Abstract
Purpose
Higher risk for birth of singletons being large for gestational age (LGA) has been revealed after in vitro fertilization (IVF) frozen–thawed embryo-transfer (FET). This phenomenon is now being investigated, since there is a speculation that these neonates could suffer from underlying epigenetic disturbances. The aim of the study was to expose independent LGA risk factors and to identify those connected to the IVF techniques.
Methods
Altogether, 4508 singleton pregnancies and births were included in the cohort case-matched study. Two hundred eleven singleton pregnancies and births after FET and 916 after fresh embryo transfer (ET) were included into two study groups. The IVF procedures were performed at the University Medical Centre Ljubljana between 2004 and 2011. For each IVF pregnancy, three matched consecutive controls after natural conception were included. Using logistic regression models, we observed LGA connection to maternal parameters (smoking, hypertension, parity, BMI, gestational diabetes, IVF conception, FET, double ET, and ICSI procedure).
Results
Singletons born after FET had a significantly higher risk for being LGA (p = 0.032; OR 1.697; 95 % CI 1.047–2.752). BMI 25–30 was a significant independent risk factor for LGA in the IVF groups (FET p = 0.041, OR 2.460, 95 % CI 1.030–5.857 and fresh ET p = 0.003; OR 2.188, 95 % CI 1.297–3.691). ICSI and double ET had no significant effect on LGA occurrence.
Conclusions
Besides maternal BMI, FET is a significant independent LGA risk factor in IVF patients. Other observed factors (smoking, hypertension, multiparity, GDM, ICSI procedure, or number of embryos transferred) do not influence LGA risk significantly.
Keywords: IVF singletons, Frozen–thawed embryo transfer, Birth weight, Large for gestation, IVF outcome
Introduction
The proportion of children born after frozen–thawed embryo transfer (FET) after the in vitro fertilization–embryo transfer (IVF–ET) procedure grows worldwide [1]. Several studies have shown that singletons born after FET have a perinatal outcome more comparable to those born after natural conception than to those born after fresh embryo transfer (fresh ET), having higher birth weight and less pregnancy complications [2–10]. However, higher risk for birth of a singleton being large for gestational age (LGA) has been revealed after FET [2, 3, 7, 8, 11–14].
The reasons for these differences are now being sought. The LGA child has an increased risk for stillbirth, asphyxia, distress, trauma, caesarian section, and metabolic disturbances at birth [15–17]. Moreover, another concern in LGA neonates born after assisted reproductive techniques (ART) is the possible underlying epigenetic pathology, since certain rare epigenetic disorders in fact occur more often after IVF–ET than after spontaneous conception [18]. For example, higher “large offspring syndrome” incidence has been noticed following ART in the cattle and sheep. This syndrome has been proven to be epigenetically and phenotypically similar to epigenetic Beckwith–Wiedemann’s syndrome in humans, which also seems to occur more often after IVF conception [19]. Besides, placental expression of certain imprinted genes has already been connected to altered LGA risk in term pregnancies [20].
There are different hypothetical mechanisms for higher LGA rate after FET to explore:
An average woman involved in FET procedure has supernumerary embryos and is therefore considered a good prognosis patient. More embryos of higher quality are indicating that a patient is more likely to conceive in an ongoing IVF cycle than in a patient without them.
The techniques of freezing and thawing procedures enable positive selection of those high-quality embryos, which survive the cryopreservation procedure. It seems reasonable to speculate that they could be more successful also in other selective events, such as implantation, invasion, and placentation or nutrition supplementation.
The FET performed in a natural cycle provides different embryo implantation and growth conditions due to a different hormonal environment in comparison to the stimulated cycle.
The cryopreservation technique itself could induce changes in the embryo on an epigenetic level.
Pinborg et al. have recently shown that siblings conceived after FET have an increased risk for being LGA when compared to the siblings born after fresh ET from the same mother [8]. The influences of the culture media [21–23], culture period [24, 25], hormonal protocols [26], and infertility [12, 14, 27] on the IVF singleton birth weight have already been considered. There are several studies confirming that IVF placentas differ from spontaneous placentas in their size, shape, weight, and abnormality rate [3, 6, 7, 28–31], and that placentation following FET differ from the placentation following fresh ET [3, 6, 7, 13]
In this study, we analyzed the connection of IVF procedure type and maternal background factors to LGA risk. The factors observed were the fertilization technique, ET type, number of embryos transferred, maternal age, smoking, hypertension, parity, BMI, and gestational diabetes mellitus (GDM).
Materials and methods
We analyzed the LGA rates in neonates, born after FET and fresh ET, in comparison to case-matched population of neonates, born after spontaneous conception. Furthermore, we analyzed the correlation of LGA rate with specific maternal and IVF factors.
Patients
We included 4507 singleton pregnancies and births in this retrospective, case-matched cohort study.
Two hundred eleven singleton pregnancies and births after FET and 916 after fresh ET were included into two study groups: the “FET group” and the “fresh ET group”. For each of the IVF pregnant women included in the two study groups, we included three consecutive controls who gave birth after natural conception and were matched by age, parity, and maternity hospital. Altogether, 3381 singleton pregnancies and births after natural conception were included in the study: 633 in the group of “FET controls” and 2748 in the group of “fresh ET controls”.
IVF–ET procedure
The IVF–ET procedures were performed at the Department of Human Reproduction, University Medical Centre Ljubljana between January 2004 and December 2011.
Short antagonist cetrorelix protocol (Cetrotide: Serono, London, UK or AstaMedica AG, Frankfurt, Germany) or long agonist buserelin protocol (Suprefact: Hoechst AG, Frankfurt/Main, Germany) was used for ovarian stimulation. The short protocol consisted of GnRH antagonist cetrorelix acetate in a dose of 0.25 mg, which was administered when the dominant follicle measured ≥14 mm in diameter, after daily stimulation with 225 IU of follicle stimulating hormone (FSH) (Gonal F: Serono, Switzerland, Puregon), from day 2 of the menstrual cycle. In the long protocol, buserelin was used from day 22 of the menstrual cycle, at a daily dose of 0.6 ml (600 pg) subcutaneously until ovarian desensitization. Afterward, 125 to 300 IU of FSH was administered daily. All patients were administered 10,000 IU of human chorionic gonadotrophin (hCG) (Pregnyl; N.V. Organon) when at least three follicles measured ≥17 mm in diameter.
Transvaginal ultrasound-guided oocyte retrieval was performed 34 h after hCG administration. Fertilization of oocytes was performed using classical in vitro fertilization or intracytoplasmic sperm injection (ICSI).
Embryos were cultured to the blastocyst stage in the sequential media M1 and M2 (Origio/MediCult, Jyllinge, Denmark) until day 5. One or two best quality blastocysts were transferred in the uterus on day 5. Blastocyst quality was evaluated according to the blastocyst grading system introduced by Gardner et al. [32]. Supernumerary blastocysts were cryopreserved on day 5 or day 6 using our original, modified two-step Menezo’s method, described by Virant-Klun et al. [33]. Blastocysts were stored in liquid nitrogen at −196 °C. Day 2 or 3 ET was performed only in patients with previous blastocyst development failure and patients with only one or two embryos developed.
Coordination between the endometrium and blastocysts in the frozen–thawed cycles was performed as described in Virant-Klun et al. [33]. Three different protocols for the FET endometrial synchronization have been used: the spontaneous cycle in 103 patients, minimal stimulation protocol using 75 IE of gonadotrophins from day 5 of the menstrual cycle in 85 patients and hormonal supplementation in 8 patients. For 15 patients, the type of protocol was unknown. The estradiol serum measurements were performed only in patients having FET performed in the spontaneous cycles for more accurate monitoring. Luteal phase support consisted of 3 × 200 mg of daily progesterone (Utrogestan: ViatrisPharma, Paris, France) until a gestational sac with a fetal heart activity was visible on day 30 after ET .
Data collection
Data on IVF procedures were collected in the IVF laboratory of our department on a daily basis (Table 1). All 14 Slovenian maternity hospitals systematically collect data on maternal demographic characteristics, medical, gynecological and reproductive history, prenatal care, pregnancy, delivery, postpartum period, and neonates for each mother–infant pair using the same definitions of variables and the same form of medical record.
The research was performed according to the Personal Data Protection Act (UL). The data obtained from National Perinatal Information System (NPIS) were depersonalized in order to ensure the anonymity of women and neonates. The research was performed according to the Personal Data Protection Act.
Definitions
Slovene reference standard curves for weight, length, and head circumference at birth for a given gestational age were used for fetal and neonatal growth estimation [34]. LGA was defined as growth above the 90th percentile (or 95th percentile when specifically mentioned in the text, see Table 4) according to the aforementioned population growth curves. Diagnosis of GDM was made following screening test with oral 50 g glucose challenge test at 24 to 28 weeks of pregnancy and confirmed by diagnostic 100 g oral glucose tolerance test [35]. Preterm delivery was defined as the delivery before completed 37 weeks of gestation.
Table 4.
Comparison of LGA rates (gestational weight above 90th and 95th centile) in FET and fresh ET group to their control groups
| FET N = 211 |
FET controls N = 633 |
Fresh ET N = 916 |
Fresh controls N = 2748 |
|
|---|---|---|---|---|
| LGA (above 90th centile) N (%) |
31 (14.7) | 55 (8.7) | 86 (9.4) | 249 (9.1) |
| p value | 0.017a | 0.791 | ||
| LGA (above 95th centile) N (%) |
21 (10.0) | 31 (4.9) | 45 (4.9) | 141 (5.1) |
| p value | 0.012a | 0.862 | ||
FET frozen–thawed embryo transfer, fresh ET fresh embryo transfer, LGA large for gestation birthweight
aStatistically significant (p < 0.05), as revealed by chi-squared test
Statistics
Statistical analysis was performed using IBM SPSS Statistics, version 19 (IBM Corp, Armonk, NY). For assessment of normality, we used normal Q–Q plot, along with the Shapiro–Wilk normality test. Student’s t test was used to compare normally distributed parametrical variables, Mann–Whitney’s test was used to compare non-normally distributed variables, and chi-squared test was used to compare categorical variables. A multivariate logistic regression was used to identify independent risk factors. p value of <0.050 was considered significant.
Results
Among all patients having IVF, 653 (58 %) women were treated for female factor infertility, 202 (18 %) for male factor infertility, and 271 (24 %) for both female and male factor infertility. In 95 % of ET cases, a blastocyst transfer was performed.
The characteristics of FET and fresh ET cycles are shown in Table 1.
Table 1.
Characteristics of IVF treatment cycles of patients conceiving after FET and fresh ET
| FET No. (%) |
Fresh ET No. (%) |
p value | |
|---|---|---|---|
| No. of patients | 211 | 916 | |
| Infertility cause | |||
| Tubal | 52 (24.6) | 253 (27.7) | 0.511 |
| Endometriosis | 43 (20.3) | 187 (20.4) | 0.982 |
| Endocrine | 44 (20.9) | 198 (21.6) | 0.931 |
| Male | 39 (18.5) | 166(18.1) | 0.277 |
| Othersb | 33 (15.6) | 111 (12.1) | |
| No. of IVF attempts (Mean ± SD) | 1.89 ± 1.2 | 2.19 ± 2.1 | a0.03 |
| Double ET N (%) | 160 (75.8) | 751 (82.1) | a0.041 |
FET frozen–thawed embryo transfer, ET embryo transfer, SD standard deviation
aStatistically significant (p < 0.05), as revealed by chi-squared or Mann–Whitney test
bOther causes include oncologic patients and idiopathic infertility
There were no significant differences in infertility cause between patients conceiving after FET and patients conceiving after fresh ET. Women conceiving after FET had significantly less previous IVF attempts and less double ET in comparison to women conceiving after fresh ET.
Mean maternal age, multiparity, smoking, irregular menstrual cycle rate, mean birth weight, hypertension rate, GDM rate, impaired glucose tolerance rate, mean gestation, preterm birth rate, fetal distress rate, cesarean section rate, and congenital malformation rate of FET and fresh ET with both control groups are shown in Table 2.
Table 2.
Maternal factors and pregnancy outcome following FET and fresh ET and comparison to control groups
| FET N = 211 |
FET controls N = 633 |
p valuea | Fresh ET N = 916 |
Fresh controls N = 2748 |
p valuea | |
|---|---|---|---|---|---|---|
| Mean age (years ± SD) | 33.5 ± 3.7 | 33.5 ± 3.7 | 1.000b | 33.4 ± 4.1 | 33.4 ± 4.1 | 1.000b |
| Multiparous N (%) | 80 (37.9) | 240 (37.9) | 1.000b | 232 (25.3) | 696 (25.3) | 1.000b |
| Irregular menstrual cycle N (%) | 2(0.9) | 8(1.3) | 1.000 | 11(1.2) | 39 (1.4) | 0.743 |
| Smoking N (%) | 19 (9.0) | 48 (7.6) | 0.556 | 69 (7.5) | 224 (8.2) | 0.574 |
| Hypertension N (%) | 3 (1.4) | 30 (4.7) | 0.036a | 43 (4.7) | 147 (5.3) | 0.491 |
| Gestational diabetes N (%) | 8(3.8) | 25(3.9) | 1.000 | 35(3.8) | 104(3.8) | 1.000 |
| Impaired glucose tolerance N (%) | 2(0.9) | 9(1.4) | 0.740 | 10 (1.1) | 22(0.8) | 0.414 |
| Birth weight (grams ± SD) | 3355 ± 626 | 3290 ± 621 | 0.191 | 3200 ± 633 | 3314 ± 579 | <0.001a |
| Mean gestation (weeks ± SD) | 38.6 ± 2.5 | 38.8 ± 2.3 | 0.545 | 38.5 ± 2.5 | 38.9 ± 2.2 | <0.001a |
| Preterm birth N (%) | 19 (9.0) | 60 (9.5) | 0.838 | 111 (12.1) | 199 (7.2) | <0.001a |
| Fetal distress N (%) | 7 (3.3) | 25 (3.9) | 0.677 | 34 (3.7) | 127 (4.6) | 0.245 |
| Caesarean section N (%) | 53 (25.1) | 152 (24) | 0.746 | 236 (25.8) | 588 (21.4) | 0.006a |
| Congenital malformation N (%) | 13 (6.2) | 37 (5.8) | 0.866 | 68 (7.4) | 180 (6.6) | 0.362 |
FET frozen–thawed embryo transfer, fresh ET fresh embryo transfer
aStatistically significant (p < 0.05) as revealed by chi-squared, Mann–Whitney test or t test
bMothers were matched according to age and parity
Significantly fewer women had hypertension in the FET group, while there were no other significant differences between the observed maternal factors and pregnancy outcome between FET group and their controls. In the fresh ET group, however, the birth weight and mean gestation were significantly lower in comparison to their control group. There were also significantly higher rates of preterm birth, fetal distress, cesarean section, and malformations in the fresh ET group, compared to their controls.
The distribution of BMI is shown in Table 3. There was a significant shift among FET and fresh ET patients from normal BMI toward overweight BMI.
Table 3.
Distribution of BMI in IVF and control groups
| BMI <18.5 Underweight |
BMI 18.5–25 Normal |
BMI 25–30 Overweight |
Bmi >30 Obese |
p value | |
|---|---|---|---|---|---|
| FET group N (%) |
2 (0.9) | 145 (68.7) | 51 (24.2) | 13 (6.2) | 0.014 |
| FET controls N (%) |
26 (4.1) | 453 (71.6) | 104 (16.4) | 50 (7.9) | |
| Fresh ET group N (%) |
36 (3.9) | 612 (66.8) | 181 (19.8) | 87 (9.5) | 0.002 |
| Fresh ET controls N (%) |
145(5.3) | 1983(72.2) | 435(15.8) | 185(6.7) |
Numbers in bold are statistically significant (p < 0.05), as revealed by chi-squared test
FET frozen-thawed embryo transfer, fresh ET fresh embryo transfer
The LGA rate (gestational weight above 90th or 95th centile) was significantly higher in the FET group in comparison to their controls. The LGA rate in the fresh ET group of singletons was similar to the LGA rate of their control group. The results are shown in Table 4.
The LGA rate did not differ significantly among the subgroups of patients having FET after different endometrial preparation: there were 17 LGA neonates born after FET in spontaneous cycle (18 %), 10 LGA neonates born after FET using minimal stimulation (12 %), and 2 LGA neonates born after hormonal replacement therapy (25 %) (p = 0.386).
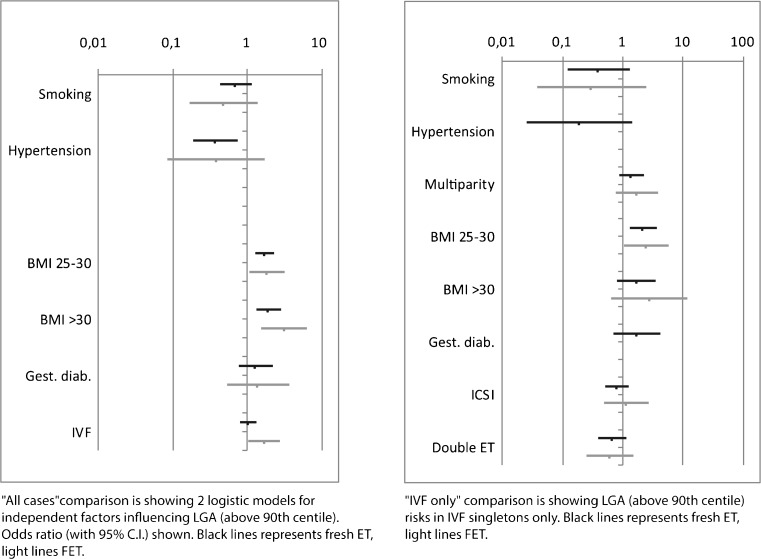
We included smoking, hypertension, multiparity, BMI, GDM, and IVF–ET procedure into an LGA (above 90th centile) logistic regression model. We analyzed the influence of the aforementioned factors on the LGA rate (above 90th centile) in four groups of patients: FET with controls, fresh ET with controls (Fig. 1, graph “All cases”), FET only, and fresh ET only (Fig. 1, graph “IVF only”).
Fig. 1.
Graph “All cases” shows a comparison of two logistic models for independent factors effecting LGA (above 90th centile) odds ratio (with 95 % C.I) light line shows FET with controls, black line shows fresh ET with controls. Graph “IVF only” is showing LGA (above 90th centile) risks in IVF singletons only, following FET (light line) or fresh ET (black line)
The LGA odds for independent factors are shown in Fig. 1.
In FET with controls, higher BMI and the IVF (FET) procedure increase the risk for birth of an LGA singleton. In the fresh ET with controls, the hypertension reduces the LGA risk, whereas higher BMI increases the LGA risk significantly. In fresh ET patients with controls, the IVF procedure has no significant LGA risk effect.
Smoking and diagnosis of GDM have no significant effect on LGA risk.
In both the FET and fresh ET group, higher BMI (25–30) increases the LGA risk significantly. Other extreme BMI classes have insignificant effect on LGA risk. Smoking, hypertension, multiparity, GDM, ICSI, and double ET have no significant effect on LGA risk in IVF groups either.
These results are shown in Table 5.
Table 5.
Independent factors influencing LGA odds ratio (as shown in Fig. 1, graph “All cases” and graph “IVF only”)
| Factor | p value | OR (95 % CI) | |
|---|---|---|---|
| All cases—fresh ET with controls (black line) | Smoking | 0.143 | 0.7 (0.43–1.13) |
| Hypertension | 0.006 | 0.38 (0.19–0.75) | |
| BMI (ref = 18.5–25) | <0.001 | ||
| BMI <18.5 | 0.099 | 0.54 (0.26–1.12) | |
| BMI 25–30 | <0.001 | 1.71 (1.29–2.26) | |
| BMI >30 | 0.001 | 1.93 (1.32–2.83) | |
| Gestational diabetes | 0.322 | 1.3 (0.77–2.19) | |
| FET procedure (IVF) | 0.882 | 0.98 (0.76–1.27) | |
| All cases—FET with controls (light line) | Smoking | 0.171 | 0.48 (0.17–1.37) |
| Hypertension | 0.209 | 0.38 (0.09–1.71) | |
| BMI (ref = 18.5–25) | 0.008 | ||
| BMI <18.5 | 0.999 | 0.00 | |
| BMI 25–30 | 0.026 | 1.85 (1.08–3.18) | |
| BMI >30 | 0.002 | 3.12 (1.52–6.42) | |
| Gestational diabetes | 0.508 | 1.38 (0.53–3.61) | |
| FET procedure (IVF) | 0.032 | 1.7 (1.05–2.75) | |
| IVF only—fresh ET (black line) | Smoking | 0.124 | 0.39 (0.12–1.29) |
| Hypertension | 0.108 | 0.19 (0.03–1.44) | |
| Multiparity | 0.178 | 1.4 (0.86–2.28) | |
| BMI (ref = 18.5–25) | 0.029 | ||
| BMI <18.5 | 0.915 | 1.07 (0.31–3.66) | |
| BMI 25–30 | 0.003 | 2.19 (1.3–3.69) | |
| BMI >30 | 0.175 | 1.68 (0.79–3.54) | |
| Gestational diabetes | 0.233 | 1.73 (0.7–4.23) | |
| ICSI | 0.331 | 0.8 (0.5–1.26) | |
| Double ET | 0.158 | 0.67 (0.39–1.17) | |
| IVF only—FET (light line) | Smoking | 0.257 | 0.3 (0.04–2.4) |
| Hypertension | 0.999 | 0.00 | |
| Multiparity | 0.182 | 1.73 (0.77–3.88) | |
| BMI (ref = 18.5–25) | 0.167 | ||
| BMI <18.5 | 0.999 | 0.00 | |
| BMI 25–30 | 0.043 | 2.46 (1.03–5.86) | |
| BMI >30 | 0.175 | 2.76 (0.64–11.91) | |
| Gestational diabetes | 0.999 | 0.00 | |
| ICSI | 0.731 | 1.16 (0.5–2.71) | |
| Double ET | 0.292 | 0.62 (0.25–1.51) |
FET—frozen–thawed embryo transfer, fresh ET—fresh embryo transfer, ICSI—intracytoplasmic sperm injection,, BMI—body mass index, double ET—embryo transfer of 2 embryos, OR—odds ratio, C.I—confidence interval statistically significant when p < 0.050, as revealed by logistic regression.
Discussion
This study investigates the connections of maternal and IVF factors in singleton pregnancies to the LGA rate.
Since both maternal age and parity correlate with higher birth weight, LGA rate, and other maternal pathology, such as hypertension or placenta previa [3, 36–39] we matched the controls to the study groups in order to exclude the possible bias.
According to Lintsen et al., the impact of smoking on the live birth rate in IVF treatment is comparable with an increase in female age of >10 years in young women [40]. In our population, the rate of patients reporting smoking was similar in all groups and did not reduce LGA rate significantly.
Both our IVF groups had higher BMI than what the control groups had, and BMI was a significant independent factor connected to LGA in all regression models. There was a significant shift from normal BMI toward overweight BMI (25–30) in the IVF patients. Data on BMI effect on IVF treatment are conflicting [41–43]. Higher BMI, in overweight as well as in obese patients, was related to lower success and live birth rates in women undergoing ART and has negative consequences for pregnancy including preterm birth and lower birth weight [43, 44]. Can the patients in the FET group differ from other patients in their lifestyle or other health parameters? It was previously shown by Pinborg et al. that FET acts as an independent risk factor for an LGA child from the same mother; however, in that study, they did not include factors such as BMI or smoking [8].
Patients having FET represent a “good prognosis” group having more quality embryos. Are there some other fertility factors that put them in the “good prognosis” group? For example, it has been shown that chances to obtain a pregnancy and live birth are threefold higher in obese infertile patients who are regularly physically active in comparison to those who are not, irrespective to their body weight loss [45]. We investigated irregular menstrual cycle rate in order to discover whether PCO syndrome represents a risk for an LGA singleton, and we found no connection to LGA. On the other hand, although GDM is a known risk factor for the birth of a macrosomic neonate, it was not significantly connected to the birth of an LGA singleton in our study population. The reason is probably in the adequate screening of the whole population of pregnant women for GDM and the active observation of those with the diagnosis of GDM.
ICSI procedure did not influence the LGA rate in our population. ICSI has mostly been used for treating male and, rarely, unexplained infertility during the study period. Some studies have already shown that ICSI does not significantly influence birth weight in comparison to IVF, and the differences between fresh and frozen ICSI birth weight are similar to those following classical IVF procedure [9]. In an Austrian study, ICSI singletons were more prone to neonatal complications lower gestation and birth weight, whereas maternal pregnancy pathology was more related to classical IVF [46].
Double ET was a factor that was insignificant for lower LGA rate. Nevertheless, it has been shown, that double ET, in comparison to single ET, results in lower birth weight of IVF singletons [11, 47]. The effect of embryo culture length on birth weight still remains a matter of debate. Prolonged embryo culture (days 5 and 6) has been linked to a higher LGA rate in comparison to day 2 or 3 ET in fresh ET singletons [3, 25, 37]. In contrast, others report an increased risk of low birth weight and preterm birth after blastocyst transfer [24, 39]. The policy of blastocyst transfer is prevailing at our center because of a presumed benefit. During the study period, over 95 % of FET and fresh blastocyst transfers have been performed; therefore, the blastocyst transfer was not included into a linear regression model. It was suggested that a protein source in human embryo culture media has significant effect on the singleton birth weight and LGA rate [21, 23]. The influence of culture media on LGA rate was not analyzed in our study, since all embryos were cultured equally, regardless of ET type or fertilization procedure used.
Several studies have confirmed that there are important differences in placental abnormality rates between FET and fresh ET singleton pregnancies [3, 6, 7, 13] and that placentas after IVF are larger and heavier [28]. In a recent study, the researchers speculated that increased placental size could promote glucose delivery to LGA fetuses [48]. The cryopreservation is known to damage some of the embryo’s cells [49], leading to a conclusion that this is a mechanism for a positive selection of “more aggressive” blastocysts. According to Veleva et al. [50], the processes of freezing and thawing cause damage to the embryos, but the extent of the damage depends on the embryo’s quality. Top quality embryos are more viable than embryos of a poorer quality, and the transfer of a frozen–thawed top quality embryo may lead to better results.
In this study, we found no explanation for FET neonates having higher LGA risk than fresh ET neonates or neonates born after spontaneous conception. Therefore, the epigenetic cause cannot be excluded. The study, however, had some limitations. One of them is the lack of the serum estradiol measurements. According to the literature, there were birth weight differences observed after fresh IVF–ET cycles according to peak estradiol serum levels [51]. However, there were no significant differences among the LGA rates in patients having FET performed in different endometrial preparation protocol. Although, the difference might become significant on a larger FET population of neonates. Another limitation of our study was the lack of the infertile population as a control group, which could strengthen the results of the study.
Conclusion
Besides maternal BMI, FET is a significant independent LGA risk factor in IVF patients. Other observed factors (smoking, hypertension, multiparity, GDM, ICSI procedure, or number of embryos transferred) do not influence LGA risk significantly.
Acknowledgments
The authors would like to thank Professor Irma Virant Klun for contributing fresh ideas. Special thanks go to the Embryology team from our IVF Laboratory for their persistent and accurate data collection.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national ethical research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of the study formal informed consent is not required.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule
Besides maternal BMI, FET is a significant independent LGA risk factor in IVF patients. Other observed factors (smoking, hypertension, multiparity, GDM, ICSI procedure, or number of embryos transferred) do not influence LGA risk significantly.
References
- 1.Sullivan EA, Zegers-Hochschild F, Mansour R, Ishihara O, de Mouzon J, Nygren KG, et al. International committee for monitoring assisted reproductive technologies (ICMART) world report: assisted reproductive technology 2004. Hum Reprod. 2013;28(5):1375–90. doi: 10.1093/humrep/det036. [DOI] [PubMed] [Google Scholar]
- 2.Wennerholm UB, Henningsen AK, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28(9):2545–53. doi: 10.1093/humrep/det272. [DOI] [PubMed] [Google Scholar]
- 3.Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. FertilSteril. 2014;101(1):128–33. doi: 10.1016/j.fertnstert.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Wennerholm UB, Söderström-Anttila V, Bergh C, Aittomäki K, Hazekamp J, Nygren KG, et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24(9):2158–72. doi: 10.1093/humrep/dep125. [DOI] [PubMed] [Google Scholar]
- 5.Henningsen AK, Pinborg A, Lidegaard Ø, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertil Steril. 2011;95(3):959–63. doi: 10.1016/j.fertnstert.2010.07.1075. [DOI] [PubMed] [Google Scholar]
- 6.Healy DL, Breheny S, Halliday J, Jaques A, Rushford D, Garrett C, et al. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Hum Reprod. 2010;25(1):265–74. doi: 10.1093/humrep/dep376. [DOI] [PubMed] [Google Scholar]
- 7.Korosec S, Ban Frangez H, Verdenik I, Kladnik U, Kotar V, Virant-Klun I, et al. Singleton pregnancy outcomes after in vitro fertilization with fresh or frozen-thawed embryo transfer and incidence of placenta previa. Biomed Res Int. 2014;2014:431797. doi: 10.1155/2014/431797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinborg A, Henningsen AA, Loft A, Malchau SS, Nyboe FJ, Andersen A. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Hum Reprod. 2014;29(3):618–627. doi: 10.1093/humrep/det440. [DOI] [PubMed] [Google Scholar]
- 9.Belva F, Henriet S, Van den Abbeel E, Camus M, Devroey P, Van der Elst J. LiebaersI, Haentjens P, Bonduelle M. Neonatal outcome of 937 children born after transfer of cryopreserved embryos obtained by ICSI and IVF and comparison with outcome data of fresh ICSI and IVF cycles. Hum Reprod. 2008;23(10):2227–38. doi: 10.1093/humrep/den254. [DOI] [PubMed] [Google Scholar]
- 10.Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari AM, Hydén-Granskog C, Martikainen H, Tiitinen A, Hartikainen AL. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995–2006. Hum Reprod. 2010;25(4):914–23. [DOI] [PubMed]
- 11.Li Z, Wang YA, Ledger W, Sullivan EA. Birthweight percentiles by gestational age for births following assisted reproductive technology in Australia and New Zealand, 2002–2010. Hum Reprod. 2014;29(8):1787–800. doi: 10.1093/humrep/deu120. [DOI] [PubMed] [Google Scholar]
- 12.Marino JL, Moore VM, Willson KJ, Rumbold A, Whitrow MJ, Giles LC, Davies MJ. Perinatal outcomes by mode of assisted conception and sub-fertility in an Australian data linkage cohort. PLoS One. 2014; 8;9(1):e80398. [DOI] [PMC free article] [PubMed]
- 13.Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum Reprod. 2012;27(5):1343–50. doi: 10.1093/humrep/des036. [DOI] [PubMed] [Google Scholar]
- 14.Hansen M, Bower C. The impact of assisted reproductive technologies on intra-uterine growth and birth defects in singletons. Semin Fetal Neonatal Med. 2014;19(4):228–33. doi: 10.1016/j.siny.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Weissmann-Brenner A, Simchen MJ, Zilberberg E, Kalter A, Weisz B, Achiron R, et al. Maternal and neonatal outcomes of large for gestational age pregnancies. Acta Obstet Gynecol Scand. 2012;91(7):844–9. doi: 10.1111/j.1600-0412.2012.01412.x. [DOI] [PubMed] [Google Scholar]
- 16.Bukowski R, Hansen NI, Willinger M, Reddy UM, Parker CB, Pinar H, et al. Fetal growth and risk of stillbirth: a population-based case-control study. PLoS Med. 2014;11(4):e1001633. doi: 10.1371/journal.pmed.1001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriksen T. The macrosomicfetus: a challenge in current obstetrics. Acta ObstetGynecol Scand. 2008;87:134–45. doi: 10.1080/00016340801899289. [DOI] [PubMed] [Google Scholar]
- 18.Grace SK, Sinclair KD. Assisted reproductive technology, epigenetics, and long-term health: a developmental time bomb still ticking. Semin Reprod Med. 2009;27(5):409–16. doi: 10.1055/s-0029-1237429. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Robbins KM, Wells KD, Rivera RM. Large offspring syndrome: a bovie model for the human loss-of-imprinting overgrowth syndrome Beckwith-Wiedemann. Epigenetics. 2013;8(6):591–601. doi: 10.4161/epi.24655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappil MA, Green BB, Armstrong DA, Sharp AJ, Lambertini L, Marsit CJ, et al. Placental expression profile of imprinted genes impacts birth weight. Epigenetics. 2015;10(9):842–9. doi: 10.1080/15592294.2015.1073881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J, Lin S, Li M, Chen L, Lian Y, Liu P, et al. Effect of in vitro culture period on birthweight of singleton newborns. Hum Reprod. 2014;29(3):448–454. doi: 10.1093/humrep/det460. [DOI] [PubMed] [Google Scholar]
- 22.Lemmen JG, Pinborg A, Rasmussen S, Ziebe S. Birthweight distribution in ART singletons resulting from embryo culture in two different culture media compared with the national population. Hum Reprod. 2014;27. [DOI] [PubMed]
- 23.Eskild A, Monkerud L, Tanbo T. Birthweight and placental weight; do changes in culture media used for IVF matter? Comparisons with spontaneous pregnancies in the corresponding time periods. Hum Reprod. 2013;28(12):3207–14. doi: 10.1093/humrep/det376. [DOI] [PubMed] [Google Scholar]
- 24.Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric andperinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. FertilSteril. 2012;98(2):368–77. doi: 10.1016/j.fertnstert.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J, Li M, Chen L, Liu P, Qiao J. The protein source in embryo culture media influences birthweight: a comparative study between G1 v5 and G1-PLUS v5. Hum Reprod. 2014;29(7):1387–92. doi: 10.1093/humrep/deu103. [DOI] [PubMed] [Google Scholar]
- 26.Griesinger G, Kolibianakis EM, Diedrich K, Ludwig M. Ovarian stimulation for IVF has no quantitative association with birthweight: a registry study. Hum Reprod. 2008;23(11):2549–54. doi: 10.1093/humrep/den286. [DOI] [PubMed] [Google Scholar]
- 27.Ban Frangez H, Korosec S, Verdenik I, Kladnik V, Kotar U, Vrtacnik BE. Preterm delivery risk factors in singletons born after in vitro fertilization procedures. Eur J Obstet Gynecol Reprod Biol. 2014;176:183–6. doi: 10.1016/j.ejogrb.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Haavaldsen C, Tanbo T, Eskild A. Placental weight in singleton pregnancies with and without assisted reproductive technology: a population study of 536,567 pregnancies. Hum Reprod. 2012;27(2):576–82. doi: 10.1093/humrep/der428. [DOI] [PubMed] [Google Scholar]
- 29.Al-Khaduri M, Kadoch IJ, Couturier B, Dubé J, Lapensée L, Bissonnette F. Vasapraevia after IVF: should there be guidelines? Report of two cases and literature review. Reprod Biomed Online. 2007;14(3):372–4. doi: 10.1016/S1472-6483(10)60881-4. [DOI] [PubMed] [Google Scholar]
- 30.Romundstad LB, Romundstad PR, Sunde A, von Düring V, Skjaerven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. 2006;21(9):2353–8. doi: 10.1093/humrep/del153. [DOI] [PubMed] [Google Scholar]
- 31.Farhi J, Ben-Haroush A, Andrawus N, Pinkas H, Sapir O, Fisch B, et al. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod Biomed Online. 2010;21(3):331–7. doi: 10.1016/j.rbmo.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 33.Virant-Klun I, Tomazevic T, Bacer-Kermavner L, Mivsek J, Valentincic-Gruden B, Meden-Vrtovec H. Successful freezing and thawing of blastocysts cultured in sequential media using a modified method. FertilSteril. 2003;79:1428–33. doi: 10.1016/s0015-0282(03)00395-9. [DOI] [PubMed] [Google Scholar]
- 34.Verdenik I. Slovene reference standards for weight, length and head circumference at birth for given gestational age of population born in years 1987–96. Zdravniškivestnik. 2000;69(3):153–156. [Google Scholar]
- 35.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–73. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 36.Schimmel MS, Bromiker R, Hammerman C, Chertman L, Ioscovich A, Granovsky-Grisaru S, et al. The effects of maternal age and parity on maternal and neonatal outcome. Arch Gynecol Obstet. 2015;291:793–8. doi: 10.1007/s00404-014-3469-0. [DOI] [PubMed] [Google Scholar]
- 37.Mäkinen S, Söderström-Anttila V, Vainio J, Suikkari AM, Tuuri T. Does long in vitro culture promote large for gestational age babies? Hum Reprod. 2013;28(3):828–34. doi: 10.1093/humrep/des410. [DOI] [PubMed] [Google Scholar]
- 38.Toshimitsu M, Nagamatsu T, Nagasaka T, Iwasawa-Kawai Y, Komatsu A, Yamashita T, et al. Increased risk of pregnancy-induced hypertension and operative delivery after conception induced by in vitro fertilization/intracytoplasmic sperm injection in women aged 40 years and older. Fertil Steril. 2014;102(4):1065–1070. doi: 10.1016/j.fertnstert.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Factors affecting obstetric outcome of singletons born after IVF. Hum Reprod. 2011;26(10):2878–86. doi: 10.1093/humrep/der241. [DOI] [PubMed] [Google Scholar]
- 40.Lintsen AM, Pasker-de Jong PC, de Boer EJ, Burger CW, Jansen CA, Braat DD, et al. Effects of subfertility cause, smoking and body weight on the success rate of IVF. Hum Reprod. 2005;20(7):1867–75. doi: 10.1093/humrep/deh898. [DOI] [PubMed] [Google Scholar]
- 41.Koning AM, Mutsaerts MA, Kuchenbecker WK, Broekmans FJ, Land JA, Mol BW, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women. Hum Reprod. 2012;27(2):457–67. doi: 10.1093/humrep/der416. [DOI] [PubMed] [Google Scholar]
- 40.Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421–39. doi: 10.1016/j.rbmo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Legge A, Bouzayen R, Hamilton L, Young D. The impact of maternal body mass index on in vitro fertilization outcomes. J Obstet Gynaecol Can. 2014;36(7):613–9. doi: 10.1016/S1701-2163(15)30541-7. [DOI] [PubMed] [Google Scholar]
- 42.Bailey AP, Hawkins LK, Missmer SA, Correia KF, Yanushpolsky EH. Effect of body mass index on in vitro fertilization outcomes in women with polycystic ovary syndrome. Am J Obstet Gynecol. 2014;211(2):163. doi: 10.1016/j.ajog.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Pinborg A, Gaarslev C, Hougaard CO, Nyboe Andersen A, Andersen PK, Boivin J, et al. Influence of female bodyweight on IVF outcome: a longitudinal multicentre cohort study of 487 infertile couples. Reprod Biomed Online. 2011;23(4):490–9. doi: 10.1016/j.rbmo.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Chavarro JE, Ehrlich S, Colaci DS, Wright DL, Toth TL, Petrozza JC, et al. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril. 2012;98(1):109–16. doi: 10.1016/j.fertnstert.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palomba S, Falbo A, Valli B, Morini D, Villani MT, Nicoli A, et al. Physical activity before IVF and ICSI cycles in infertile obese women: an observational cohort study. RBM Online. 2014;29(1):72–9. doi: 10.1016/j.rbmo.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Nouri K, Ott J, Stoegbauer L, Pietrowski D, Frantal S, Walch K. Obstetric and perinatal outcomes in IVF versus ICSI-conceived pregnancies at a tertiary care center—a pilot study. Reprod Biol Endocrinol. 2013;11:84. doi: 10.1186/1477-7827-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grady R, Alavi N, Vale R, Khandwala M, McDonald SD. Elective single embryo transfer and perinatal outcomes: a systematic review and meta-analysis. FertilSteril. 2012;97(2):324–31. doi: 10.1016/j.fertnstert.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 48.Acosta O, Ramirez VI, Lager S, Gaccioli F, Dudley DJ, Powell TL, et al. Increased glucose and placental GLUT-1 in large babies of obese non-diabetic mothers. Am J Obstet Gynecol. 2015;212(2):227.e1–7. doi: 10.1016/j.ajog.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Van Landuyt L, Van de Velde H, De Vos A, Haentjens P, Blockeel C, Tournaye H, et al. Influence of cell loss after vitrification or slow-freezing on further in vitro development and implantation of human Day 3 embryos. Hum Reprod. 2013;28(11):2943–9. doi: 10.1093/humrep/det356. [DOI] [PubMed] [Google Scholar]
- 50.Veleva Z, Orava M, Nuojua-Huttunen S, Tapanainen JS, Martikainen H. Factors affecting the outcome of frozen-thawed embryo transfer. Hum Reprod. 2013;28(9):2425–31. doi: 10.1093/humrep/det251. [DOI] [PubMed] [Google Scholar]
- 51.Pereira N, Reichman DE, Goldschlag DE, Lekovich JP, Rosenwaks Z. Impact of elevated peak serum estradiol levels during controlled ovarian hyperstimulation on the birth weight of term singletons from fresh IVF-ET cycles. J Assist Reprod Genet. 2015;32(4):527–32. doi: 10.1007/s10815-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]