Abstract
AIM: To evaluate the change of growth characteristics and radiosensitivity of irradiated primary cultured human hepatocarcinoma cells.
METHODS: All tumor tissue samples were obtained from 39 hepatocarcinoma patients with a mean age of 49.6 years (range 22-76 years). We divided the samples into irradiated group and non-irradiated group and measured their plating efficiency (PE), population doubling time (PDT), radiosensitivity index SF2 and cell cycle.
RESULTS: The PDT of primary culture of hepatocarcinoma cells was 91.0±6.6 h, PE was 12.0±1.4%, SF2 was 0.41±0.05%. The PDT of their irradiated progeny was 124.8±5.8 h, PE was 5.0±0.7%, SF2 was 0.65±0.09%. The primary cultured human hepatocarcinoma cells showed significant S reduction and G2 arrest in a dose-dependent manner. The progeny of irradiated primary cultured hepatocarcinoma cells grew more slowly and its radiosensitivity increased.
CONCLUSION: The progeny of irradiated primary cultured human hepatocarcinoma cells grows more slowly and its radiosensitivity increases.
Keywords: Hepatocarcinoma, Cell cycle, Population doubling time, Radiosensitivity
INTRODUCTION
Hepatocarcinoma cells have a better reaction to X-rays. The mechanism of radiosensitivity of hepatocarcinoma cells after radiotherapy is not very clear. In this study, we have used primary cultured human hepatocarcinoma cells in vitro to evaluate the change of growth characteristics and radiosensitivity of progeny of irradiated primary hepatocarcinoma cells.
MATERIALS AND METHODS
Specimens
All tumor tissue specimens were obtained from 39 hepatocarcinoma patients with a mean age of 49.6 years (range 22-76 years).
Cell culture
Primary hepatocarcinoma tissue specimens were obtained from hepatocarcinoma patients. The volume of the sample was 1.5 cm3. Cells were grown in Dulbecco’s minimal essential medium (DMEM) containing 25% fetal calf serum and incubated at 37 °C in 50 mL/L CO2. Most cells were anchored after inoculation for 16 h and the cell growth could be seen after inoculation for 48 h. Then the primary cultured human hepatocarcinoma cells entered the experimental growth phase. The proliferation was very productive and ended 7-8 d after incubation. The density of cells was 50-95% in flask with a few fibroblasts. The time was the best opportunity for irradiation in flask.
Irradiation condition
The samples were divided into irradiated group and non-irradiated group. Cells in the experimental growth phase were irradiated with 2-8 Gy of X-ray at a dose rate of 200 cGy/min and divided into five groups (0-8 Gy). The lucite was placed in dishes.
Population doubling time (PDT)
Cells in the experimental growth phase were digested into single-cell suspension by 2.5 g/L trypsin. In vitro transduction was performed by plating 1×105 cells in 6-cm2 flasks. Cells were digested after being irradiated for 48, 72, 96, 120, 144 h and counted. The experiment was repeated thrice. The cell growth curve was drawn and the cell PDT was calculated.
Colony assay
Cells in the experimental growth phase were digested into single-cell suspension by 2.5 g/L trypsin. In vitro transduction was performed by plating 1×105 cells in 3-cm2 flasks. Cells were irradiated with 2-8 Gy. After 12-15 d of plating, the culture was ended. Cells were fixed by formaldehyde and stained with Giemsa. The number of colonies exceeding 50 was defined as positive. The survival fraction rate was the colony rate. Three parallel samples were set in each dosage point and the experiment was repeated thrice. The formulations [SF2 = e-(αD+βD2)] (the secondary equation) and [(S = 1-(1–e-KD)N)] (the multi-target click model) were used to simulate the cell survival curve of hepatocarcinoma cells. The radiosensitivity parameters such as SF2, α, D0, and N were calculated.
Detection of cell cycle
Cells in the experimental growth phase were irradiated with 2-8 Gy (Linear Accelerator Saturn 43 type). Cells after being irradiated were retrieved at five time points (0, 6, 12, 24, 36 h) and centrifuged (800 r/min). Then the cells were washed twice with PBS. The single-cell suspension was fixed in 80% ethanol and then treated with RNase. At last, the cells were resuspended and incubated for 30 min at 4 °C. Cellular fluorescence was measured by FASort flow cytometry (Becton Dickinson). The data were analyzed by CELLQuest software.
Statistical analysis
Using the SPSS 10.0 statistical analysis software, cell growth curve and cell survival curve were simulated by square regression. The cell cycle ratio and radiosensitivity parameters were expressed as mean±SD. Student’s t-test was used to compare the difference between the two groups.
RESULTS
Plating efficiency (PE) and cell population doubling time of irradiated progeny
The plating efficiency (PE) of primary cultured human hepatocarcinoma cells was 12.0±1.4%, the irradiated progeny was 5.0±0.7%, being significantly higher than that of non-irradiated cells (t = 8.547, P<0.01). The cell PDT of progeny of irradiated human hepatocarcinoma cells was longer than that of non-irradiated ones. The cell PDT of primary cultured human hepatocarcinoma cells was 91.0±6.6 h. The PDT difference between irradiated progeny and non-irradiated cells was significant (t = 3.672, P<0.05; Table 1 and Figure 1).
Table 1.
PDT and PE between irradiated and non-irradiated human hepatocarcinoma cells (mean±SD)
| Dose (Gy) | PDT | PE |
| (PDT) (t/h) | (%) | |
| 0 | 91.0±6.6 | 12.0±1.4 |
| 2 | 104.7±2.1 | 10.2±0.6 |
| 4 | 120.4±2.8 | 8.2±0.4 |
| 6 | 133.5±1.4 | 6.0±1.1 |
| 8 | 141.8±5.8a | 5.0±0.7b |
aP<0.05, bP<0.01 vs 0 Gy.
Figure 1.
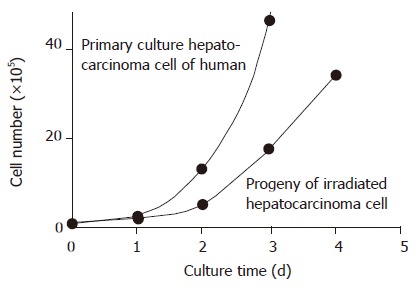
Cell growth curve and irradiated progeny of primary cultured human hepatocarcinoma cells.
Radiosensitivity of irradiated progeny
The survival curve and irradiated progeny of human hepatocarcinoma cells are shown in Figure 2. SF2, α, D0, and N of primary human hepatocarcinoma cells were 0.41±0.05, 0.37/Gy, 0.43 Gy, 2.52, respectively. SF2, α, D0, and N of irradiated progeny were 0.65±0.09, 0.10/Gy, 0.61 Gy, 1.08, respectively (t = 3.863, P<0.05). The difference was significant. Both SF2 and radiosensitivity of hepatocarcinoma cells were increased in human hepatocarcinoma cells and irradiated progeny.
Figure 2.
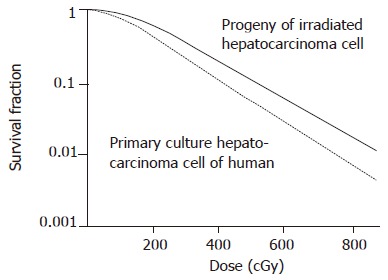
Cell survival curve of primary human hepatocarcinoma cells and irradiated progeny.
Detection of cell cycle
Cells in the experimental growth phase were irradiated with 2-8 Gy (Linear Accelerator Saturn 43 type). The cells decreased in S phase and increased in G2/M phase in a dose-dependent manner (P<0.05). The DNA synthesis time was shorter and mitosis was delayed. The cells after mitosis entering G0/G1 phase decreased in a dose-dependent manner (Table 2).
Table 2.
Cells in S and G2/M phase of irradiated progeny (mean±SD)
| Dose (Gy) | SPF (%) | G2/M (%) |
| 0 | 66.10±0.75 | 0.91±0.19 |
| 2 | 61.52±0.22 | 1.73±0.53 |
| 4 | 50.15±0.68 | 3.32±0.68 |
| 6 | 3.35±0.24 | 4.51±0.92 |
| 8 | 33.32±0.51a | 6.48±0.57a |
P<0.05 vs oGy.
DISCUSSION
The results of radiosensitivity in vitro of carcinoma cells are closely related with the clinical effect of carcinoma radiotherapy. But most studies showed that there have been great differences in radiosensitivity of identical carcinomas[1-2]. Studies indicate that radiation can lead to the instability of cell genes and survived progeny will occur[3]. It was reported that irradiated progeny produces resistance after radiation or DNA damage[4-5].
There is evidence that carcinoma is a cell cycle disease. The malignant level of carcinoma is closely related with cell cycle. The carcinoma cells in different cell cycle phases have different radiosensitivity to chemotherapy drugs. Proliferating cells have a good radiosensitivity. Radiotherapy can change the processes of cell cycle. It displays G0, G2/M, and S arrest. To study the alteration of cell cycle after radiation is of great significance in choosing suitable chemotherapy drugs after radiotherapy.
Our study indicated that the growth characteristics of progeny of human hepatocarcinoma cells were changed after being irradiated with 2-8 Gy. The growth speed was delayed, the PE was decreased and the radiosensitivity was increased. The radiosensitivity of irradiated progeny was related with G2/M arrest and cells in S phase reduced in a dose-dependent manner. The radiosensitivity of cells in S phase was the highest due to decreased progeny of irradiated human hepatocarcinoma cells and PE as well as delayed PDT cell reaction in S phase and G2/M arrest[6].
In conclusion, stereotactic radiotherapy can achieve better results in the treatment of hepatocarcinoma.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Xia YF, Li MZ, Huang B, Chen JJ, Li ZQ, Wang HM, Brook WA. Cellular radiobiological characteristics of human nas- opharyngeal carcinoma cell lines. Ai Zheng. 2001;20:683. [Google Scholar]
- 2.Hu B, Zhou XY, Wang X, Zeng ZC, Iliakis G, Wang Y. The radioresistance to killing of A1-5 cells derives from activation of the Chk1 pathway. J Biol Chem. 2001;276:17693–17698. doi: 10.1074/jbc.M009340200. [DOI] [PubMed] [Google Scholar]
- 3.Shi WM, Fan YX, Chen LH. The study of radiosensitivity of two human being hepatocacinoma cell lines in vitro. Zhongguo Xiandai Yixue. 2001;11:6–7. [Google Scholar]
- 4.Pitot HC, Hikita H, Dragan Y, Sargent L, Haas M. Review article: the stages of gastrointestinal carcinogenesis--application of rodent models to human disease. Aliment Pharmacol Ther. 2000;14 Suppl 1:153–160. doi: 10.1046/j.1365-2036.2000.014s1153.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XW, Zhang LZ, Zhou XM, Wu SY, Wang YP, Zhang W. The relationship between hydatidiform mole canceration and Y chromatosome. Beijing Yixue. 2002;24:82–83. [Google Scholar]
- 6.Ku JL, Yoon KA, Kim IJ, Kim WH, Jang JY, Suh KS, Kim SW, Park YH, Hwang JH, Yoon YB, et al. Establishment and characterisation of six human biliary tract cancer cell lines. Br J Cancer. 2002;87:187–193. doi: 10.1038/sj.bjc.6600440. [DOI] [PMC free article] [PubMed] [Google Scholar]


