Abstract
Purpose
The purpose of the study was to investigate changes in adiponectin system expression in granulosa cells (GCs) and high molecular weight adiponectin levels in serum and follicular fluid (FF) of 40 women with polycystic ovary syndrome (PCOS) compared to those in 40 women with normal ovary function.
Methods
Adiponectin (Adipo), adiponectin receptor 1 (AdipoR1), and adiponectin receptor 2 (AdipoR2) messenger RNA (mRNA) expression levels were measured using quantitative real-time polymerase chain reaction (qRT-PCR). High molecular weight (HMW) adiponectin protein concentration was evaluated by ELISA method. Data were analyzed using Student’s t test and one-way ANOVA in SPSS 21 software. At oocyte retrieval, FF was aspirated and GCs were obtained from a pooled collection of FF per each patient.
Results
PCR results showed expression of adiponectin, AdipoR1, AdipoR2, follicle-stimulating hormone receptor (FSHR), and luteinizing hormone receptor (LHR) in GCs. After controlling body mass index (BMI) values, qRT-PCR demonstrated a decreased expression of adiponectin system in GCs of PCOS patients compared to those in controls (p = 0.001). There was a strong positive correlation among AdipoR1 and AdipoR2 expression and also among FSH and LH receptor expression. (Both r = 0.8, p = 0.001). There were low levels of high molecular weight adiponectin in the serum of PCOS patients with controlled ovarian hyperstimulation (30.19 ± 4.3 ng/ml) compared to the controls (48.47 ± 5.9 ng/ml) and in the FF of PCOS patients with controlled ovarian hyperstimulation (7.86 ± 1.44 ng/ml) compared to the controls (14.22 ± 2.01 ng/ml; p = 0.02).
Conclusions
Lower expression of adiponectin and its receptors in GCs might be an important manifestation in gonadotropin-stimulated PCOS patients which could influence the physiologic adiponectin roles such as interaction with insulin and LH in induction of GC gene expression.
Keywords: Adiponectin, AdipoR1, AdipoR2, Follicular fluid, Granulosa cell (GC), HMW adiponectin
Introduction
Polycystic ovary syndrome (PCOS) as one of the most prevalent endocrine-metabolic disorders affects an estimated 5–10 % of women in reproductive age [1, 2]. Over the last decade, adipose tissue was introduced as a key element in the regulation of the endocrine system which controls appetite, lipid metabolism, and endocrine functions. Adiponectin is an important adipocytokine expressed by adipocytes [3]. It seems that the main active form of adiponectin, high molecular weight (HMW), has an insulin-sensitizing role, and circulating adiponectin concentrations are negatively correlated with body fat and tend to decrease in obesity [4, 5]. Moreover, adiponectin displays anti-inflammatory and anti-atherogenic properties. It is an insulin-sensitizer factor, and several studies have shown that high adiponectin levels may protect against the development of type 2 diabetes, metabolic syndrome, and cardiovascular disease [6–8]. Two adiponectin receptors, adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2), have been identified in many reproductive tissues such as the ovaries, placenta, endometrium, and oviduct [9, 10].
A growing body of evidence has shown that these receptors have important functions in granulosa cells (GCs). Low expression of AdipoR1 can induce apoptosis of human KGN (human ovarian granulosa-like tumor cell line) in GCs, while reduction of AdipoR2 may decrease steroid production [11, 12].
Ledoux et al. demonstrated that adiponectin, alone or with insulin and gonadotropins, induces production of prostaglandin and vascular endothelial growth factor (VEGF) in ovarian GCs. Adiponectin modulates the expression of steroid synthetic protein [13] and interacts with insulin and luteinizing hormone (LH) in induction of GC gene expression. Recent studies suggest that the effects of adiponectin signaling and adiponectin-mediated insulin sensitivity are linked to APPL1. This adaptor protein also attributed to gonadotropin signaling by association with follicle-stimulating hormone (FSH) receptor on the GCs [14, 15]. These findings indicate the remarkable interactions between gonadotropin, insulin, and adiponectin in the ovary [16]. In human preovulatory follicles, LH receptor (LHR) is also expressed in GCs and contributes to the follicular production of progesterone and estradiol [17, 18]. Follicle growth and oocyte development are closely regulated by an orderly complex series of signaling events during folliculogenesis [19, 20]. There is ample evidence that reduced insulin sensitivity plays a role in impairment of final maturation and ovulation of the follicles in PCOS ovaries. PCOS ovaries contain twice the number of growing follicles during all folliculogenesis stages [21] which suggest all stages may be disordered and there may be an intrinsic ovarian abnormality in PCOS. It remains unclear whether the resistance to atresia or arrest in the selection of follicles leads to the accumulation of multiple small antral follicles in polycystic ovaries [22]. In this study, expression of adiponectin, AdipoR1, and AdipoR2 receptors in GCs of PCOS patients were assessed in order to evaluate possible associations between the adiponectin system and gonadotropin receptors. In addition, high molecular weight (HMW) adiponectin levels in follicular fluid (FF) and serum of PCOS women and the possible influence of obesity on their expression were investigated.
Materials and methods
Subjects
This cross-sectional study was reviewed and approved by the Iran University of Medical Sciences Ethics Committee (IUMS, No 16169 on 40 PCOS women and 40 women with normal ovary function). An informed consent was obtained from each participant. PCOS women were tested and visited by a gynecologist who confirmed PCOS according to the 2003 Rotterdam criteria. At least two out of three of the following criteria were necessary for the diagnosis of PCOS: oligo ovulation and/or anovulation, an irregular menstrual cycle, clinical and/or biochemical signs of hyperandrogenism, and polycystic ovaries by transvaginal ultrasound. Patients with the following disorders were excluded: Cushing’s syndrome, non-classic congenital adrenal hyperplasia, androgen-secreting neoplasm, and any systemic disease that could possibly affect their reproductive physiology. The control group comprised infertile women with tubal obstruction and/or whose partners were diagnosed with male factor infertility. These women had no clinical or biochemical signs of hyperandrogenism or polycystic ovaries. Women with PCOS and controls were classified into four subgroups: obese (body mass index (BMI) ≥30) PCOS (n = 20), obese control (n = 20), non-obese (body mass index (BMI) <30) PCOS (n = 20), and non-obese control (n = 20). The mean age of the study population was 28.94 ± 5.1 years (range 19–42 years).
Patients and controls were treated with a standard long protocol with a GnRH agonist in the mid-luteal phase of the preceding menstrual cycle. Ovarian stimulation was initiated with recombinant FSH (Gonal-F, Merck Serono, Switzerland). Transvaginal sonography scans of the ovaries and blood sampling for estradiol (E2) determinations were performed every 1–3 days. The daily dose could be adjusted based on ovarian response after 3–5 treatment days. Human chorionic gonadotropin (hCG, Choriomon, IBSA, Lugano, Switzerland) in doses of 5000–10,000 IU was administered when more than two dominant follicles reached to a diameter of 18–20 mm. Transvaginal ultrasound-guided oocyte retrieval was performed 34–36 h after hCG injection.
Collection of GCs and FF
Granulosa cells (GCs) were obtained from a pooled collection of FF per patient. Immediately after isolation of the cumulus-oocyte complexes, purification was initiated. The pooled FF was centrifuged at 400g for 10 min. The supernatant was collected and stored at −80 °C for the ELISA assay analysis. GC cells were washed with 5 ml of Ham’s F-10 medium (Gibco, Life Technologies, Grand Island, NY, USA) and separated from the red blood cells with a 40–80 % Percoll (Amersham Biosciences, Uppsala, Sweden) density gradient. GCs were also separated from leukocytes with MicroBeads conjugated to the monoclonal anti-human CD45 antibodies (Miltenyi Biotec, MACS, USA). Purified GCs were washed with phosphate-buffered saline (PBS) (Sigma-Aldrich Germany) and resuspended for RNA isolation [23–27].
RNA isolation and cDNA synthesis
Total RNA was extracted from each GC pool using 1 ml of TRI reagent (Sigma-Aldrich, Pool, UK), according to the manufacturer’s procedure. Total RNA was treated with DNase I (Fermentas, Vilnius, Lithuania) to remove genomic DNA contamination from the samples. RNA purity and concentration were evaluated by optical density measurement with a NanoDrop spectrophotometer (BioTeK, USA). First-strand complementary DNA (cDNA) synthesis was performed using oligo-dT primers and reverse transcription by SuperScript II (Fermentas, Vilnius, Lithuania).
Primers
Primers were designed with AlleleID 6 software (Premier Bio soft Corporation, USA), and their efficacy was checked by preliminary tests on positive and negative controls. The primer sequences are shown in Table 1.
Table 1.
Adiponectin system, Gn-Rs, and r18s primer sequences used to amplify human adiponectin, AdipoR1, AdipoR2, FSHR, and LHR mRNA with RT-PCR and real-time PCR
| Gene | Forward primer (5′–3′) | Reverse primer (3′–5′) | Accession number | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|---|---|
| Adipo | GCCTACCACATCACAGTC | TCAGCATAGAGTCCATTACG | 001177800 | 46.6 | 200 |
| AdipoR1 | CAGGAAGAAGAGGAGGAG | TATGAATGCGGAAGATGC | 015999 | 53.8 | 226 |
| AdipoR2 | TGGTATTGCTCTTCTGATTATGG | AACACTCCTGCTCTTACTCC | 024551 | 45.8 | 183 |
| FSHR | GAGAGCAAGGTGACAGAGATTC | TTGATGTAGAGCAGGTTGTTGG | 000145 | 56.8 | 236 |
| LHR | CCGGTCTCACTCGACTATCA | ATGCTCCGGGCTCAATGTAT | 000069 | 52.3 | 210 |
| r18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | X03205 | 53.5 | 152 |
Adiponectin system: adiponectin, AdipoR1, and AdipoR2; Gn-Rs: FSHR and LHR
Reverse transcription–polymerase chain reaction
Reverse transcription polymerase chain reaction (RT-PCR) was performed with the constructed cDNAs, PCR Master Mix (Fermentas, Vilnius, Lithuania), and primers (Bioneer Corporation). The amplification was run for 35 cycles under the following conditions: 95 °C for 45 s, 45.8–56.8 °C for 45 s, 72 °C for 45 s with an initial denaturing step at 95 °C for 10 min, and a final extension step at 72 °C for 7 min. 18S ribosomal RNA (rRNA) was used as a positive control, and a PCR reaction without cDNA was used as a negative control. There was no difference in relative expression of 18S rRNA between the PCOS and control groups. The PCR product was visualized in a 1.2 % agarose gel and purified using an AccuPrep Gel Purification Kit (Bioneer, South Korea) sequenced in reverse (Bioneer, South Korea). The PCR product characteristics were determined using the Basic Local Alignment Search Tool (nBLAST, http://www.ncbi.nlm.nih.gov).
Quantitative real-time PCR
A quantitative real-time PCR (qRT-PCR) was performed in triplicate with the constructed cDNAs and the same primers that were used in the PCR reactions. QRT-PCR reactions were performed with a CFX96 real-time PCR detection system (BioRad, USA) in a total volume of 20 μl containing the following: 10 μl of SYBER Green (Takara, Japan), 6 μl of water, 5 pmol gene-specific primers, and 250 ng of cDNA. The cycle threshold (CT) values were normalized against the CT values of human 18S rRNA relative expression. The thermal cycle profile followed 40 cycles at 95 °C for 0.05 s, 45.8–56.8 °C for 30 s, and 72 °C for 30 s. The specificity of the PCR fragments was determined using melting curve analysis. All melting curves were generated on a peak per each PCR product. A logarithmic dilution series of total RNA was used to achieve standard curves.
The results were analyzed by the comparative CT method [28, 29]. In brief, the difference in cycle time (ΔCT) was calculated as the difference between the number of cycles required for the amplification of the target gene and the reference housekeeping gene, r18s. Then, ΔΔCT was calculated by finding the difference between the groups. The fold change was computed as Fc = 2− ΔΔCT.
Immunoassay
Serum and follicular fluid samples from each patient were centrifuged at 300g for 10 min. The supernatant was used to determine the concentration of high molecular weight (HMW) adiponectin measured by the sandwich enzyme immunoassay ELISA based on a monoclonal antibody specific for human HMW adiponectin (R&D Systems, USA), according to the manufacturer’s instructions. Briefly, a microplate coated with mouse monoclonal antibody specific for HMW adiponectin, a monoclonal antibody against HMW adiponectin conjugated to HRP (R&D System, USA), and the HRP substrate tetramethylbenzidine (R&D System, USA) as a substrate are used. Color change was measured spectrophotometrically at a wavelength of 450 nm. Concentration proteins in the samples are determined by comparing the OD of the samples to the standard curve. Serum concentrations of FSH and LH were measured using electrochemiluminescent immunoassay (ECLIA) method (Cobas, Roche, England).
Statistical analysis
The real-time PCR results were expressed as mean ± SEM. Statistical analysis was performed using the Student’s t test and one-way ANOVA with Tukey’s multiple comparisons in SPSS 21 software. A p value of <0.05 was considered statistically significant. Correlation between gene expression levels of adiponectin system and FSH receptor (FSHR) and LHR, as well as HMW adiponectin, was evaluated by Spearman’s correlation coefficient.
Results
Demographic characteristics and the basal hormonal and clinical parameters of the PCOS and control groups are summarized in Table 2. PCOS patients and controls had a similar mean age, BMI, and duration of infertility. As with most studies, LH levels and the LH/FSH ratio were higher in PCOS patients compared to controls (p = 0.001 and p = 0.01, respectively). There were no significant differences between the groups for FSH basal levels and total rFSH dose received by each patient (p = 0.57 and p = 0.23, respectively). The mean number of oocytes retrieved in the PCOS group was significantly higher than that in the control group (p = 0.04).
Table 2.
The clinical, demographical, and hormonal variables in normoovulatory controls and polycystic ovary syndrome (PCOS) patients undergoing IVF
| Variable | Control group N = 40 |
PCOS group N = 40 |
p value | ||
|---|---|---|---|---|---|
| BMI < 30 N = 20 |
BMI ≥30 N = 20 |
BMI <30 N = 20 |
BMI ≥30 N = 20 |
||
| Age (years) | 27.5 ± 4.3 | 27.55 ± 5.4 | 0.9 | ||
| 30.73 ± 5.6 | 30.38 ± 4.6 | 0.8 | |||
| BMI (kg/m2) | 25.61 ± 2.84 | 25.57 ± 2.88 | 0.96 | ||
| 32.18 ± 2.0 | 32.30 ± 2.8 | 0.87 | |||
| Duration of infertility | 4.84 ± 3.2 | 6.90 ± 4.4 | 4.69 ± 3.7 | 6.58 ± 3.8 | 0.15 |
| Basal serum FSH level (mIU/ml) | 6.54 ± 1.9 | 6.87 ± 2.5 | 0.57 | ||
| Basal serum LH level (mIU/ml) |
3.76 ± 1.7 | 8.1 ± 4.3 | 0.001 | ||
| LH/FSH ratio | 0.62 ± 0.39 | 1.03 ± 0.65 | 0.01 | ||
| Units of FSH administered | 1816.5 (1231.5–2401.5) |
1646.25 (757–2535) |
1783.5 (1063.5–2503.5) |
1567.5 (600–2535) |
0.23 |
| Numbers of oocytes retrieved | 10.04 | 14.21 | 0.04 | ||
| 7.57 | 11.88 | 0.04 | |||
Reference gene expression
In order to evaluate reference gene expression in the PCOS and control groups, the CT value of housekeeping gene was determined in each sample. The reactions were performed in triplicate, and the average of three values was considered as a CT value for 18S rRNA, an index of 18S rRNA gene expression. The calculated average of CT values for 18S rRNA was 19.45 ± 0.02 in the PCOS and 19.44 ± 0.02 in the control groups. There was no statistically significant difference in 18S RNA gene expression between the groups (p = 0.9).
Expression of adiponectin, AdipoR1, AdipoR2, FSHR, and LHR in GCs
Classic RT-PCR showed the expression profiles of adiponectin and AdipoR1 and AdipoR2 in GCs of control and PCOS patients. Moreover, FSHR and LHR messenger RNA (mRNA) expression was observed in purified GCs of PCOS and control groups. Control experiments with non-reverse transcribed RNA of each sample confirmed a lack of contamination by human DNA in the samples. The identity of all amplicons was confirmed by sequencing.
Alterations in adiponectin, AdipoR1, and AdipoR2 expression levels in GCs
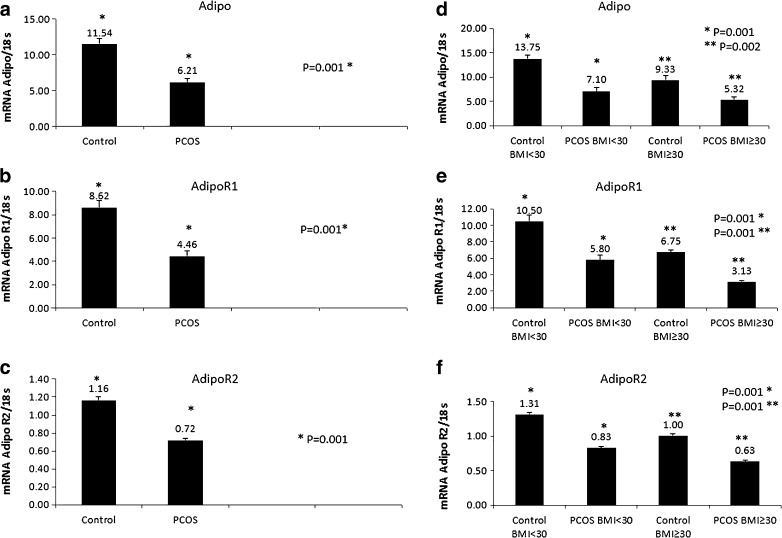
Subsequently, the relative expression of adiponectin, AdipoR1, and AdipoR2 in the GCs was measured by quantitative real-time PCR. As shown in Fig. 1, AdipoR2 expression was lower than adiponectin and AdipoR1 expression levels. Adiponectin system expression was significantly lower in PCOS patients compared to controls (p = 0.001, Fig. 1a–c). The adiponectin and AdipoR1 expression was almost twofold higher in GCs of control subjects compared to PCOSs. On the other hand, remarkable changes were observed in adiponectin, AdipoR1, and AdipoR2 gene expression in GCs based on BMI levels (Fig. 1d–f). Adiponectin system expression in obese PCOS women was significantly lower (BMI ≥30 kg/m²) than that in non-obese control women with BMI <30 kg/m² (Fig. 1). In addition, non-obese PCOS patients had statistically higher AdipoR2 expression levels compared to obese PCOS patients (p = 0.001). Adiponectin and AdipoR1 expression in obese PCOS subjects were also lower than those in non-obese PCOS subjects (Fig. 1).
Fig. 1.
Expression of adiponectin (Adipo), AdipoR1, and AdipoR2 mRNA levels in granulosa cells (GCs) of polycystic ovary syndrome (PCOS) and control groups. a Expression of Adipo in PCOS and control groups (p = 0.001). b Expression of AdipoR1 in PCOS and control groups (p = 0.001). c Expression of AdipoR2 in PCOS and control groups (p = 0.001). d Comparison of Adipo expression in patients with BMI <30 for control and PCOS groups (p = 0.001) and those with BMI ≥30 in the control and PCOS groups (p = 0.002). e Comparison of AdipoR1 expression among control and PCOS groups and in patients with BMI <30 and BMI ≥30 (p = 0.001, p = 0.001, respectively). f Comparison of AdipoR2 expression among PCOS and control groups and in patients with BMI <30 and BMI ≥30 (p = 0.001, p = 0.001, respectively). Data were analyzed by Student’s t test and one-way ANOVA with Tukey’s multiple comparison tests
Correlation between adiponectin levels and its receptors and expression of FSHR and LHR in GCs
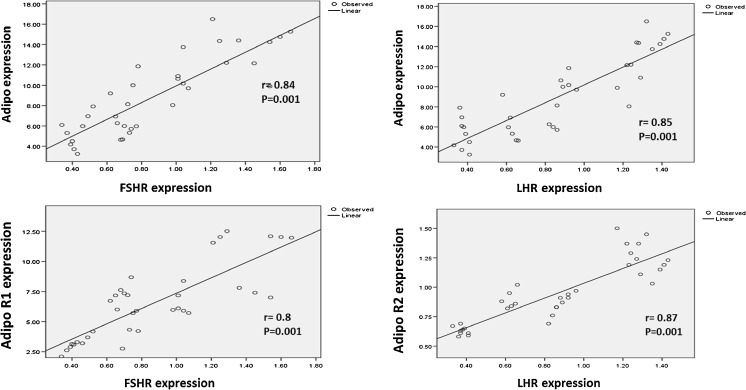
Adiponectin expression in GCs showed a positive correlation with FSHR expression (r = 0.84, p = 0.001) and with LHR expression (r = 0.85, p = 0.001). Moreover, a strong positive correlation was found between the expression of AdipoR1 and FSH receptor (r = 0.8, p = 0.001) and between the expression of AdipoR2 and LH receptor (r = 0.87, p = 0.001) (Fig. 2).
Fig. 2.
Linear regression shows significant correlations between adiponectin, AdipoR1, and AdipoR2 with FSH and LH receptors in the control and PCOS study groups
Low levels of HMW adiponectin in the serum and FF of women with PCOS undergoing controlled ovarian hyperstimulation
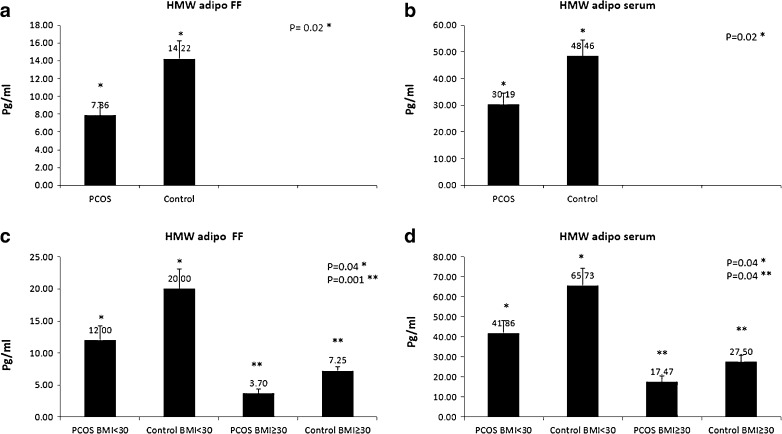
HMW adiponectin in serum and FF of PCOS and control groups was evaluated by ELISA technique. As shown in Fig. 3a, b, there were significantly lower HMW adiponectin values in the FF of PCOS women undergoing controlled ovarian hyperstimulation (30.19 ± 4.3 ng/ml) compared to controls (48.47 ± 5.9 ng/ml, p = 0.02) and in the serum of the PCOS women undergoing controlled hyperstimulation (7.86 ± 1.44 ng/ml) compared to control (14.22 ± 2.01 ng/ml, p = 0.02) women. Considering BMI groups (Fig 3c), HMW adiponectin levels in the FF of PCOS patients with both BMI <30 and ≥30 were significantly lower than those in controls with the same BMI (p = 0.04 and p = 0.001, respectively), and, also, the adiponectin levels in the serum samples of PCOS patients with BMI <30 and ≥30 were significantly lower than those in controls with BMI <30 and BMI ≥30 (Fig. 3d, in both p = 0.04). Compared to the HMW adiponectin levels in FF, the HMW adiponectin levels in the serum were significantly higher in the PCOS (p = 0.007) and control groups (p=0.002). Considering all subjects with and without PCOS undergoing controlled ovarian hyperstimulation, there was a strong positive correlation between the HMW adiponectin levels in serum and FF samples (r = 0.81, p = 0.001).
Fig. 3.
HMW adiponectin protein concentration in the follicular fluid (FF) and in the serum of PCOS and control groups evaluated by ELISA method. a, b FF and serum HMW adiponectin levels of PCOS patients were significantly lower than that of controls (p = 0.02). c Comparison of HMW adiponectin levels in FF of control and PCOS groups with BMI <30 (p = 0.04) and in those with BMI ≥30 (p = 0.001). d Comparison of serum HMW adiponectin levels between control and PCOS groups with BMI <30 (p = 0.04) and in those with BMI ≥30 (p = 0.04). Data were analyzed by Student’s t test and one-way ANOVA with Tukey’s multiple comparison tests
Discussion
Adiponectin has been extensively accepted as a main modulator of insulin sensitivity and metabolism [30–32], while physiological regulation and the role of adiponectin receptors, AdipoR1 and AdipoR2, and glucose intolerance in PCOS remain unresolved. This study showed a lower expression of adiponectin and its receptors in GCs of PCOS women compared to the normovulatory women undergoing IVF which seems to be an important manifestation in gonadotropin-stimulated PCOS women. Carmina et al. also demonstrated a lower level of adiponectin and HMW adiponectin in patients with PCOS compared to weight-matched controls [33–35]. However, some studies have not confirmed this finding [36–39]. Arriving at a definite answer as to whether adiponectin levels are lower (as expected), higher, or the same in the women with PCOS compared to the women with normal ovary function provides useful information regarding the pathogenesis and prognosis of PCOS. The results of the present study showed a detectable level of adiponectin expression in human GCs. Previous studies indicated very low levels or undetectable levels of adiponectin in mouse or human GCs which could be due to the different methodology or distinct demographic characteristics of study population. [11, 40]. According to a number of reports, adiponectin and its receptors AdipoR1 and AdipoR2 can be expressed in the ovarian cells [7, 41, 42]. Some researches have shown that adiponectin can modulate GC steroid genesis [13, 41] and the expression of genes associated with ovulation in both humans and animals [13, 43]. In the present study, AdipoR2 mRNA expression in GCs was significantly lower than AdipoR1 mRNA expression. Several studies suggest different roles for AdipoR1 and AdipoR2 in human in GCs [12, 44]. Reduction in AdipoR1 led to the apoptosis of human KGN GCs, whereas reduction in AdipoR2 decreases steroid synthesis [43, 45]. However, the mechanisms by which adiponectin modulates fertility and whether the disruption of AdipoR1/R2 causes any changes in ovarian function have not been addressed. Based on our finding, the obese women exhibit a reduced adiponectin receptor mRNA expression compared to non-obese control women. Adiponectin system expression was known to be inversely related to BMI in both PCOS and control women. Madsen et al. showed that weight loss should exceed 10 % in order to induce significant improvement in adiponectin [46]. However, our results which paralleled other investigations showed that increased BMI in PCOS women is associated with hypoadiponectinemia [47–49]. In line with our findings, García et al. in 2015 demonstrated that obese PCOS women have a significantly lower adiponectin serum levels compared to control women with obesity. Moreover, they showed a twofold higher AdipoR1 protein and gene expression in the obese group compared to obese PCOS. On the other hand, AdipoR2 protein and mRNA expression were similar in both groups. Indeed, adiponectin is downregulated by increased adipose tissue in obesity [36, 50]. It has been reported that low adiponectin levels are associated with progression towards type 2 diabetes mellitus [51, 52] and the possibility of increased risk for cardiovascular disease in women [53]. Moreover, both type 2 diabetes and cardiovascular disease are considered to be the major long-term health risks for women with PCOS [54]. Given the fact that low adiponectin levels could serve as a predictor to progression to type 2 diabetes and cardiovascular disease in women, it is reasonable to hypothesize that the adiponectin levels in PCOS may help recognize high-risk PCOS patients or possibly carry out an early treatment initiation. According to the current study results, there was no room for causality to be established and further investigation should be undertaken to confirm causality between the adiponectin system and expression of specific genes in GCs of IVF patients.
This study revealed a strong positive correlation between FSHR and LHR mRNA expression levels and adiponectin receptors. FSH stimulates follicular development via FSHR activation and induces GC proliferation, which play a crucial role in the selection of dominant follicle along with the expression levels of FSHR. It seems that these findings did not confirm the functional link between adiponectin system expression and FSHR expression; however, the findings greatly could suggest coordinate regulation of these genes. Meanwhile, functional links between the FSHR pathway, the adiponectin/AdipoR1/R2 pathways, and insulin receptor were observed in GCs, which suggest similar mechanisms might be involved in GCs [43]. Collectively, these results suggest that a decrease in cellular adiponectin system may alter the gonadotropin activities or vice versa. The relationship between LH signaling pathway and PCOS is still unknown. Beside the two-cell–two-gonadotropin theory, LH plays a critical role in folliculogenesis. Chen et al. demonstrated a strong association between PCOS and the LHR gene loci, and PCOS had a lower frequency of the LHR18inslq genotype compared to controls (24.9 vs. 0.28 %). Other studies, however, find contrasting results: significantly higher LHR [55, 56] and FSHR mRNA levels in PCOS compared to controls in GCs from both small and large follicles [57]. Several explanations for the alleged disparity in these findings can be hypothesized. Differences in the demographic features of the study populations (specifically ethnicity, different PCOS criteria, and phenotype), different clinical characteristics, and assisted reproductive technology (ART) may contribute to this disparity. In the present study, AdipoR2 expression was significantly associated with LH receptor. Indeed, AdipoR2 basal protein level is low and it will be upregulated in human GCs via activation of the PKA/cAMP pathway [58]. Moreover, significant positive correlation was observed between the low HMW adiponectin levels in the serum of women undergoing IVF and intrafollicular HMW adiponectin levels. Our findings support previous reports in humans proposing that decreased HMW adiponectin in the FF is associated with an increased number of follicles in normovulatory and PCOS [59].
Conclusions
In summary, PCOS women have a lower expression of adiponectin and its receptors in GCs compared to the normovulatory women undergoing IVF. This reduction may affect the follicular development and the selection of dominant follicle. Significantly low levels of HMW multimer form of adiponectin in FF and serum of PCOS women as a useful marker also could be implicated in the pathogenesis of ovulatory dysfunction. Moreover, reduction in gonadotropin and steroid receptors in GCs of PCOS women could provide further evidence of granulosa cell dysfunction in these patients. However, further investigations are required to determine the mechanisms of the potential contributions of alterations in the adiponectin system on ovulatory dysfunction in PCOS women.
Acknowledgments
We express our appreciation to the Research Center for Endometrium and Endometriosis and Research Center for Molecular Medicine, Hamadan University of Medical Sciences, for their technical assistance.
This work was supported by the Research and Technology Deputy of Iran University of Medical Sciences as a Ph.D. thesis (grant no. 16169).
Compliance with ethical standards
This cross-sectional study was reviewed and approved by the Iran University of Medical Sciences Ethics Committee. An informed consent was obtained from each participant.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Capsule The low expression of adiponectin and its receptors in PCOS patients could influence the oocyte quality and may lead to intrinsic oocyte abnormalities.
References
- 1.Webber LJ, Stubbs SA, Stark J, Margara RA, Trew GH, Lavery SA, et al. Prolonged survival in culture of preantral follicles from polycystic ovaries. J Clin Endocrinol Metab. 2007;92(5):1975–8. doi: 10.1210/jc.2006-1422. [DOI] [PubMed] [Google Scholar]
- 2.Sartor BM, Dickey RP. Polycystic ovarian syndrome and the metabolic syndrome. Am J Med Sci. 2005;330(6):336–42. doi: 10.1097/00000441-200512000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 4.Takemura Y, Osuga Y, Yamauchi T, Kobayashi M, Harada M, Hirata T, et al. Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology. 2006;147(7):3203–10. doi: 10.1210/en.2005-1510. [DOI] [PubMed] [Google Scholar]
- 5.Guerre-Millo M. Adiponectin: an update. Diabetes Metab. 2008;34(1):12–8. doi: 10.1016/j.diabet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Maddineni S, Metzger S, Ocon O, Hendricks G, 3rd, Ramachandran R. Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology. 2005;146(10):4250–6. doi: 10.1210/en.2005-0254. [DOI] [PubMed] [Google Scholar]
- 7.Chabrolle C, Tosca L, Crochet S, Tesseraud S, Dupont J. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) in chicken ovary: potential role in ovarian steroidogenesis. Domest Anim Endocrinol. 2007;33(4):480–7. doi: 10.1016/j.domaniend.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Chappaz E, Albornoz MS, Campos D, Che L, Palin MF, Murphy BD, et al. Adiponectin enhances in vitro development of swine embryos. Domest Anim Endocrinol. 2008;35(2):198–207. doi: 10.1016/j.domaniend.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 10.Michalakis KG, Segars JH. The role of adiponectin in reproduction: from polycystic ovary syndrome to assisted reproduction. Fertility and Sterility. 2010;94(6). [DOI] [PMC free article] [PubMed]
- 11.Richards JS, Liu Z, Kawai T, Tabata K, Watanabe H, Suresh D, et al. Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human. Fertil Steril. 2012;98(2):471–9. doi: 10.1016/j.fertnstert.2012.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierre P, Froment P, Negre D, Rame C, Barateau V, Chabrolle C et al. Role of adiponectin receptors, AdipoR1 and AdipoR2, in the steroidogenesis of the human granulosa tumor cell line, KGN. Human reproduction. 2009:dep292. [DOI] [PubMed]
- 13.Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology. 2006;147(11):5178–86. doi: 10.1210/en.2006-0679. [DOI] [PubMed] [Google Scholar]
- 14.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8(5):516–23. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 15.Nechamen CA, Thomas RM, Cohen BD, Acevedo G, Poulikakos PI, Testa JR, et al. Human follicle-stimulating hormone (FSH) receptor interacts with the adaptor protein APPL1 in HEK 293 cells: potential involvement of the PI3K pathway in FSH signaling. Biol Reprod. 2004;71(2):629–36. doi: 10.1095/biolreprod.103.025833. [DOI] [PubMed] [Google Scholar]
- 16.Mihm M, Evans A. Mechanisms for dominant follicle selection in monovulatory species: a comparison of morphological, endocrine and intraovarian events in cows, mares and women. Reprod Domest Anim. 2008;43(s2):48–56. doi: 10.1111/j.1439-0531.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- 17.Jeppesen JV, Kristensen SG, Nielsen ME, Humaidan P, Dal Canto M, Fadini R, et al. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97(8):E1524–31. doi: 10.1210/jc.2012-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yding Andersen C, Bungum L, Nyboe Andersen A, Humaidan P. Preovulatory progesterone concentration associates significantly to follicle number and LH concentration but not to pregnancy rate. Reprod biomed online. 2011;23(2):187–95. doi: 10.1016/j.rbmo.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11(4):391–410. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- 20.Voronina E, Lovasco LA, Gyuris A, Baumgartner RA, Parlow AF, Freiman RN. Ovarian granulosa cell survival and proliferation requires the gonad-selective TFIID subunit TAF4b. Dev Biol. 2007;303(2):715–26. doi: 10.1016/j.ydbio.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra‐ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10(2):107–17. doi: 10.1093/humupd/dmh010. [DOI] [PubMed] [Google Scholar]
- 22.Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, et al. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(3):881–7. doi: 10.1210/jc.2007-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrero H, Delgado-Rosas F, Garcia-Pascual CM, Monterde M, Zimmermann RC, Simón C, et al. Efficiency and purity provided by the existing methods for the isolation of luteinized granulosa cells: a comparative study. Hum Reprod. 2012;27(6):1781–9. doi: 10.1093/humrep/des096. [DOI] [PubMed] [Google Scholar]
- 24.Acosta E, Peña Ó, Naftolin F, Ávila J, Palumbo A. Angiotensin II induces apoptosis in human mural granulosa-lutein cells, but not in cumulus cells. Fertil Steril. 2009;91(5):1984–9. doi: 10.1016/j.fertnstert.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Quinn M, McGregor S, Stanton J, Hessian P, Gillett W, Green D. Purification of granulosa cells from human ovarian follicular fluid using granulosa cell aggregates. Reprod Fertil Dev. 2006;18(5):501–8. doi: 10.1071/RD05051. [DOI] [PubMed] [Google Scholar]
- 26.Chilvers R, Bodenburg Y, Denner L, Urban R. Development of a novel protocol for isolation and purification of human granulosa cells. J Assist Reprod Genet. 2012;29(6):547–56. doi: 10.1007/s10815-012-9739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centurione L, Giampietro F, Sancilio S, Piccirilli M, Artese L, Tiboni GM, et al. Morphometric and ultrastructural analysis of human granulosa cells after gonadotrophin-releasing hormone agonist or antagonist. Reprod biomed online. 2010;20(5):625–33. doi: 10.1016/j.rbmo.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2< sup>− ΔΔCT</sup> method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 30.Blüher M, Williams CJ, Klöting N, Hsi A, Ruschke K, Oberbach A, et al. Gene expression of adiponectin receptors in human visceral and subcutaneous adipose tissue is related to insulin resistance and metabolic parameters and is altered in response to physical training. Diabetes Care. 2007;30(12):3110–5. doi: 10.2337/dc07-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol. 2008;22(5):1023–31. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27(7):762–78. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 33.Carmina E, Bucchieri S, Mansueto P, Rini G, Ferin M, Lobo RA. Circulating levels of adipose products and differences in fat distribution in the ovulatory and anovulatory phenotypes of polycystic ovary syndrome. Fertil Steril. 2009;91(4):1332–5. doi: 10.1016/j.fertnstert.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Aroda V, Ciaraldi TP, Chang S-A, Dahan MH, Chang RJ, Henry RR. Circulating and cellular adiponectin in polycystic ovary syndrome: relationship to glucose tolerance and insulin action. Fertil Steril. 2008;89(5):1200–8. doi: 10.1016/j.fertnstert.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N, Shi Y-H, Hao C-F, Gu HF, Li Y, Zhao Y-R, et al. Association of +45G15G (T/G) and +276 (G/T) polymorphisms in the ADIPOQ gene with polycystic ovary syndrome among Han Chinese women. Eur J Endocrinol. 2008;158(2):255–60. doi: 10.1530/EJE-07-0576. [DOI] [PubMed] [Google Scholar]
- 36.Orio F, Jr, Palomba S, Cascella T, Milan G, Mioni R, Pagano C, et al. Adiponectin levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(6):2619–23. doi: 10.1210/jc.2002-022033. [DOI] [PubMed] [Google Scholar]
- 37.Panidis D, Kourtis A, Farmakiotis D, Mouslech T, Rousso D, Koliakos G. Serum adiponectin levels in women with polycystic ovary syndrome. Hum Reprod. 2003;18(9):1790–6. doi: 10.1093/humrep/deg353. [DOI] [PubMed] [Google Scholar]
- 38.Shroff R, Kerchner A, Maifeld M, Van Beek EJ, Jagasia D, Dokras A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab. 2007;92(12):4609–14. doi: 10.1210/jc.2007-1343. [DOI] [PubMed] [Google Scholar]
- 39.Barber TM, Hazell M, Christodoulides C, Golding SJ, Alvey C, Burling K, et al. Serum levels of retinol-binding protein 4 and adiponectin in women with polycystic ovary syndrome: associations with visceral fat but no evidence for fat mass-independent effects on pathogenesis in this condition. J Clin Endocrinol Metab. 2008;93(7):2859–65. doi: 10.1210/jc.2007-2759. [DOI] [PubMed] [Google Scholar]
- 40.Chabrolle C, Tosca L, Rame C, Lecomte P, Royere D, Dupont J. Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil Steril. 2009;92(6):1988–96. doi: 10.1016/j.fertnstert.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Chabrolle C, Tosca L, Dupont J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction. 2007;133(4):719–31. doi: 10.1530/REP-06-0244. [DOI] [PubMed] [Google Scholar]
- 42.Maillard V, Uzbekova S, Guignot F, Perreau C, Ramé C, Coyral-Castel S, et al. Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod Biol Endocrinol. 2010;8(23):10.1186. doi: 10.1186/1477-7827-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campos DB, Palin MF, Bordignon V, Murphy BD. The ‘beneficial’ adipokines in reproduction and fertility. Int J Obes (Lond) 2008;32(2):223–31. doi: 10.1038/sj.ijo.0803719. [DOI] [PubMed] [Google Scholar]
- 44.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 45.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145(1):367–83. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 46.Madsen EL, Rissanen A, Bruun JM, Skogstrand K, Tonstad S, Hougaard DM, et al. Weight loss larger than 10% is needed for general improvement of levels of circulating adiponectin and markers of inflammation in obese subjects: a 3-year weight loss study. Eur J Endocrinol. 2008;158(2):179–87. doi: 10.1530/EJE-07-0721. [DOI] [PubMed] [Google Scholar]
- 47.Spranger J, Möhlig M, Wegewitz U, Ristow M, Pfeiffer AF, Schill T, et al. Adiponectin is independently associated with insulin sensitivity in women with polycystic ovary syndrome. Clin Endocrinol. 2004;61(6):738–46. doi: 10.1111/j.1365-2265.2004.02159.x. [DOI] [PubMed] [Google Scholar]
- 48.Orio F, Palomba S, Zullo F, Colao A, Lombard G. Are serum adiponectin levels really reduced in obese women with polycystic ovary syndrome? Hum Reprod. 2004;19:215–6. doi: 10.1093/humrep/deh013. [DOI] [PubMed] [Google Scholar]
- 49.Ardawi MS, Rouzi AA. Plasma adiponectin and insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 2005;83(6):1708–16. doi: 10.1016/j.fertnstert.2004.11.077. [DOI] [PubMed] [Google Scholar]
- 50.Garcia V, Orostica L, Poblete C, Rosas C, Astorga I, Romero C, et al. Endometria from obese PCOS women with hyperinsulinemia exhibit altered adiponectin signaling. Horm Metab Res. 2015 doi: 10.1055/s-0035-1555806. [DOI] [PubMed] [Google Scholar]
- 51.Schwarz PE, Towers GW, Fischer S, Govindarajalu S, Schulze J, Bornstein SR, et al. Hypoadiponectinemia is associated with progression toward type 2 diabetes and genetic variation in the ADIPOQ gene promoter. Diabetes Care. 2006;29(7):1645–50. doi: 10.2337/dc05-2123. [DOI] [PubMed] [Google Scholar]
- 52.Jalovaara K, Santaniemi M, Timonen M, Jokelainen J, Kesäniemi YA, Ukkola O, et al. Low serum adiponectin level as a predictor of impaired glucose regulation and type 2 diabetes mellitus in a middle-aged Finnish population. Metabolism. 2008;57(8):1130–4. doi: 10.1016/j.metabol.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Zyriax B-C, Algenstaedt P, Hess UF, Schöffauer M, Bamberger C, Boeing H, et al. Factors contributing to the risk of cardiovascular disease reflected by plasma adiponectin: data from the coronary risk factors for atherosclerosis in women (CORA) study. Atherosclerosis. 2008;200(2):403–9. doi: 10.1016/j.atherosclerosis.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 54.Rotterdam E, ASRM-Sponsored P. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Human reprod (Oxford, England) 2004;19(1):41. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 55.Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries 1. J Clin Endocrinol Metab. 2001;86(3):1318–23. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- 56.Liu N, Ma Y, Wang S, Zhang X, Zhang Q, Zhang X, et al. Association of the genetic variants of luteinizing hormone, luteinizing hormone receptor and polycystic ovary syndrome. Reprod Biol Endocrinol. 2012;10(1):36. doi: 10.1186/1477-7827-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catteau-Jonard S, Jamin SP, Leclerc A, Gonzalès J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(11):4456–61. doi: 10.1210/jc.2008-1231. [DOI] [PubMed] [Google Scholar]
- 58.Wickham EP, 3rd, Tao T, Nestler JE, McGee EA. Activation of the LH receptor up regulates the type 2 adiponectin receptor in human granulosa cells. J Assist Reprod Genet. 2013;30(7):963–8. doi: 10.1007/s10815-013-0012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Amn J Physiology-Endocrinol Metab. 2009;296(1):E22–36. doi: 10.1152/ajpendo.90731.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]