Abstract
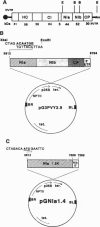
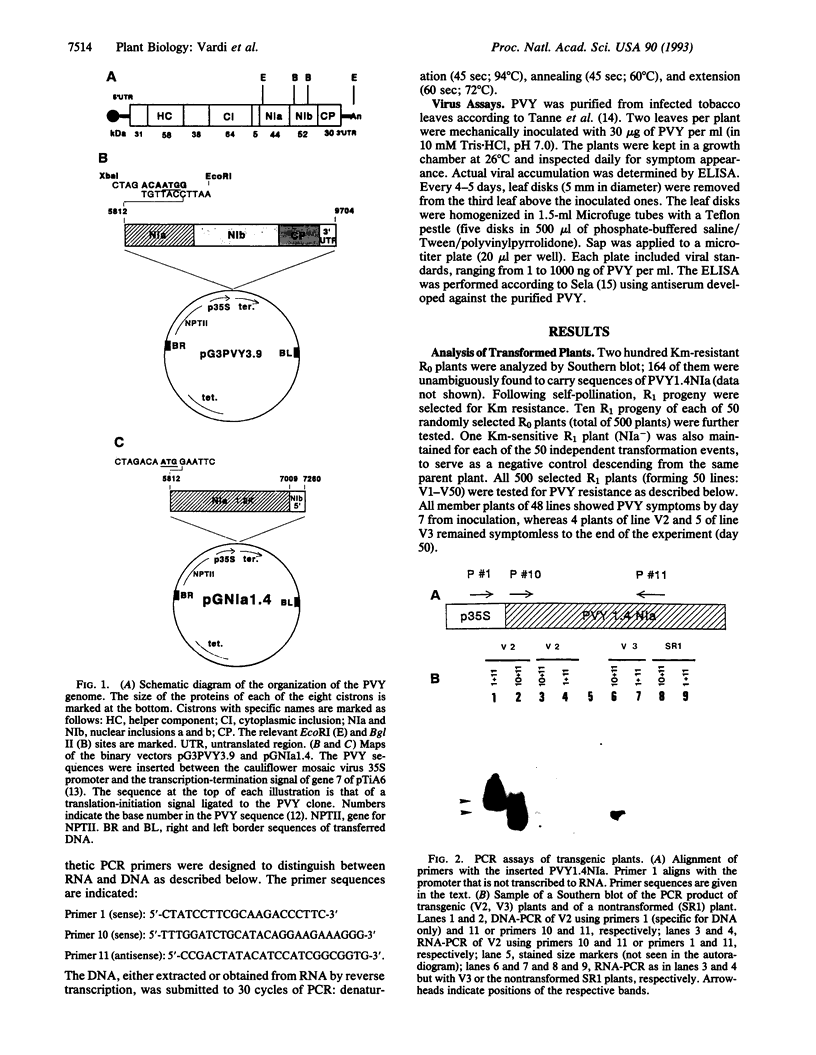
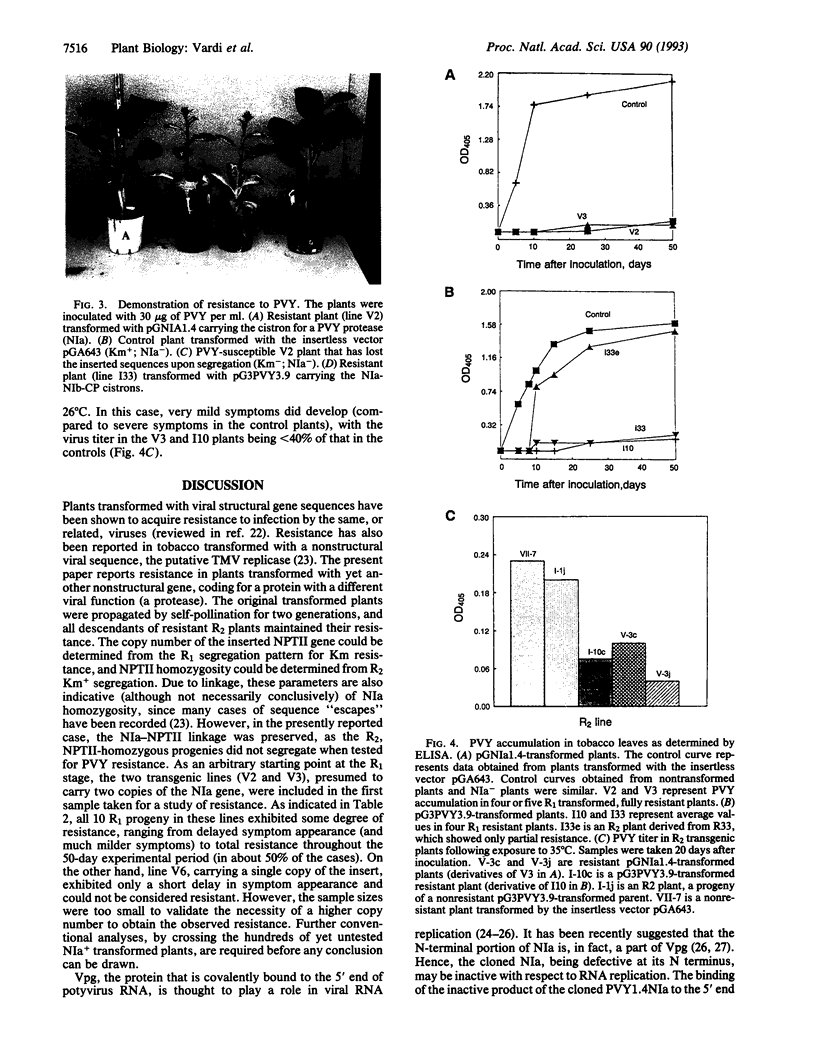
An oligonucleotide carrying signals for translation initiation in plants was engineered upstream to a cDNA clone containing nucleotides 5812-7260 of the potato virus Y (PVY) genome. This fragment contains all but the first 100 5' terminal bases of the cistron encoding one of the PVY proteases (NIa) as well as the first 251 bases of the next cistron (NIb). Nicotiana tabacum cv. SR1 plants were transformed with this fragment. The presence of the NIa sequences in transformed plants was determined by hybridization or PCR, and its expression was ascertained by reverse transcription coupled to PCR. Plants expressing NIa were self-pollinated, and the R1 kanamycin-resistant progeny were rechecked for NIa expression. Several of these plants were found to be resistant to PVY infection, inasmuch as they did not develop symptoms for at least 50 days (the duration of the experiments), and no viral accumulation could be detected in their leaves by ELISA. All of the descendents of resistant homozygous R2 plants were also resistant. Several of the plants transformed with the last three cistrons of PVY (bases 5812-9704; NIa-NIb-coat protein) were also resistant to PVY. None of the transformed plants exhibited resistance to tobacco mosaic virus. Exposure of the plants to 35 degrees C for 48 hr prior to inoculation lowered, but did not abolish, resistance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Palukaitis P., Zaitlin M. A defective replicase gene induces resistance to cucumber mosaic virus in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8759–8763. doi: 10.1073/pnas.89.18.8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J. P., Marsh L. E., Lomonossoff G. P., Sekiya M. E., Zaitlin M. Resistance to tobacco mosaic virus induced by the 54-kDa gene sequence requires expression of the 54-kDa protein. Mol Plant Microbe Interact. 1992 Sep-Oct;5(5):397–404. doi: 10.1094/mpmi-5-397. [DOI] [PubMed] [Google Scholar]
- Carrington J. C., Cary S. M., Dougherty W. G. Mutational analysis of tobacco etch virus polyprotein processing: cis and trans proteolytic activities of polyproteins containing the 49-kilodalton proteinase. J Virol. 1988 Jul;62(7):2313–2320. doi: 10.1128/jvi.62.7.2313-2320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Cary S. M., Parks T. D., Dougherty W. G. A second proteinase encoded by a plant potyvirus genome. EMBO J. 1989 Feb;8(2):365–370. doi: 10.1002/j.1460-2075.1989.tb03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Herndon K. L. Characterization of the potyviral HC-pro autoproteolytic cleavage site. Virology. 1992 Mar;187(1):308–315. doi: 10.1016/0042-6822(92)90319-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dougherty W. G., Parks T. D. Post-translational processing of the tobacco etch virus 49-kDa small nuclear inclusion polyprotein: identification of an internal cleavage site and delimitation of VPg and proteinase domains. Virology. 1991 Aug;183(2):449–456. doi: 10.1016/0042-6822(91)90974-g. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J. A., Riechmann J. L., Laín S. Proteolytic activity of the plum pox potyvirus NIa-like protein in Escherichia coli. Virology. 1989 Jun;170(2):362–369. doi: 10.1016/0042-6822(89)90426-1. [DOI] [PubMed] [Google Scholar]
- Golemboski D. B., Lomonossoff G. P., Zaitlin M. Plants transformed with a tobacco mosaic virus nonstructural gene sequence are resistant to the virus. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6311–6315. doi: 10.1073/pnas.87.16.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann G. M., Shaw J. G., Rhoads R. E. In vitro analysis of tobacco vein mottling virus NIa cistron: evidence for a virus-encoded protease. Virology. 1988 Apr;163(2):554–562. doi: 10.1016/0042-6822(88)90296-6. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Karchi M., Sela I., Edelbaum O., Ori A., Raccah B., Rosner A., Stram Y. Cloning and mapping of the potato virus Y genome and its in vitro expression. Virus Genes. 1990 Sep;4(3):215–224. doi: 10.1007/BF00265631. [DOI] [PubMed] [Google Scholar]
- Lindbo J. A., Dougherty W. G. Pathogen-derived resistance to a potyvirus: immune and resistant phenotypes in transgenic tobacco expressing altered forms of a potyvirus coat protein nucleotide sequence. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):144–153. doi: 10.1094/mpmi-5-144. [DOI] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane S. A., Davies J. W. Plants transformed with a region of the 201-kilodalton replicase gene from pea early browning virus RNA1 are resistant to virus infection. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5829–5833. doi: 10.1073/pnas.89.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L. E., Pogue G. P., Connell J. P., Hall T. C. Artificial defective interfering RNAs derived from brome mosaic virus. J Gen Virol. 1991 Aug;72(Pt 8):1787–1792. doi: 10.1099/0022-1317-72-8-1787. [DOI] [PubMed] [Google Scholar]
- Robaglia C., Durand-Tardif M., Tronchet M., Boudazin G., Astier-Manifacier S., Casse-Delbart F. Nucleotide sequence of potato virus Y (N Strain) genomic RNA. J Gen Virol. 1989 Apr;70(Pt 4):935–947. doi: 10.1099/0022-1317-70-4-935. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sela I. Preparation and measurement of an antiviral protein found in tobacco cells after infection with tobacco mosaic virus. Methods Enzymol. 1986;119:734–744. doi: 10.1016/0076-6879(86)19099-9. [DOI] [PubMed] [Google Scholar]
- Verchot J., Koonin E. V., Carrington J. C. The 35-kDa protein from the N-terminus of the potyviral polyprotein functions as a third virus-encoded proteinase. Virology. 1991 Dec;185(2):527–535. doi: 10.1016/0042-6822(91)90522-d. [DOI] [PubMed] [Google Scholar]
- Wimmer E. Genome-linked proteins of viruses. Cell. 1982 Feb;28(2):199–201. doi: 10.1016/0092-8674(82)90335-x. [DOI] [PubMed] [Google Scholar]