Abstract
Individuals with heterozygous 16p11.2 deletions reportedly suffer from a variety of difficulties with speech and language. Indeed, recent copy-number variant screens of children with childhood apraxia of speech (CAS), a specific and rare motor speech disorder, have identified three unrelated individuals with 16p11.2 deletions. However, the nature and prevalence of speech and language disorders in general, and CAS in particular, is unknown for individuals with 16p11.2 deletions. Here we took a genotype-first approach, conducting detailed and systematic characterization of speech abilities in a group of 11 unrelated children ascertained on the basis of 16p11.2 deletions. To obtain the most precise and replicable phenotyping, we included tasks that are highly diagnostic for CAS, and we tested children under the age of 18 years, an age group where CAS has been best characterized. Two individuals were largely nonverbal, preventing detailed speech analysis, whereas the remaining nine met the standard accepted diagnostic criteria for CAS. These results link 16p11.2 deletions to a highly penetrant form of CAS. Our findings underline the need for further precise characterization of speech and language profiles in larger groups of affected individuals, which will also enhance our understanding of how genetic pathways contribute to human communication disorders.
Introduction
Deletions of a ~600-kb region in human chromosomal band 16p11.2 occur in ~1/2000 individuals,1, 2 and have been associated with a number of neurodevelopmental disorders, including autism.3 Individuals with heterozygous 16p11.2 deletions have increased head circumference,1, 4 high rates of obesity,3, 5 and seizures.6 Cognitively, these individuals have a reduced intelligence quotient (IQ), approximately two SDs below that of familial noncarriers.6 Furthermore, affected individuals have a variety of speech and/or language difficulties.6, 7 However, the precise nature of these difficulties remains unknown. Here, we focus on the speech domain, testing whether 16p11.2 deletions confer a risk of developing a specific speech disorder: Childhood Apraxia of Speech (CAS).8
CAS is a rare motor speech disorder that affects the production, sequencing and timing of sounds, syllables and words,8 with a diagnosis distinct from other speech (e.g., stuttering) and language disorders (e.g., specific language impairment). Although CAS has been linked to a number of genes and genomic pathways, including FOXP2 and ELKS/ERC1,9, 10, 11 the confirmed genetic risk factors explain only a small proportion of cases.12 Recently, however, three unrelated individuals with 16p11.2 deletions were identified in children diagnosed with CAS and screened for copy-number variations,13, 14 thus implicating 16p11.2 deletions as one of the genetic causes of CAS. However, a crucial question remains: how common is CAS among individuals with 16p11.2 deletions?
No standardized or systematic testing of speech abilities has been reported in a group of individuals with 16p11.2 deletions. Further, in existing studies, the terms ‘speech' (the perception and production of speech sounds) and ‘language' (understanding and use of syntax, morphology, semantics and pragmatics) are often used interchangeably. Yet these terms refer to broad multicomponent domains, and deficits within each encompass many distinct disorders. Precise phenotyping, with the use of correct terminology, diagnostic tools and criteria accessible and hence replicable to others, is thus critical to elucidate genotype–phenotype relations in this relatively common deletion syndrome. Here, we conduct the first systematic differential diagnostic assessment of speech disorders in a cohort of individuals with 16p11.2 deletions. In contrast to prior phenotype-driven studies of CAS,13, 14 we have taken a genotype-first approach15 and recruited participants with 16p11.2 deletions to conduct speech and neuropsychological assessment.
Subjects and methods
Participants
Eleven children (8 males) ages 5.4–18.1 (mean 10.8) with 16p11.2 deletions from the Simons Variation In Individuals Project (Simons VIP) cohort (http://sfari.org/resources/simons-vip) participated (see Supplementary Table 1 for SFARIbase IDs). Participants were recruited from attendees of the 2013 Simons VIP Connect Family Meeting in Orlando, Florida. The study was advertised to attendees as a 'language study'. Inclusion criteria were: presence of a 16p11.2 deletion, an IQ of at least 80, based on previously performed phenotyping (cognitive testing is challenging for individuals with lower IQ), and age ≤18 years. The presentation of CAS is known to change across the lifespan, with little or no information available on core features of the disorder to guide informed diagnosis in mid- to late adulthood. Hence, we excluded individuals older than 18 years. Thirteen individuals met these criteria, and 11 agreed to be tested. As described previously, all 11 children carry the canonical deletion (∼600 kb, chr16.hg18:g.29557497_30107356del) at the locus, and do not have any other copy-number variant that is known to affect function, or another neurogenetic or neurological diagnosis unrelated to 16p11.2 (e.g., tuberous sclerosis).16 Written consent and assent was provided, in accordance with the requirements of the Internal Review Board at MIT. A testing battery of ~1 h was conducted. Families were compensated with Amazon.com gift cards and other prizes.
Speech sampling and analysis
Speech samples for transcription were derived from audio (Sony Digital ICD-PX312, Sony, San Diego, CA, USA) and video (Sony HDR-CX260V) recordings of conversation with the examiner and/or parent/guardian during and between standardized tests, and also from three cognitive tasks (syllable repetition task (SRT), Comprehensive Test of Phonological Processing (CTOPP) and rapid automatized naming (RAN); see below). Conversations were elicited for a total of at least 10 min to seek a sample of at least 100 words.17 Samples were transcribed by a speech language pathologist (EM) using broad phonetic transcription with supplemental diacritics to note distortion errors.
Samples were analyzed for speech features associated with articulation disorder, phonological disorder, dysarthria and CAS. A phonetic inventory was determined, including initial, medial and final consonant and vowel inventories. Percentage phoneme correct scores were calculated based on conversational speech. An overall impression of intelligibility was provided by EM and AM using a perceptual intelligibility rating scale.18 Ability to repeat pronounceable nonsense words was additionally examined using nonword repetition and the SRT (see below). Phonological process analysis was conducted (Table 1). Traditional articulation errors were noted where present (e.g., interdental or lateral /s/ or /z/, distorted /r/). Motor speech features (encompassing symptoms of dysarthria and apraxia) were rated using the ‘Mayo Clinic Motor Speech Characteristics Rating Scale'19 (Table 1) and further diagnostic criteria for CAS were adapted from Murray et al.20, 21 based on the three American Speech and Hearing Association (ASHA)8 consensus-based criteria (Table 2). Two SLPs (EM and AM) examined the resultant data to make a final diagnosis by consensus.
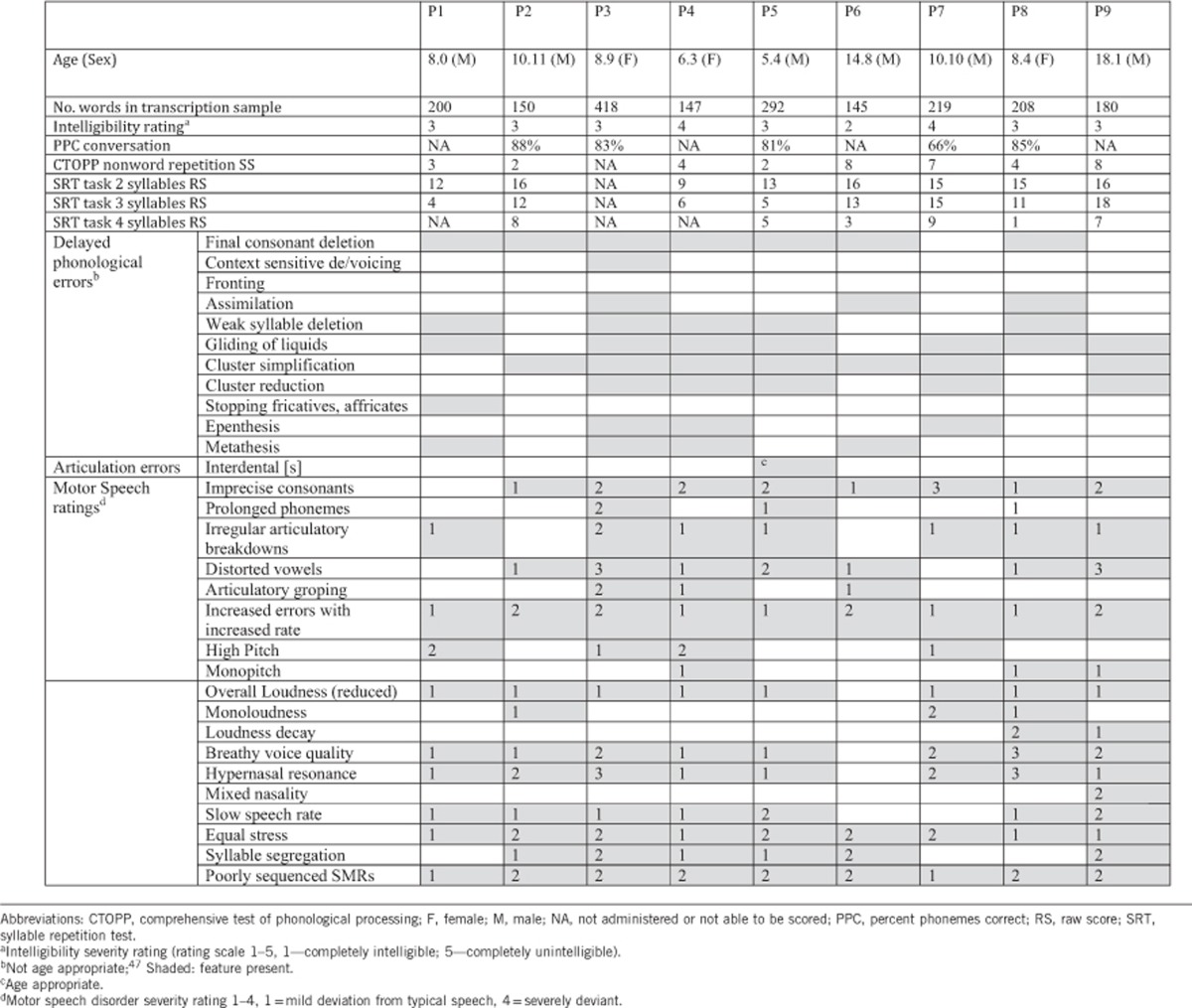
Table 1. Speech (phonetic, phonemic and intelligibility) features for the participants with 16p11.2 deletion.
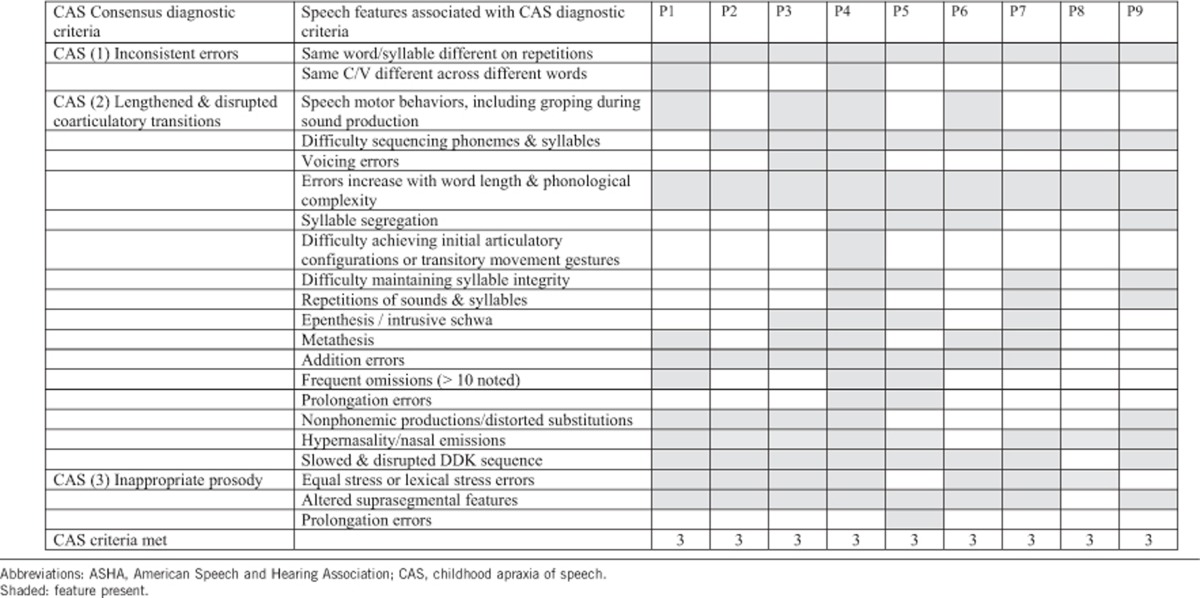
Table 2. Speech features categorized under the three consensus diagnostic criteria for CAS diagnosis (ASHA 2007).
Neuropsychological tests
Not every participant was able to perform all of the tasks because of general unwillingness to perform particular tasks, fatigue, insufficient time, and because two (male) participants were mostly nonverbal, as confirmed by parent report. Eight participants were tested on the SRT,22 the nonword repetition subtest from the CTOPP,23 and RAN tasks.24 In the syllable and nonword repetition tasks, participants are presented with spoken syllable sequences or nonwords, respectively, of increasing length and are asked to repeat each one. The SRT22 and nonword repetition tasks are particularly sensitive for detecting errors in children with speech sound disorder, including CAS. Poor performance on the SRT in particular, a tool that overcomes some of the methodological limitations of standard nonword repetition tasks (see the report by the American Speech-Language-hearing association8 for a review), has been suggested to have diagnostic accuracy for identifying the signature transcoding deficits seen in CAS.25 The RAN task requires participants to name in order, as quickly as possible, sequences of letters, digits, pictures of objects or colored dots. Seven participants were tested on the Peabody Picture Vocabulary Task (PPVT),26 and four on the Test for Reception of Grammar (TROG).27 In these tasks, participants are shown sets of four pictures accompanied by a word (PPVT) or sentence (TROG) and have to choose the picture that corresponds to the word/sentence. Non-verbal IQ was assessed in 10 of the 11 participants with the matrices subtest of the Kaufman Brief Intelligence Test.28 Fine and gross motor skills were evaluated in 10 participants with the Bruininks–Oseretsky Test of motor proficiency (BOT),29 which requires participants to string beads and copy and draw figures (fine motor skills), as well as catch a ball, walk on a straight line and jump on one leg (gross motor skills). Raw scores, computed for all tasks following the instructions in the manuals, were converted into standardized scores and percentiles. The parents/guardians of nine participants completed the Children's Communication Checklist (CCC).30
The phenotyping data have been uploaded to the Simons Foundation Autism Research Initiative (SFARI) database (available at http://sfari.org/resources/sfari-base).
Results
Speech results and diagnosis
The limited verbal output for two participants precluded speech diagnosis (Tables 1 and 2). All nine remaining participants met the three ASHA-based consensus diagnostic criteria for CAS8 (Table 2). All nine also had additional motor speech deficits that could be associated with either dysarthria or CAS, including phoneme imprecision (8/9), hypernasality (7/9), slow speech rate (6/9) and equal stress (9/9), characterized by the Mayo clinic rating scale.19 Further, some features more commonly associated with dysarthria than CAS included mildly reduced overall loudness (7/9) and a breathy voice (6/9). The majority also demonstrated phonological errors (Table 1), with final consonant deletion, gliding, weak syllable deletion, cluster reduction and cluster simplification most common. Some of these error patterns could reflect motor features of CAS rather than being purely phonological in nature. Poorer performance with increasing stimulus length was noted on the SRT and nonword repetition tasks (Table 1), a hallmark characteristic of CAS. Participants scored in the 7th percentile (SEM=1.22) on nonword repetition. Conversational speech intelligibility was reduced in all, with most rated as ‘somewhat intelligible', with corroborating percent phoneme correct ratings (range 66–88%, Table 1) falling well below the expected intelligibility range for this age range.31
Neuropsychological results
Here we include the two largely nonverbal participants excluded from the speech analyses (Table 3; Supplementary Table 2). Consistent with prior reports (e.g., see Zufferey et al.6), nonverbal IQ (n=10) was about two SDs below the general population mean (average standard score=78.8, SEM=5.57; average percentile=18.4, SEM=4.25). Participants performed poorly on tasks assessing higher levels of language processing. In particular, on lexical knowledge (PPVT), participants (n=7) scored on average in the 36th percentile (SEM=10.2), and on grammatical comprehension (TROG), participants (n=4) scored on average in the 21st percentile (SEM=15.4). Participants (n=8) scored relatively higher on the RAN task: in the 57th percentile (SEM=12.7), with a large range. Finally, on the motor-skills assessment, participants (n=10) scored on average in the 22nd percentile (SEM=9.33), again with a large range. The summed CCC scores were in the 11th percentile on average, with the speech subscale revealing the lowest score (6th percentile on average, SEM=3.59).
Table 3. Neuropsychological test results.
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (sex) | 8.0 (M) | 10.11 (M) | 8.9 (F) | 6.3 (F) | 5.4 (M) | 14.8 (M) | 10.10 (M) | 8.4 (F) | 18.1 (M) | 9.11 (M) | 16.3 (M) |
| KBIT matrices percentile | 6 | 27 | 37 | 32 | 16 | 19 | NA | 1 | 27 | 1 | <0.1 |
| PPVT percentile | NA | 77 | 16 | NA | NA | 45 | 58 | NA | 50 | 6 | 0.5 |
| TROG percentile | NA | 66 | NA | NA | NA | 16 | NA | NA | NA | 1 | 1 |
| RAN percentile | 40 | 85.3 | 92 | 16 | NA | 97.8 | 76 | 48.5 | 2 | NA | NA |
| BOT percentile | 14 | 7 | 31 | 1 | 1 | 99 | 27 | 31 | 12 | NA | 1 |
Abbreviations: BOT, Bruininks–Oseretsky Test of motor proficiency; F, female; KBIT, Kaufmann Brief Intelligence Test; M, male; NA, not administered; PPVT, Peabody Picture Vocabulary Task; RAN, rapid automatized naming; TROG, Test for Reception of Grammar. NA: Not administered.
In terms of inter-task relationships (Supplementary Table 2), parental assessments of children's speech abilities (CCC speech subscale) predicted nonword repetition scores (r=0.52), as did the overall CCC scores (r=0.46). Interestingly, IQ did not show a strong relationship with nonword repetition scores (r=0.34), although it did predict performance on PPVT (r=0.57) and TROG (r=0.89), consistent with prior reports revealing such correlations in both healthy individuals (e.g., see Hodapp and Gerken32 and Bell et al.33) and individuals with neuropsychiatric disorders (e.g., see Beck and Black34). PPVT and TROG were also highly correlated with each other (r=0.94). BOT performance was weakly correlated with nonword repetition scores (r=0.23) and not correlated with IQ (r<0.1).
Discussion
Systematic differential diagnostic assessment of speech disorders in a cohort of children with 16p11.2 deletions revealed a high number of individuals with features of CAS: of eleven children, two were mostly nonverbal, and the remaining nine met the core consensus-based ASHA criteria for CAS.8 This finding is striking, given that CAS is a specific speech disorder, distinct from—though often co-morbid with—other speech and language disorders, and exhibiting low prevalence in the general population (for e.g., one study estimates prevalence of ~0.01–0.02%35). Nonetheless, the cognitive profile associated with 16p11.2 deletion is not characterized by a selective deficit in speech production. Our participants were additionally intellectually impaired and had difficulties with lexical and syntactic processing and general motor coordination. The relatively low inter-task correlations, however, suggest that these language and general cognitive deficits are at least somewhat independent from the speech production difficulties.
Our findings are in line with a handful of previous reports of CAS13(2 cases);14 (1 case); dysarthria36 (2/3 cases);37 (1/10 cases); and articulation difficulties37 (2/10 cases);38 (1/15 cases);39 (1/18 cases) in individuals with 16p11.2 deletions (Supplementary Table 3). However, most studies of individuals with 16p11.2 deletions have used the non-specific term ‘speech delay', making it impossible to identify the precise sub-type(s) of speech disorder. Our data go beyond these earlier reports by establishing, with detailed phenotyping, that many individuals with 16p11.2 deletions do not just have generalized speech and language disorders, but features of CAS in particular. Ours is the first genotype-driven study to apply the systematic analyses necessary to distinguish features of CAS from other speech and language disorders in individuals with 16p11.2 deletions.
One limitation in our speech phenotyping was the absence of an oral peripheral exam. An oral exam can determine the presence of altered tone (spasticity, flaccidity), reduced range and rate of movement of the articulators, or structural deficits (high arched palate, submucous cleft, bifid uvula). These data can assist in more precisely differentiating between dysarthria, apraxia and structural speech deficits.
As in many other clinical reports, our sample may not be fully free of ascertainment bias; genetic anomalies in these children were discovered after they presented cognitive and/or behavioral problems and were evaluated by medical professionals. Yet, any such bias is unlikely to be specific to CAS and is instead a general bias toward neurodevelopmental problems. Although we found that 9/9 of the 16p11.2 individuals we tested met criteria for CAS (two additional participants were nonverbal and thus not tested for CAS), we do not conclude that features of CAS will be found in every individual with a 16p11.2 deletion. Nevertheless, given the rarity of CAS in the general population, and the high proportion of features of CAS cases in our sample, it seems likely that CAS is a reasonably frequent component of the phenotype of the 16p11.2 deletion syndrome.
One important aim for future research will be to test a larger number of individuals with 16p11.2 deletions, to precisely quantify the penetrance of this genomic rearrangement with respect to features of CAS. Another important direction would be to attempt to link CAS-related deficits to some aspects of brain structure and function. Some recent studies have reported neurological markers associated with the 16p11.2 deletion syndrome (e.g., increased brain volume, including increased gray and white matter, subcortical and cerebellar volumes,40, 41 some regional differences,41 and aberrant white matter microstructure42). However, the neurobiological mechanisms underlying these effects are not yet well understood.43, 44 Also, there have been no consistent reports of relationships between speech/language problems in 16p11.2 deletion cases and neuroanatomical abnormalities4, 36, 37, 38, 39 and no investigations into potential dysfunction of language networks45 or brain regions related to speech articulation.46 Finally, it will be of considerable interest to eventually determine which of the 25 or so genes that are contained within the deleted region contribute to the different neurodevelopmental aspects of this major chromosomal rearrangement.
Acknowledgments
We thank the participants and their families for taking part in our study. We are also grateful to the Simons Foundation team (especially Alexandra Bowe, Debra Olchick, Nicole Takahashi, and Jennifer Tjernagel) for help with recruiting and scheduling participants. We thank Heather Mabie and Larry Shriberg for sharing the audio materials for the SRT. We thank Zuzanna Balewski, Cara Borelli, Jenelle Feather, Jeanne Gallée, Kyle Mahowald, Caitlin Malloy, and Terri Scott for help in preparing everything for testing and analyzing the data. We thank Kim Leeds for administrative support. This work was supported by a grant to NK from the Simons Foundation. EF is supported by the R00 award HD-057522 from NICHD. SEF is supported by the Max Planck Society. HTF's contribution to this research is supported by NIDCD (P50 DC 13027). AM is supported by the NHMRC (#607315). Preparation of this paper was also supported by the Victorian's Government's Operational Infrastructure Support Program, Australia.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- 1Jacquemont S, Reymond A, Zufferey F et al: Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature 2011; 478: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Stefansson H, Meyer-Lindenberg A, Steinberg S et al: CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature 2014; 505: 361–366. [DOI] [PubMed] [Google Scholar]
- 3Sanders SJ, Ercan-Sencicek AG, Hus V et al: Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams Syndrome region, are strongly associated with autism. Neuron 2011; 70: 863–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Shinawi M, Liu P, Kang S-HL et al: Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size. J Med Genet 2010; 47: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Walters RG, Jacquemont S, Valsesia A et al: A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature 2010; 463: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Zufferey F, Sherr EH, Beckmann ND et al: A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J Med Genet 2012; 49: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Hanson E, Bernier R, Porche K et al: The cognitive and behavioral phenotype of the 16p11.2 deletion in a clinically ascertained population. Biol Psychiatry 2014; 77: 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8American Speech-Language-Hearing Association. Childhood apraxia of speech (technical report). 2007. Available from http://www.asha.org/policy/.
- 9Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP: A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 2001; 413: 519–523. [DOI] [PubMed] [Google Scholar]
- 10Thevenon J, Callier P, Andrieux J et al: 12p13.33 microdeletion including ELKS/ERC1, a new locus associated with childhood apraxia of speech. Eur J Hum Genet 2013; 21: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Worthey EA, Raca G, Laffin JJ et al: Whole-exome sequencing supports genetic heterogeneity in childhood apraxia of speech. J Neurodev Disord 2013; 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Deriziotis P, Fisher SE: Neurogenomics of speech and language disorders: the road ahead. Genome Biol 2013; 14: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Raca G, Baas BS, Kirmani S et al: Childhood Apraxia of Speech (CAS) in two patients with 16p11.2 microdeletion syndrome. Eur J Hum Genet 2013; 21: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Newbury DF, Mari F, Sadighi Akha E et al: Dual copy number variants involving 16p11 and 6q22 in a case of childhood apraxia of speech and pervasive developmental disorder. Eur J Hum Genet 2013; 21: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Stessman HA, Bernier R, Eichler EE: A genotype-first approach to defining the subtypes of a complex disease. Cell 2014; 156: 872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Spiro JE, Chung WK: Simons Variation in Individuals Project (Simons VIP): a genetics-first approach to studying autism spectrum and related neurodevelopmental disorders. Neuron 2012; 73: 1063–1067. [DOI] [PubMed] [Google Scholar]
- 17Barnes E, Roberts J, Long SH et al: Phonological accuracy and intelligibility in connected speech of boys with Fragile X Syndrome or Down Syndrome. J Speech Lang Hear Res 2009; 52: 1048–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Bleile KM: Manual of Articulation and Phonological Disorders. San Diego, CA, USA: Singular, 1995. [Google Scholar]
- 19Duffy J: Motor Speech Disorders, 3rd edn. St Louis, MO, USA: Elsevier, 2013. [Google Scholar]
- 20Murray E, McCabe P, Ballard KJ: A systematic review of treatment outcomes for children with childhood apraxia of speech. Am J Speech Lang Pathol 2014; 23: 486–504. [DOI] [PubMed] [Google Scholar]
- 21Murray E, McCabe P, Heard R, Ballard KJ: Differential diagnosis of children with suspected childhood apraxia of speech. J Speech Lang Hear Res 2015; 58: 43–60. [DOI] [PubMed] [Google Scholar]
- 22Shriberg LD, Lohmeier HL, Campbell TF, Dollaghan CA, Green JR, Moore CA: A nonword repetition task for speakers with misarticulations: the Syllable Repetition Task (SRT). J Speech Lang Hear Res 2009; 52: 1189–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Wagner R, Torgesen J, Rashotte C, Pearson NA: Comprehensive Test of Phonological Processing—Second Edition 2013.
- 24Wolf M, Denckla MB: Rapid Automatized Naming and Rapid Alternating Stimulus Tests (RAN/RAS). Pro-Ed: Austin, TX, USA, 2005. [Google Scholar]
- 25Shriberg LD, Lohmeier HL, Strand EA, Jakielski KJ: Encoding, memory, and transcoding deficits in Apraxia of Speech. Clin Linguist Phon 2012; 26: 445–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Dunn LM, Dunn DM: Peabody Picture Vocabulary Test, 4th edn. Pearson: Minneapolis, MN, USA, 2007. [Google Scholar]
- 27Bishop D: Test for Reception of Grammar: TROG-2 version 2. Pearson Assessment: London, UK, 2003. [Google Scholar]
- 28Kaufman AS, Kaufman NL: Kaufman Brief Intelligence Test, 2nd edn. American Guidance Services: : Circle Pines, MN, USA, 2004. [Google Scholar]
- 29Bruininks BD, Bruininks RH: Bruininks-Oseretsky Test of Motor Proficiency, 2nd edn. Pearson: Bloomington, MN, USA, 2005. [Google Scholar]
- 30Bishop D: The Children's Communication Checklist (CCC-2): CCC-2 Manual. London, UK: The Psychological Corporation, 2003. [Google Scholar]
- 31Flipsen P: Measuring the intelligibility of conversational speech in children. Clin Linguist Phon 2006; 20: 303–312. [DOI] [PubMed] [Google Scholar]
- 32Hodapp AF, Gerken KC: Correlations between scores for Peabody Picture Vocabulary Test-III and the Wechsler Intelligence Scale for Children-III. Psychol Rep 1999; 84: 1139. [Google Scholar]
- 33Bell N, Lassiter K, Matthews T, Hutchinson M: Comparison of the Peabody Picture Vocabulary Test-Third Edition and Wechsler Adult Intelligence Scale-Third Edition with university students. J Clin Psychol 2001; 57: 417–422. [DOI] [PubMed] [Google Scholar]
- 34Beck FW, Black FL: Comparison of PPVT-R and WISC-R in a mild/moderate handicapped sample. Percept Mot Skills 1986; 62: 891–894. [DOI] [PubMed] [Google Scholar]
- 35Shriberg LD, Aram DM, Kwiatkowski J: Developmental apraxia of speech: I. descriptive and theoretical perspectives. J Speech Lang Hear Res 1997; 40: 273–285. [DOI] [PubMed] [Google Scholar]
- 36Schaaf CP, Goin-Kochel RP, Nowell KP et al: Expanding the clinical spectrum of the 16p11.2 chromosomal rearrangements: three patients with syringomyelia. Eur J Hum Genet 2011; 19: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Al-Kateb H, Khanna G, Filges I et al: Scoliosis and vertebral anomalies: additional abnormal phenotypes associated with chromosome 16p11.2 rearrangement. Am J Med Genet Part A 2014; 164: 1118–1126. [DOI] [PubMed] [Google Scholar]
- 38Bijlsma EK, Gijsbers ACJ, Schuurs-Hoeijmakers JHM et al: Extending the phenotype of recurrent rearrangements of 16p11.2: deletions in mentally retarded patients without autism and in normal individuals. Eur J Med Genet 52: 77–87. [DOI] [PubMed] [Google Scholar]
- 39Rosenfeld JA, Coppinger J, Bejjani BA et al: Speech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11.2 microdeletions and microduplications. J Neurodev Disord 2010; 2: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Qureshi AY, Mueller S, Snyder AZ et al: Opposing brain differences in 16p11.2 deletion and duplication carriers. J Neurosci 2014; 34: 11199–11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Maillard AM, Ruef A, Pizzagalli F et al: The 16p11.2 locus modulates brain structures common to autism, schizophrenia and obesity. Mol Psychiatry 2015; 20: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Owen JP, Chang YS, Pojman NJ et al: Aberrant white matter microstructure in children with 16p11.2 deletions. J Neurosci 2014; 34: 6214–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Pucilowska J, Vithayathil J, Tavares EJ, Kelly C, Karlo JC, Landreth GE: The 16p11.2 deletion mouse model of autism exhibits altered cortical progenitor proliferation and brain cytoarchitecture linked to the ERK MAPK pathway. J Neurosci 2015; 35: 3190–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Lin GN, Corominas R, Lemmens I et al: Spatiotemporal 16p11.2 protein network implicates cortical late mid-Fetal brain development and KCTD13-Cul3-RhoA pathway in psychiatric diseases. Neuron 2015; 85: 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Fedorenko E, Hsieh PJ, Neto-Castañon A, Whitfield-Gabrieli S, Kanwisher N: A new method for fMRI investigations of language: defining ROIs functionally in individual subjects. J Neurophys 2010; 104: 1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Bohland JW, Guenther FH: fMRI investigation of syllable sequence production. Neuroimage 2006; 32: 821–841. [DOI] [PubMed] [Google Scholar]
- 47McLeod S, Bleile KM: Neurological and developmental foundations of speech acquisition. Paper presented at the Amer Speech-Lang-Hear Assoc Convention, Chicago, IL, USA Invited Seminar Presentation retrieved from www.speech-language-therapy.com/pdf/docs/ASHA03McLeodBleile.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.