Abstract
AIM: To study the effects of perioperative administration of cimetidine (CIM) on peripheral blood lymphocytes, natural killer (NK) cells and tumor infiltrating lymphocytes (TIL) in patients with gastrointestinal (GI) cancer.
METHODS: Forty-nine GI cancer patients were randomized into treatment group, who took CIM in perioperative period, and control group, who did not take the drug. The treatment was initiated 7 days before operation and continued for 10 days after surgery. At baseline examination before operation, on the 2nd and 10th postoperative days, total T lymphocytes, T helper cells, T suppressor cells, and NK cells in peripheral blood were measured respectively by immunocytochemical method using mouse-anti human CD3, CD4, CD8 and CD57 monoclonal antibodies. Blood samples from 20 healthy volunteers were treated in the same way as normal controls. Surgical specimens were examined during routine histopathological evaluation for the presence of TIL in tumor margin. Immunohistochemical study was performed to measure the proportion of T and B lymphocytes in TIL population. T and B lymphocytes were detected respectively using mouse-anti-human CD3 and CD20 monoclonal antibodies.
RESULTS: In comparison with normal controls, both the treatment and control groups had decreased T cells, T helper cells and NK cells at baseline. In control group, total T cells, T helper cells and NK cells declined continuously with the disease progression and the decrease became more obvious after operation. From baseline to the 2nd postoperative day, the proportion of total T cells, T helper cells, and NK cells went down from 60.5 ± 4.6% to 56.2 ± 3.8%, 33.4 ± 3.7% to 28.1 ± 3.4%, and 15.0 ± 2.8% to 14.2 ± 2.2%, respectively. On the other hand, there were significant improvements in these parameters after CIM treatment. On the 10th postoperative day, the treatment group had significantly higher percentages of total T cells, T helper cells and NK cells than control group. Moreover, CIM treatment also boosted TIL response, as was reflected by findings that 68%(17/25) of the patients in treatment group had significant TIL responses and only 25% (6/24) of the cases had discernible TIL responses (P < 0.01).
CONCLUSION: Perioperative application of CIM to GI cancer patients could help restore the diminished cellular immunity induced by tumor burden and surgical maneuver. The drug could also boost TIL responses to tumor. These effects suggest that the drug be used as an immunomodulator for GI cancer patients.
INTRODUCTION
Gastric and colorectal cancers are the most common cancers in China, with their incidence ranking number one and number four respectively. Although surgery, chemotherapy and radiotherapy are major treatment options for GI cancer, the long-term survival is low. Treatment failure is mainly due to recurrence and metastasis. One major cause for such an adverse outcome is the patients’ diminished immunity against residual tumor cells after surgery. Therefore, how to restore and improve the patients’ immunity against cancer has been an area of active study.
There has been much progress in understanding the relationship between the immune system and GI cancer, which has led to the use of immunomodulatory therapy as an adjuvant and palliative treatment. Many non-specific immunomodulatory agents such as levamisole, CIM, alpha interferon, n-3 fatty acids, polysaccharide K (PSK), supplementary diet with glutamine, arginine and omega-3-fatty acids, and Bacillus Calmette-Guerin (BCG) have been tried[1-4]. CIM is a type 2 histamine receptor antagonist widely used for the treatment of peptic ulcers. It also has important effects on immune system. Administration of CIM has been found to preserve, to some degree, the patients’ perioperative immunity[5,6], to improve the survival of patients with colorectal cancer, melanoma, and renal cell cancer[7-15]. Although it is not clear whether this effect of CIM on cancer is direct or indirect, it has been proposed that CIM may act by enhancing the host immune response to tumor cells[16,17] or by blocking the cell growth-promoting activity of histamine in cancer cell lines[14,16-20].
In this study, we used CIM in the perioperative period as an adjuvant immunomodulatory agent, and studied its effects on peripheral blood lymphocytes, NK cells and TIL in a randomized controlled clinical trial in patients with GI cancer.
MATERIALS AND METHODS
Study design
This was a prospective, randomized clinical trial. The subjects included in this study were selected from patients with pathologically confirmed GI cancer who were admitted to the Department of Oncology, Zhongnan Hospital of Wuhan University, from Sept.1997 to May 1998. The entry criteria were: primary GI cancers indicative of surgery, no preoperative evidence of distant metastasis, no history of previous immunity-impairing chronic diseases such as diabetes mellitus, and no history of preoperative chemotherapy, radiotherapy or immunotherapy. From a total of 125 patients admitted during this period, 49 eligible patients were recruited and staged according to the International Union against Cancer Classification. After signing informed consent forms, the patients were randomized into treatment group (n = 25) and control group (n = 24). The clinico-pathological characteristics of the two groups were comparable and balanced. The patients in treatment group started oral CIM treatment (Tagamet, Tianjin Smith Kline and French Laboratories Ltd.) at the dose of 400 mg, tid, 7 days before operation until the operation day. During and after operation, they were given CIM at 600 mg, i.v. drip, bid, until the 10th postoperative day. The patients in control group received similar routine treatment except for perioperative CIM intervention. All the patients in both groups underwent curative resection of cancer.
Separation of peripheral blood mononuclear cells (PBMCs) and immunocytochemical staining
From all the patients in both groups, 2 mL of venous blood was taken and heparinized at admission, before operation, on the 2nd and the 10th postoperative days, respectively. PBMCs were obtained immediately by standard Ficoll-Hypague gradient centrifugation at 2 000 rpm for 20 min at 4 °Cand smeared onto slides, dried and fixed for immunocytochemical staining. The primary antibodies were mouse-anti-human CD3, CD4, CD8 and CD57 monoclonal antibodies (Sigma Chemical Company, St Louis, MO, USA) for the detection of total T lymphocytes, helper T lymphocytes, inhibitor T lymphocytes and natural killer (NK) cells, respectively. The primary antibody was visualized with avidin-biotin-peroxidase supersensitive kit (Wuhan Boster Bioengineering Co. Ltd., Wuhan, China). The slides were counterstained with methyl green. PBMCs from 20 healthy controls were processed in the same way as normal controls.
The slides were mounted and viewed under binocular microscope (Olympus, Japan) by an independent viewer. Positive cells were stained green in nuclei and yellow-brown in cytoplasm and cell membrane (Figure 1). A total of 200 cells were counted on each slide and positive cells were recorded. The immunocytochemical staining procedure was repeated 3 times and the percentage of each cell subpopulation was calculated and expressed as mean ± standard deviation ((-χ ± s).
Figure 1.
Immunocytochemical staining of PBMCs with monoclonal antibodies to CD3, CD4, CD8 and CD57. Positive cells were stained green in the nuclei and yellow-brown in cytoplasm and cell membrane. The microphoto showed CD3 positive cells.
Immunohistochemical study on TIL in surgical specimens
Immediately after resection, the specimens were cut open and washed clean. For each patient, three pieces of tumor samples were taken at different sites from the peripheral margin of the tumor, fixed in 10% neutral formalin and processed in standard histopathology procedure. For observation of TIL responses, conventional HE staining was performed on the 4 μm thick tissue sections. Immunohistochemical staining on the sections was conducted for subpopulation study of TIL, with anti-CD3 monoclonal antibody to recognize T lymphocytes and anti-CD20 monoclonal antibody to recognize B lymphocytes (both from Sigma). The immunohistochemical staining procedure followed a standard protocol. The sections were counterstained by hemotoxylin, mounted and interpreted under microscope. The number of TIL was recorded in five high power (HP, 200 × ) view fields randomly chosen at the tumor border. The degree of TIL response was determined based on a modified grading system by Jass[21]. Grade I ( ± ): no TIL response, in which there were less than 10 infiltrating lymphocytes per HP view field; grade II (+): mild TIL response, in which there were 10-100 infiltrating lymphocytes per HP view field; grade III (++): intermediate TIL response, in which there were 101-200 infiltrating lymphocytes per HP view field; and grade IV (+++): prominent TIL response in which there were over 201 infiltrating lymphocytes per HP view field. Grades I and II TIL responses were defined as poor responses and grades III and IV lymphocyte responses as significant responses.
Statistical analysis
All data were expressed as mean ± standard deviation (-χ ± s). Analysis of variance (ANOVA) was used to process the data within groups. Student’s t test and chi-square test were used to evaluate the differences between groups. For the comparison in TIL response among different tumor TNM stages, Fisher’s exact test was used. All the tests were two-tailed with a level of significance P = 0.05.
RESULTS
Clinico-pathological characteristics of patients
A total of 49 eligible patients were enrolled in this study, 25 of whom were randomized into treatment group and 24 into control group. Their clinico-pathological characteristics are detailed in Table 1. There were no statistically significant differences in demographic and histopathologic variables between the two groups (P > 0.05) (Table 1).
Table 1.
Clinico-pathological characteristics of 49 GI cancer patients
| Parameters | Treatment group (n = 25) | Control group (n = 24) |
| Age (y) | ||
| Mean (range) | 50 (25-73) | 53 (27-78) |
| Gender | ||
| Male | 13 | 16 |
| Female | 12 | 8 |
| Tumor sites | ||
| Stomach | 6 | 5 |
| Colon | 3 | 3 |
| Rectum | 16 | 16 |
| Pathological types | ||
| Tubular adenocarcinoma | 14 | 12 |
| Papillary adenocarcinoma | 3 | 3 |
| Villous adenocarcinoma | 2 | 1 |
| Signet-ring-cell carcinoma | 2 | 3 |
| Mucous adenocarcinoma | 4 | 5 |
| TNM stages | ||
| I | 3 | 5 |
| II | 7 | 9 |
| III | 9 | 6 |
| IV | 6 | 4 |
| Tumor differentiation | ||
| Well differentiated | 5 | 6 |
| Moderately differentiated | 8 | 7 |
| Poorly differentiated | 12 | 11 |
All the variables showed no statistically significant differences between the two groups (P > 0.05).
Percentages of lymphocyte subpopulations at baseline
At randomization, the percentages of CD3+, CD4+, CD57+ cells and the CD4+/CD8+ ratio in both treatment and control groups were lower than those in normal control (P < 0.001), and the percentage of CD8+ cells was higher in treatment and control groups than in normal control (P < 0.05) (Table 2). There were no statistically significant differences between the treatment and control groups in the percentages of the above parameters, implying that the patients were well balanced with regard to peripheral lymphocyte subpopulations at randomization. The results indicated that the cellular immunity was significantly decreased in patients with GI cancer in this study population.
Table 2.
Baseline values of lymphocyte subpopulations in pe-ripheral blood mononuclear cells of normal control, treatment and control groups (%, (-χ ± s)
| Groups | n | CD3+ | CD4+ | CD8+ | CD57+ | CD4+/CD8+ |
| Normal | 20 | 67.1 ± 6.3 | 40.2 ± 5.1 | 27.7 ± 5.0 | 18.5 ± 2.31 | 1.49/0.24 |
| control | ||||||
| Treatment | 25 | 60.8 ± 6.3b | 33.6 ± 4.2b | 30.6 ± 5.2 | 14.8 ± 4.4a | 1.15 ± 0.34b |
| Control | 24 | 60.5 ± 4.6b | 33.4 ± 3.7b | 31.0 ± 3.9a | 15.0 ± 2.8a | 1.11 ± 0.25b |
aP < 0.05 vs normal control, bP < 0.01 vs normal control
Changes in lymphocyte subpopulations in PBMCs during CIM treatment
During the perioperative period, dynamic changes in the percentages of peripheral blood lymphocyte subpopulations were observed in both treatment and control groups. Preoperative treatment with CIM for 1 wk had positive effects on the percentages of CD3+, CD4+ lymphocytes, and CD4+/CD8+ ratio. CD3+ cells were increased from 60.8 ± 6.3% at randomization to 63.0 ± 4.9% after CIM treatment for 1 wk. After operation, CD3+ cells were decreased to 60.3 ± 5.4% on the 2nd postoperative day, and recovered gradually thereafter until it reached 64.2 ± 3.9% on the 10th postoperative day, which was higher than the pretreatment level. In contrast, the percentage of CD3+ cells in control group continued declining during the perioperative period, and became significantly lower than that in the treatment group on both the 2nd and 10th postoperative days. The changes in CD4+ and CD57+ cells followed a similar pattern (Table 3, Figure 2, Figure 3, Figure 4).
Table 3.
Changes of lymphocytes and NK cells in perioperative period (%, -χ ± s)
| Items Groups |
Perioperative period |
||||
| A | B | C | D | ||
| CD3 | Treatment | 60.8 ± 6.3 | 63.0 ± 4.9d | 60.3 ± 5.4a | 64.2 ± 3.9d |
| Control | 60.5 ± 4.6 | 61.0 ± 2.7 | 56.2 ± 3.8bd | 58.6 ± 4.0ab | |
| CD4 | Treatment | 33.6 ± 4.2 | 36.3 ± 3.4a | 32.8 ± 4.0d | 36.6 ± 6.2d |
| Control | 33.4 ± 3.7 | 32.8 ± 3.3b | 28.1 ± 3.4bd | 30.4 ± 3.3ab | |
| CD8 | Treatment | 30.6 ± 5.2 | 29.6 ± 4.3 | 31.1 ± 4.3 | 29.4 ± 3.6 |
| Control | 31.0 ± 3.9 | 31.2 ± 4.8 | 32.9 ± 4.4a | 32.1 ± 5.3 | |
| CD57+ | Treatment | 14.8 ± 4.4 | 15.7 ± 3.8 | 17.2 ± 3.7 | 21.1 ± 4.5b |
| Control | 15.0 ± 2.8 | 13.1 ± 2.5 | 14.2 ± 2.2b | 15.6 ± 1.7b | |
| CD4 | Treatment | 1.15 ± 0.34 | 1.25 ± 0.23a | 1.08 ± 0.21d | 1.27 ± 0.30d |
| /CD8 | Control | 1.11 ± 0.25 | 1.08 ± 0.22b | 0.87 ± 0.17bd | 0.98 ± 0.24ab |
A: at admission, B: before operation, C: on the 2nd postopera-tive day, D: on the 10th postoperative day.
P < 0.05,
P < 0.01, B vs A, C vs B, D vs C;
P < 0.01 vs treatment group.
Figure 2.
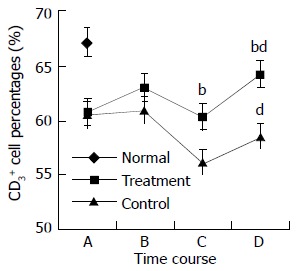
Changes in CD3+ cell percentages in perioperative period. A: at randomization; B: before operation; C: on the 2nd postoperative day; and D: on the 10th postoperative day. The difference in CD3+ percentages between treatment and control groups at time points C and D was statistically significant, bP < 0.01. The difference in CD3+ percentages on the 10th postoperative day and the randomization day was statistically significant for both treatment and control groups, dP < 0.01.
Figure 3.
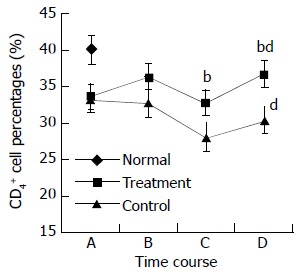
Changes in CD4+ cell percentages in perioperative period. A: at randomization; B: before operation; C: on the 2nd postoperative day; and D: on the 10th postoperative day. The difference in CD4+ percentages between treatment and control groups at time points C and D was statistically significant, bP < 0.01. The differences in CD4+ percentages on the 10th postoperative day and the randomization day was statistically significant for both treatment and control groups, dP < 0.01.
Figure 4.
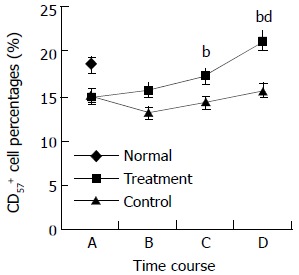
Changes in CD57+ cell percentages in perioperative period. A: at randomization; B: before operation; C: on the 2nd postoperative day; and D: on the 10th postoperative day. The difference in CD57+ percentages between treatment and con-trol groups at time points C and D was statistically significant, bP < 0.01. The differences in CD57+ percentages between treat-ment group and normal control on the 10th postoperative day was statistically significant, dP < 0.01.
Effects of CIM treatment on TIL
In addition to routine histopathological examinations of resected specimens, all the tumor sections were reviewed for the presence of peritumor lymphocytes and TIL responses. Six out of 24 patients (25%) in control group had discernible lymphocyte infiltration in the peritumor area, whereas 17 out of 25 cases (68%) in CIM treatment group had obvious TIL responses (P < 0.01) (Table 4).
Table 4.
TIL responses in treatment and control groups
| Groups |
SR |
PR |
Total | Rate (%) | ||
| +++ | ++ | + | + | |||
| Treatment | 9 | 8 | 6 | 2 | 25 | 68b |
| Control | 2 | 4 | 9 | 9 | 24 | 25 |
| Total | 23 | 26 | 49 | |||
SR = significant response, PR = poor response.
P < 0.01 vs control group, chi-square test.
There was a negative correlation between TIL response and the clinico-pathological stages of the tumor (Table 5). In control group, TIL responses were mainly observed in TNM stages I and II cases, and there were very few peritumor lymphocyte responses in stages III and IV cases. In contrast, there were obvious TIL responses in 78% (7/9) of TNM stage III cases and 83% (5/6) of stage IV cases in CIM treatment group, both of which were significantly higher than those in control group (P < 0.05 and P < 0.01, respectively, Fisher’s exact test) (Figure 4). Immunohistochemical studies of the specimens revealed that most of the TILs were CD3+ T lymphocytes clustered around tumor tissues. There were few CD20+ cells in TIL population.(Figure 5)
Table 5.
Comparison in TIL responses by TNM stages between control and treatment groups
| TNM stages | Groups | Poor response | Significant response | Response rate (%) |
| I | Treatment | 2 | 1 | 33 |
| Control | 3 | 2 | 40 | |
| II | Treatment | 3 | 4 | 57 |
| Control | 6 | 3 | 33 | |
| III | Treatment | 2 | 7 | 78a |
| Control | 5 | 1 | 17 | |
| IV | Treatment | 1 | 5 | 83b |
| Control | 4 | 0 | 0 |
P < 0.05,
P < 0.01, vs control (Fisher’s exact test).
Figure 5.

TIL responses in surgical specimens in treatment and control groups. In control group, there was a progressive de-crease in TIL responses with increase of TNM staging. On the other hand, the treatment group showed a steady increase in TIL responses after CIM treatment for 1 wk. The differences in TIL response rates were statistically significant between treat-ment and control groups for TNM stages III and IV diseases (aP < 0.05 and bP < 0.01, respectively, Fisher’s exact test).
DISCUSSION
To date, surgical resection remains the only approach that can offer possible cure for GI cancer patients. However, operation itself is a double-edged sword to cancer patients in terms of tumor immunology. On the one hand, a successful operation can remove tumor burden which is immuno-suppressive. This will help bring about an improved clinical outcome for most patients. On the other hand, the operation itself is a major blow to the immune system. Lines of evidence suggest that surgical patients undergo a period of immunodepression immediately after operation, the length of which depends on many factors such as the general status of the patients, extent of the operation itself, pre-operative treatment. Previous studies demonstrated that T helper cells decreased and T suppressor cells increased significantly as early as 1 day after surgery[22-24]. Many subsequent studies also confirmed that surgery for patients with lung cancer[25], esophageal cancer[26], gastric cancer[27,28] and colorectal cancer[29,30] induced immediate severe immunosuppression. For cancer patients, an important function adversely affected by this immunosuppression is the anti-tumor response itself. This immunosuppression might increase the chance of accelerated growth of residual tumors or micro-metastases already present at the time of surgical resection. As a result, postoperative immunosuppression maybe one of the major contributing factors for post-operative recurrence and metastases. Indeed our previous study found a local recurrent rate as high as 34.27% for rectal cancer 5 years after curative resection, most of which (89.04%) occurred 3 years after operation[31]. Other studies also revealed that 7% to 65% of rectal cancer patients would develop local recurrence after curative surgery[32-34]. Therefore, how to effectively improve peri-operative immunity of GI cancer patients remains a major challenge of significant clinical importance.
Many researches have been conducted to tackle this problem, and one approach is to use small molecular immunomodulator drugs such as CIM, a histamine H2 receptor antagonist. It has long been observed that histamine was a growth factor for certain cancers and could, by itself, stimulate tumor cell proliferation[18-20]. As one of the many important chemo-mediators involved in immune responses, histamine had inhibitory effects on immune response[35-38] via its H2 receptors[39]. It was discovered that T suppressor cells, which are part of the regulatory arm of the immune system, could express histamine receptors on their surface[40-42], and histamine was capable of suppressing the immune response by activating these T suppressor cells[43]. Many tumors, particularly colorectal cancer, secrete histamine resulting in elevated histamine levels within the tumor. Histamine is also often secreted in response to surgical resection of colorectal cancers. All these factors working together will create an immuno-suppressive environment both in the area of tumor growth and in the whole body, facilitating tumor growth.
Several clinical studies have shown that administration of CIM could help reduce the immuno-suppression due to increased histamine level in the tumor environment[15,16,44-46]. In vitro studies also demonstrated that CIM could inhibit the adhesion of some breast cancer cells[47], and the adhesion of human colon cancer cells to human umbilical cord cells[48], a very important step in tumor growth and progression. In vivo experimental studies also showed that daily administration of CIM to a mouse model of colon cancer significantly retarded tumor growth by up-regulating the expression of tumor-suppressive cytokines[49], and the use of CIM also retarded the growth of human melanoma in a nude mouse model and prolonged the survival of tumor bearing SCID mice by directly inhibiting the proliferation of tumor cells and indirectly promoting the infiltration of activated macrophages to tumor site[50,51].
In line with previous findings, our study discovered the diminished cellular immunity in GI cancer patients. Compared with normal controls, the total T lymphocytes (CD3+), T helper lymphocytes (CD4+), and NK cells (CD57+) were significantly decreased in GI cancer patients. This reflects the nature that GI tumor burden exerts inhibitory effects on immune system. Moreover, the percentages of these immune cells showed a continuously declining trend with the progression of the disease, as reflected by the finding that patients with advanced TNM stage tumors had a more profound decrease in these immune cells than those with early stage tumors. Surgery itself, while removing the tumor burden, did have a temporary negative impact on host cellular immunity, as reflected by the marked decline in total T lymphocytes, T helper cells and NK cells in the immediate post-operative period. This decline continued with a downhill course in control group, in which no immunity-boosting therapies were given besides conventional surgical treatment. It is noteworthy that total T cells, T helper cells and NK cells did not return to baseline level 10 days after curative resection. This again highlighted the fact that while most patients in control group were physically recovered from operation at this time, they were far from immunologically recovered, and some interventional measures should be warranted to facilitate the recovery of cellular immune functions.
In contrast to control group, patients in treatment group showed favorable responses to CIM therapy in terms of cellular immunity parameters. After CIM treatment for 1 wk, total T cells, T helper cells and NK cells showed a slow but steady increase, although the increments did not reach statistical significance for NK cells, they did reach statistical significance for both total T cells and T helper cells. However, their levels were still lower than those found in normal controls, implying that while GI cancer patients have reduced their cellular immunity, they were nevertheless responsive to immunity-boosting measures, although such measures alone could not efficiently restore the hosts’ cellular immunity to the level of normal controls. The data demonstrated again that surgery itself reduced cellular immunity, but to a much less degree, as a result of CIM treatment. Moreover, such reductions were quickly addressed and a positive balance was reached 10 days after CIM treatment, as reflected by the fact that the total T cells, T helper cells and NK cells on the 10th postoperative day were significantly higher than the baseline level (at randomization). Although total T cells, T helper cells were not up to the level of normal controls, NK cells did exceed the level of normal controls. If the cellular immunity parameters for both treatment and control groups on the 10th postoperative day were analyzed, it would be obvious that CIM treatment did exert remarkable boosting effects on cellular immunity parameters.
The current study also demonstrated that immunity-enhancing effects of CIM treatment were not limited to peripheral blood immune cells alone, it also enhanced TIL response at the tumor site, as was revealed by the findings that while 25% of the patients in the control group had discernible TIL responses in the peri-tumor area, as high as 68% of cases in the treatment group showed obvious TIL responses. As demonstrated by immunohistochemical studies, most of the TILs were T lymphocytes. TILs have been found to be the highly effective tumoricidal lymphocytes[52-54], and TIL treatment could decrease the relapse rate and prolong the survival of stage III melanoma patients with one positive lymph node[55,56], and the overall survival of stage IV gastric and colorectal cancers[57,58], and induce regression of metastatic tumors in the lung, liver and lymph nodes in patients with advanced melanoma after lymphodepletion[59]. The enhanced TIL response at the tumor site induced by CIM treatment, therefore, might help reduce tumor aggressiveness and promote local control.
In summary, the current randomized clinical trial demonstrated that perioperative administration of CIM to GI cancer patients helped accelerate the recovery of cellular immunity and boost TIL responses at the tumor sites. This has potential therapeutic implications. Since circulating T lymphocytes and NK cells are major defense mechanisms against tumor cells released into the circulation, and TIL is one of the most crucial factors restricting local tumor growth and progression, it is desirable to use it as an immunomodulator in the perioperative period. Moreover, since CIM has many other favorable effects besides immunomodulation on lymphocytes, such as activating macrophages, increasing tumor inhibitory cytokines[49], enhancing the antigen presenting capacity of dendritic cells[60], reducing tumor cell proliferation, inhibiting tumor cell metastasis via anti-adhesion mechanisms[49] and increasing the overall survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis-A epitope expression on tumor cells[61], it is therefore advisable to use this low cost, convenient and almost nontoxic drug as a practical immunity-enhancing measure for GI cancer patients.
Footnotes
Co-correspondents: Cong-Yao Lin and Yan Li a
Supported by the Science Progress Project of Hubei Province, No. 2001AA301C35
Edited by Zhu LH and Wang XL
References
- 1.Yip D, Strickland AH, Karapetis CS, Hawkins CA, Harper PG. Immunomodulation therapy in colorectal carcinoma. Cancer Treat Rev. 2000;26:169–190. doi: 10.1053/ctrv.1999.0160. [DOI] [PubMed] [Google Scholar]
- 2.Weiss G, Meyer F, Matthies B, Pross M, Koenig W, Lippert H. Immunomodulation by perioperative administration of n-3 fatty acids. Br J Nutr. 2002;87 Suppl 1:S89–S94. doi: 10.1079/bjn2001461. [DOI] [PubMed] [Google Scholar]
- 3.Shibata M, Nezu T, Kanou H, Nagata Y, Kimura T, Takekawa M, Ando K, Fukuzawa M. Immunomodulatory effects of low dose cis-Diaminedichloroplatinum (cisplatin) combined with UFT and PSK in patients with advanced colorectal cancer. Cancer Invest. 2002;20:166–173 doi: 10.1081/CNV-120001142. doi: 10.1081/cnv-120001142. [DOI] [PubMed] [Google Scholar]
- 4.Wu GH, Zhang YW, Wu ZH. Modulation of postoperative immune and inflammatory response by immune-enhancing enteral diet in gastrointestinal cancer patients. World J Gastroenterol. 2001;7:357–362. doi: 10.3748/wjg.v7.i3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tayama E, Hayashida N, Fukunaga S, Tayama K, Takaseya T, Hiratsuka R, Aoyagi S. High-dose cimetidine reduces proinflammatory reaction after cardiac surgery with cardiopulmonary bypass. Ann Thorac Surg. 2001;72:1945–1949. doi: 10.1016/s0003-4975(01)03225-8. [DOI] [PubMed] [Google Scholar]
- 6.Bai DJ, Yang GL, Yuan HY, Li Y, Wang K. Effects of cimetidine on T lymphocyte subsets in perioperative gastrointestinal cancer patients. Shijie Huaren Xiaohua Zazhi. 2000;8:147–149. [Google Scholar]
- 7.Adams WJ, Morris DL. Short-course cimetidine and survival with colorectal cancer. Lancet. 1994;344:1768–1769. [PubMed] [Google Scholar]
- 8.Matsumoto S. Cimetidine and survival with colorectal cancer. Lancet. 1995;346:115. doi: 10.1016/s0140-6736(95)92136-2. [DOI] [PubMed] [Google Scholar]
- 9.Svendsen LB, Ross C, Knigge U, Frederiksen HJ, Graversen P, Kjaergård J, Luke M, Stimpel H, Sparsø BH. Cimetidine as an adjuvant treatment in colorectal cancer. A double-blind, randomized pilot study. Dis Colon Rectum. 1995;38:514–518. doi: 10.1007/BF02148852. [DOI] [PubMed] [Google Scholar]
- 10.Hellstrand K, Naredi P, Lindner P, Lundholm K, Rudenstam CM, Hermodsson S, Asztély M, Hafström L. Histamine in immunotherapy of advanced melanoma: a pilot study. Cancer Immunol Immunother. 1994;39:416–419. doi: 10.1007/BF01534430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creagan ET, Ahmann DL, Green SJ, Long HJ, Frytak S, Itri LM. Phase II study of recombinant leukocyte A interferon (IFN-rA) plus cimetidine in disseminated malignant melanoma. J Clin Oncol. 1985;3:977–981. doi: 10.1200/JCO.1985.3.7.977. [DOI] [PubMed] [Google Scholar]
- 12.Sagaster P, Micksche M, Flamm J, Ludwig H. Randomised study using IFN-alpha versus IFN-alpha plus coumarin and cimetidine for treatment of advanced renal cell cancer. Ann Oncol. 1995;6:999–1003. doi: 10.1093/oxfordjournals.annonc.a059097. [DOI] [PubMed] [Google Scholar]
- 13.Morris DL, Adams WJ. Cimetidine and colorectal cancer--old drug, new use. Nat Med. 1995;1:1243–1244. doi: 10.1038/nm1295-1243. [DOI] [PubMed] [Google Scholar]
- 14.Sasson AR, Gamagami R, An Z, Wang X, Moossa AR, Hoffman RM. Cimetidine: an inhibitor or promoter of tumor growth. Int J Cancer. 1999;81:835–838. doi: 10.1002/(sici)1097-0215(19990531)81:5<835::aid-ijc27>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Kelly MD, King J, Cherian M, Dwerryhouse SJ, Finlay IG, Adams WJ, King DW, Lubowski DZ, Morris DL. Randomized trial of preoperative cimetidine in patients with colorectal carcinoma with quantitative assessment of tumor-associated lymphocytes. Cancer. 1999;85:1658–1663. doi: 10.1002/(sici)1097-0142(19990415)85:8<1658::aid-cncr3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Hansbrough JF, Zapata-Sirvent RL, Bender EM. Prevention of alterations in postoperative lymphocyte subpopulations by cimetidine and ibuprofen. Am J Surg. 1986;151:249–255. doi: 10.1016/0002-9610(86)90080-2. [DOI] [PubMed] [Google Scholar]
- 17.Adams WJ, Morris DL, Ross WB, Lubowski DZ, King DW, Peters L. Cimetidine preserves non-specific immune function after colonic resection for cancer. Aust N Z J Surg. 1994;64:847–852. doi: 10.1111/j.1445-2197.1994.tb04562.x. [DOI] [PubMed] [Google Scholar]
- 18.Adams WJ, Lawson JA, Morris DL. Cimetidine inhibits in vivo growth of human colon cancer and reverses histamine stimu-lated in vitro and in vivo growth. Gut. 1994;35:1632–1636. doi: 10.1136/gut.35.11.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson JA, Adams WJ, Morris DL. Ranitidine and cimetidine differ in their in vitro and in vivo effects on human colonic cancer growth. Br J Cancer. 1996;73:872–876. doi: 10.1038/bjc.1996.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds JL, Akhter J, Morris DL. In vitro effect of histamine and histamine H1 and H2 receptor antagonists on cellular proliferation of human malignant melanoma cell lines. Melanoma Res. 1996;6:95–99. doi: 10.1097/00008390-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Jass JR, Ajioka Y, Allen JP, Chan YF, Cohen RJ, Nixon JM, Radojkovic M, Restall AP, Stables SR, Zwi LJ. Assessment of invasive growth pattern and lymphocytic infiltration in colorectal cancer. Histopathology. 1996;28:543–548. doi: 10.1046/j.1365-2559.1996.d01-467.x. [DOI] [PubMed] [Google Scholar]
- 22.Hansbrough JF, Bender EM, Zapata-Sirvent R, Anderson J. Altered helper and suppressor lymphocyte populations in surgical patients. A measure of postoperative immunosuppression. Am J Surg. 1984;148:303–307. doi: 10.1016/0002-9610(84)90459-8. [DOI] [PubMed] [Google Scholar]
- 23.Nichols PH, Ramsden CW, Ward U, Sedman PC, Primrose JN. Perioperative immunotherapy with recombinant interleukin 2 in patients undergoing surgery for colorectal cancer. Cancer Res. 1992;52:5765–5769. [PubMed] [Google Scholar]
- 24.Espí A, Arenas J, García-Granero E, Martí E, Lledó S. Relationship of curative surgery on natural killer cell activity in colorectal cancer. Dis Colon Rectum. 1996;39:429–434. doi: 10.1007/BF02054059. [DOI] [PubMed] [Google Scholar]
- 25.Leaver HA, Craig SR, Yap PL, Walker WS. Lymphocyte responses following open and minimally invasive thoracic surgery. Eur J Clin Invest. 2000;30:230–238. doi: 10.1046/j.1365-2362.2000.00622.x. [DOI] [PubMed] [Google Scholar]
- 26.van Sandick JW, Gisbertz SS, ten Berge IJ, Boermeester MA, van der Pouw Kraan TC, Out TA, Obertop H, van Lanschot JJ. Immune responses and prediction of major infection in patients undergoing transhiatal or transthoracic esophagectomy for cancer. Ann Surg. 2003;237:35–43. doi: 10.1097/00000658-200301000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato N, Endo S, Kimura Y, Ikeda K, Aoki K, Iwaya T, Akiyama Y, Noda Y, Saito K. Influence of a human protease inhibitor on surgical stress induced immunosuppression. Dig Surg. 2002;19:300–305. doi: 10.1159/000064578. [DOI] [PubMed] [Google Scholar]
- 28.Yao XX, Yin L, Sun ZC. The expression of hTERT mRNA and cellular immunity in gastric cancer and precancerosis. World J Gastroenterol. 2002;8:586–590. doi: 10.3748/wjg.v8.i4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braga M, Vignali A, Zuliani W, Radaelli G, Gianotti L, Martani C, Toussoun G, Di Carlo V. Metabolic and functional results after laparoscopic colorectal surgery: a randomized, controlled trial. Dis Colon Rectum. 2002;45:1070–1077. doi: 10.1007/s10350-004-6362-2. [DOI] [PubMed] [Google Scholar]
- 30.Wang YX, Ruan CP, Li L, Shi JH, Kong XT. Clinical significance of changes of perioperative T cell and expression of its activatedantigen in colorectal cancer patients. World J Gastroenterol. 1999;5:181–182. doi: 10.3748/wjg.v5.i2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan HY, Li Y, Yang GL, Bei DJ, Wang K. Study on the causes of local recurrence of rectal cancer after curative resection: analysis of 213 cases. World J Gastroenterol. 1998;4:527–529. doi: 10.3748/wjg.v4.i6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballantyne GH, Quin J. Surgical treatment of liver metastases in patients with colorectal cancer. Cancer. 1993;71:4252–4266. doi: 10.1002/1097-0142(19930615)71:12+<4252::aid-cncr2820711815>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Vaughn DJ, Haller DG. Nonsurgical management of recurrent colorectal cancer. Cancer. 1993;71:4278–4292. doi: 10.1002/1097-0142(19930615)71:12+<4278::aid-cncr2820711817>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 34.Turk PS, Wanebo HJ. Results of surgical treatment of nonhepatic recurrence of colorectal carcinoma. Cancer. 1993;71:4267–4277. doi: 10.1002/1097-0142(19930615)71:12+<4267::aid-cncr2820711816>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 35.Beer DJ, Rocklin RE. Histamine-induced suppressor-cell activity. J Allergy Clin Immunol. 1984;73:439–452. doi: 10.1016/0091-6749(84)90353-1. [DOI] [PubMed] [Google Scholar]
- 36.Lima M, Rocklin RE. Histamine modulates in vitro IgG production by pokeweed mitogen-stimulated human mononuclear cells. Cell Immunol. 1981;64:324–336. doi: 10.1016/0008-8749(81)90484-6. [DOI] [PubMed] [Google Scholar]
- 37.Rocklin RE, Blidy A, Kamal M. Physiochemical characterization of human histamine-induced suppressor factor. Cell Immunol. 1983;76:243–252. doi: 10.1016/0008-8749(83)90367-2. [DOI] [PubMed] [Google Scholar]
- 38.Uotila P. Inhibition of prostaglandin E2 formation and histamine action in cancer immunotherapy. Cancer Immunol Immunother. 1993;37:251–254. doi: 10.1007/BF01518519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Black JW, Duncan WA, Durant CJ, Ganellin CR, Parsons EM. Definition and antagonism of histamine H 2 -receptors. Nature. 1972;236:385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- 40.Melmon KL, Bourne HR, Weinstein J, Sela M. Receptors for histamine can be detected on the surface of selected leukocytes. Science. 1972;177:707–709. doi: 10.1126/science.177.4050.707. [DOI] [PubMed] [Google Scholar]
- 41.Osband M, McCaffrey R. Solubilization, separation, and partial characterization of histamine H1 and H2 receptors from calf thymocyte membranes. J Biol Chem. 1979;254:9970–9972. [PubMed] [Google Scholar]
- 42.Burtin C, Scheinmann P, Salomon JC, Lespinats G, Canu P. Decrease in tumour growth by injections of histamine or serotonin in fibrosarcoma-bearing mice: influence of H1 and H2 histamine receptors. Br J Cancer. 1982;45:54–60. doi: 10.1038/bjc.1982.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rocklin RE, Greineder DK, Melmon KL. Histamine-induced suppressor factor (HSF): further studies on the nature of the stimulus and the cell which produces it. Cell Immunol. 1979;44:404–415. doi: 10.1016/0008-8749(79)90015-7. [DOI] [PubMed] [Google Scholar]
- 44.Adams WJ, Lawson JA, Nicholson SE, Cook TA, Morris DL. The growth of carcinogen-induced colon cancer in rats is inhibited by cimetidine. Eur J Surg Oncol. 1993;19:332–335. [PubMed] [Google Scholar]
- 45.Nishiguchi S, Tamori A, Shiomi S, Enomoto M, Tatsumi N, Koh N, Habu D, Sakaguchi H, Takeda T, Seki S, et al. Cimetidine reduces impairment of cellular immunity after transcatheter arterial embolization in patients with hepatocellular carcinoma. Hepatogastroenterology. 2003;50:460–462. [PubMed] [Google Scholar]
- 46.Zhu Q, Si F, Xu JY, Wu YL. Effect of Cimetidine against diges-tive tract tumors. Huaren Xiaohua Zazhi. 1998;6:68–69. [Google Scholar]
- 47.Bobek V, Boubelik M, Kovarík J, Taltynov O. Inhibition of adhesion breast cancer cells by anticoagulant drugs and cimetidine. Neoplasma. 2003;50:148–151. [PubMed] [Google Scholar]
- 48.Kobayashi K, Matsumoto S, Morishima T, Kawabe T, Okamoto T. Cimetidine inhibits cancer cell adhesion to endothelial cells and prevents metastasis by blocking E-selectin expression. Cancer Res. 2000;60:3978–3984. [PubMed] [Google Scholar]
- 49.Takahashi K, Tanaka S, Ichikawa A. Effect of cimetidine on intratumoral cytokine expression in an experimental tumor. Biochem Biophys Res Commun. 2001;281:1113–1119. doi: 10.1006/bbrc.2001.4487. [DOI] [PubMed] [Google Scholar]
- 50.Szincsák N, Hegyesi H, Hunyadi J, Falus A, Juhász I. Different h2 receptor antihistamines dissimilarly retard the growth of xenografted human melanoma cells in immunodeficient mice. Cell Biol Int. 2002;26:833–836. doi: 10.1016/s1065-6995(02)90934-0. [DOI] [PubMed] [Google Scholar]
- 51.Szincsak N, Hegyesi H, Hunyadi J, Martin G, Lazar-Molnar E, Kovacs P, Rivera E, Falus A, Juhasz I. Cimetidine and a tamoxifen derivate reduce tumour formation in SCID mice xenotransplanted with a human melanoma cell line. Melanoma Res. 2002;12:231–240 12140379 doi: 10.1097/00008390-200206000-00006. doi: 10.1097/00008390-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 53.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 54.Wang XZ, Li B, Zheng XX, Qu YZ, Lin GZ, Tang NH, Chen ZX. Experimental and clinical study on tumor infiltrating lympho-cytes in solid tumor. Xin Xiaohuabingxue Zazhi. 1997;5:481–482. [Google Scholar]
- 55.Dréno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S, Cassidanius A, Lemarre P, Billaudel S, Labarrière N, et al. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2002;51:539–546. doi: 10.1007/s00262-002-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Labarrière N, Pandolfino MC, Gervois N, Khammari A, Tessier MH, Dréno B, Jotereau F. Therapeutic efficacy of melanoma-reactive TIL injected in stage III melanoma patients. Cancer Immunol Immunother. 2002;51:532–538. doi: 10.1007/s00262-002-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kono K, Takahashi A, Ichihara F, Amemiya H, Iizuka H, Fujii H, Sekikawa T, Matsumoto Y. Prognostic significance of adoptive immunotherapy with tumor-associated lymphocytes in patients with advanced gastric cancer: a randomized trial. Clin Cancer Res. 2002;8:1767–1771. [PubMed] [Google Scholar]
- 58.Liu SC, Yuan SZ. Relationship between infiltration of dendritic cells, pericancerous lymphocytic reaction and prognosis in colorectal carcinomas. Xin Xiaohuabingxue Zazhi. 1997;5:156–157. [Google Scholar]
- 59.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kubota T, Fujiwara H, Ueda Y, Itoh T, Yamashita T, Yoshimura T, Okugawa K, Yamamoto Y, Yano Y, Yamagishi H. Cimetidine modulates the antigen presenting capacity of dendritic cells from colorectal cancer patients. Br J Cancer. 2002;86:1257–1261. doi: 10.1038/sj.bjc.6600233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsumoto S, Imaeda Y, Umemoto S, Kobayashi K, Suzuki H, Okamoto T. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis-A epitope expression on tumour cells. Br J Cancer. 2002;86:161–167. doi: 10.1038/sj.bjc.6600048. [DOI] [PMC free article] [PubMed] [Google Scholar]


