Abstract
Screening for founder mutations in BRCA1 and BRCA2 has been discussed as a cost-effective testing strategy in certain populations. In this study, comprehensive BRCA1 and BRCA2 testing was performed in a routine diagnostic setting. The prevalence of the BRCA1 stop mutation c.4183C>T, p.(Gln1395Ter), was determined in unselected breast and ovarian cancer patients from different regions in the Tyrol. Cancer registry data were used to evaluate the impact of this mutation on regional cancer incidence. The mutation c.4183C>T was detected in 30.4% of hereditary BRCA1-associated breast and ovarian cancer patients in our cohort. It was also identified in 4.1% of unselected (26% of unselected triple negative) Tyrolean breast cancer patients and 6.8% of unselected ovarian cancer patients from the Lower Inn Valley (LIV) region. Cancer incidences showed a region-specific increase in age-stratified breast and ovarian cancer risk with standardized incidence ratios of 1.23 and 2.13, respectively. We, thus, report a Tyrolean BRCA1 founder mutation that correlates to a local increase in the breast and ovarian cancer risks. On the basis of its high prevalence, we suggest that targeted genetic analysis should be offered to all women with breast or ovarian cancer and ancestry from the LIV region.
Introduction
A central aim of preventive oncology is the identification of patients with a heritable predisposition to cancer. An estimated 1–7% of unselected breast cancer patients1, 2, 3, 4 and 6–17% of women with ovarian cancer5, 6 have hereditary breast and ovarian cancer (HBOC) disposition caused by variants in either BRCA1 or BRCA2 that confer a high risk of developing cancer. In agreement with a widely used terminology, such variants will be called mutations throughout this paper. Because of high costs, comprehensive molecular analyses for HBOC are usually restricted to individuals with a suggestive family history, young age, or several independent tumours. Tumour characteristics (triple-negative breast cancer; TNBC) are also used for the identification of patients with a high probability of carrying a BRCA mutation.7, 8
Reduced costs of novel sequencing methods and better knowledge of the clinical relevance of alterations in BRCA1 and BRCA2 will increase the number of individuals eligible for sequence analysis, although some selection process will still remain necessary in the foreseeable future. Identification of common founder mutations in certain ethnicities allows integration of genetic testing strategies in the routine care of all cancer patients. Here we report the identification of a highly prevalent BRCA1 founder mutation in a particular region of the Tyrol and consider the implications for a screening programme offered to all breast and ovarian cancer patients in this region.
Materials and methods
Patients
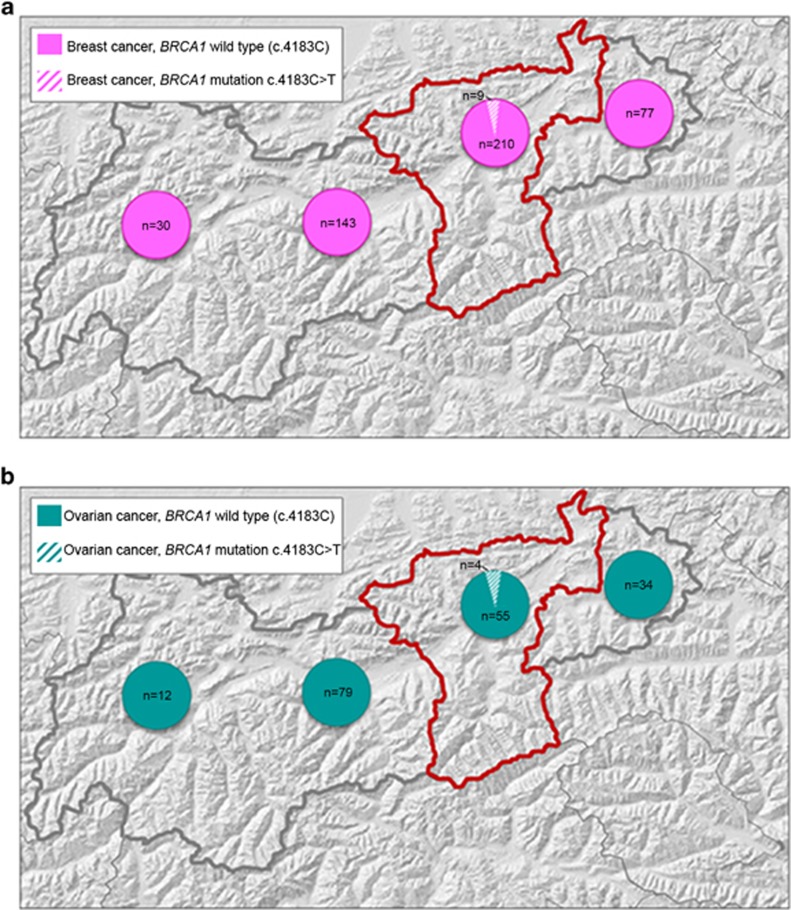
The diagnostic cohort of this report consists of 238 apparently independent index individuals. Details are described in Supplementary Information. Presence of BRCA1 stop mutation c.4183C>T was investigated in tumour samples from a research cohort of unselected Tyrolean individuals with breast cancer (n=471) and ovarian cancer (n=186). Details are described in Supplementary Table S1. Breast and ovarian cancer samples were selected to fall each into two matching sub-groups based on patients' home address: one sub-group comprised 219 breast and 59 ovarian cancer patients from the Lower Inn Valley (LIV) region (districts Schwaz and Kufstein). The other sub-group included 250 breast and 125 ovarian cancer patients from other districts of the North Tyrol. Number of analysed cases according to the districts can be seen in Figures 1a and b.
Figure 1.
(a) Number and distribution of breast cancer cases genotyped for the BRCA1 mutation c.4183C>T in the North Tyrol. (b) Number and distribution of ovarian cancer cases genotyped for the BRCA1 mutation c.4183C>T in the North Tyrol. Grey outline: the Austrian federal state of the Tyrol; red outline: the Lower Inn Valley region (districts Schwaz and Kufstein). Filled parts of circles: number of cases without the mutation. Hatched parts of circles: number of mutation carriers.
The study was approved by the Ethics Committee of the Medical University Innsbruck (reference number: UN4848).
A detailed version of Materials and Methods can be found in Supplementary Information and Supplementary Table S2.
Results
Identification of a Tyrolean BRCA1 founder mutation
BRCA1 and BRCA2 mutations were identified in 70 index individuals from 238 seemingly unrelated families with suspected HBOC (29.4%). Forty-six index patients (65.7%) carried 21 different BRCA1 mutations and 24 patients (34.3%) carried 23 different BRCA2 mutations (Supplementary Table S3). The same BRCA1 mutation NM_007294.2: c.4183C>T was identified in 14 seemingly unrelated families, representing 30.4% of BRCA1-associated HBOC families in our cohort (cases of ovarian/lower abdominal cancer were reported in 8/13 families for which information was available). Evaluation of the regional origin of these families revealed that 11 of them were from the LIV region, with the majority from the Zillertal, a large connected side valley of this region. Assessment of sequencing data in informative c.4183C>T mutation carriers showed that with the exception of a patient from Germany, all 13 c.4183C>T mutation carriers from the Tyrol had SNV genotypes compatible with a haplotype described as B1 allele by Frosk et al.9 Genotyping of all 13 Tyrolean c.4183C>T mutation carriers in the diagnostic cohort was compatible with a common haplotype c.[2082 T; 2311C; 2612 T; 3113G] D17S1323[171] D17S1322[136] D17S855[145] on one allele. Segregation analysis in three informative families confirmed this haplotype on the mutant allele (data not shown).
High prevalence of BRCA1 mutation c.4183C>T in unselected Tyrolean breast and ovarian cancer patients
More than 75% (11/14) of molecularly confirmed HBOC families from the LIV region carried the founder mutation c.4183C>T. To assess the prevalence of this mutation in a larger number of individuals, we examined a total of 219 breast and 59 ovarian cancer samples from unselected patients living in that region. Mutation frequencies were compared with matching breast (n=250) and ovarian (n=125) cancer samples from patients living in other regions of the Tyrol. Although the mutation was detected in 4.1% (9/219) of breast cancer and 6.8% (4/59) of ovarian cancer samples from patients of the LIV, it was absent in all other Tyrolean samples (Figures 1a and b). This difference was highly significant (P=0.001, respectively, P=0.011, using χ2-test). SNV genotyping in all tumours with the BRCA1 mutation c.4183C>T was compatible with at least one B-allele present.
Increased breast and ovarian cancer incidence in regions with a high prevalence of BRCA1 mutation c.4183C>T
Considering that the majority of known families with the BRCA1 c.4183C>T mutation originate from the Zillertal, we subdivided the LIV region into the Zillertal (denoted LIV-Z) and the main LIV (denoted LIV-M) for statistical analysis of breast and ovarian cancer incidence. Age-stratified evaluation of data from the Tyrolean Cancer Registry based on incidence data from 2001 to 2010 revealed incremental incidence numbers of breast and ovarian cancer from the North Tyrol to LIV-M and LIV-Z (Table 1). The risk increase did not meet statistical significance for breast cancer, with an age-adjusted SIR of 1.05 (0.93–1.19) in LIV-M (only marginal increase) and 1.23 (0.93–1.58) in the Zillertal region (LIV-Z). Age-adjusted SIR for ovarian cancer below 65 years was significantly increased in both, LIV-M with a SIR of 1.34 (1.05–1.69) and LIV-Z with a SIR of 2.13 (1.29–3.33). Notably, women from the Zillertal below the age of 65 years had a cumulative risk of 1 in 99 for ovarian cancer and thus were twice as likely to be diagnosed with ovarian cancer than women of this age group in the North Tyrol outside the LIV region (1 in 198).
Table 1. Breast and ovarian cancer incidence and risk by geographic region.
| Cases observed | SIR age standardized | Cumulative risk | |
|---|---|---|---|
| Breast cancer risk (<50) | |||
| LIV-Z | 59 | 1.23 (0.93–1.58) | 1 in 46 |
| LIV-M | 260 | 1.05 (0.93–1.19) | 1 in 53 |
| North Tyrol | 915 | 1 | 1 in 57 |
| Ovarian cancer risk (<65) | |||
| LIV-Z | 19 | 2.13 (1.29–3.33) | 1 in 99 |
| LIV-M | 74 | 1.34 (1.05–1.69) | 1 in 146 |
| North Tyrol | 230 | 1 | 1 in 198 |
Abbreviations: LIV-Z, Zillertal; LIV-M, Lower Inn Valley excluding the Zillertal; SIR, standardized incidence ratio.
95% confidence interval is given in brackets. Significant values in bold.
Clinical and tumour characteristics in BRCA1 c.4183C>T mutation carriers
There were no significant differences in clinical and tumour characteristics between the breast or ovarian cancer research cohorts from the LIV and the other Tyrolean regions (Supplementary Table S1). Clinicopathological features of LIV patients with and without the BRCA1 mutation c.4183C>T are shown in Table 2. The mutation was identified in 16.7% (7/42) and 15.1% (8/53) of oestrogen or progesterone receptor negative tumours, respectively. The majority of breast cancers from c.4183C>T mutation carriers were triple-negative tumours (7/9); vice versa, the mutation was detected in 7 out of 27 triple-negative tumours (25.9%). TNBC in a patient from the LIV was calculated to predict the BRCA1 founder mutation with a sensitivity of 77.8% and a specificity of 90.5% (Pearson's χ2-test: P<0.0001).
Table 2. Comparison of BRCA1 c.4183C>T mutation carriers and non-carriers from the Lower Inn Valley region.
| Non-carriers (n=210) | BRCA1 c.4183C>T carriers (n=9) | P-valuea | |
|---|---|---|---|
| A. Breast cancer patients | |||
| Patient age at diagnosis (years) | |||
| Median | 56.7 | 41.0 | 0.002 |
| Range | 29.5–84.0 | 22.3–65.7 | |
| Sample age (years) | |||
| Median | 4.8 | 3.9 | NS |
| Range | 1.4–11.1 | 2.3–7.0 | |
| Tumour grade | |||
| I | 28 | 0 | 0.018 |
| II | 159 | 5 | |
| III | 22 | 4 | |
| Unknown | 1 | 0 | |
| Histology | |||
| Invasive lobular carcinoma | 1 | 0 | NS |
| Invasive ductalcarcinoma | 151 | 7 | |
| Mixed: invasive ductalcarcinoma and DCIS | 54 | 1 | |
| Medullary carcinoma | 4 | 1 | |
| MP | |||
| Premenopausal | 74 | 7 | 0.035 |
| Postmenopausal | 135 | 2 | |
| Unknown | 1 | 0 | |
| HER2 | |||
| Neg | 180 | 9 | NS |
| Pos | 28 | 0 | |
| Unknown | 2 | 0 | |
| ER | |||
| Neg | 35 | 7 | <0.001 |
| Pos | 175 | 2 | |
| Unknown | 0 | 0 | |
| PR | |||
| Neg | 45 | 8 | <0.001 |
| Pos | 165 | 1 | |
| Unknown | 0 | 0 | |
| HER2-ER-PR | |||
| Neg (triple-negative tumours) | 20 | 7 | <0.001 |
| Pos | 190 | 2 | |
| Unknown | 0 | 0 | |
| Non-carriers (n=55) | BRCA1 c.4183C>T carriers (n=4) | P-valuea | |
| B. Ovarian cancer patients | |||
| Patient age at diagnosis (years) | |||
| Median | 59.1 | 56.7 | NS |
| Range | 39.5–84.1 | 50.3–62.4 | |
| Sample age (years) | |||
| Median | 7.9 | 11.0 | NS |
| Range | 1.4–13.8 | 8.8–13.9 | |
| FIGO | |||
| I | 10 | 0 | NS |
| II | 2 | 0 | |
| III | 36 | 4 | |
| IV | 7 | 0 | |
| Unknown | 0 | 0 | |
Abbreviations: Neg, negative; NS, not significant; Pos, positive. Bold P-values are significant at the 0.05 level.
The χ2-test was used for testing associations between clinicopathological features and the mutation status and the Mann–Whitney U-test was used for the analysis of associations between the age and the mutation status.
Discussion
We identified BRCA1 or BRCA2 mutations in 30% of families at risk for HBOC, with a BRCA1:BRCA2 ratio of 1.9:1. Six truncating mutations have not been published previously (Supplementary Table S3). Three alterations listed as mutations are classified as variants of unknown significance in the Breast Cancer Information Core database.10 Nevertheless, there is strong evidence to consider them as deleterious; see Supplementary Table S3.
The BRCA1 mutations c.3018_3021delTTCA and c.4183C>T were the most frequent mutations, found seven and 14 times, respectively. Mutation c.3018_3021delTTCA has been previously reported as an Austrian founder mutation11 but is infrequent in persons of Tyrolean origin.
The BRCA1 mutation c.4183C>T represented 30.4% of all identified BRCA1 mutation alleles in our cohort. This recurrent mutation4 is recorded in several databases10, 12, 13 and has been previously observed in Austria, albeit with lower frequencies than in our cohort.11, 14 We found this mutation only in individuals who lived in the Tyrol, an alpine province characterized by confined valleys separated by huge mountain ranges historically forming isolates. Intriguingly, the c.4183C>T mutation was responsible for >75% of seemingly unrelated HBOC families in the LIV region, and in particular the major side valley Zillertal.
The high frequency and relatively restricted geographic origin of c.4183C>T mutation carriers in our diagnostic patients suggested a regional founder effect that was supported by a common haplotype shared among all Tyrolean c.4183C>T carriers. To further substantiate this assumption, we determined the prevalence of the BRCA1 mutation c.4183C>T in unselected breast and ovarian cancer samples from different Tyrolean regions. The mutation was found in 4% of breast and 7% of ovarian cancer samples from the LIV region but was not detected in samples from other regions of the Tyrol. The highest prevalence was found in patients from the Zillertal, confirming the observation in our diagnostic cohort. Even considering the common mutation only, breast cancer patients from the LIV have a much higher probability of being affected by HBOC than patients in other regions. Comprehensive analysis of BRCA1 in unselected breast cancer cohorts found mutation prevalence rates of 0.7–1.3% in British studies1, 2 and 3.3% in a US-based study.3 Twenty-six percentage of breast cancer patients with TNBC from the LIV region and 50% of patients from the Zillertal carried the BRCA1 mutation c.4183C>T. This is a much higher percentage than the 10–20% seen in other studies of patients with TNBC unselected for family history.7, 8, 15
Considering the results from both our diagnostic and research cohorts, we wondered whether the high prevalence of mutation c.4183C>T in the Zillertal and LIV region is associated with an increased incidence of cancer. Detailed regional data from the Tyrolean Cancer Registry confirmed an increased risk for breast and ovarian cancer especially in the region of Zillertal with SIRs of 1.23 for breast cancer below the age of 50 and 2.13 years for ovarian cancer below the age of 65 years.
Our data suggest that HBOC due to the prevalent founder mutation c.4183C>T is an important health problem in breast and ovarian cancer patients and their families from the LIV and should be addressed in the care of patients from this region. Prompt and cost efficient mutation identification is becoming clinically highly relevant and will increasingly be so, considering the trend towards individualised tumour therapy such as PARP inhibitors.16, 17 Ancestry-informed testing of a few common BRCA1/2 mutations has been proposed as a cost-effective approach in a number of populations including Ashkenazi Jews.18, 19, 20, 21, 22, 23, 24, 25 Diagnosing BRCA mutations triggers preventive measures in relatives, which contributes to the value of such screening approaches.21 The identification of a prevalent founder mutation in a very specific part of the Tyrol may facilitate a long-term study to examine the true sensitivity, usefulness, and cost-effectiveness of routine testing of breast or ovarian cancer patients without any selection based on established HBOC criteria.
Acknowledgments
This project was supported by a grant by the Tyrolean Wissenschaftsfonds UNI-0404/1458 to LP. Work in the Division of Human Genetics was supported by the Propter Homines Foundation, Liechtenstein. Work in the Department of Obstetrics and Gynaecology was supported by the Competence Center Oncotyrol. We thank I. Gaugg and A. Ruetz-Sapinsky for their excellent technical assistance. We also wish to acknowledge the expert pathology assistance and long-term contributions of the late Professor Elisabeth Müller-Holzner, Department of Obstetrics and Gynaecology, Medical University of Innsbruck.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- 1Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer 2000; 83: 1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Peto J, Collins N, Barfoot R et al: Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 1999; 91: 943–949. [DOI] [PubMed] [Google Scholar]
- 3Newman B, Mu H, Butler LM, Millikan RC, Moorman PG, King MC: Frequency of breast cancer attributable to BRCA1 in a population-based series of American women. JAMA 1998; 279: 915–921. [DOI] [PubMed] [Google Scholar]
- 4Langston AA, Malone KE, Thompson JD, Daling JR, Ostrander EA: BRCA1 mutations in a population-based sample of young women with breast cancer. N Engl J Med 1996; 334: 137–142. [DOI] [PubMed] [Google Scholar]
- 5Ramus SJ, Gayther SA: The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol Oncol 2009; 3: 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Cancer Genome Atlas Research Network: Integrated genomic analyses of ovarian carcinoma. Nature 2011; 474: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Robertson L, Hanson H, Seal S et al: BRCA1 testing should be offered to individuals with triple-negative breast cancer diagnosed below 50 years. Br J Cancer 2012; 106: 1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Hartman AR, Kaldate RR, Sailer LM et al: Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer 2012; 118: 2787–2795. [DOI] [PubMed] [Google Scholar]
- 9Frosk P, Burgess S, Dyck T, Jobse R, Spriggs EL: The use of ancestral haplotypes in the molecular diagnosis of familial breast cancer. Genet Test 2007; 11: 208–215. [DOI] [PubMed] [Google Scholar]
- 10World Wide Web: The Breast Cancer Information Core mutation database. (Online). Available at: http://research.nhgri.nih.gov/bic/index.shtml. Accessed on April 2015.
- 11Wagner TM, Moslinger RA, Muhr D et al: BRCA1-related breast cancer in Austrian breast and ovarian cancer families: specific BRCA1 mutations and pathological characteristics. Int J Cancer 1998; 77: 354–360. [DOI] [PubMed] [Google Scholar]
- 12Caputo S, Benboudjema L, Sinilnikova O et al: Description and analysis of genetic variants in French hereditary breast and ovarian cancer families recorded in the UMD-BRCA1/BRCA2 databases. Nucleic Acids Res 2012; 40: D992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Fokkema IF, Taschner PE, Schaafsma GC, Celli J, Laros JF, den Dunnen JT: LOVD v.2.0: the next generation in gene variant databases. Hum Mutat 2011; 32: 557–563. [DOI] [PubMed] [Google Scholar]
- 14Singer CF, Muhr D, Rappaport C et al: Clinical implications of genetic testing for BRCA1 and BRCA2 mutations in Austria. Clin Genet 2014; 85: 72–75. [DOI] [PubMed] [Google Scholar]
- 15Sharma P, Klemp JR, Kimler BF et al: Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat 2014; 145: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Tutt A, Robson M, Garber JE et al: Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010; 376: 235–244. [DOI] [PubMed] [Google Scholar]
- 17Audeh MW, Carmichael J, Penson RT et al: Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010; 376: 245–251. [DOI] [PubMed] [Google Scholar]
- 18Abeliovich D, Kaduri L, Lerer I et al: The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am J Hum Genet 1997; 60: 505–514. [PMC free article] [PubMed] [Google Scholar]
- 19Thorlacius S, Sigurdsson S, Bjarnadottir H et al: Study of a single BRCA2 mutation with high carrier frequency in a small population. Am J Hum Genet 1997; 60: 1079–1084. [PMC free article] [PubMed] [Google Scholar]
- 20Rubinstein WS, Jiang H, Dellefave L, Rademaker AW: Cost-effectiveness of population-based BRCA1/2 testing and ovarian cancer prevention for Ashkenazi Jews: a call for dialogue. Genet Med 2009; 11: 629–639. [DOI] [PubMed] [Google Scholar]
- 21Weitzel JN, Clague J, Martir-Negron A et al: Prevalence and type of BRCA mutations in Hispanics undergoing genetic cancer risk assessment in the southwestern United States: a report from the Clinical Cancer Genetics Community Research Network. J Clin Oncol 2013; 31: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Filippini S, Blanco A, Fernandez-Marmiesse A et al: Multiplex SNaPshot for detection of BRCA1/2 common mutations in Spanish and Spanish related breast/ovarian cancer families. BMC Med Genet 2007; 8: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Gorski B, Byrski T, Huzarski T et al: Founder mutations in the BRCA1 gene in Polish families with breast-ovarian cancer. Am J Hum Genet 2000; 66: 1963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Menkiszak J, Gronwald J, Gorski B et al: Hereditary ovarian cancer in Poland. Int J Cancer 2003; 106: 942–945. [DOI] [PubMed] [Google Scholar]
- 25Van Der Looij M, Szabo C, Besznyak I et al: Prevalence of founder BRCA1 and BRCA2 mutations among breast and ovarian cancer patients in Hungary. Int J Cancer 2000; 86: 737–740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.