the intratumoral distribution of enzymatically enhanced suicide genes. Other promising approaches rely on the employment of strong hepatoma-specific promoters which are suitable to achieve high levels of hepatoma-specific SuperCD expression in vivo (e.g. the cell growth-related midkine promoter)[37]. Most importantly, gene transfer systems have to be identified, which are suitable to provide a significant increase of the accessibility of liver tumor cells to systemically applied gene transfer vectors. In a recent study, herpes thymidine kinase suicide gene transducing liposomes, based on the hemagglutinating virus of Japan, were injected directly into the portal vein of severe combined immunodeficiency mice, and reported to be as an efficient tool for the transduction of multilocalized HUH7 human hepatocellular carcinomas in this animal model[38]. Thus, transduction properties of hemagglutinating virus of Japan (also defined as Sendai virus) seem to favor transduction of liver tumors. Concluding from this, also recombinant Sendai virus vectors[39,40] encoding optimized suicide genes might be suitable to overcome the liver tumor transduction barrier[41,42]. HIV-derived lentiviral vectors have also been successfully used for suicide gene therapy in hepatocellular carcinoma[43]. In addition, newly developed replication-competent vectors/oncolytic vectors[42,44-47] could be used to cope with the liver tumor transduction barrier.
Concerning our current knowledge on severe gene therapeutic side effects employing state-of-the-art retroviral[48], adenoviral[49], or adeno-associated virus vectors[50], a reduction of vector dosages might become possible with the SuperCD suicide gene. This would constitute an important additional safety factor for further clinical gene therapy applications. Subsequent in vivo studies will compare application of the therapeutic SuperCD suicide gene by virtue of direct (injection), systemic (iv) or regional (ia) gene transfer and treatment with 5-FC.
In summary, a combination of synergistic strategies as described above may allow a further enhancement of the suicide gene effect in the near future. Thus, further refinements may help to make suicide gene therapy a feasible treatment modality for solid malignant tumors in human beings.
Footnotes
Supported by grants from German Research Foundation (LA649-20-2), Federal Ministry of Education, Science, Research and Technology (Fö. 01KS9602, Fö. 01KV9532), Interdisciplinary Clinical Research Center (IZKF) Tübingen, and the fortüne-program of the Medical Faculty of Eberhard-Karls-University Tübingen (F.1281127). W.A.W. supported by a scholarship from Pinguin Foundation (Henkel KGaA)
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
References
- 1.Danielsen S, Kilstrup M, Barilla K, Jochimsen B, Neuhard J. Characterization of the Escherichia coli codBA operon encoding cytosine permease and cytosine deaminase. Mol Microbiol. 1992;6:1335–1344. doi: 10.1111/j.1365-2958.1992.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 2.Austin EA, Huber BE. A first step in the development of gene therapy for colorectal carcinoma: cloning, sequencing, and expression of Escherichia coli cytosine deaminase. Mol Pharmacol. 1993;43:380–387. [PubMed] [Google Scholar]
- 3.Kanai F, Lan KH, Shiratori Y, Tanaka T, Ohashi M, Okudaira T, Yoshida Y, Wakimoto H, Hamada H, Nakabayashi H, et al. In vivo gene therapy for alpha-fetoprotein-producing hepatocellular carcinoma by adenovirus-mediated transfer of cytosine deaminase gene. Cancer Res. 1997;57:461–465. [PubMed] [Google Scholar]
- 4.Ohwada A, Hirschowitz EA, Crystal RG. Regional delivery of an adenovirus vector containing the Escherichia coli cytosine deaminase gene to provide local activation of 5-fluorocytosine to suppress the growth of colon carcinoma metastatic to liver. Hum Gene Ther. 1996;7:1567–1576. doi: 10.1089/hum.1996.7.13-1567. [DOI] [PubMed] [Google Scholar]
- 5.Richards CA, Austin EA, Huber BE. Transcriptional regulatory sequences of carcinoembryonic antigen: identification and use with cytosine deaminase for tumor-specific gene therapy. Hum Gene Ther. 1995;6:881–893. doi: 10.1089/hum.1995.6.7-881. [DOI] [PubMed] [Google Scholar]
- 6.Adachi Y, Tamiya T, Ichikawa T, Terada K, Ono Y, Matsumoto K, Furuta T, Hamada H, Ohmoto T. Experimental gene therapy for brain tumors using adenovirus-mediated transfer of cytosine deaminase gene and uracil phosphoribosyltransferase gene with 5-fluorocytosine. Hum Gene Ther. 2000;11:77–89. doi: 10.1089/10430340050016175. [DOI] [PubMed] [Google Scholar]
- 7.Miller CR, Williams CR, Buchsbaum DJ, Gillespie GY. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002;62:773–780. [PubMed] [Google Scholar]
- 8.Uckert W, Kammertöns T, Haack K, Qin Z, Gebert J, Schendel DJ, Blankenstein T. Double suicide gene (cytosine deaminase and herpes simplex virus thymidine kinase) but not single gene transfer allows reliable elimination of tumor cells in vivo. Hum Gene Ther. 1998;9:855–865. doi: 10.1089/hum.1998.9.6-855. [DOI] [PubMed] [Google Scholar]
- 9.Hirschowitz EA, Ohwada A, Pascal WR, Russi TJ, Crystal RG. In vivo adenovirus-mediated gene transfer of the Escherichia coli cytosine deaminase gene to human colon carcinoma-derived tumors induces chemosensitivity to 5-fluorocytosine. Hum Gene Ther. 1995;6:1055–1063. doi: 10.1089/hum.1995.6.8-1055. [DOI] [PubMed] [Google Scholar]
- 10.Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci USA. 1994;91:8302–8306 DOI : 10.1073/pnas.91.17.8302. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinh QT, Austin EA, Murray DM, Knick VC, Huber BE. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995;55:4808–4812. [PubMed] [Google Scholar]
- 12.Tiraby M, Cazaux C, Baron M, Drocourt D, Reynes JP, Tiraby G. Concomitant expression of E. coli cytosine deaminase and uracil phosphoribosyltransferase improves the cytotoxicity of 5-fluorocytosine. FEMS Microbiol Lett. 1998;167:41–49. doi: 10.1111/j.1574-6968.1998.tb13205.x. [DOI] [PubMed] [Google Scholar]
- 13.Trübenbach J, Graepler F, Pereira PL, Ruck P, Lauer U, Gregor M, Claussen CD, Huppert PE. Growth characteristics and imaging properties of the morris hepatoma 3924A in ACI rats: a suitable model for transarterial chemoembolization. Cardiovasc Intervent Radiol. 2000;23:211–217. doi: 10.1007/s002700010045. [DOI] [PubMed] [Google Scholar]
- 14.Kern L, de Montigny J, Jund R, Lacroute F. The FUR1 gene of Saccharomyces cerevisiae: cloning, structure and expression of wild-type and mutant alleles. Gene. 1990;88:149–157. doi: 10.1016/0378-1119(90)90026-n. [DOI] [PubMed] [Google Scholar]
- 15.Wybranietz WA, Lauer U. Distinct combination of purification methods dramatically improves cohesive-end subcloning of PCR products. Biotechniques. 1998;24:578–580. doi: 10.2144/98244bm13. [DOI] [PubMed] [Google Scholar]
- 16.Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–982, 980-982, 980-982. [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen CA, Kilstrup M, Blaese RM. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: a negative selection system. Proc Natl Acad Sci USA. 1992;89:33–37. doi: 10.1073/pnas.89.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wybranietz WA, Prinz F, Spiegel M, Schenk A, Bitzer M, Gregor M, Lauer UM. Quantification of VP22-GFP spread by direct fluorescence in 15 commonly used cell lines. J Gene Med. 1999;1:265–274. doi: 10.1002/(SICI)1521-2254(199907/08)1:4<265::AID-JGM48>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 19.Rogulski KR, Kim JH, Kim SH, Freytag SO. Glioma cells transduced with an Escherichia coli CD/HSV-1 TK fusion gene exhibit enhanced metabolic suicide and radiosensitivity. Hum Gene Ther. 1997;8:73–85. doi: 10.1089/hum.1997.8.1-73. [DOI] [PubMed] [Google Scholar]
- 20.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 21.Carlsson G, Gullberg B, Hafström L. Estimation of liver tumor volume using different formulas - an experimental study in rats. J Cancer Res Clin Oncol. 1983;105:20–23. doi: 10.1007/BF00391826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erbs P, Regulier E, Kintz J, Leroy P, Poitevin Y, Exinger F, Jund R, Mehtali M. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813–3822. [PubMed] [Google Scholar]
- 23.Maron DJ, Tada H, Moscioni AD, Tazelaar J, Fraker DL, Wilson JM, Spitz FR. Intra-arterial delivery of a recombinant adenovirus does not increase gene transfer to tumor cells in a rat model of metastatic colorectal carcinoma. Mol Ther. 2001;4:29–35. doi: 10.1006/mthe.2001.0417. [DOI] [PubMed] [Google Scholar]
- 24.Yoon SK, Armentano D, Wands JR, Mohr L. Adenovirus-mediated gene transfer to orthotopic hepatocellular carcinomas in athymic nude mice. Cancer Gene Ther. 2001;8:573–579. doi: 10.1038/sj.cgt.7700345. [DOI] [PubMed] [Google Scholar]
- 25.Mullen CA, Kilstrup M, Blaese RM. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: a negative selection system. Proc Natl Acad Sci USA. 1992;89:33–37. doi: 10.1073/pnas.89.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kievit E, Nyati MK, Ng E, Stegman LD, Parsels J, Ross BD, Rehemtulla A, Lawrence TS. Yeast cytosine deaminase improves radiosensitization and bystander effect by 5-fluorocytosine of human colorectal cancer xenografts. Cancer Res. 2000;60:6649–6655. [PubMed] [Google Scholar]
- 27.Diasio RB, Lakings DE, Bennett JE. Evidence for conversion of 5-fluorocytosine to 5-fluorouracil in humans: possible factor in 5-fluorocytosine clinical toxicity. Antimicrob Agents Chemother. 1978;14:903–908. doi: 10.1128/aac.14.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roncarolo MG, Levings MK, Mangia P, Bordignon C, Marktel S, Bonini C, Traversar C. Characterization and modulation of the undesirable T cell-mediated responses to transgenes. Mol Ther. 2002. p. S399. [Google Scholar]
- 29.Kanyama H, Tomita N, Yamano T, Aihara T, Miyoshi Y, Ohue M, Sekimoto M, Sakita I, Tamaki Y, Kaneda Y, et al. Usefulness of repeated direct intratumoral gene transfer using hemagglutinating virus of Japan-liposome method for cytosine deaminase suicide gene therapy. Cancer Res. 2001;61:14–18. [PubMed] [Google Scholar]
- 30.Kievit E, Bershad E, Ng E, Sethna P, Dev I, Lawrence TS, Rehemtulla A. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59:1417–1421. [PubMed] [Google Scholar]
- 31.Chung-Faye GA, Chen MJ, Green NK, Burton A, Anderson D, Mautner V, Searle PF, Kerr DJ. In vivo gene therapy for colon cancer using adenovirus-mediated, transfer of the fusion gene cytosine deaminase and uracil phosphoribosyltransferase. Gene Ther. 2001;8:1547–1554. doi: 10.1038/sj.gt.3301557. [DOI] [PubMed] [Google Scholar]
- 32.Hamstra DA, Rice DJ, Fahmy S, Ross BD, Rehemtulla A. Enzyme/prodrug therapy for head and neck cancer using a catalytically superior cytosine deaminase. Hum Gene Ther. 1999;10:1993–2003. doi: 10.1089/10430349950017356. [DOI] [PubMed] [Google Scholar]
- 33.Block A, Freund CT, Chen SH, Nguyen KP, Finegold M, Windler E, Woo SL. Gene therapy of metastatic colon carcinoma: regression of multiple hepatic metastases by adenoviral expression of bacterial cytosine deaminase. Cancer Gene Ther. 2000;7:438–445. doi: 10.1038/sj.cgt.7700131. [DOI] [PubMed] [Google Scholar]
- 34.Wybranietz WA, Gross CD, Phelan A, O'Hare P, Spiegel M, Graepler F, Bitzer M, Stähler P, Gregor M, Lauer UM. Enhanced suicide gene effect by adenoviral transduction of a VP22-cytosine deaminase (CD) fusion gene. Gene Ther. 2001;8:1654–1664. doi: 10.1038/sj.gt.3301564. [DOI] [PubMed] [Google Scholar]
- 35.Liu CS, Kong B, Xia HH, Ellem KA, Wei MQ. VP22 enhanced intercellular trafficking of HSV thymidine kinase reduced the level of ganciclovir needed to cause suicide cell death. J Gene Med. 2001;3:145–152. doi: 10.1002/jgm.164. [DOI] [PubMed] [Google Scholar]
- 36.Ford KG, Souberbielle BE, Darling D, Farzaneh F. Protein transduction: an alternative to genetic intervention? Gene Ther. 2001;8:1–4. doi: 10.1038/sj.gt.3301383. [DOI] [PubMed] [Google Scholar]
- 37.Tomizawa M, Yu L, Wada A, Tamaoki T, Kadomatsu K, Muramatsu T, Matsubara S, Watanabe K, Ebara M, Saisho H, et al. A promoter region of the midkine gene that is frequently expressed in human hepatocellular carcinoma can activate a suicide gene as effectively as the alpha-fetoprotein promoter. Br J Cancer. 2003;89:1086–1090. doi: 10.1038/sj.bjc.6601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirano T, Kaneko S, Kaneda Y, Saito I, Tamaoki T, Furuyama J, Tamaoki T, Kobayashi K, Ueki T, Fujimoto J. HVJ-liposome-mediated transfection of HSVtk gene driven by AFP promoter inhibits hepatic tumor growth of hepatocellular carcinoma in SCID mice. Gene Ther. 2001;8:80–83. doi: 10.1038/sj.gt.3301355. [DOI] [PubMed] [Google Scholar]
- 39.Yonemitsu Y, Kitson C, Ferrari S, Farley R, Griesenbach U, Judd D, Steel R, Scheid P, Zhu J, Jeffery PK, et al. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat Biotechnol. 2000;18:970–973. doi: 10.1038/79463. [DOI] [PubMed] [Google Scholar]
- 40.Sedlmeier R, Neubert WJ. The replicative complex of paramyxoviruses: structure and function. Adv Virus Res. 1998;50:101–139. doi: 10.1016/s0065-3527(08)60807-6. [DOI] [PubMed] [Google Scholar]
- 41.Bilbao R, Bustos M, Alzuguren P, Pajares MJ, Drozdzik M, Qian C, Prieto J. A blood-tumor barrier limits gene transfer to experimental liver cancer: the effect of vasoactive compounds. Gene Ther. 2000;7:1824–1832. doi: 10.1038/sj.gt.3301312. [DOI] [PubMed] [Google Scholar]
- 42.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 2002;62:1063–1068. [PubMed] [Google Scholar]
- 43.Gerolami R, Uch R, Faivre J, Garcia S, Hardwigsen J, Cardoso J, Mathieu S, Bagnis C, Brechot C, Mannoni P. Herpes simplex virus thymidine kinase-mediated suicide gene therapy for hepatocellular carcinoma using HIV-1-derived lentiviral vectors. J Hepatol. 2004;40:291–297 DOI : 10.1016/j.jhep.2003.10.019. doi: 10.1016/j.jhep.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Ohashi M, Kanai F, Tateishi K, Taniguchi H, Marignani PA, Yoshida Y, Shiratori Y, Hamada H, Omata M. Target gene therapy for alpha-fetoprotein-producing hepatocellular carcinoma by E1B55k-attenuated adenovirus. Biochem Biophys Res Commun. 2001;282:529–535. doi: 10.1006/bbrc.2001.4573. [DOI] [PubMed] [Google Scholar]
- 45.Rogulski KR, Wing MS, Paielli DL, Gilbert JD, Kim JH, Freytag SO. Double suicide gene therapy augments the antitumor activity of a replication-competent lytic adenovirus through enhanced cytotoxicity and radiosensitization. Hum Gene Ther. 2000;11:67–76. doi: 10.1089/10430340050016166. [DOI] [PubMed] [Google Scholar]
- 46.Bitzer M, Lauer UM. [Oncolytic viruses for genetic therapy of gastrointestinal tumors] Z Gastroenterol. 2003;41:667–674. doi: 10.1055/s-2003-40543. [DOI] [PubMed] [Google Scholar]
- 47.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 48.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, Thrasher AJ, Wulffraat N, Sorensen R, Dupuis-Girod S, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 49.Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1:91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 50.Arruda VR, Fields PA, Milner R, Wainwright L, De Miguel MP, Donovan PJ, Herzog RW, Nichols TC, Biegel JA, Razavi M, et al. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]