Abstract
AIM: To construct the recombinant Lactococcus lactis as oral delivery vaccination against malaria.
METHODS: The C-terminal 19-ku fragments of MSP1 (MSP-119) of Plasmodium yoelii 265-BY was expressed in L. lactis and the recombinant L. lactis was administered orally to BALB/c and C57BL/6 mice. After seven interval vaccinations within 4 wk, the mice were challenged with P. yoelii 265-BY parasites of erythrocytic stage. The protective efficacy of recombinant L. lactis was evaluated.
RESULTS: The peak parasitemias in average for the experiment groups of BALB/c and C57BL/6 mice were 0.8±0.4% and 20.8±26.5%, respectively, and those of their control groups were 12.0±0.8% and 60.8±9.6%, respectively. None of the BALB/c mice in both experimental group and control group died during the experiment. However, all the C57BL/6 mice in the control group died within 23 d and all the vaccinated mice survived well.
CONCLUSION: The results imply the potential of recombinant L. lactis as oral delivery vaccination against malaria.
Keywords: Lactococcus lactis, Oral delivery vaccination, Malaria
INTRODUCTION
The development of efficacious vaccines against malaria is one of the greatest challenges for the application of current life sciences in infectious diseases. The easiness of administration of a vaccine provides an attractive alternative to continue drug treatments in a population exceeding hundreds of millions of people with limited health care resources. Merozoite surface protein 1 (MSP1) is present in all species of Plasmodium[1,2], and has been widely studied as the major candidate for vaccine against malaria[3-5]. The high level expression of MSP1 by Plasmodium in the asexual stage is closely related to its invasion into erythrocytes. MSP1 can be proteolytically cleaved into five fragments by two processing steps after the maturation of merozoite, with the carboxyl-terminal 19-ku fragment (MSP-119) remaining on the merozoite surface[6-8]. The MSP-119 comprises two epidermal growth factor (EGF)-like modules. Antibodies directed to this fragment have been shown to inhibit the invasion of Plasmodium falciparum into erythrocyte in vitro[9,10] and intranasal or subcutaneous immunization may protect mice against the challenge of Plasmodium yoelii asexual blood-stage parasite[11]. The recombinant MSP-119 has been expressed in several host organisms, such as Escherichia coli[12], Saccharomyces cerivisae[11], Bacillus Calmette-Guerin (BCG)[13], and Baculovirus[14]. It has also been proven to be immuno-effective against the challenge of parasite. In vaccination experiments with recombinant MPS-119 from P. yoelii, immunized mice were protected against challenge with blood-stage parasites, and the protection was confirmed to be largely mediated by antibodies[15-17].
The development of efficient mucosal vaccines is one of the hotspots in modern vaccinology. One approach to deliver the protective antigens to the mucosal surfaces is to use live bacteria carrying plasmids responsible for the expression of specific antigen. Until recently most of these are derived from attenuated pathogenic microorganisms, such as Salmonella typhi[18] and chlorella[19]. As an alternative to this strategy, non-pathogenic food grade bacteria such as lactic acid bacteria are being focused for their efficacy as live antigen carriers[20]. Lactococcus lactis has a long history of being used in food fermentations and has been, therefore, generally regarded as a safe (GRAS) status[21]. This food-grade lactic acid bacterium is able to survive through the gastrointestinal tract of human beings and other animals, with a retention time of 2-3 d, but it does not invade or colonize the mucous and does not evoke strong host immune responses[21]. The availability of various food-grade genetic engineering systems for L. lactis[22] makes the bacteria a potentially functional food or medicines by expressing heterogeneous peptides. Recent report using L. lactis preloaded with a bacterial antigen, tetanus toxin fragment C of Clostridium tetanus, demonstrated the feasibility of this approach: a protective systemic antibody response was elicited after nasal or oral immunization of mice[21]. Similar study was carried out by Lee et al[23], in which urease subunit B (UreB) gene of Helicobacter pylori was expressed in L. lactis MG1363 and the recombinant bacterium was used as an oral vaccine against H pylori infection in mice. However, in this case no protective effect was observed, which implied that the adjuvant effects of L. lactis are likely to be insufficient to produce an effective immune response to protect against H pylori challenge, when used to deliver a weak immunogen like UreB.
Since the use of oral (or other mucosal) routes for immunization against malaria is also desirable due to the easiness of administration, we attempted L. lactis as the live vehicle for vaccine development against malaria. In this work, we showed that the oral immunization, with recombinant L. lactis constitutively expressing MSP-119 antigen, could protect BALB/c and C57BL/6 mice against malaria parasites challenge.
MATERIALS AND METHODS
Genes, plasmids, bacteria, and malarial parasites
The DNA fragment encoding for MSP-119 domain was amplified from the genomic DNA of P. yoelii. Plasmid pTRKL2 was from Prof. Todd R. Klaenhammer at Food Science Center, North California University, USA[24]. Cloning vector pBluescriptSKII(+) was from Strategene (La Jolla, CA, USA), and fusion-protein expression vector pGEX-5X-3 was from Amersham Pharmacia. L. lactis LM2345 was from Prof. Keith Thompson at Agriculture and Food Science Center, Newforge Lane, Northern Ireland. Lactobacillus brevis (ATCC8287) and Bacillus subtilis BR151 (ATCC33677) were purchased from the American Type Culture Collection. P. yoelii 265-BY was by Professor Weibin Guan at Second Military Medical University, Shanghai, China.
Construction of expression vector
The plasmid for the expression of MSP-119 fragment was constructed by conventional DNA recombination manipulation. The promoter region and the first five amino acids of the signal peptide-coding region of S-layer protein A (SlpA) gene (nucleotides 1-282, GenBank Z14250) was amplified by polymerase chain reaction (PCR) from genomic DNA of L. brevis with forward primer GCTGAGCTCGATTACAAAGGCTTTAAGCAGGTTAGTGAC (with SacI site) and reverse primer GTCGGATCCTAAACTTGATTGCATAATCTTTCTTCCTCC (with BamHI site). The DNA fragment encoding MSP-119 (nucleotides 5 040-5 451, GenBank AF165928) was amplified from the genomic DNA of P. yoelii 265BY, with forward primer ACGGGATCCAA CACATAGCCTCAATAGCT (with BamHI site) and reverse primer ACGGAATTCTAGCTGG AAGAACTACAGAA (with EcoRI site). The terminator of N-acetylmuramoyl-L-alanine amidase (cwlB) (nucleotides 2 187-2 471, GenBank M81324) was amplified from Bacillus subtilis BR151 by PCR with forward primer CTCGAGCTCCACAAGCTATTCATGAC (with XhoI site) and reverse primer GGTACCTCTCT GCACTCACTG ACACA (with KpnI site). The PCR products were cloned on T-vector (Promega, USA) first, and then joined together in a tandem way on pBluescriptSKII(+) with restriction enzyme pairs of SacI/BamHI, BamHI/EcoRI, and XhoI/KpnI respectively to obtain plasmid pSK-PSGT. For expression of MSP-119 in L. lactis, pSK-PSGT was then digested with PvuII to release the 1.6-kb blunt-end fragment, which was then inserted into the EcoRV site of shuttle vector pTRKL2[24]. The final construct was referred as pL2-PSGT. For expression of fusion protein GST-MSP-119 in E. coli, the DNA fragment encoding MSP-119 was derived by PCR and joined to fusion protein expression vector pGEX-5X-3 with BamHI and EcoRI sites. The derived plasmid was noted as pGEX-MSP-119.
Transformation
Electroporation[25] was used to transform L. lactis LM2345 with pL2-PSGT. CaCl2 method was used to transform E. coli BL21 with plasmid pGEX-5X-3.
Preparation of antiserum against P. yoelii 265-BY
The antiserum against P. yoelii 265-BY was prepared from BALB/c mice infected with 104 asexual blood stage parasites. The serum was collected from the eye veins 4 d after burst with 104 parasites one month after infection, and stored at 4 °C.
Expression and analysis of MSP-119 in E. coli and L. lactis
The BL21 transformant harboring plasmid pGEX-MSP-119 was cultured in L-broth to A600 around 1.0, and was treated with supplement containing 1.0 mmol/L of isopropylthio-β-galactoside (IPTG) for 3 h. The fusion protein GST-MSP-119 was purified from cell lysate by affinity chromatography of Glutathione Sepharose-4B according to manual instruction provided by the manufacturer. The purified fusion protein was used as positive control of immunoblotting. To express MSP-119 in L. lactis, the transformant harboring plasmid pL2-PSGT was cultured overnight in MRS medium[26] supplemented with 10 mg/L erythromycin. The cells were collected by centrifuge, and the lysates were analyzed by using SDS-polyacrylamide gel electrophoresis analysis followed with immunoblotting by using antiserum against P. yoelii 265-BY (1:500 dilution). All other operations were performed following standard protocols[27].
Oral immunization of BALB/c and C57BL/6 mice with recombinant L. lactis
Oral immunization experiments were performed according to the protocol described by Robinson et al[21]. The BALB/c and C57BL/6 mice were divided into groups of 10 mice and fed for 6-8 wk. The test group was administered with recombinant L. lactis constitutively expressing MSP-119, and the control groups were administered with free L. lactis bacteria or phosphate-buffered saline (PBS). For BALB/c mice, every dose containing 5×109 bacterial cells in the suspension buffer (0.2 mol/L sodium bicarbonate, 5% Casino acids, and 0.5% glucose); and for C57BL/6 mice (two dosage groups), every dose containing 5×109 or 1×108 cells were administered. All the groups were administered with recombinant L. lactis on d 1, 2, 3, 29, 30, 31, and 36, respectively.
Challenge infections and evaluation of protective efficacy
Mice in each group were challenged on d 49 with 1×105 asexual blood stage P. yoelii parasites obtained from a donor mouse. Parasitemia was monitored every two days after challenge using microscopic examination of blood film with Giemsa staining.
RESULTS
Expression of fusion protein GST-MSP-119 in E. coli
The fusion protein GST-MSP-119 was expressed in E. coli BL21 transformed with plasmid pGEX-MSP-119. The transformed BL21 cells were cultured in L-broth until the A600 reached 1.0, and then induced with 1.0 mmol/L IPTG for 3 h. Total proteins of BL 21 E. coli cells were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). As shown in lane 4 of Figure 1, the thick band of the expressed protein showed a molecular weight of 45 ku, matching well with the theoretical value of fusion protein GST-MSP-119. The expressed fusion protein was about 40% of the total protein of E. coli cells. Most of the fusion protein was found in inclusion body, but a small fraction was soluble. Glutathione Sepharose-4B affinity chromatography was carried out with the soluble fraction of E. coli cell lysate, and the derived fusion protein was used as the positive control of immunoblotting.
Figure 1.
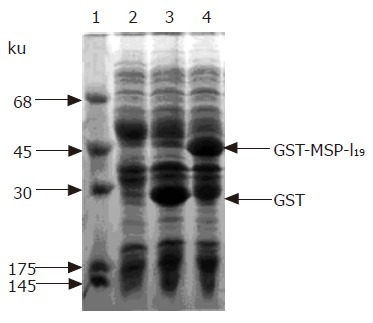
Expression of fusion protein in E. coli BL21 cells. Coomassie brilliant blue-stained 12% SDS-polyacrylamide gel. lane 1, protein markers; lane 2, total protein of BL21 cells; lane 3, total protein of E. coli BL21 transformed with plasmid pGEX-5X-3 with IPTG induction; lane 4, total protein of E. coli BL21 cells harboring plasmid pGEX-MSP-119 after with IPTG induction. The arrows indicate the positions of GST and fusion protein GST-MSP-119.
Expression of MSP-119 in L. lactis
For the expression of MSP-119 in L. lactis, the transformant harboring plasmid pL2-PSGT was cultured overnight at 30 °C in MRS medium[26] supplemented with 10 mg/L erythromycin. The cells were collected by centrifuge, and the lysates were analyzed with SDS-PAGE. However, no obvious protein bands around 19 ku were detected. Immunoblotting with antiserum against P. yoelii 265-BY was applied to check if there was low-level expression of MSP-119. As a result, the presence of MSP-119 was confirmed by immunoblotting as shown in lane 3 of Figure 2. The stained protein band at 19 ku was a little bit broad. This might be the result of partial degradation of MSP-119 in E coli cells. At least part of the expressed MSP-119 was in its native structure, since the antiserum prepared by P. yoelii parasites infection is considered to preferentially recognize the MSP-119 fragment located on erythrocyte membrane with the native conformation.
Figure 2.
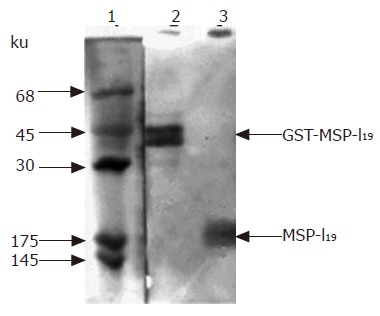
Immunoblotting analysis of MSP-119 expressed in L. lactis. Protein samples were first analyzed on 12% SDS-polyacrylamide gel and then transferred on nitrocellulose membrane followed by immunostaining with antiserum prepared by infecting mouse with P. yoelii parasites. lane 1, protein markers stained by amido black; lane 2, positive control of fusion protein GST-MSP-119 purified from E. coli cell lysate expressing the fusion protein by pGEX-MSP-119; lane 3, total protein of L. lactis cells harboring plasmid pL2-PSGT. The arrows indicate the position of fusion protein GST-MSP-119 and MSP-119.
In lane 2 of the positive control, two protein bands were stained: one was at 45 ku, and the other was slightly below 45 ku, but not detectable on SDS-PAGE with Coomassie brilliant blue staining. When fusion protein is isolated from inclusion body of corresponding E. coli and refolded by rapid dilution method, only one protein band could be stained (data not shown). Therefore, we concluded that the low molecular weight protein was the degraded fusion protein present in the soluble fraction of E. coli.
Evaluation of protective immunity induced by recombinant L. lactis
Previous work of Tian et al[27] indicated that mice with different genetic backgrounds may have quite different responses to P. yoelii infection. C57BL/6 mice showed the highest level against challenge with infected erythrocytes after immunization with recombinant proteins consisting of the PyMSP-1 C terminus in adjuvants. In this work, two strains of mice, BALB/c and C57BL/6, were used for oral immunization for comparison.
For BALB/c mice, two control groups were designed. Mice were administered with phosphate-buffered saline in control group 1, with 5×109 per dose of original L. lactis cells in control group 2, and with 5×109 per dose of L. lactis cells carrying pL2-PSGT construct in the test group, respectively. After seven doses of vaccination, each mouse was challenged with 1×105 asexual blood stage parasites. The parasitemias were measured from the next day of parasite challenging. The average and standard deviation of each group are shown in Figure 3. The peak p-arasitemias were 0.8±0.4% at d 4 for test group, 16.0±1.2% at d 8 for control group 1, and 12.0±0.8% at d 8 for control group 2, respectively. There was little difference between the peak parasitemias of the two control groups. Therefore, the non-specific immunity caused by the adjuvanticity of L. lactis was little. It should be noted that the appearance of parasitemia in the test group was one-day delayed compared with the control groups. None of the mice in any of the three groups died during the experiment. Overall, the BALB/c mice in all the three groups had the ability to scavenge P. yoelii parasites from their bodies by themselves.
Figure 3.
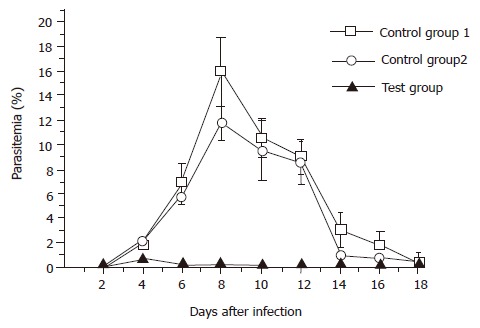
Blood-stage parasitemia of immunized BALB/c mice challenged with P. yoelii 265BY parasites. Mice were orally administered with PBS in the control group 1 (-□-), with 5×109 per dose of free L. lactis cells in the control group 2
For C57BL/6 mice, one control group and two test groups were designed. Mice were administered with 5×109 plasmid-harbored L. lactis cells per dose in test group 1, and 1×108 cells per dose in test group 2. Vaccination was performed by the same protocol for BALB/c mice, and the parasitemias were measured from the next day of parasite challenging. As shown in Figure 4A, the average peak parasitemias for the three groups were 60.8±9.6% for the control group, 41.6±8.8% for test group 1, and 20.8±26.5% for test group 2, respectively. Surprisingly, test group 2 administered with 1×108 bacterial cells per dose gained significantly stronger immune protection than test group 1, which was administered with 15 times more bacterial cells per dose. However, the standard deviation of test group 2 was remarkably bigger than the other two groups. This was largely due to the difference between individuals. Another important fact that should be noted was that all the mice in the control group died within 23 d after parasite infection, whereas all the vaccinated mice survived despite the high parasitemias (Figure 4B). After 30 d counting from the day of parasite challenging, malarial parasites were no more detectable in both of the two test groups (data not shown), implicating the complete elimination of the P. yoelii parasites.
Figure 4.
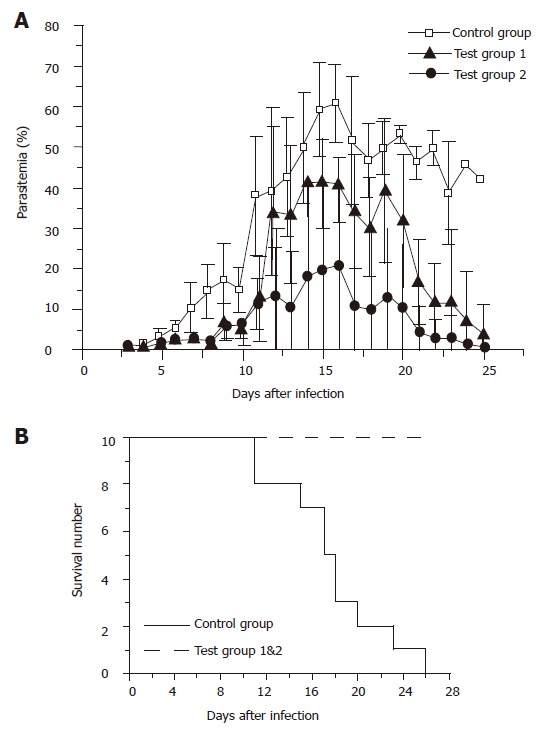
(A) Blood-stage parasitemia of immunized C57BL/6 mice challenged with P. yoelii 265BY parasites. Mice were orally administered with 5×109 per dose of free L. lactis cells in the control group (-□-), with 5×109 pL2-PSGT plasmid-harbored L. lactis cells per dose in the test group 1 (-▲-), and with 1×108 cells per dose of pL2-PSGT plasmid-harbored L. lactis cells in the test group 2 (-●-). Immunization procedure is described in Materials and Methods, and each mouse was challenged with 1×105 asexual blood-stage P. yoelii parasites. (B) Time course of survivals of the mice in the three groups is indicated in A.
We also checked the duration of recombinant bacteria in mice gut by investigation of the titers of recombinant bacterial cells in mice feces. After feeding of a single dose of 5×109 recombinant cells, the recombinant L. lactis reached a peak at 6 h with a density of 1×107 cells/g of feces, and then gradually decreased with time. The density of recombinant cells decreased to 1×104/g at 48 h, and 1×103/g at 72 h. Therefore, the interaction between the host and recombinant bacterial cell could be as long as 3 d per dose.
DISCUSSION
It is known that the immunogenicity of soluble protein is low when administered orally but when expressed by genetically engineered bacteria and can be considerably enhanced. To achieve this goal, promoters that can drive the expression of a gene constitutively are essential. S-layer protein is a protein that forms regular crystalline arrays on prokaryotic cell surface. The slpA promoter can express the β-lactamase constitutively at a high level in L. lactis[27]. However, it failed to express MSP-119 with high efficiency in this study. The expression of MSP-119 in L. lactis could be detected only by immunoblotting; therefore, it was estimated at the level of several nanograms per 107 bacterial cells.
Unexpectedly, the low-level expression of MSP-119 in L. lactis was still able to elicit strong protection against P. yoelii infection on both BALB/c and C57BL/6 mice by oral administration. Although BALB/c mice seemed to be able to scavenge the malarial parasites by themselves, the parasitemia was reduced more than 10-folds (less than 1%) in the test group compared with the control groups. In the case of C57BL/6 strain, all the members in the control group died within 26 d after the infection, whereas all the members in the two test groups survived, and the parasites disappeared from both groups one month after the parasite challenging.
The different immune responses of the two C57BL/6 test groups also support the point of view that the expression level of MSP-119 is not critical for the elicitation of immune response, i.e., the group administered with a low dose of the recombinant bacterium gained stronger protection than the group administered with a high dose. The reason for the difference between the two groups is not clear at present; however, this might be partially due to immune tolerance caused by overdose. In the study by Robinson et al[21], expression of tetanus toxin fragment C of C. tetanus in the intracellular accumulation of L. lactis was up to 3% of soluble cellular protein, and 5×109 cells were orally administered per dose to gain complete protection of mice from tetanus. Therefore, the optimal dose and the time schedule for oral administration should be carefully determined for each antigen.
It is striking to find the difference in immune response between the two strains of mice, BACL/c and C57BL/6. Our results partially support the report by Tian et al[27] that C57BL/6 mice are most sensitive to P. yoelii infection. In most cases, BALB/c and C3H/He mice are used for protection test of a vaccine. Our results suggest that at least for protection experiments, C57BL/6 mice are better to be used in parallel.
In vaccination experiments with recombinant MPS-119 of P. yoelii, the protection effect has been found to be mediated by humoral immune response[15-17]. Robinson et al[21] also reported high titers of IgG against tetanus toxin. We also tried immunoblotting and ELISA test with sera from the survivals of C57BL/6 mice in the test groups (data not shown). Nearly all the mice generated IgG against MSP-119, and there were no significant differences between the two test groups at the titers of IgG. In general, the titers were between 5×102 and 3×103, lower than the titers reported by Robinson et al. On the other hand, Lee et al[23] measured the antigen-specific IgG titers in monkeys immunized with recombinant L. lactis bacterium expressing H pylori UreB gene, and titers as high as 1×105 were detected. However, despite the presence of high-titer antigen-specific IgG, all monkeys were infected after H pylori challenge, and there were no differences in the density of colonization. Taking our results obtained from low-dose test group of C57BL/6, we suggest that high-titer antigen-specific IgG should not be considered as the major indicator of the protective immune response. The role of cell-mediated immunity played in live bacteria vaccine should be focused.
ACKNOWLEDGMENTS
The authors thank Professor Weibin Guan of Second Military Medical University for his advices and kind instruction on P. yoelii culture. The authors are also grateful to Dr WeiQing Pan of Second Military Medical University for providing monoclonal antibody.
Footnotes
Supported by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), No. 980198
Science Editor Wang XL and Li WZ Language Editor Elsevier HK
References
- 1.Cooper JA. Merozoite surface antigen-I of plasmodium. Parasitol Today. 1993;9:50–54. doi: 10.1016/0169-4758(93)90031-a. [DOI] [PubMed] [Google Scholar]
- 2.Holder AA, Blackman MJ. What is the function of MSP-I on the malaria merozoite? Parasitol Today. 1994;10:182–184. doi: 10.1016/0169-4758(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 3.Holder AA, Freeman RR. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981;294:361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui WA, Tam LQ, Kramer KJ, Hui GS, Case SE, Yamaga KM, Chang SP, Chan EB, Kan SC. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1987;84:3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian JH, Kumar S, Kaslow DC, Miller LH. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect Immun. 1997;65:3032–3036. doi: 10.1128/iai.65.8.3032-3036.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holder AA, Sandhu JS, Hillman Y, Davey LS, Nicholls SC, Cooper H, Lockyer MJ. Processing of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Parasitology. 1987;94(Pt2):199–208. doi: 10.1017/s0031182000053889. [DOI] [PubMed] [Google Scholar]
- 7.Blackman MJ, Ling IT, Nicholls SC, Holder AA. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991;49:29–33. doi: 10.1016/0166-6851(91)90127-r. [DOI] [PubMed] [Google Scholar]
- 8.Blackman MJ, Heidrich HG, Donachie S, McBride JS, Holder AA. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller LH, Roberts T, Shahabuddin M, McCutchan TF. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1) Mol Biochem Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 10.Kang Y, Long CA. Sequence heterogeneity of the C-terminal, Cys-rich region of the merozoite surface protein-1 (MSP-1) in field samples of Plasmodium falciparum. Mol Biochem Parasitol. 1995;73:103–110. doi: 10.1016/0166-6851(95)00102-7. [DOI] [PubMed] [Google Scholar]
- 11.Rénia L, Ling IT, Marussig M, Miltgen F, Holder AA, Mazier D. Immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect Immun. 1997;65:4419–4423. doi: 10.1128/iai.65.11.4419-4423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto S, Yukitake H, Kanbara H, Yamada T. Recombinant Mycobacterium bovis bacillus Calmette-Guerin secreting merozoite surface protein 1 (MSP1) induces protection against rodent malaria parasite infection depending on MSP1-stimulated interferon gamma and parasite-specific antibodies. J Exp Med. 1998;188:845–854. doi: 10.1084/jem.188.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian JH, Kumar S, Kaslow DC, Miller LH. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect Immun. 1997;65:3032–3036. doi: 10.1128/iai.65.8.3032-3036.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perera KL, Handunnetti SM, Holm I, Longacre S, Mendis K. Baculovirus merozoite surface protein 1 C-terminal recombinant antigens are highly protective in a natural primate model for human Plasmodium vivax malaria. Infect Immun. 1998;66:1500–1506. doi: 10.1128/iai.66.4.1500-1506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvo PA, Daly TM, Long CA. Plasmodium yoelii: the role of the individual epidermal growth factor-like domains of the merozoite surface protein-1 in protection from malaria. Exp Parasitol. 1996;82:54–64. doi: 10.1006/expr.1996.0007. [DOI] [PubMed] [Google Scholar]
- 16.Daly TM, Long CA. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J Immunol. 1995;155:236–243. [PubMed] [Google Scholar]
- 17.Ling IT, Ogun SA, Holder AA. The combined epidermal growth factor-like modules of Plasmodium yoelii Merozoite Surface Protein-1 are required for a protective immune response to the parasite. Parasite Immunol. 1995;17:425–433. doi: 10.1111/j.1365-3024.1995.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 18.Hackett J. Use of Salmonella for heterologous gene expression and vaccine delivery systems. Curr Opin Biotechnol. 1993;4:611–615. doi: 10.1016/0958-1669(93)90085-b. [DOI] [PubMed] [Google Scholar]
- 19.Holmgren J, Czerkinsky C. Cholera as a model for research on mucosal immunity and development of oral vaccines. Curr Opin Immunol. 1992;4:387–391. doi: 10.1016/s0952-7915(06)80027-0. [DOI] [PubMed] [Google Scholar]
- 20.Mercenier A, Müller-Alouf H, Grangette C. Lactic acid bacteria as live vaccines. Curr Issues Mol Biol. 2000;2:17–25. [PubMed] [Google Scholar]
- 21.Robinson K, Chamberlain LM, Schofield KM, Wells JM, Le Page RW. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol. 1997;15:653–657. doi: 10.1038/nbt0797-653. [DOI] [PubMed] [Google Scholar]
- 22.de Vos WM. Gene expression systems for lactic acid bacteria. Curr Opin Microbiol. 1999;2:289–295. doi: 10.1016/S1369-5274(99)80050-2. [DOI] [PubMed] [Google Scholar]
- 23.Lee MH, Roussel Y, Wilks M, Tabaqchali S. Expression of Helicobacter pylori urease subunit B gene in Lactococcus lactis MG1363 and its use as a vaccine delivery system against H. pylori infection in mice. Vaccine. 2001;19:3927–3935. doi: 10.1016/s0264-410x(01)00119-0. [DOI] [PubMed] [Google Scholar]
- 24.O'Sullivan DJ, Klaenhammer TR. High- and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene. 1993;137:227–231. doi: 10.1016/0378-1119(93)90011-q. [DOI] [PubMed] [Google Scholar]
- 25.Posno M, Leer RJ, van Luijk N, van Giezen MJ, Heuvelmans PT, Lokman BC, Pouwels PH. Incompatibility of Lactobacillus Vectors with Replicons Derived from Small Cryptic Lactobacillus Plasmids and Segregational Instability of the Introduced Vectors. Appl Environ Microbiol. 1991;57:1822–1828. doi: 10.1128/aem.57.6.1822-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Lab press 198827. Savijoki K, Kahala M, Palva A. High level heterologous protein production in Lactococcus and Lactobacillus using a new secretion system based on the Lactobacillus brevis S-layer signals. Gene. 1997;186:255–262. doi: 10.1016/s0378-1119(96)00717-2. [DOI] [PubMed] [Google Scholar]
- 27.Tian JH, Miller LH, Kaslow DC, Ahlers J, Good MF, Alling DW, Berzofsky JA, Kumar S. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J Immunol. 1996;157:1176–1183. [PubMed] [Google Scholar]


