Abstract
Purpose
The purpose of the study was to establish the mechanism by which the estrogen concentration difference between the follicular fluid and the serum is maintained.
Methods
We used dialysis membrane with a pore size of <3 KD to characterize the estrogen-binding capacity of the follicular fluid. We performed PCR, western blot, and ELISA on luteinized granulosa cells to determine if sex hormone-binding globulin (SHBG) is produced by granulosa cells, and finally we used affinity columns and mass spectrometry to identify the estrogen-binding protein in the follicular fluid.
Results
We found that a significant estrogen concentration difference is maintained in a cell-free system and is lost with proteolysis of the follicular fluid proteins. Luteinized granulosa cells are likely not a source of SHBG, as we were not able to detect expression of SHBG in these cells. Perlecan was the most highly enriched follicular fluid protein in the affinity columns.
Conclusions
We were able to identify perlecan as the most likely candidate for the major estrogen-binding protein in the follicular fluid.
Keywords: Estrogen binding, Follicular fluid, SHBG, Perlecan
Introduction
Sex steroids, as all other steroids, are lipophilic derivatives of cholesterol and as such can readily cross cell membranes to bind to their intracellular receptor. While circulating in the blood, a majority of the principal sex steroids estradiol and testosterone is bound to a protein carrier known as sex hormone-binding globulin (SHBG). Another 30 % is loosely bound to albumin, leaving only about 1 % unbound and bioavailable [1]. A very small percentage also binds to the corticosteroid-binding globulin [2]. Estradiol is the most potent estrogen. It is produced predominantly by the aromatization of testosterone by granulosa cells (GC) of the ovarian follicle. In the follicular phase of the cycle, the increased rate of secretion of FSH induces expression of the aromatase enzyme in GC. As a result, estrogen production in the follicle increases rapidly. The rising concentration of serum FSH leads also to an accelerated growth of the follicle largely due to the rapid secretion and accumulation of follicular fluid (FF). The FF is also formed by GC. It is a complex biologic mixture of lipids and proteins similar in composition to the serum. The accumulation of the FF is attributed to the secretion of the high molecular weight glycosaminoglycans and proteoglycans that generate a high osmotic pressure drawing fluid into the antrum [3]. The FF provides a carefully controlled microenvironment designed to support the final stages of oocyte maturation. It also serves as a reservoir for sex steroids produced by the follicle estrogen, progesterone, and androgens.
Control over serum concentration of estrogen is of immense importance. Transient uncontrolled increase in estradiol serum concentration may interfere with phasic secretion of FSH and consequently may prevent proper development of a dominant pre-ovulatory follicle leading to anovulation and dysfunctional uterine bleeding. Furthermore, chronically elevated unopposed estrogen may result in endometrial hyperplasia, dysplasia, and carcinogenesis [1]. Thus, finding out how the source (e.g., follicle) maintains control over estrogen release is a key to understanding the intricate network of steroid hormone physiology. The concentration difference of estradiol between the pre-ovulatory follicle and the serum can be in the order of thousands [4]. However, the rate of release of steroids from the follicle is very slow. Younglai et al. established that the hourly transfer of estrogen from the follicle to the circulation is only 3–9 % [5]. Interestingly, despite the significant concentration difference of estradiol and slow rate of release, the concentration of the known estrogen-binding proteins SHBG and albumin is similar to or even lower in the FF in comparison to the serum [4]. Therefore, it is unclear how such a high concentration of estradiol is maintained in the FF. The aim of this study was to identify the mechanism that prevents leakage of estradiol from the ovarian follicle.
Methods
Patients
Participants included in the study were patients undergoing in vitro fertilization (IVF) at the Toronto Center for Advanced Reproductive Technology (Toronto, Canada). The project was approved by the institutional Research Ethics Board of Mount Sinai Hospital in Toronto, and participants provided informed consent. Patients were treated with either a long protocol or a short microdose flare protocol, including a gonadotropin-releasing hormone agonist (Superfact, Sanofi-Aventis Canada Inc.) and ovarian stimulation with either human menopausal gonadotropin (Menopur, Ferring Pharmaceuticals, Canada) or recombinant follicle-stimulating hormone (Gonal-F® Serono, Oakville, Ontario, Canada, or Puregon®, Merck, Scarborough, Ontario, Canada). Follicular growth was monitored by pelvic ultrasound and serum estradiol determination, and final maturation was induced by human chorionic gonadotropin (human chorionic gonadotropin, Pregnyl®, Merck, Scarborough, Ontario, Canada) given 36 h before follicular aspiration. FF and oocyte aspiration was transvaginal ultrasound guided, under conscious sedation.
Sample collection
Follicular fluid was obtained from follicle aspirates after the removal of the egg from 13 women (average age 34.9, range 29–43) undergoing oocyte retrieval as part of an in vitro fertilization treatment. Follicular fluid from a single follicle was aspirated into a tube devoid of culture media. Only clear follicular fluid (without blood) was used for further analysis. After removal of the egg by the IVF laboratory, the fluid was centrifuged at 700g for 10 min to remove cells and cellular debris and frozen shortly after at −800 C until the time of analysis. Granulosa cells (GCs) were collected from pooled FF, as described previously with minor modifications [6]. Briefly, the pooled follicular aspirate was centrifuged at 800g for 10 min, and the resulting cell slurry was diluted twofold with Hanks’ balanced salt solution (HBSS) and then gently layered on top of 3 ml of a Ficoll-paque Plus 50 % (GE Health Care Life Sciences) gradient cushion, and then centrifuged at 700g for 20 min. GCs were collected from the cell layer at the interface, resuspended in 10 ml HBSS, and centrifuged. The resulting cell pellets were resuspended in 0.5 ml of culture media and cell number and viability were assessed in a hemocytometer by a Trypan blue exclusion test. The cells (∼50,000 cells per well) were plated in a 24-well with Complete Media 199 (Life Technologies), supplemented with 10 % FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 % fetal calf serum, and 0.25 μg/ml amphotericin B, incubated in 37 °C, 5.5 % CO2. Hormones used were recombinant FSH (Puregon®, Merck, Canada) in a concentration of 1, 5, and 10 IU per well, insulin (Humulin R®, Eli Lilly, Indianapolis USA) 0.5, 1, 5, and 10 units per well, hCG (Pregnyl®, Merck, Canada) 0.5, 1, 5, and 10 units per well, triiodothyronine (T3) (Sigma-Aldrich) 10 and 20 nmol per well, hydrocortisone (Sigma-Aldrich) 250 and 500 nmol per well, and 17β-estradiol (Sigma-Aldrich) 250 and 500 nmol per well [7]. Following incubation for 48 h, the cells were harvested for either RNA or protein extraction and medium was used to measure SHBG by ELISA (MX52001, IBL Hamburg, Germany).
Reverse transcriptase–PCR for SHBG gene
Unless specified, all reagents were purchased from Qiagen (Toronto, Canada) and were used according to the manufacturer instructions. RNA was extracted from granulosa cells using the RNAeasy mini kit. Commercial human liver and testis RNA (Zyagen, San Diego, CA) and pooled RNA samples from three individuals were used for reverse transcriptase (RT) reaction using the QuantiTect Reverse Transcription Kit. PCR amplification was carried with the Taq PCR Master Mix Kit using the following primers: SHBG exon 1—forward 5′-TGCTGCTGTTGCTACTACTG; exon 6—reverse 5′-CAAGATGGGTTCTCTGGTGTC; exon 3—forward 5′-AGGATGACTGGTTTATGCTG; exon 6—forward 5′-GACACCAGAGAACCCATCTTG; and exon 8—reverse 5′-ATCTCATGGCTTCTGTTCAGG. PCR reaction products were separated by electrophoresis on a 1 % agarose gel and visualized with SYBR safe DNA gel stain (Life Technologies).
Real-time PCR for SHBG
Quantitative PCR (qPCR) was performed as described before (Perumalsamy et al., 2010), utilizing the QuantiTect SYBR® Green PCR kit (Qiagen) on the LiteCycler (Roche, Mississauga, ON, Canada). The reaction conditions were as follows: 95 °C for 10 min and then 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. Comparisons of expression levels were determined by delta CT method normalized to β-actin.
Western blot for SHBG
Granulosa cells and ovarian protein lysates were prepared in 1 % SDS-radioimmunoprecipitation assay (RIPA) buffer containing a complete protease inhibitor cocktail (Roche). Protein concentration was determined using the bicinchoninic acid (BCA) protein assay and protein samples resolved through 12 % acrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking, the blots were probed with rabbit anti-CoQ6 or anti-PDSS2 (1:400 and 1:600, respectively; Proteintech Group Inc).
Follicular fluid aspirates obtained from patients undergoing oocyte retrieval were centrifuged and treated with RBC lysis buffer (0.16 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) to remove any red blood cells, and cell pellet was washed with PBS. GC pellet and OVCAR3 cells were prepared with 1 % SDS-RIPA buffer containing protease inhibitor cocktail (Roche Diagnostics), and protein concentrations were determined using the BCA protein assay. FF was prepared as follows: 9 μl of RIPA lysis buffer with protease inhibitors was added to 3 μl of follicular fluid. The protein lysates were run on a 12 % SDS-PAGE gel and transferred onto PVDF membrane. The membrane was blocked with 5 % powdered milk in TBS with 0.05 % Tween-20 for 1 h at room temperature and incubated overnight in 4 °C with mouse anti-human SHBG monoclonal antibody (1:500 dilution, R&D Systems), followed by incubation with goat anti-mouse HRP-conjugated secondary antibody (1:5000, BioRad Lab., Inc.). The membrane was then washed and incubated with appropriate HRP-conjugated secondary antibodies and developed by the ECL Plus kit (GE Healthcare). The membrane was stripped by incubation with stripping buffer (0.1 M glycine, 1 % SDS, pH 2.5) for 20 min at ∼55 °C. The membrane was re-blocked and incubated overnight at 4 °C in goat anti-actin polyclonal antibody (1:500 dilution, from Santa Cruz Biotechnology), washed, and incubated for 1 h at room temperature in horseradish peroxidase-conjugated anti-goat secondary antibody (1:10,000 Jackson ImmunoResearch Lab., Inc.).
Dialysis experiments
To test the hypothesis that follicular fluid estrogen-binding proteins are responsible for the slow release of estradiol from the follicle to the serum, we used a dialysis model. A tube made of dialysis membrane (Float-A-Lyzer Spectrum Laboratories Inc., Rancho Dominguez, CA, USA) with a pore size of <3 KD was placed in a container with water. This pore size is large enough to allow free passage of small molecules such as steroids but no significant passage of most proteins. The test solution was placed in the inner chamber and a control solution in the outer chamber. The volume of the inner chamber was 1–5 ml and the outer chamber was 200 ml. The solutions placed in the inner and outer chambers were sampled to test for estradiol concentration at baseline. The device was left in the outer chamber with continuous stirring for 24 h before retesting for estrogen concentration. The concentration of 17β-estradiol was measured using the Vitros ECiQ Immunodiagnostic System (Ortho-Clinical diagnostics, Johnson and Johnson, Rochester, NY 14626-5101). The concentration range of the assay is 23.4–14,000 pmol/l (6.360–3813.6 pg/ml), analytical sensitivity is 23.4 pmol/l, the intra-assay variability is 1.3–6.6 %, and inter-assay variability is 4.8–9.3 %.
Biotin avidin column
Follicular fluid samples were stripped using 0.5 % charcoal-coated dextran in TEMG buffer per 1 ml of FF and incubated overnight on a shaker. The solution was then centrifuged for 15 min at 3000g and the supernatant was aspirated into a fresh tube. We then incubated 1.0 ml of FF with 0.017 mg of biotinylated 17-β-estradiol (6-keto-17β-estradiol-6-carboxymethyloxime-NH-propyl-biotin, Steraloids Inc. Newport R.I 02840, USA) for 4 h. The SoftLink Avidin (Promega, USA) column was prepared by pouring 2 ml of Avidin resin solution into a 5-ml chromatography column with a reservoir (Econo-Column, Bio-Rad labs, USA). All the processes were carried out at 4 °C. The column was equilibrated with 0.1 M NaHPO4 (pH 7.0) followed by pre-adsorption with a 5 mM biotin solution (Sigma-Aldrich). The SoftLink Avidin resin is a rigid methacrylate polymeric gel filtration matrix covalently bound to monomeric avidin. Native or tetrameric avidin binds biotin with a very strong affinity (Kd = 10−15) and requires strong denaturing conditions to elute bound materials. Monomeric avidin, on the other hand, binds biotin with a Kd = 10−7 allowing reversible binding of bound biotinylated proteins under mild elution conditions. The purpose of the pre-adsorption phase is to saturate all the tetrameric avidin that may still exist in the resins with biotin before exposing the resins to the biotinylated proteins. This allows for an optimal recovery of these proteins at the purification stage. The following is a sample of papers that used this protocol to recover biotinylated proteins: [7–25]. Following the pre-adsorption phase, the column was then washed with 16 ml of 10 % acetic acid and 0.1 M NaHPO4 until the pH of the flow-through was >6.8. The column was equilibrated with Tris–HCl 50 mM (pH 7.8). The samples of FF with biotinylated estradiol were loaded onto the columns with no flow for 30 min. We collected the flow-through and then washed the column with 10 ml Tris–HCl 50 mM buffer to remove non-specifically bound proteins. The flow-through was collected in fractions of 0.5 ml. We then eluted proteins bound to the estrogen using biotin solution. The protein content of each fraction was quantified using NanoDrop (Thermo-Fisher Scientific, Wilmington, DE, USA). The native follicular fluid, flow-through fractions, and biotin washes were frozen at −80 °C until analyzed with mass spectrometry (MS).
Mass spectrometry analysis
Samples were first digested in solution by trypsin, and the digested peptides were loaded onto a 150-μm ID pre-column (Magic C18, Michrom Biosciences) at 4 μl/min and separated over a 75-μm ID analytical column packed into an emitter tip containing the same packing material. The peptides were eluted over 120 min at 300 nl/min using a 0 to 40 % acetonitrile gradient in 0.1 % formic acid using an EASY n-LC nano-chromatography pump (Proxeon Biosystems, Odense, Denmark). The peptides were eluted into a LTQ-Orbitrap hybrid mass spectrometer (Thermo-Fisher Scientific, Bremen, Germany) operated in a data dependent mode. MS was acquired at 60,000 FWHM resolution in the FTMS and MS/MS was carried out in the linear ion trap. 6 MS/ MS scans were obtained per MS cycle. The raw data files were searched using Sequest (Thermo-Fisher Scientific, San Jose, CA) using a parent ion accuracy of 5 ppm and a fragment accuracy of 0.5 Da. A fixed modification of carbamidomethyl cysteine and variable modification of oxidized methionine were included in the search.
Results
An estradiol solution of a known concentration placed in the dialysis tube had equilibrated with the water in the outer chamber within 24 h. Next, a FF sample of a known estradiol concentration was placed in the inner chamber of the dialysis device. Following 24 h of incubation with continuous stirring, there was no significant change in estradiol concentration of the FF (Table 1). Similarly, a FF sample placed in the a dialysis tube incubated for 24 h with an albumin solution of increasing concentration in the outer chamber showed no significant change in estradiol concentration. Despite a concentration of albumin in the outer chamber solution as high as 8 g/dl, double the average concentration of albumin in the follicular fluid, FF estradiol had only changed from 390 to 240 nmol/l [26]. However, pretreatment of the FF with trypsin (trypsin to protein ratio 1:50 at 37 °C for 4 h) or heat (to 85 °C for 10 min) resulted in equilibration of estradiol concentration between the two chambers within 24 h (Table 1).
Table 1.
The concentration of 17β-estradiol (nmol/l) in the inner chamber of the dialysis apparatus containing either native follicular fluid or trypsin treated before and after a 24-h incubation
| Type | Native | Trypsin treated | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Follicular fluid | Water | Follicular fluid | Water | ||||
| AVG | SEM | AVG | SEM | AVG | SEM | AVG | SEM | |
| Pre-incubation estradiol concentration (nmol/l) | 682.12 | 319.16 | 2.68 | 1.43 | 615.41 | 352.51 | 2.55 | 1.52 |
| Post incubation estradiol concentration (nmol/l) | 253.65 | 207.97 | 3.55 | 2.05 | 1.16 | 0.50 | 5.55 | 1.73 |
The estradiol concentration is also shown for the outer water-containing chamber. The results show that the ability of the follicular fluid to maintain an estradiol gradient was lost following treatment with trypsin, suggesting that it is maintained by the action of a binding protein. The average and standard error represent three separate experiments
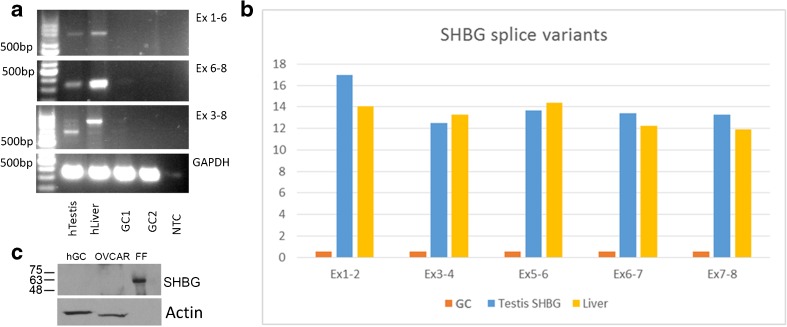
In order to determine if SHBG is responsible for binding the estrogen in follicular fluid, we first set to determine if SHBG is expressed by GC. Using a primer design that was published previously by Forges et al. [27] and other primers that we predesigned, we performed RT-PCR as well as real-time PCR of the full- and partial-length messenger RNA (mRNA). We were not able to show any expression of SHBG mRNA in either GC or cultured GC while showing a clear expression of several SHBG transcripts with both primer sets (Fig. 1a, b) in liver and testis samples. Western blot for SHBG and actin showed a clear signal for SHBG in FF but none in human GC while actin was clearly detected in the GC extract (Fig. 1c).
Fig. 1.
SHBG is not expressed in granulosa cells. a RT-PCR for multiple segments of the sex hormone-binding globulin (SHBG) mRNA in human granulosa cells, liver, and testis. Results show no expression of any part of the SHBG mRNA in granulosa cells. The top panel shows SHBG exons 1–6, second panel shows SHBG exons 6–8, and the third panel shows SHBG exons 3–8 with the human testis and liver but not with two pools of GC. The bottom panel shows human GAPDH as positive control. hTestis human testis, hLiver human liver, GC granulosa cells, NTC no template control. b Real-time PCR for multiple segments of SHBG mRNA in the human granulosa cells, liver, and testis. Results show no expression of any part of the SHBG mRNA in granulosa cells. c Western blot analysis for the SHBG protein in human granulosa cells as well as ovarian carcinoma cell line and follicular fluid and actin as a positive control. Results show a clear detection of SHBG in follicular fluid but none in granulosa cells and ovarian carcinoma cell line
Affinity column and mass spectrometry analysis
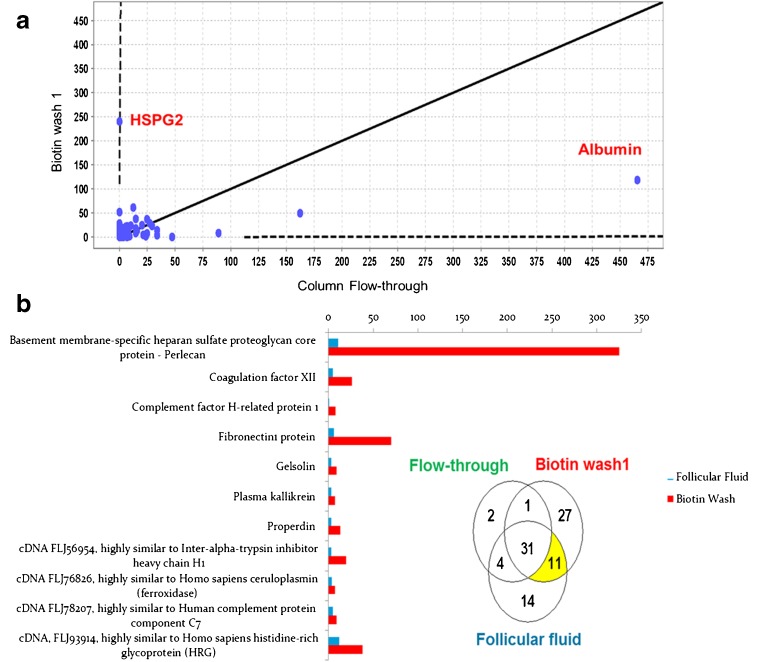
Analysis of the total protein concentration in the stripped FF, flow-through, buffer wash, and biotin wash fractions showed that the concentration of protein dropped from 3.79 mg/ml in the flow-through to 0.14 mg/ml in the last biotin wash. The FF, flow-through, and biotin washes were then analyzed with MS at the Advanced Protein Technology center in Toronto. The results were analyzed with the scaffold 3.2 software, and a 95 % probability was specified as a threshold for protein detection. With these criteria, we were able to identify a total of 100 proteins. Figure 2a shows the concentration of proteins in the flow-through fraction on the x-axis and the biotin wash on the y-axis. The flow-through contains the proteins that had passed through the avidin column without binding (non-specific binding) while the biotin wash fraction is enriched with proteins that were bound to the avidin beads and were released when the column was washed with a concentrated biotin solution (specific binding). Albumin, the most common protein in the FF, was also the most abundant in the flow-through. While heparan sulfate proteoglycan 2 (HSPG2; also known as perlecan) was hardly detectable in the flow-through fraction, it was the most enriched protein in the biotin wash. Figure 2b shows a Venn diagram of the number of proteins found in the stripped FF, flow-through, and biotin wash. There were 11 proteins that were detected in the FF and in the biotin wash but not in the flow-through, suggesting that they bound to the avidin column and were released with the biotin wash. These proteins are shown in the bar graph that details their concentration in the FF and the biotin wash. The largest enrichment between the FF and biotin wash occurred with perlecan that showed a tenfold increase between the two fractions. Both MS data and RT-PCR analysis of GC support a full-length expression of the perlecan gene (data not shown).
Fig. 2.
Mass spectrometry analysis of avidin biotin fractions. a This figure illustrates the concentration of the proteins in the flow-through (the native follicular fluid that passed through the column before any washes were made) on the x-axis and the first biotin wash on the y-axis. Albumin was the most abundant protein in the flow-through fraction that contains the proteins that had passed the avidin column without binding. At the other end the HSPG2 protein that could not be detected in the flow-through but had the highest concentration in the biotin wash which is enriched with specifically bound proteins. b This Venn diagram represents the distribution of the detected proteins in the different fractions. In yellow is the group of proteins that were detected in the follicular fluid but not in the flow-through and were released from the column with the biotin wash. The bar graph represents the relative concentration of the 11 proteins exhibiting specific binding to the avidin biotin-bound estradiol. HSPG2, also known as perlecan, had the highest enrichment following the biotin wash
Discussion
The FF is a complex solution that constitutes the microenvironment of the developing follicle. It is formed by the GC and contains their secretory products. The main hormone secreted by GC during the follicular phase is estradiol. It is a potent hormone in comparison to other steroids, exerting its action in a concentration several orders of magnitude lower. Disruption of the control of estradiol serum concentration may cause infertility, thrombosis, and even cancer [28]. Only the unbound fraction of estradiol, about 2 %, is bioactive while the rest of the estradiol in serum is bound to proteins, predominantly SHBG and albumin. In the FF, the concentration of estradiol is a thousand times higher than in the serum while the concentrations of its known binding proteins is similar to or lower than the concentrations in the serum. The question this study is trying to answer is what are the forces that keep the estradiol in the FF and maintain such a high concentration?
There could be several potential explanations to the maintenance of the high FF-serum estradiol concentration differences: a higher rate of production and secretion to the FF, an intracellular estrogen-binding protein with a unidirectional secretion of estrogen through the apical membrane, and a different soluble estrogen-binding protein or the action of cellular tight junctions between granulosa cells that prevent estrogen leakage from the follicle.
As mentioned earlier, the rate of release of estradiol from the follicle is very slow; therefore, a rapid rate of production with a significant but slower leakage to the serum could not be the explanation [5].
In this study, we were able to show that the high concentration of FF estradiol is maintained in a cell-free system in a dialysis membrane system that allows free passage of estradiol but blocks peptides and proteins. We also showed that the gradient is lost after protein denaturation, suggesting that the estradiol gradient is maintained by a binding protein.
SHBG is a homodimeric glycoprotein produced mostly by hepatocytes [29]. It is a highly conserved steroid-binding protein. It binds dihydrotestosterone (DHT) with the highest affinity (Ka = 1 × 1010 M) and testosterone and estradiol with 100-fold lower affinity [30]. The SHBG/ABP gene has been localized to 17p13-12 and has two distinct forms: one that is secreted mainly by the liver into the circulation and the other a non-secreted form, known as the androgen-binding protein (ABP), identified in Sertoli cells of the testis and in the brain and liver. SHBG/ABP is considered as the major binding protein for estradiol in the serum. In contrast to our results, Forges et al. [27] described the presence of full-length SHBG mRNA in luteinized GC. They were also able to show the presence of the SHBG/ABP protein in the cytoplasm of luteinized GC using immunohistochemistry. We, however, were not able to show that the main serum estrogen-binding protein, SHBG, is produced in either form by GC. Our finding was validated by RT-PCR and qPCR using the published primers as well as newly designed primers. It was also validated by Western blot of GC extract. Due to the discrepancy of our finding with the previous publications, we requested the group of Dr. G.L Hammond [31–38], a leading expert on SHBG/ABP, to validate our findings. They also were not able to demonstrate any expression of SHBG in granulosa cells. In addition, SHBG production could not be induced in cultured GC by the hormonal treatments that are known to control its production in liver cells or control the function of GC. Therefore, in light of the known low concentration of SHBG in follicular fluid and the lack of an intracellular non-secreted form of SHBG, the contribution of this protein to the estrogen binding in FF is likely non-significant. Furthermore, if SHBG was the main sex steroid-binding protein in the ovarian follicle, its higher affinity to DHT would sequester more of this potent androgen. Since DHT, unlike testosterone, cannot be aromatized to estradiol, its accumulation in the FF could potentially lead to follicular atresia [39].
Albumin is the most abundant protein in both the follicular fluid and the serum. It is the universal ligand carrier of the serum with mostly non-specific binding for most of the ligands.
In the serum, 61 % of the estradiol is bound to SHBG, 37 % to albumin, and 2 % is free [40]. The concentration of albumin in the FF is similar to that of the serum.
In our dialysis system experiment, highly concentrated albumin solutions were not capable of creating a significant leak of estradiol from FF. This suggests that the binding protein of estradiol in FF is likely not albumin. Hence, after showing that the FF-serum estradiol concentration difference is kept by the action of a binding protein other than SHBG or albumin, we tried to target the binding protein using an affinity column. We reduced the amount of estradiol in the FF in order to increase the binding capability of any estrogen-binding proteins and then added biotinylated estradiol. After binding to its carrier protein, the complex of estradiol-binding protein was captured by the avidin resins, followed by its release from avidin by a concentrated biotin solution. Analysis of the specifically bound proteins with mass spectrometry identified heparan sulfate proteoglycan 2 (HSPG2) as the protein with the highest enrichment and the most likely FF estrogen-binding protein. HSPG2, also known as perlecan, is an exceptionally large, highly conserved, secreted extracellular matrix proteoglycan with a modular structure that supports its many functions. It is referred to as a depot of essential proteins combined together into a single large storage protein that is placed in the extracellular matrix. This “cartridge” of proteins allows for immediate protein action by cleaving a certain active component with a specific protease, thereby skipping several time-consuming stages in protein production [41]. HSPGs are defined as proteins containing one or more covalently attached heparan sulfate chains supporting its function as a natural anticoagulant. HSPGs can be divided into three major classes: (1) lipid-anchored, e.g., the glypicans; (2) transmembrane, e.g., syndecans; and (3) extracellular or secreted, a group that includes perlecan [42]. HSPGs had been found in cartilage [43], bone stroma [44], uterine decidua [45], and follicular fluid [46]. Perlecan is particularly abundant in the ovary with a level of expression that is stronger than the kidney—a known rich source of this protein. Labeling of perlecan using 125I-anti-thrombin in ovarian tissue had shown it to be expressed only in endothelial and granulosa cells. In the follicle, the fate of perlecan was shown to be tightly regulated by gonadotropins; its turnover, synthesis, internalization, and release are all regulated by FSH and LH [47].
Expression of perlecan was limited to the larger follicles and peaked in the pre-ovulatory follicle, decreasing in concentration in luteinized GC after ovulation [48]. The high expression of perlecan in GC and its closely regulated production and secretion into the FF instigated research looking for a potential physiological role for perlecan in the development of the ovarian follicle.
In order for a follicle to complete its final stages of growth and maturation, several key factors need to exist: (1) the follicle needs to rapidly accumulate FF in its antrum and as a result expand the follicular antrum and grow; (2) the protein-rich and viscous FF must not coagulate so that expulsion of the cumulus oocyte complex (COC) can occur during ovulation; (3) the follicle must remain avascular before ovulation and rapidly grow new blood vessels immediately after luteinization; and (4) the follicle needs to be able to produce and store large amounts of estrogen.
HSPG as a group are actively secreted to the antrum of the follicle and as a result increase the osmotic pressure of the FF and drive its formation. The degradation of these proteins allows for the removal of the FF in atretic follicles. Perlecan was shown to be essential for alteration of the permeability of the basal lamina, a vital stage in FF formation [49]. In addition, perlecan core protein is covalently bound to heparan sulfate (HS) chains. HS binds anti-thrombin III and endows with HSPG anticoagulant properties. The presence of an anticoagulant in the FF allows the maintenance of fluidity of the oocyte’s microenvironment despite the influx of procoagulant proteins due to inflammatory vascular permeabilisation prior to ovulation [50]. The sharp drop in HSPG concentration post ovulation allows for the coagulation of the remaining content of follicle and the formation of the matrix of the corpus luteum [51].
One of the active components created by proteolytic cleavage of the fifth domain of perlecan is endorepellin [52]. This is a powerful angiostatic agent capable of inhibiting several aspects of angiogenesis: endothelial cell migration, collagen-induced endothelial tube morphogenesis, and blood vessel growth [53]. The presence of perlecan in the FF and GC is likely the mechanism that keeps the follicle avascular until ovulation. With the expulsion of the FF during ovulation and the drop in perlecan content, a rapid process of angiogenesis ensues into the luteinized follicle to form the corpus luteum.
These findings established a central role for perlecan in follicular development. The data we present in this study may suggest another role for perlecan in the ovarian follicle physiology: serving as the estrogen-binding protein of the FF. This added role will center perlecan as one of the most important proteins in the formation and function of the ovarian follicle and will coincide with its developmental stage-specific expression.
FSH stimulation of GC promotes the liberation of cell bound perlecan and its secretion to the FF mirroring the increase in estradiol secretion [54]. Similarly to the expression of FSH receptor in the developing follicle, the expression of perlecan shows very little or no expression in primordial and pre-antral follicles and peak concentration in the pre-ovulatory follicle [55].
In the follicle, perlecan is part of the follicular basal lamina, a specialized extracellular matrix that enables the granulosa cells to orient their polarity and control their microenvironment. Irving-Rodgers et al. [56] identified perlecan as part of a developmentally regulated novel extracellular matrix, termed “focimatrix.” They showed a gradual increase in the amount of the focimatrix with increased follicular size. Interestingly, the appearance of the focimatrix was shown to closely precede the expression of the enzymes involved with sex steroid synthesis, the main secretory product being estradiol.
In this study, we showed that perlecan was the most enriched protein in the specifically bound fraction of the FF, making it the most likely FF estradiol-binding protein. One of the major functions of GC during the follicular phase is the production of estrogen. Estrogen is important for endothelial proliferation, regulation of FSH secretion, and the selection of the dominant follicle. It is unclear whether estrogen has a local function in the follicle parallel to the essential role of testosterone in Sertoli cells. However, mice null for estrogen receptor α and aromatase form large cystic hemorrhagic follicles instead of the normal follicles [57, 58]. The structure of the perlecan protein also supports its potential role as a high-affinity estrogen-binding protein. The estrogen-binding component in the SHBG protein is attributed to a tandem repeat of two laminin G-like domains. These were shown to form a hydrophobic pocket that binds steroids [30]. Domain V of perlecan contains a tandem repeat of three laminin G-like domains [41]. This might be the potential site of estrogen-binding on the perlecan molecule.
The lack of an alternative mechanism for estradiol sequestration in FF and the multiple characteristics of perlecan control of expression and structure add to the findings presented in this study and suggest that this protein is the most likely candidate for the estradiol-binding protein in the FF. However, more research is needed to characterize the estrogen-binding characteristics of perlecan and its control.
Compliance with ethical standards
Ethics approval and consent to participate
The project was approved by the institutional Research Ethics Board of Mount Sinai Hospital in Toronto, and participants provided informed consent.
Footnotes
Capsule We were able to identify perlecan as the most likely candidate for the major estrogen-binding protein in the follicular fluid.
References
- 1.Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility. 7. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 2.Fritz MA, Speroff L. Clinical gynecologic endocrinology and infertility. 8. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 3.Clarke HG, Hope SA, Byers S, Rodgers RJ. Formation of ovarian follicular fluid may be due to the osmotic potential of large glycosaminoglycans and proteoglycans. Reproduction. 2006;132(1):119–31. doi: 10.1530/rep.1.00960. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Rafael Z, Mastroianni L, Jr, Meloni F, Lee MS, Flickinger GL. Total estradiol, free estradiol, sex hormone-binding globulin, and the fraction of estradiol bound to sex hormone-binding globulin in human follicular fluid. J Clin Endocrinol Metab. 1986;63(5):1106–11. doi: 10.1210/jcem-63-5-1106. [DOI] [PubMed] [Google Scholar]
- 5.Younglai EV, Short RV. Pathways of steroid biosynthesis in the intact graafian collicle of mares in oestrus. J Endocrinol. 1970;47(3):321–31. doi: 10.1677/joe.0.0470321. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal R, Jacobs H, Payne N, Conway G. Concentration of vascular endothelial growth factor released by cultured human luteinized granulosa cells is higher in women with polycystic ovaries than in women with normal ovaries. Fertil Steril. 2002;78(6):1164–9. doi: 10.1016/S0015-0282(02)04242-5. [DOI] [PubMed] [Google Scholar]
- 7.Loukovaara M, Carson M, Palotie A, Adlercreutz H. Regulation of sex hormone-binding globulin production by isoflavonoids and patterns of isoflavonoid conjugation in HepG2 cell cultures. Steroids. 1995;60(9):656–61. doi: 10.1016/0039-128X(95)00089-9. [DOI] [PubMed] [Google Scholar]
- 8.Arakawa S, Kuramitsu HK. Cloning and sequence analysis of a chymotrypsinlike protease from Treponema denticola. Infect Immun. 1994;62(8):3424–33. doi: 10.1128/iai.62.8.3424-3433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahrer J, Kranaster R, Altmeyer M, Marx A, Bürkle A. Quantitative analysis of the binding affinity of poly(ADP-ribose) to specific binding proteins as a function of chain length. Nucleic Acids Res. 2007;35(21) doi: 10.1093/nar/gkm944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields S, Ternyak K, Gao H, Ostraat R, Akerlund J, Hagman J. The ‘zinc knuckle’ motif of early B cell factor is required for transcriptional activation of B cell-specific genes. Mol Immunol. 2008;45(14):3786–96. doi: 10.1016/j.molimm.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallahan TW, Shapiro R, Vallee BL. Dual site model for the organogenic activity of angiogenin. Proc Natl Acad Sci U S A. 1991;88(6):2222–6. doi: 10.1073/pnas.88.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He MM, Voss J, Hubbell WL, Kaback HR. Arginine 302 (helix IX) in the lactose permease of Escherichia coli is in close proximity to glutamate 269 (helix VIII) as well as glutamate 325. Biochemistry. 1997;36(44):13682–7. doi: 10.1021/bi971531b. [DOI] [PubMed] [Google Scholar]
- 13.Hoke DE, Egan S, Cullen PA, Adler B. LipL32 is an extracellular matrix-interacting protein of Leptospira spp. and Pseudoalteromonas tunicata. Infect Immun. 2008;76(5):2063–9. doi: 10.1128/IAI.01643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan RA, Preissler MT, Banas JA, Gosselin EJ. Production of genetically engineered biotinylated interleukin-2 and its application in a rapid nonradioactive assay for T-cell activation. Clin Diagn Lab Immunol. 2003;10(3):339–44. doi: 10.1128/CDLI.10.3.339-344.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawasaki H, Suyama E, Iyo M, Taira K. siRNAs generated by recombinant human Dicer induce specific and significant but target site-independent gene silencing in human cells. Nucleic Acids Res. 2003;31(3):981–7. doi: 10.1093/nar/gkg184. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Kernstock RM, Girotti AW. Lipid transfer protein binding of unmodified natural lipids as assessed by surface plasmon resonance methodology. Anal Biochem. 2007;365(1):111–21. doi: 10.1016/j.ab.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosovský J, Durmanová V, Kúdelová M, Rezuchová I, Tkáciková L, Rajcáni J. A simple procedure for expression and purification of selected non-structural (alpha and beta) herpes simplex virus 1 (HSV-1) proteins. J Virol Methods. 2001;92(2):121–9. doi: 10.1016/S0166-0934(00)00281-0. [DOI] [PubMed] [Google Scholar]
- 18.La Gruta NL, Liu H, Dilioglou S, Rhodes M, Wiest DL, Vignali DA. Architectural changes in the TCR:CD3 complex induced by MHC:peptide ligation. J Immunol. 2004;172(6):3662–9. doi: 10.4049/jimmunol.172.6.3662. [DOI] [PubMed] [Google Scholar]
- 19.Lapik YR, Misra JM, Lau LF, Pestov DG. Restricting conformational flexibility of the switch II region creates a dominant-inhibitory phenotype in Obg GTPase Nog1. Mol Cell Biol. 2007;27(21):7735–44. doi: 10.1128/MCB.01161-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovrich SD, La Fleur RL, Jobe DA, Johnson JC, Asp KE, Schell RF, et al. Borreliacidal OspC antibody response of canines with Lyme disease differs significantly from that of humans with Lyme disease. Clin Vaccine Immunol. 2007;14(5):635–7. doi: 10.1128/CVI.00431-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller BM, Kistner U, Kindler S, Chung WJ, Kuhlendahl S, Fenster SD, et al. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron. 1996;17(2):255–65. doi: 10.1016/S0896-6273(00)80157-9. [DOI] [PubMed] [Google Scholar]
- 22.Nossal NG, Makhov AM, Chastain PD, Jones CE, Griffith JD. Architecture of the bacteriophage T4 replication complex revealed with nanoscale biopointers. J Biol Chem. 2007;282(2):1098–108. doi: 10.1074/jbc.M606772200. [DOI] [PubMed] [Google Scholar]
- 23.Pichaud F, Roux S, Frendo JL, Delage-Mourroux R, Maclouf J, de Vernejoul MC, et al. 1,25-Dihydroxyvitamin D3 induces NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase in human neonatal monocytes. Blood. 1997;89(6):2105–12. [PubMed] [Google Scholar]
- 24.Richter S, Lamppa GK. A chloroplast processing enzyme functions as the general stromal processing peptidase. Proc Natl Acad Sci U S A. 1998;95(13):7463–8. doi: 10.1073/pnas.95.13.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas NS, Pizzey AR, Tiwari S, Williams CD, Yang J. p130, p107, and pRb are differentially regulated in proliferating cells and during cell cycle arrest by alpha-interferon. J Biol Chem. 1998;273(37):23659–67. doi: 10.1074/jbc.273.37.23659. [DOI] [PubMed] [Google Scholar]
- 26.Velazquez A, Reyes A, Chargoy J, Rosado A. Amino acid and protein concentrations of human follicular fluid. Fertil Steril. 1977;28(1):96–100. doi: 10.1016/s0015-0282(16)42324-1. [DOI] [PubMed] [Google Scholar]
- 27.Forges T, Gerard A, Hess K, Monnier-Barbarino P, Gerard H. Expression of sex hormone-binding globulin (SHBG) in human granulosa-lutein cells. Mol Cell Endocrinol. 2004;219(1-2):61–8. doi: 10.1016/j.mce.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Yang CH, Almomen A, Wee YS, Jarboe EA, Peterson CM, Janat-Amsbury MM. An estrogen-induced endometrial hyperplasia mouse model recapitulating human disease progression and genetic aberrations. Cancer Med. 2015;4(7):1039–50. doi: 10.1002/cam4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond GL. Access of reproductive steroids to target tissues. Obstet Gynecol Clin N Am. 2002;29(3):411–23. doi: 10.1016/S0889-8545(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 30.Munell F, Suarez-Quian CA, Selva DM, Tirado OM, Reventos J. Androgen-binding protein and reproduction: where do we stand? J Androl. 2002;23(5):598–609. [PubMed] [Google Scholar]
- 31.Berube D, Seralini GE, Gagne R, Hammond GL. Localization of the human sex hormone-binding globulin gene (SHBG) to the short arm of chromosome 17 (17p12–p13) Cytogenet Cell Genet. 1990;54(1-2):65–7. doi: 10.1159/000132958. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson AL, Lorentzon M, Mellstrom D, Vandenput L, Swanson C, Andersson N, et al. SHBG gene promoter polymorphisms in men are associated with serum sex hormone-binding globulin, androgen and androgen metabolite levels, and hip bone mineral density. J Clin Endocrinol Metab. 2006;91(12):5029–37. doi: 10.1210/jc.2006-0679. [DOI] [PubMed] [Google Scholar]
- 33.Fortunati N, Raineri M, Cignetti A, Hammond GL, Frairia R. Control of the membrane sex hormone-binding globulin-receptor (SHBG-R) in MCF-7 cells: effect of locally produced SHBG. Steroids. 1998;63(5-6):282–4. doi: 10.1016/S0039-128X(98)00021-X. [DOI] [PubMed] [Google Scholar]
- 34.Hammond GL, Langley MS. Identification and measurement of sex hormone binding globulin (SHBG) and corticosteroid binding globulin (CBG) in human saliva. Acta Endocrinol (Copenh) 1986;112(4):603–8. doi: 10.1530/acta.0.1120603. [DOI] [PubMed] [Google Scholar]
- 35.Hammond GL, Langley MS, Robinson PA. A liquid-phase immunoradiometric assay (IRMA) for human sex hormone binding globulin (SHBG) J Steroid Biochem. 1985;23(4):451–60. doi: 10.1016/0022-4731(85)90192-X. [DOI] [PubMed] [Google Scholar]
- 36.Lahteenmaki PL, Hammond GL, Luukkainen T. Serum non-protein bound percentage and distribution of the progestin ST-1435: no effect of ST-1435 treatment on plasma SHBG and CBG binding capacities. Acta Endocrinol (Copenh) 1983;102(2):307–13. doi: 10.1530/acta.0.1020307. [DOI] [PubMed] [Google Scholar]
- 37.Murayama Y, Hammond GL, Sugihara K. The SHBG gene and hormone dependence of breast cancer: a novel mechanism of hormone dependence of MCF-7 human breast cancer cells based upon SHBG. Breast Cancer (Tokyo, Japan) 1999;6(4):338–43. doi: 10.1007/BF02966450. [DOI] [PubMed] [Google Scholar]
- 38.Niemi S, Maentausta O, Bolton NJ, Hammond GL. Time-resolved immunofluorometric assay of human SHBG. Steroids. 1988;52(4):413–4. doi: 10.1016/0039-128X(88)90173-0. [DOI] [PubMed] [Google Scholar]
- 39.Speroff L, Fritz M. The preantral follicle. In: Fritz MA, editor. Clinical gynecologic endocrinology and infertility. 8. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011. pp. 204–5. [Google Scholar]
- 40.Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981;53(1):58–68. doi: 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- 41.Farach-Carson MC, Warren CR, Harrington DA, Carson DD. Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol. 2013 doi: 10.1016/j.matbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farach-Carson MC, Carson DD. Perlecan—a multifunctional extracellular proteoglycan scaffold. Glycobiology. 2007;17(9):897–905. doi: 10.1093/glycob/cwm043. [DOI] [PubMed] [Google Scholar]
- 43.Farach-Carson MC, Hecht JT, Carson DD. Heparan sulfate proteoglycans: key players in cartilage biology. Crit Rev Eukaryot Gene Expr. 2005;15(1):29–48. doi: 10.1615/CritRevEukaryotGeneExpr.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- 44.Schofield KP, Gallagher JT, David G. Expression of proteoglycan core proteins in human bone marrow stroma. Biochem J. 1999;343(Pt 3):663–8. doi: 10.1042/bj3430663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.French MM, Smith SE, Akanbi K, Sanford T, Hecht J, Farach-Carson MC, et al. Expression of the heparan sulfate proteoglycan, perlecan, during mouse embryogenesis and perlecan chondrogenic activity in vitro. J Cell Biol. 1999;145(5):1103–15. doi: 10.1083/jcb.145.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eriksen GV, Carlstedt I, Morgelin M, Uldbjerg N, Malmstrom A. Isolation and characterization of proteoglycans from human follicular fluid. Biochem J. 1999;340(Pt 3):613–20. doi: 10.1042/bj3400613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yanagishita M. Proteoglycans and hyaluronan in female reproductive organs. EXS. 1994;70:179–90. doi: 10.1007/978-3-0348-7545-5_10. [DOI] [PubMed] [Google Scholar]
- 48.Princivalle M, Hasan S, Hosseini G, de Agostini AI. Anticoagulant heparan sulfate proteoglycans expression in the rat ovary peaks in preovulatory granulosa cells. Glycobiology. 2001;11(3):183–94. doi: 10.1093/glycob/11.3.183. [DOI] [PubMed] [Google Scholar]
- 49.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82(6):1021–9. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 50.Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- 51.de Agostini A. An unexpected role for anticoagulant heparan sulfate proteoglycans in reproduction. Swiss Med Wkly. 2006;136(37-38):583–90. doi: 10.4414/smw.2006.11368. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B, et al. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J Biol Chem. 2005;280(8):7080–7. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 53.Mongiat M, Fu J, Oldershaw R, Greenhalgh R, Gown AM, Iozzo RV. Perlecan protein core interacts with extracellular matrix protein 1 (ECM1), a glycoprotein involved in bone formation and angiogenesis. J Biol Chem. 2003;278(19):17491–9. doi: 10.1074/jbc.M210529200. [DOI] [PubMed] [Google Scholar]
- 54.Hosseini G, Liu J, de Agostini AI. Characterization and hormonal modulation of anticoagulant heparan sulfate proteoglycans synthesized by rat ovarian granulosa cells. J Biol Chem. 1996;271(36):22090–9. doi: 10.1074/jbc.271.36.22090. [DOI] [PubMed] [Google Scholar]
- 55.McArthur ME, Irving-Rodgers HF, Byers S, Rodgers RJ. Identification and immunolocalization of decorin, versican, perlecan, nidogen, and chondroitin sulfate proteoglycans in bovine small-antral ovarian follicles. Biol Reprod. 2000;63(3):913–24. doi: 10.1095/biolreprod63.3.913. [DOI] [PubMed] [Google Scholar]
- 56.Irving-Rodgers HF, Harland ML, Rodgers RJ. A novel basal lamina matrix of the stratified epithelium of the ovarian follicle. Matrix Biol. 2004;23(4):207–17. doi: 10.1016/j.matbio.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 57.Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, et al. An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology. 2000;141(7):2614–23. doi: 10.1210/endo.141.7.7578. [DOI] [PubMed] [Google Scholar]
- 58.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90(23):11162–6. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]