Abstract
AIM: To observe the interaction between the expression of telomerase activity (TA) and its associate genes in regulation of the terminal restriction fragment length (TRFL) in esophageal squamous cell carcinoma (SCC).
METHODS: Seventy-four specimens of esophageal SCC were examined. The TA was measured by telomeric repeat amplification protocol (TRAP) assay, and the associated genes [human telomerase-specific reverse transcriptase (hTERT), hTERC, TP1, c-Myc, TRF1, and TRF2] were detected using RT-PCR method. The TRFL was measured by Telomere Length Assay Kit and Southern blotting. The correlations between the expression of telomerase and its associated genes with the TRFL and survivals were examined.
RESULTS: Expressions of the TA, hTERT, hTERC, TP1, c-Myc, TRF1, and TRF2 genes were observed in 85.1%, 64.9%, 79.7%, 100.0%, 94.6%, 82.4%, and 91.9% of the tumor tissues, respectively. The TRFL of the tumor and normal esophageal tissues were 2.70±1.42 and 4.93±1.74 kb, respectively (P<0.0001). The TRFL of the telomerase positive and telomerase negative tumor tissues were 2.72±1.44 and 2.58±1.32 kb, respectively (P = 0.767). The TRFL ratios (TRFLR) of the telomerase positive and telomerase negative tumor tissues were 0.55±0.22 and 0.59±0.41, respectively (P = 0.742). The expression rates of h-TERT (P = 0.0002), hTERC (P<0.0001), and TRF1 (P = 0.002) in the tumor tissues are higher than those of the normal paired tissues. Though TA is markedly activated in tumor tissues (P<0.0001), its expression is not related to clinicopathological parameters including gender, tumor differentiation, and TNM stages. The cumulative 4-year survival rates of telomerase positive and telomerase negative cases were 35.86% and 31.2%, respectively (P = 0.8442). The cumulative 4-year survival rates of patients with their TRFLR ≤85% and >85% were 38.7% and 15.7%, respectively (P = 0.1307).
CONCLUSION: Though telomerase expression is not related to tumor stages and prognosis, our data support that the TA increased as the TRFL decreased, probably under the control of hTERT, hTERC, and TRF1. When telomerase expression was activated, only TRF2 overexpression persisted to stabilize T-loop formation. Furthermore, as the TRFLR decreased to 85%, a trend of better prognosis was observed. Cox model analysis indicates a higher t/n TRFLR and distant metastasis are independent poorer prognostic factors (P = 0.035 and P = 0.042, respectively).
Keywords: Telomere, Telomerase, hTERT, Terminal restriction fragment length, Esophageal cancer
INTRODUCTION
Telomerase is a ribonucleoprotein, which is responsible for the synthesis and maintenance of the telomeric repeats at the distal ends of human chromosomes. These end structures, named telomeres, serve as protective caps and consist of specific tandem repeats (5’-TTAGGG-3’) with an average length of 5-20 kb[1-3]. Upon each cell division, the chromosomal ends shorten at a rate of 50-200 bp[4] This molecular erosion sets a physical limit to the potential number of cell divisions and serves as a “mitotic clock” defining the lifespan of somatic cells. Unlike somatic cells, new telomeric repeats are added to the chromosomal end of the germline cells to maintain their stability and also preserve their full genomic information for the next generation[5]. Similarly, immortalized cell lines and more than 85% of the cancer cells can prevent the telomere from progressive shortening by telomerase activation. This phenomenon is regulated by a length-sensing feedback mechanism when the critical point is reached[6]. Telomerase contains a catalytic human telomerase-specific reverse transcriptase (hTERT) and a RNA template (hTERC) for the telomere, provides the cancer cells unlimited replicative capacity and prevents lethal chromosomal instability. Other telomerase-independent mechanism called as alternative lengthening of telomeres (ALT) may ensure the same chromosome ends replication functions.
Normally, the 3’ DNA terminal protein-DNA complexes of the telomeres form capping structures to stabilize chromosomal ends and prevent them from being recognized as DNA double-strand breaks by the cells. The current model for chromosome capping is that telomeres form a higher-order chromatin structure that physically hides the 3’-chromosome end from cellular activities. This protective structure could be provided by the ability of the 3’-overhang to fold back and invade the double-strand region of the telomere forming the so-called T-loop and D-loop with the help of TRF1 and TRF2[7,8]. If these checkpoints fail, chromosomal instability may ensue leading to oncogenic mutations.
Since 1994, the telomeric repeat amplification protocol (TRAP) assay was extensively used for the detection of TA. Our previous report has demonstrated good correlations between the expressions of hTERT (not telomerase) and its associated genes such as c-Myc, TRF1 and TRF2[9]. We also found that the expression of the TA may indicate poorer prognosis[10]. A tumor-to-normal telomere restriction fragment length ratio (t/n TRFLR) ≤75% indicates a better prognosis[11]. In addition, we found a negative linear correlation between the t/n TRFLR and expression of TA, suggesting a negative feedback mechanism in the maintenance of TRFL[11]. In this study, we investigated for correlations between the changes of t/n TRFLR and expression of the telomerase associated genes including c-Myc, TRF1 and TRF2 in squamous cell carcinoma of the esophagus.
MATERIALS AND METHODS
Patients and follow-up
Between June 1999 and December 2003, we included 74 cases of squamous cell carcinoma of the esophagus who underwent surgical resection in this prospective study. Patients who received pre-operative chemotherapy or radiotherapy were excluded. Whole body bone scan and liver sonography were performed for all of the patients to rule out systemic metastasis. The tumor differentiation included well-differentiated carcinoma in none, moderately differentiated carcinoma in 49, and poorly differentiated carcinoma in 25. Tumor staging was performed according to the AJCC (6th edition) criteria[12]. The p-TNM stages included stage I in 2, stage II in 25, stage III in 33, and stage IV in 14. The clinicopathological characteristics of the patients are summarized in Table 1.
Table 1.
Clinical characteristics of 74 patients with esophageal cancer
| Numbers of patients | |
| Age (mean), years | 36-79 (59.5) |
| Sex | |
| Male | 71 |
| Female | 3 |
| Differentiation | |
| Well | 0 |
| Moderate | 49 |
| Poor | 25 |
| Tumor site | |
| T1 | 3 |
| T2 | 10 |
| T3 | 50 |
| T4 | 11 |
| Lymph node | |
| N0 | 24 |
| N1 | 50 |
| Metastasis | |
| M0 | 60 |
| M1 | 14 |
| Stage | |
| I | 2 |
| II | 25 |
| III | 33 |
| IV | 14 |
Preparation of cell extracts
Twenty milligrams of frozen tissue samples were lysed with 200 μL lysis buffer and homogenized by polytron. Samples were then incubated in ice for 30 min and the lysate was centrifuged at 16 000 g at 4 °C for 20 min. The supernatant was transferred to a fresh tube and the protein concentration was determined by the Bradford assay (Bio-Rad Protein Assay Kit, Bio-Rad Lab., Hercules, CA, USA).
DNA isolation from tissues
Twenty-five milligrams of fresh frozen tissue was lysed with 800 μL lysis buffer containing 0.5% sodium dodecyl sulfate (SDS), 2 mmol/L EDTA, 0.5 mol/L NaCl, 10 mmol/L MgCl2, 10 mmol KCl and 10 mmol Tris-HCl (pH 76), and digested with proteinase K at 50 μg/mL at 50 °C for at least 2 h. High molecular weight DNA was extracted with phenol/chloroform.
Assay for telomerase activity
TA was measured twice in independent experiments using 1-3 μg of total protein. Assays were performed using Telomerase PCR ELISA Kit (Boehringer Mannheim GmbH, Mannheim, Germany) including TRAP assay and detection by ELISA in two steps. In the first step, using TRAP, cell extracts were incubated with biotinylated telomerase substrate oligonucleotide (P1-TS) at 25 °C for 30 min, followed by 94 °C for 10 min to inactivate the telomerase. The extended products were amplified by PCR using Taq polymerase, the P1-TS, P2 primers and nucleotides. The PCR conditions were 33 cycles of 94 °C for 30 s on a DNA thermocycler (GeneAmp PCR System 9700, Perkin Elmer, Norwalk, CT, USA). In the second step, using the ELISA method, the amplified products were immobilized onto streptavidin-coated microtiter plates via biotin-streptavidin interaction, and then detected by anti-digoxigenin (DIG) antibody conjugated to peroxidase. After the addition of the peroxidase substrate (3,3’,5,5’-tetramethyl benzidine), the amount of TRAP products were determined by measurement of their absorbance at 450 nm (with a reference wavelength of 690 nm). Negative control reactions were performed by incubating cell extracts with 1 μg/μL RNase for 20 min at 37 °C. The results were interpreted as negative, 1+, 2+, and 3+ when the optic density (OD) values were <0.2, 0.2-1, 1-2, and >2, respectively.
Moreover, to confirm the ELISA results, amplified products were systemically run on 15% non-denaturing polyacrylamide gel. After transferring the PCR products onto a positively charged nylon membrane, Southern blotting was performed by the semi-dry electrophoretic blotting instrument (Multiphore II NovaBlot Unit, Amersham Pharmacia Biotech, Buckinghamshire, UK). The membrane was then incubated with a streptavidin alkaline phosphatase conjugate (1:5 000 dilute in blocking solution), and after rinsing, blotted products were visualized by Biotin Luminescence Detection Kit (Boehringer Mannheim). In addition, all telomerase-negative tumor specimens were re-checked by additional TRAP assay using a 150 bp internal telomerase assay standard to exclude the possibility of Taq DNA polymerase inhibition in the tumor extracts[13].
Reverse transcription-polymerase chain reaction (RT-PCR) for telomerase-associated genes
Total RNA was isolated from tissue by SV Total RNA Isolation System (Promega Corporation, USA). First strand complementary DNA (cDNA) was synthesized using 5 mg of total cellular RNA with reverse transcriptase (Invitrogen Tech-Line SM, USA) and random primers (Protech Technology Enterprise Co. Ltd). PCR was performed using RT-MPCR* Kits for Human Telomerase Genes (Maxim Biotech, Inc., USA). RTMPCR* Kits included PCR primers for human 18S (hTELS-18S, 554 bp), PCR primers for human TRF-1 (hTELS-TRF1, 433 bp), PCR primers for human c-Myc (hTELS-MYC, 381 bp), PCR primers for human TRF-2 (hTELS-TRF2, 337 bp), PCR primers for human TP-1 (hTELS-TP1, 292 bp), PCR primers for hTERT (hTELS-TERT, 255 bp), and PCR primers for human TER (hTELS-TER, 191 bp). PCR reaction mixture contained RT-MPCR buffer, 200 mM each of dATP, dCTP, dTTP, and dGTP, 5U Taq DNA polymerase and 1 mL primers. The thermal cycles of PCR were performed as follows: 3 cycles at 95 °C for 1 min and 56 °C for 4 min followed by 30 cycles at 94 °C for 1 min and 55 °C for 2.5 min and then an extension of 1 cycle at 70 °C for 10 min. PCR products were subjected to electrophoresis through 3% agarose gel stained with ethidium bromide.
Terminal restriction fragment (TRF) length measurement and tumor-to-normal TRFL ratio (t/n TRFLR)
TRFL measurement was performed using TeloTAGGG Telomere Length Assay Kit (Roche, Mannheim, Germany). Eight micrograms of genomic DNA was digested with each 30U Hinf 1/Rsa l at 37 °C for 16 h. The resulting fragments were fractionated by electrophoresis on 0.8% agarose gel and transferred to nylon membrane using Southern blotting. After transfer, the transferred DNA was fixed on the membrane by UV-crosslinking (120 mJ). The membrane was first pre-hybridized at 42 °C for 30 min and then hybridized with telomere-specific DIG-labeled probe at 42 °C for 3 h. After washing the membrane in 2× SSC, the membrane was incubated with anti-DIG-alkaline phosphatase (1:5 000 dilute in blocking solution). Finally, the immobilized telomere probe was visualized by alkaline phosphatase metabolizing CDP-Star, a highly sensitive chemiluminescent substrate. The membrane was then exposed to X-ray film, and the average TRFL was determined by comparing the signals relative to a molecular weight standard (using BIO-PROFIL Bio-1D Software, Version 99, Vilber Lourmat, France), and the mean of three measured TRFLs deducted by 2.5 kb was used as the presented telomere length[14,15]. Furthermore, the TRFLR was defined as the ratio between the length of tumor tissue TRF (t-TRF) and their paired normal tissue TRF (n-TRF) from the same patient.
Statistical analysis
All probabilities were two-tailed, with a P-value less than 0.05 regarded as statistically significant. The statistical calculations were conducted with SPSS software (v10.5, SPSS Inc., Chicago, IL, USA).
RESULTS
Expression of telomerase activity and its associated genes
Positive TAs were observed in 63 of 74 (85.1%) tumor tissue samples, and 24 of 74 (32.4%) normal tissue samples, respectively. Expressions of hTERT, hTERC, TP1, c-Myc, TRF1 and TRF2 genes were observed in 64.9%, 79.7%, 100.0%, 94.6%, 82.4%, and 91.9% of the tumor tissues, respectively. Representative samples showing the expression of the TA by TRAP assay, and the associated genes in paired tumor (T) and normal (N) tissues are shown in Figure 1. Expression of TA according to the patient’s clinicopathological characteristics are listed in Table 2. Expression of the telomerase associate genes in normal and tumor tissues are listed in Table 3. Expression of the telomerase associate genes in tumor tissues according to the TA are listed in Table 4.
Figure 1.
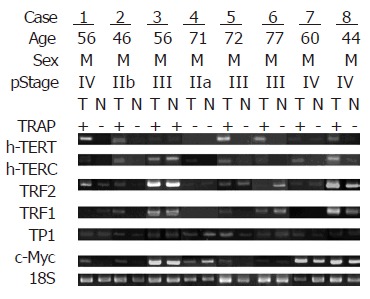
Representative samples showing expression of the telomerase activity by TRAP assay, and the associated genes in paired tumor (T) and normal (N) tissues.
Table 2.
Expression of telomerase activity according to the clinicopathological characteristics of 74 esophageal cancer patients
| Telomerase (+) | Telomerase (-) | P value1 | |
| Gender | 1.0 | ||
| Female | 3 | 0 | |
| Male | 60 | 11 | |
| Differentiation | 0.492 | ||
| Well to moderate | 20 | 5 | |
| Poor | 43 | 6 | |
| Tumor Size | 1.0 | ||
| T1+T2 | 11 | 2 | |
| T3+T4 | 52 | 9 | |
| Lymph node | 0.321 | ||
| N0 | 19 | 5 | |
| N1 | 44 | 6 | |
| Metastasis | 0.11 | ||
| M0 | 49 | 11 | |
| M1 | 14 | 0 | |
| Stage | 0.194 | ||
| I+II | 21 | 6 | |
| III+IV | 42 | 5 |
Fisher's exact test (if expectation<5) or Yate's correction of contingency.
Table 3.
Expression of telomerase associate genes of the tumor and normal tissues in 74 esophageal cancer patients
| Expression | Positive | Negative | P-values1 |
| TRF1 | 0.002 | ||
| Tumor | 61 | 13 | |
| Normal | 43 | 31 | |
| TRF2 | 1.0 | ||
| Tumor | 68 | 6 | |
| Normal | 67 | 7 | |
| c-Myc | 0.16 | ||
| Tumor | 70 | 4 | |
| Normal | 64 | 10 | |
| hTERT | 0.0002 | ||
| Tumor | 48 | 26 | |
| Normal | 24 | 50 | |
| hTERC | <0.0001 | ||
| Tumor | 59 | 14 | |
| Normal | 30 | 44 | |
| TP1 | N/A | ||
| Tumor | 74 | 0 | |
| Normal | 74 | 0 | |
| Tissue | 0.288 | ||
| Tumor | 63 | 11 | |
| Normal | 24 | 50 | <0.0001 |
Pearson’s χ2-test.
Table 4.
Expression of telomerase associate genes of the tumor tissues according to the telomerase activity in 74 esophageal cancer patients
| Telomerase (+) | Telomerase (-) | P-value1 | |
| hTERT | 0.737 | ||
| Positive | 40 | 8 | |
| Negative | 23 | 3 | |
| hTERC | 0.684 | ||
| Positive | 51 | 8 | |
| Negative | 12 | 3 | |
| c-Myc | 0.103 | ||
| Positive | 61 | 9 | |
| Negative | 2 | 2 | |
| TRF1 | 0.396 | ||
| Positive | 53 | 8 | |
| Negative | 10 | 3 | |
| TRF2 | 0.0391 | ||
| Positive | 60 | 8 | |
| Negative | 3 | 3 | |
| TP1 | N/A | ||
| Positive | 63 | 11 | |
| Negative | 0 | 0 |
Fisher's exact test (if expectation<5) or Yate's correction of contingency.
Terminal restriction fragment (TRF) length and tumor-to-normal TRFL ratio (t/n TRFLR)
The mean TRFL of the tumor and normal esophageal tissues were 2.70±1.42 and 4.93±1.74 kb, respectively (P<0.0001). The mean TRFL of the telomerase positive and telomerase negative tumor tissues were 2.72±1.44 and 2.58±1.32 kb, respectively (P = 0.767). The TRFLR of the telomerase positive and telomerase negative tumor tissues were 0.55±0.22 and 0.59±0.41, respectively (P = 0.742). The mean TRFL were 3.04±0.42 kb in stage I tumor, 2.65±1.44 kb in stage II tumor, 2.83±1.44 kb in stage III tumor, and 2.41±1.49 kb in stage IV tumor, respectively (stage I+II vs stage III+IV, P = 0.936). The t/n TRFLR were 0.818±0.019 in stage I tumor, 0.686±0.184 in stage II tumor, 0.729±0.265 in stage III tumor, and 0.649±0.185 in stage IV tumor, respectively (stage I+II vs stage III+IV, P = 0.867). Table 5 lists the TRFL and t/n TRFLR data of our patients. The representative samples showing TRFL in paired T and N tissues are shown in Figure 2.
Table 5.
TRFL and t/n TRFLR of the tumor tissues according to the telomerase expression and TNM stages
| Variables | TRFL | P- values | t/n TRFLR | P- values |
| Tissues | <0.00011 | |||
| Normal | 4.93±1.74 kb | |||
| Tumor | 2.70±1.42 kb | |||
| Telomerase | 0.7671 | 0.7422 | ||
| Positive | 2.72±1.44 kb | 0.55±0.22 | ||
| Negative | 2.58±1.32 kb | 0.59±0.41 | ||
| Tumor stages | 0.9362 | 0.8672 | ||
| Stage I | 3.04±0.42 kb | 0.818±0.019 | ||
| Stage II | 2.65±1.44 kb | 0.686±0.184 | ||
| Stage III | 2.83±1.44 kb | 0.729±0.265 | ||
| Stage IV | 2.41±1.49 kb | 0.649±0.185 | ||
| Total | 2.70±1.42 kb | 0.73±0.24 |
P values:
Paired t-test,
Independent t-test.
Figure 2.
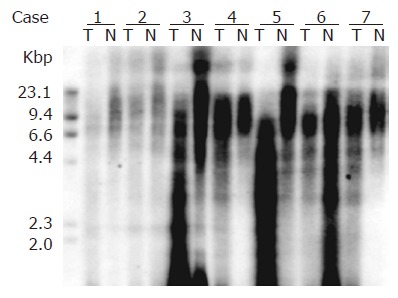
Representative samples showing TRFL by telomere length assay kit in paired tumor (T) and normal (N) tissues are shown.
Survival analysis
The influence of TA expression was evaluated by cumulative survival period. The 5-year cumulative survival rates of the patients by tumor stages and presence of distant metastasis are shown in Figures 3 and 4. As shown in Figure 5, the 4-year cumulative survival rates of telomerase-positive and telomerase-negative patients were 35.8, and 31.2%, respectively (P = 0.8442). When survival analyses were performed based on the change of TRFL in the tumor tissues, a cut-off value of 50% demonstrated a trend in survival difference. As shown in Figure 6, the 4-year cumulative survival rates of lower t/n TRFLR (≤85%) and higher t/n TRFLR (>85%) patients were 38.7, and 15.7%, respectively (P = 0.1307). Multivariate survival analysis using Cox proportional hazards model revealed independent prognostic factors that includes t/n TRFLR (P = 0.035), and M-status (P = 0.042) of the tumor (Table 6).
Figure 3.
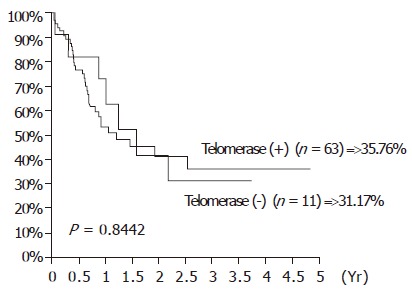
Cumulative survival rates according to the expression of telomerase activity in 74 SCC of esophagus.
Figure 4.
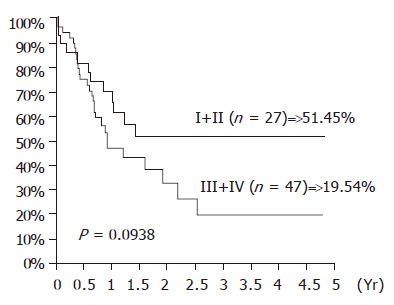
Cumulative survival rates according to the TNM stages in 74 SCC of esophagus.
Figure 5.
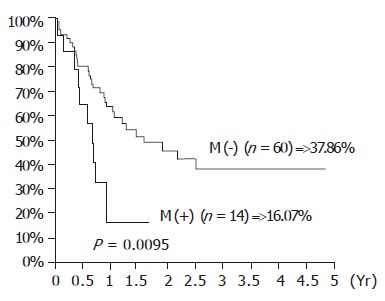
Cumulative survival rates according to the distant metastasis in 74 SCC of esophagus.
Figure 6.
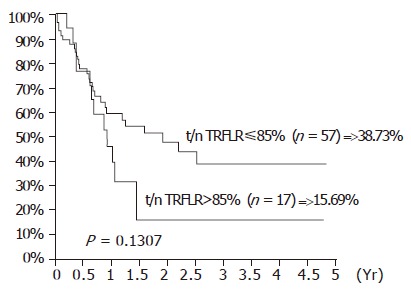
Cumulative survival rates according to the t/n TRFLR in 74 SCC of esophagus.
Table 6.
Multivariate survival analysis for Cox proportional hazards model
| Risk factors (# Patients) | Coefficients (SE) | Relative risk (95%CI) | P-values1 |
| t/n TRFLR | 0.78 (0.37) | (1.06-4.48) | 0.035 |
| ≤85% (n = 57) | 1 | ||
| >85% (n = 17) | 2.18 | ||
| T-status | 1.17 (0.78) | (0.70-15.06) | 0.134 |
| T1+T2 (n = 13) | 1 | ||
| T3+T4 (n = 61) | 3.24 | ||
| N-status | 1.06 (0.66) | (0.79-10.48) | 0.108 |
| N0 (n = 24) | 1 | ||
| N1+N2 (n = 50) | 2.88 | ||
| M-status | 0.87 (0.43) | (1.03-5.49) | 0.042 |
| M0 (n = 60) | 1 | ||
| M1 (n = 14 ) | 2.38 | ||
| Stage | -0.90 (0.78) | (0.09-1.89) | 0.251 |
| I+II (n = 27) | 1 | ||
| III+IV (n = 47) | 0.41 | ||
| Differentiation | 0.33 (0.35) | (0.70-2.76) | 0.352 |
| W+M (n = 25) | 1 | ||
| P (n = 49) | 1.39 | ||
| Telomerase expression | -0.11 (0.46) | (0.36-2.22) | 0.811 |
| Negative (n = 11) | 1 | ||
| Positive (n = 63) | 0.9 |
Wald statistic
DISCUSSION
Cell numbers are vigorously controlled within the body and, in human adults, only a few cell types are capable of continued division. Cultured cells in vitro can undergo only a limited number of cell divisions, known as the Hayflick limit, before entering a state of senescence where they remain metabolically active but have lost their capacity to replicate (M1 crisis). Reduction in telomere length could provide the signal to cause growth arrest. Cultured cells can be induced to continue to divide beyond the Hayflick limit by inactivation of p53 or p16INK4a genes. During this process of oncogenesis, their telomeres continue to shorten with each division and at a certain point cells enter a crisis where the majority will die (M2 crisis). Rare immortalized clones that emerge from crisis express the enzyme telomerase[16]. The regulation of TA is a complex issue, involving the transcriptional activity of the hTERC (telomerase RNA component gene), and the hTERT, as well as the interaction of telomerase with other telomerase-associated proteins, such as TP1/c-Myc/TRF1/TRF2/Tankyrase. TP1, which is expressed ubiquitously, may play a role in coordinating telomerase holoenzyme tertiary or/and quaternary structures and/or serve as a docking/scaffold protein in recruiting telomerase regulatory factors. The ability of c-Myc to function as a transcription factor has been shown to depend upon its dimerization with the protein Max[17]. In addition to the formation of stable complexes with c-Myc, Max also heterodimerizes with proteins of the Mad(Mxi1) family[18]. These Mad/Max complexes act in an antagonistic manner to c-Myc/Max-induced transactivation and result in potent repression of gene expression. Two proteins that bind to the double-stranded region of mammalian telomeres have been identified: TTAGGG repeat binding factor 1 (TRF1) and factor 2 (TRF2). These proteins are related and have a similar domain organization. Both proteins are associated with telomeres throughout the cell cycle and bind to the cognate telomeric sequence as homodimers using a carboxy-terminal myb-type DNA binding domain. TRF1 regulates telomere length and TRF2 protects chromosome ends. These two paralogs bind to double stranded telomeric DNA with high affinity, but no interaction between TRF1 and TRF2 has been observed so far[19,20]. However, TRF1 and TRF2 interact with other proteins in regulating telomeric repair. Together with tankyrase, TRF1 is involved in telomere length regulation via negative feedback mechanism; overexpression results in shortened telomeres, and mutation of telobox causes elongated telomeres[20,21]. Removal of TRF2 from the telomere results in the loss of the 3’-overhang, covalent fusion of telomeres, and the induction of ATM and p53 dependent apoptosis. Overexpression of TRF2 in telomerase negative cells prevents critically short telomeres from fusion and delays the onset of senescence[19] .
The expression rates of telomerase and hTERT were 85.1% and 64.9%, which were consistent with other reports. We also found telomerase expression in 32.4% of the paired normal esophageal mucosa. This had been attributed to actively dividing basal layer cells[22] or submucosal tumor infiltration[2]. A higher telomerase and hTERT expression rate in the normal esophageal mucosa makes it a distinct finding as compared with other digestive tract mucosa[23]. The telomerase expression of the tumor is not related to the clinicopathological parameters including gender, tumor differentiation, and TNM stages of the patients (see Table 2). Controversy in interpretation of the clinical significance of telomerase expression may be related to the presence of the alternative telomere lengthening (ATL) mechanism. ALT cells have long heterogeneous telomeres thought to be generated by a recombination-based mechanism[24]. Interestingly, tumor cells may simultaneously obtain both telomerase and ATL mechanisms in maintaining telomere length[25]. This will cause more complexity in analyzing the relation between the telomerase expression, telomere maintenance, and their impact on prognosis.
In a previous study in non-small cell lung cancers, we found that c-Myc, TRF1, and TRF2 expression was closely related to hTERT expression, although there was no association with telomerase expression[9]. We also found that when the TRFL decreased to a critical level, the TA could be elicited[11]. This hypothesis was further confirmed by the establishment of a negative linear association between the t/n TRFLR and the expression of TA in NSCLC tumor tissues[11] In the current study, we found a higher expression rate of the hTERT, hTERC, and TRF1 in the tumor tissues (Table 3). This suggests that hTERT, hTERC, and TRF1 are incorporated in the regulation of telomerase expression in the tumor tissues as the TRFL becomes progressively shortened. However, once the telomerase expression was activated, TRF1 expression becomes suppressed to prevent interference with telomerase binding. Instead, TRF2 overexpression persisted (see Table 4), which increases the number of TRF2 molecules binding on the telomeric DNA, and subsequently leads to more efficient and stable T-loop formation as described in the in vitro study[26]. Therefore, it is not only the telomere length, but also the TRF2 that determines whether senescence ensues or not.
Though a decreased TRFL was observed in the tumor tissues, there were no significant changes in TRFL between different tumor stages or different telomerase expression. Also, the t/n TRFLR did not change accordingly in different tumor stages. But when the t/n TRFLR decreased to a critical level (≤85%), a better survival was observed (Figure 6). This may be due to the failure of the tumor cells to regain an adequate telomere length, which subsequently triggers the apoptosis pathway. Cox model analysis also confirmed t/n TRFLR as an independent prognostic factor in addition to distant metastasis.
In summary, our data suggest that telomerase expression in esophageal cancers is not related to tumor stages and patient’s prognosis. This may be due to a high telomerase expression in the normal esophageal mucosa which makes telomerase not a reliable biomarker in esophageal tumors. However, TA can be elicited as the TRFL decreased in the tumor tissues, probably under the control of hTERT, hTERC, and TRF1 (but not TRF2). Once the telomerase expression was elicited, TRF1 expression becomes suppressed to prevent interference with telomerase binding. Instead, TRF2 overexpression persisted, which increases the number of TRF2 molecules binding on the telomeric DNA, and subsequently leads to more efficient and stable T-loop formation. Moreover, as the t/n TRFLR decreased to 85%, a trend of poorer prognosis was observed. These findings further confirm our previous proposal using the t/n TRFLR as an indicator of chromosome ends replication ability[11]. The complex interweaving of the regulatory pathway for telomere maintenance and the mechanism involved in the detection of telomere loss by tumor cells, which subsequently activates telomerase expression require further study.
ACKNOWLEDGMENTS
The authors thank Ms SW Tang, and Ms HC Ho (Biostatistics Task Force of Taichung Veterans General Hospital) for their assistance in tissue preparation, data recording, and statistical analysis.
Footnotes
Supported by grant from NSC (NSC-92-2314-B-075A-011), Taipei, Taiwan, China
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Allshire RC, Dempster M, Hastie ND. Human telomeres contain at least three types of G-rich repeat distributed non-randomly. Nucleic Acids Res. 1989;17:4611–4627. doi: 10.1093/nar/17.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross SH, Allshire RC, McKay SJ, McGill NI, Cooke HJ. Cloning of human telomeres by complementation in yeast. Nature. 1989;338:771–774. doi: 10.1038/338771a0. [DOI] [PubMed] [Google Scholar]
- 4.Vaziri H, Schächter F, Uchida I, Wei L, Zhu X, Effros R, Cohen D, Harley CB. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993;52:661–667. [PMC free article] [PubMed] [Google Scholar]
- 5.Buchkovich KJ, Greider CW. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell. 1996;7:1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256:271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 7.Stansel RM, de Lange T, Griffith JD. T-loop assembly in vitro involves binding of TRF2 near the 3' telomeric overhang. EMBO J. 2001;20:5532–5540. doi: 10.1093/emboj/20.19.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CP, Miaw J, Hsia JY, Shai SE, Chen CY. Concordant expression of the telomerase-associated genes in non-small cell lung cancer. Eur J Surg Oncol. 2003;29:594–599. doi: 10.1016/s0748-7983(03)00108-2. [DOI] [PubMed] [Google Scholar]
- 10.Wu TC, Lin P, Hsu CP, Huang YJ, Chen CY, Chung WC, Lee H, Ko JL. Loss of telomerase activity may be a potential favorable prognostic marker in lung carcinomas. Lung Cancer. 2003;41:163–169. doi: 10.1016/s0169-5002(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CP, Miaw J, Shai SE, Chen CY. Correlation between telomerase expression and terminal restriction fragment length ratio in non-small cell lung cancer--an adjusted measurement and its clinical significance. Eur J Cardiothorac Surg. 2004;26:425–431. doi: 10.1016/j.ejcts.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL, Page DL Fleming ID Fritz AG Balch CM Haller DG Morrow M. AJCC Cancer Staging Handbook. 6th ed. New York, Springer-Verlag Inc. 2002. pp. 191–203. [Google Scholar]
- 13.Wright WE, Shay JW, Piatyszek MA. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995;23:3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE. Structure and variability of human chromosome ends. Mol Cell Biol. 1990;10:518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright WE, Shay JW. Cellular senescence as a tumor-protection mechanism: the essential role of counting. Curr Opin Genet Dev. 2001;11:98–103. doi: 10.1016/s0959-437x(00)00163-5. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber-Agus N, DePinho RA. Repression by the Mad(Mxi1)-Sin3 complex. Bioessays. 1998;20:808–818. doi: 10.1002/(SICI)1521-1878(199810)20:10<808::AID-BIES6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 18.Ayer DE, Kretzner L, Eisenman RN. Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell. 1993;72:211–222. doi: 10.1016/0092-8674(93)90661-9. [DOI] [PubMed] [Google Scholar]
- 19.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 20.van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- 21.Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 22.Ikeguchi M, Unate H, Maeta M, Kaibara N. Detection of telomerase activity in esophageal squamous cell carcinoma and normal esophageal epithelium. Langenbecks Arch Surg. 1999;384:550–555. doi: 10.1007/s004230050242. [DOI] [PubMed] [Google Scholar]
- 23.Koyanagi K, Ozawa S, Ando N, Kitagawa Y, Ueda M, Kitajima M. Clinical significance of telomerase activity in peripheral blood of patients with esophageal squamous cell carcinoma. Ann Thorac Surg. 2002;73:927–932. doi: 10.1016/s0003-4975(01)03435-x. [DOI] [PubMed] [Google Scholar]
- 24.Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 26.Karlseder J, Smogorzewska A, de Lange T. Senescence induced by altered telomere state, not telomere loss. Science. 2002;295:2446–2449. doi: 10.1126/science.1069523. [DOI] [PubMed] [Google Scholar]


