Abstract
Objective
We investigated the prognostic value of intratumoral [18F]fluorodeoxyglucose (FDG) uptake heterogeneity (IFH) derived from positron emission tomography/computed tomography (PET/CT) in patients with cervical cancer.
Methods
Patients with uterine cervical cancer of the International Federation of Obstetrics and Gynecology (FIGO) stage IB to IIA were imaged with [18F]FDG PET/CT before radical surgery. PET/CT parameters such as maximum and average standardized uptake values (SUVmax and SUVavg), metabolic tumor volume (MTV), total lesion glycolysis (TLG), and IFH were assessed. Regression analyses were used to identify clinicopathological and imaging variables associated with progression-free survival (PFS).
Results
We retrospectively reviewed clinical data of 85 eligible patients. Median PFS was 32 months (range, 6 to 83 months), with recurrence observed in 14 patients (16.5%). IFH at an SUV of 2.0 was correlated with primary tumor size (p<0.001), SUVtumor (p<0.001), MTVtumor (p<0.001), TLGtumor (p<0.001), depth of cervical invasion (p<0.001), and negatively correlated with age (p=0.036). Tumor recurrence was significantly associated with TLGtumor (p<0.001), MTVtumor (p=0.001), SUVLN (p=0.004), IFH (p=0.005), SUVtumor (p=0.015), and FIGO stage (p=0.015). Multivariate analysis identified that IFH (p=0.028; hazard ratio, 756.997; 95% CI, 2.047 to 279,923.191) was the only independent risk factor for recurrence. The Kaplan-Meier survival graphs showed that PFS significantly differed in groups categorized based on IFH (p=0.013, log-rank test).
Conclusion
Preoperative IFH was significantly associated with cervical cancer recurrence. [18F]FDG based heterogeneity may be a useful and potential predicator of patient recurrence before treatment.
Keywords: FDG PET/CT, Intratumoral, Population Characteristics, Uterine Cervical Neoplasms
INTRODUCTION
Cervical cancer rates have decreased dramatically in recent years due to cytological screening. However, this disease continues to be a considerable health problem, with 500,000 new cases and 250,000 deaths annually worldwide [1]. Cervical cancer is the only major gynaecological malignancy that is clinically staged according to the International Federation of Obstetrics and Gynecology (FIGO) recommendations.
The National Cancer Comprehensive Network practice guidelines for cervical cancer work-up include chest radiography, [18F]fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT), and magnetic resonance imaging (MRI) as indicated . [18F]FDG uptake is an indicator of tumor metabolism that is usually measured semi-quantitatively using a standardized uptake value (SUV). Specifically, the maximum SUV (SUVmax) reflects the highest metabolic activity within the tumor. Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are measures of tumor metabolic activity that are determined on [18F]FDG PET/CT images [2].
There has been increasing interest in assessing the intratumoral [18F]FDG uptake heterogeneity (IFH) due to the association between IFH measures on baseline [18F]FDG PET images and overall patient outcome [3]. This association has been seen in studies of head and neck cancer, cervical cancer, esophageal cancer and lung cancer [3,4,5]. IFH characterization can be performed on the global (whole tumor), regional (tumor subvolumes), and local (a few voxels) levels. It is hypothesized that the heterogeneous distribution of [18F]FDG PET activity correlates with several physiological processes, including glucose metabolism, necrosis, vascularization, and angiogenesis [6,7]. Within this context, a robust analysis of IFH has the potential to be useful in assessing a tumor’s physiological characteristics.
However, studies of the clinical significance of IFH are limited, even though IFH is a potential prognostic factor. Objective criteria from [18F]FDG PET images can be used to assess IFH, and one study suggests that [18F]FDG heterogeneity is associated with prognosis in cervical cancer [8].
Although tumor heterogeneity has been demonstrated within tumor microenvironments, IFH across the entire volume of a primary tumor has not been quantified or analysed for its association with outcome measures in humans.
Accordingly, we investigated the relationship between the IFH and various clinical and PET/CT parameters and then evaluated the prognostic value of IFH in early stage uterine cervical cancer. The objectives of this study were to quantitatively measure cervical metabolic IFH in pretreatment PET/CT and to explore the relationship of IFH with treatment outcome.
MATERIALS AND METHODS
1. Patients
This study involved patients with histopathologically confirmed invasive cervical cancer (FIGO stage IB to IIA). All included patients had undergone a preoperative work-up with PET/CT scan, followed by radical hysterectomy with pelvic and/or para-aortic lymphadenectomy between February 2004 and January 2013. All patient clinical, histological and imaging data were collected and stored in a computerized database. The Institutional Review Board approved this study, and the requirement to obtain informed consent was waived because of its retrospective nature.
2. Study procedures and lesion status determination
Each PET/CT scan was performed within 1 week before radical surgery. If distant metastasis was confirmed histologically prior to surgical treatment, the surgery was postponed and concurrent chemoradiotherapy or palliative systemic chemotherapy was administered according to current clinical standards.
3. PET/CT
The patients were examined using a dedicated Biograph PET/CT scanner (Siemens Medical Solutions, Malvern, PA, USA). Each patient was asked to fast for at least 4 hours prior to undergoing PET/CT. One hour before imaging, 125 mL of a barium sulphate solution (EZCT: 1.5% weight-volume barium sulphate suspension; Taejoon Pharm, Seoul, Korea) was administered orally to opacify the bowel for the CT portion of the study. Diuretics were not used for preparation. In addition, 0.15 mCi FDG/kg body weight was administered intravenously 1 hour prior to imaging. Prior to PET, CT was performed using the following parameters: 80 mAs, 120 kV, 5-mm section thickness, 0.5 second per rotation and reconstruction onto a 512×512 matrix. The CT results were used to generate an attenuation correction map for PET, and the PET images were reconstructed. Each PET scan was acquired from the skull base to the proximal thigh in three-dimensional row-action maximum-likelihood algorithm mode, with four iterations, eight subsets and 4.8-mm full-width half-maximum reconstruction onto a 512×512 matrix. A total of 7 to 9 bed positions were examined for PET acquisition, with 2.5-minute acquisition per bed.
4. Assessment of [18F]FDG PET/CT imaging
From the attenuation-corrected [18F]FDG PET/CT images, MTV (cm3) were measured using commercial software (Syngo.via, VA 30, Siemens Healthcare, Erlangen, Germany) including an SUV-based automated contouring program (VOI isocontour). This program automatically produces a contour around the target lesions inside the boundaries. Nuclear medicine physicians used the interactive graphical user interface to select each hypermetabolic lesion by clicking on its projection. To define metabolically significant tumor volume, the contouring margin enclosed voxels within each SUV threshold at the interval of 0.5 from 2 to 4 in the volume of interest. Cases with FDG uptake in PET/CT other than cervix such as pelvic lymph node (LN) were included in the analysis.
The maximum and average standardized uptake values (SUVmax and SUVavg) were then used to quantitatively determine [18F]FDG avidity. SUV was defined as the [18F]FDG concentration divided by the injected dose, corrected for the patient’s body weight and the radioactive decay at scanning time: SUV=activity concentration/(injected dose/body weight). TLG was calculated by multiplying the SUVavg of the primary tumor by the metabolic volume of the tumor [9,10]. The pretreatment [18F]FDG PET/CT data were used to determine the following functional criteria for each lesion in a given patient: SUVmax, SUVavg, MTV, and TLG. Parameters that were considered in the analysis included the highest SUVmax and SUVavg values, the MTV and the TLG values of primary tumor for a given patient. In addition, we evaluated the IFH in uterine cervical cancer primary tumors. Towards this end, we evaluated the coefficient of variation (CV) as follows.
5. Intratumoral FDG heterogeneity analysis
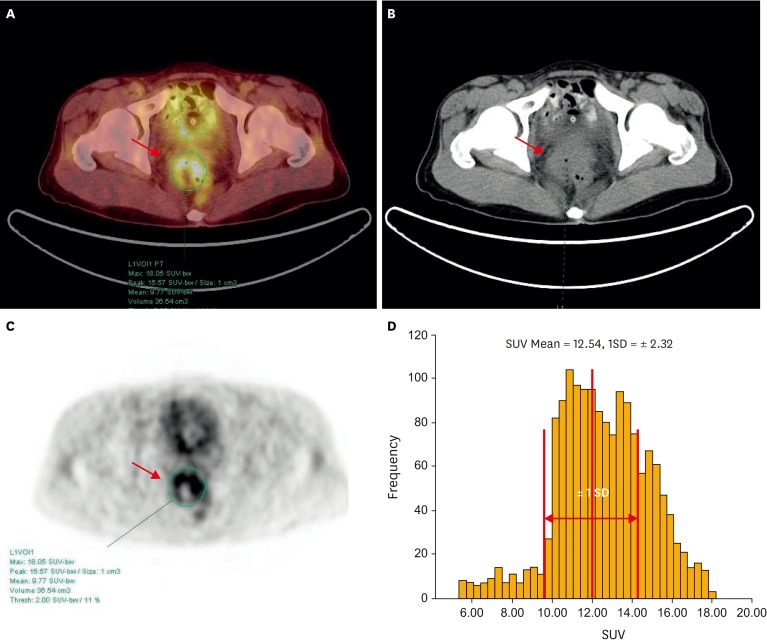
There are several ways to calculate heterogeneity by statistics-based methods. Statistics-based methods explain the distribution of grey levels of voxels. The statistical heterogeneity parameters are categorized based on the scale of analysis as global, regional, and local levels. In this analysis, CV which is one of representative parameters of global level that has been reported to predict therapy response and prognosis in other cancer was chosen to measure IFH [11]. CV is an intuitive and feasible parameter as against complexity of other statistics-based heterogeneity parameters for local and regional levels. CV was defined as the ratio between the standard deviation (SD) of the SUVmax and the SUVavg within the automatically delineated tumor volume calculated using each SUV threshold from 2 to 4. Fig. 1 demonstrates actual calculation procedures obtaining CV.
Fig. 1.
Positron emission tomography/computed tomography (PET/CT) (A), CT (B), PET (C) images, and three-dimensional (3D)-VOI based standardized uptake value (SUV)-histogram (D) for measurement of intratumoral [18F]fluorodeoxyglucose (FDG) heterogeneity (IFH) using a SUV-based automated contouring program in a 49-year-old female patient. Transaxial PET/CT image (A-C) shows a mass lesion in the pelvic cavity (arrow); axial PET image (C) shows a heterogeneous distribution of FDG. 3D-VOI based SUV-histogram of this single tumor (D) shows heterogeneous FDG uptake. Thus, the coefficient of variation (CV) of this mass is 0.185 as following the calculation of standard deviation (SD) of SUVs (2.32) divided by SUVavg (12.54).
6. Clinical endpoints and follow-up
Data were collected from medical records to determine recurrence or death and other clinical characteristics. The time to recurrence was defined as the time from the date of surgery to the date of histological or imaging evidence of recurrence. Recurrent tumor and distant metastasis were diagnosed based on either a positive biopsy or unequivocal clinical or radiographic evidence of progression. Progression-free survival (PFS) ended at the time of recurrence or death.
7. Statistical analysis
We wished to determine the prognostic significance of pretreatment SUVmax, MTV, TLG, and IFH for PFS. Time to event was calculated as the time from the date of diagnosis to the date of the first clinical or imaging finding suggestive of local, regional, or distant disease recurrence that led to additional confirmatory testing (e.g., biopsy or additional imaging). The threshold value that was most discriminating in terms of differentiating between two groups of patients was selected using receiver operating characteristic (ROC) methodology. Kaplan-Meier estimates and the log-rank test were used to assess survival function equality across variables in the PFS analysis. The Cox proportional hazard model was used to evaluate prognostic variables, and an estimated hazard ratio (HR) with 95% CI was presented. Independent sample t-tests were used to compare the means of clinicopathological parameters in the non-recurrent and recurrent groups. Correction for multiple testing was performed using the false discovery rate Benjamini-Hochberg step-up procedure. A p<0.05 was considered statistically significant. All analyses were performed using SPSS ver. 19.0 (IBM Co., Armonk, NY, USA).
RESULTS
1. Patient characteristics
During the study period, 235 patients at our institution were newly diagnosed with stage IB to IIA uterine cervical cancer, and 132 of these patients underwent PET/CT prior to primary treatment. Twenty-six patients were suspected of having distant metastases at the PET/CT scan, and scans from 21 patients were not eligible for the image analysis (no FDG avid lesion in 15 patients, and data error in 6 patients). Scans from 85 of these patients (median age, 47 years; range, 27 to 80 years) were eligible for MTV, TLG, and IFH analysis. Table 1 presents the clinic-pathological characteristics of these patients. The median follow-up was 35 months (range, 6 to 83 months), and the median PFS was 32 months (range, 6 to 83 months). Of the patients, 69.4% were FIGO stage IB1, and 69.4% of the tumors were squamous cell carcinoma. Supplementary Table 1 presents the PET/CT parameters of the enrolled patients.
Table 1. Clinicopathological characteristics of patients who underwent positron emission tomography/computed tomography before operation for cervical cancer (n=85).
| Characteristic | No. (%) |
|---|---|
| Age (yr), median (range) | 47 (27-80) |
| Progression-free survival (mo), median (range) | 32 (6-83) |
| FIGO stage | |
| IB1 | 59 (69.4) |
| IB2 | 11 (12.9) |
| IIA | 15 (17.6) |
| Histology | |
| Squamous cell carcinoma | 59 (69.4) |
| Adenocarcinoma | 16 (18.8) |
| Adenosquamous carcinoma | 5 (5.9) |
| Others | 5 (5.9) |
| Tumor diameter, median (range) | 3.2 (0.5-9.5) |
| Lymph node metastasis | 20 (23.5) |
| Parametrium invasion | 15 (17.7) |
| Recurrence | 14 (16.5) |
FIGO, International Federation of Gynecology and Obstetrics.
2. Correlation between parameters
Our results showed that IFH at an SUV of 2.0 was correlated with SUVtumor (Pearson coefficient (r)=0.811, p<0.001), MTVtumor (r=0.685, p<0.001), TLGtumor (r=0.624, p<0.001), primary tumor size (r=0.460, p<0.001), depth of cervical stromal invasion (r=0.385, p<0.001), and negatively correlated with age (r=–0.227, p=0.036). IFH at an SUV of 2.5 was correlated with SUVtumor (r=0.269, p=0.013), MTVtumor (r=0.474, p<0.001), TLGtumor (r=0.435, p<0.001), and parametrium (PM) invasion (r=0.245, p=0.024). IFH at an SUV of 3.0 was correlated with SUVtumor (r=0.865, p<0.001), MTVtumor (r=0.730, p<0.001), TLGtumor (r=0.641, p<0.001), primary tumor size (r=0.455, p<0.001), depth of cervical stromal invasion (r=0.425, p<0.001), and PM invasion (r=0.227, p=0.039). Supplementary Table 2 depicts Descriptive statistics for each MTV by SUV threshold.
3. Cut-off value for tumor heterogeneity
The ROC curves used to analyse the IFH in relation to PFS. IFH at an SUV of 2.0 was used for ROC analysis, and the area under the curve was 0.661, and 0.418 was determined to be the cut-off value.
4. Tumor heterogeneity and recurrence
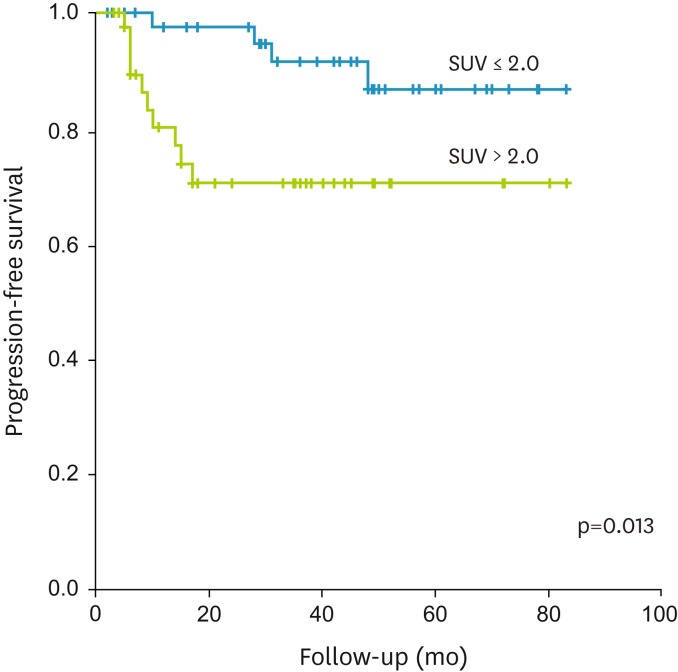
Table 2 summarizes the prognostic values of all of the parameters investigated in the current study. Except FIGO stage which was calculated as categorized variable, other parameters were calculated as continuous variables. Cox proportional hazard analysis revealed that recurrence was significantly associated with TLGtumor (p<0.001), MTVtumor (p=0.001), SUVtumor (p=0.015), FIGO stage (p=0.015), SUVLN (p=0.004), and IFH (p=0.005). The Kaplan-Meier survival graphs (Fig. 2) showed that PFS differed significantly in groups of subjects categorized based on IFH (p=0.013, log-rank test).
Table 2. Analyses of prognostic factors for progression-free survival in patients with cervical cancer.
| Variable | Test for PFS | HR | 95% CI | p-value |
|---|---|---|---|---|
| Age (yr) | 1.000 | 0.946-1.057 | 0.999 | |
| FIGO stage | II vs. I | 3.746 | 1.290-10.881 | 0.015 |
| Tumor size | 1.177 | 0.924-1.500 | 0.188 | |
| LN metastasis | Present vs. absent | 1.716 | 0.536-5.497 | 0.363 |
| PM invasion | Present vs. absent | 1.899 | 0.594-6.066 | 0.279 |
| SUVtumor | 1.040 | 1.008-1.072 | 0.015 | |
| MTVtumor | 1.020 | 1.008-1.032 | 0.001 | |
| TLGtumor | 1.001 | 1.001-1.002 | <0.001 | |
| SUVLN | 1.105 | 1.031-1.184 | 0.004 | |
| IFH | 34.586 | 2.853-419.310 | 0.005 |
FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; IFH, intratumoral [18F]fluorodeoxyglucose (FDG) uptake heterogeneity; LN, lymph node; MTV, metabolic tumor value; PFS, progression-free survival; PM, parametrium; SUV, standardized uptake value; TLG, total lesion glycolysis.
Fig. 2.
Kaplan-Meier survival graph shows significantly different progression-free survival between the groups categorized by intratumoral [18F]fluorodeoxyglucose uptake heterogeneity above (blue line) and below (green line) cut-off value (0.418; p=0.013, log-rank test).
5. Prediction of recurrence
Table 3 presents the multivariate regression analysis of the prognostic values of the parameters determined to be significant in univariate analysis. Multivariate analysis identified IFH (HR, 756.997; 95% CI, 2.047 to 279,923.191; p=0.028) was the only independent risk factor for recurrence in the current study.
Table 3. Multivariate analyses of prognostic factors for progression-free survival.
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| IFH | 756.997 | 2.047-279,923.191 | 0.028 |
| FIGO stage* | 1.817 | 0.418-7.911 | 0.426 |
| MTVtumor | 0.981 | 0.946-1.017 | 0.293 |
| TLGtumor | 1.005 | 0.999-1.011 | 0.118 |
| SUVtumor | 0.832 | 0.657-1.055 | 0.129 |
| SUVLN | 1.056 | 0.956-1.165 | 0.283 |
FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; IFH, intratumoral [18F]fluorodeoxyglucose (FDG) uptake heterogeneity; LN, lymph node; MTV, metabolic tumor value; PFS, progression-free survival; SUV, standardized uptake value; TLG, total lesion glycolysis.
*Test for PFS: II vs. I.
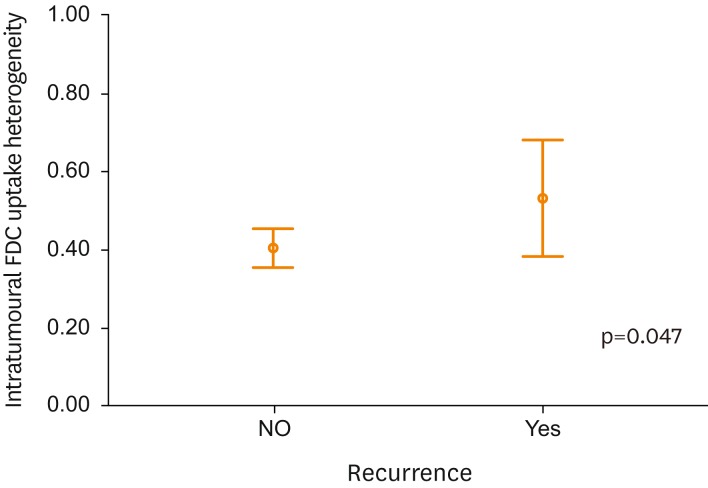
Table 4 summarizes the clinico-pathological and PET/CT derived characteristics of patients without and with recurrence. There were significant differences of PFS, MTVtumor, TLGtumor, SUVLN, and IFH between patients with and without recurrence. There was significant difference (p=0.047) between the mean IFH values of non-recurrent and recurrent groups (Fig. 3).
Table 4. Clinicopathological and positron emission tomography/computed tomography derived characteristics of patients without and with recurrence (n=85).
| Variable | Recurrence (-) | Recurrence (+) | p-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (yr) | 47.859 | 9.266 | 49.000 | 14.049 | 0.702 |
| PFS (mo) | 36.916 | 22.495 | 15.214 | 12.392 | 0.001 |
| Tumor size (cm) | 3.394 | 1.987 | 3.936 | 1.753 | 0.346 |
| SUVtumor | 11.476 | 9.493 | 17.376 | 19.116 | 0.065 |
| MTVtumor | 32.028 | 30.248 | 58.446 | 54.542 | 0.012 |
| TLGtumor | 170.307 | 223.006 | 494.586 | 676.236 | 0.001 |
| SUVLN | 1.350 | 1.589 | 4.090 | 8.646 | 0.014 |
| IFH | 0.404 | 0.212 | 0.533 | 0.255 | 0.047 |
IFH, intratumoral [18F]fluorodeoxyglucose (FDG) uptake heterogeneity; LN, lymph node; MTV, metabolic tumor value; PFS, progression-free survival; SD, standard deviation; SUV, standardized uptake value; TLG, total lesion glycolysis.
Fig. 3.
Intratumoral [18F]fluorodeoxyglucose (FDG) uptake heterogeneity (IFH) value distribution between patients with and without recurrence. There was significant difference (p=0.047) between the mean IFH values of non-recurrent and recurrent groups.
DISCUSSION
In this study, we investigated the prognostic value of IFH in early stage uterine cervical cancer, and also the relationship between preoperative IFH and various clinical and PET/CT parameters. These results depict that [18F]FDG based heterogeneity may be a useful and potential predicator of patient recurrence before treatment in patients with stage IB to IIA cervical cancer.
Malignant tumor cells are heterogeneous in several respects. Not only do the biologic constituents vary among tumor cells, but so can gene expression and the metabolic and behavioural characteristics. There is heterogeneity within the same cancer type and even within the same stage due to differences in properties such as growth rate, vascularity and necrosis in the same tumor cell population [12]. Importantly, FDG uptake is not homogeneous throughout a tumor. Factors that contribute to IFH include necrosis [13], cellular proliferation [14], blood flow [15], microvessel density [16], and hypoxia [17,18]. Since uptake of a tracer is not usually homogeneously distributed across the tumor, it may be useful to quantify IFH before, during and after treatment.
The FDG uptake pattern within a tumor has the potential to provide information that is useful for treatment planning, as the information could be used for targeted treatment of specific areas within the tumor. In addition, FDG uptake may provide additional information when monitoring treatment response by revealing a mixed response within a single tumor. Finally, differences between CT anatomical volumes and PET metabolic volumes could be characterized. In current clinical practice, there is no simple method for quantification of IFH [7].
A few methods have been proposed to quantify IFH [4,19]. Notably, methods used to characterize heterogeneity detect both partial volume effects and noise as heterogeneity; accordingly, partial volume correction and image denoising must be performed prior to calculating IFH. As the IFH increased, as measured using the CV of PET/CT images, the risk of recurrence increased significantly. This result highlights the importance of the metabolic complexity of tumors and the possibility that IFH is a novel biomarker of prognosis for early stage cervical cancer. We are developing a large multicentre database to confirm the results of the present study and to further analyse the relationship between IFH and patient outcome in advanced cervical cancer.
Recent studies evaluated the heterogeneity in FDG uptake as shown by textural features at a regional scale [20,21]. Yang et al. [20] concluded that the temporal change in the heterogeneity of intratumoral FDG distribution using image-based textural feature may provide treatment prediction in cervical cancer. Hatt et al. [21] demonstrated that textural features included entropy and dissimilarity for calculating co-occurrence matrices, and high-intensity large-area emphasis and zone percentage for calculating size-zone matrices. These studies suggested that image-derived regional textural features may allow characterization of intratumoral FDG distribution, and may also provide valuable complementary information for functional tumor volumes. Texture analysis may be a useful methodology investigating tumor heterogeneity and providing prognostic information. Hatt et al. [21], commented that most studies using textural features considered volumes greater than 3 to 5 cm3, assuming that PET cannot characterize heterogeneity in smaller volumes because of its spatial resolution. However, the correlation between MTV and textural features tended to decrease with ranges of increasing volumes. These findings imply that adequate MTV is necessary to ensure the efficacy of textural feature in the analysis.
IFH involves important parameters, such as cell proliferation, tumor vascularization and multidrug resistance. Increased knowledge of IFH before treatment could facilitate the planning of individualized therapies. Accurate visualization of IFH could help reduce tumor-sampling bias, could have implications for the development of new targeted therapies and might ameliorate drug resistance [22]. Further, visualizing IFH could be valuable during response monitoring, especially in patients with an unfavourable or heterogeneous response. Baseline heterogeneity determination would improve tumor characterization, image segmentation and help improve prediction of therapy response and survival [23].
To the best of our knowledge, this is the first study to characterize and quantify IFH in which the CV was shown to be a predictor of patient outcome in uterine cervical cancer. Additional studies are needed to address the potential clinical value of the CV. Specifically, test-retest studies need to be performed, and application of the method should be tested using larger clinical data sets. Further studies are also needed to assess the value of IFH in clinical practice. Nevertheless, the present preliminary results suggest the potential value of CV characterization of metabolic IFH.
In the current study, IFH at an SUV of 2.0 correlated with SUVtumor, MTVtumor, TLGtumor, primary tumor size, depth of cervical stromal invasion, and negatively correlated with age, which are well-known prognostic factors of cervical cancer. Interestingly, these results are in line with the concept that tumor heterogeneity is greater in tumors with aggressive clinicopathological factors. In this respect, IFH can be considered a novel prognostic biomarker for uterine cervical cancer. The recurrence rate in this study was 16.5%, and this relatively low event rate may be due to the early stage of the enrolled patient. As the recurrence rate was low, the meaning of IFH might have been underestimated. IFH in advanced disease would have more clinical impact, and we are conducting IFH analysis in patients with locally advanced cervical cancer.
This study has several limitations. First, it was retrospective and was performed at a single institution with a small number of patients (n=85). As the CI is the function of sample size, SD and the significance level, the wide range of the CI for IFH implies wide SD and small number of sample size. The results may not be generalizable to all patients with cervical cancer, because not all scans were available for IFH measurement and only measurable scans were included in the analysis. Additional large prospective studies are needed to confirm the predictive value of IFH in clinical practice. Second, the IFH observed in this study was not confirmed histopathologically. Therefore, we do not know the extent to which genetic profiles or immunohistochemical features may differ. In addition, there are other PET radiotracers that are may be more suitable than [18F]FDG for determining the proliferation index, although such radiotracers may be less widely available [24]. The use of new specific radiotracers and high-resolution devices has the potential to biologically characterize tumors in vivo [25]. Third, we could not perform partial volume correction as the segmentation software of the system does not support the option. Partial volume effect is known to increase the number of unique intensities measured, and it can cause distributions of measured intensities to appear more heterogeneous. Partial volume effect may underestimate the SUV measure, and it might limit the use of SUV as an independent parameter. In this aspect, CV may be less influenced by partial volume effect, and can be a better candidate for prognostic parameter. Partial volume correction could affect the calculations, and such a correction will alter the overall distribution of measured [18F]FDG PET intensities. Future research may be beneficial to compare the efficacy of CV and the heterogeneity parameter with partial volume correction. Finally, heterogeneous [18F]FDG uptake may arise from differences in blood supply and vascularization rather than from cellular heterogeneity per se. This notion merits further investigation that includes histopathological evaluation [26]. Future studies should focus on detection of a heterogeneous metabolic response during chemotherapy, on correlation of IFH with MRI findings and on the use of quantitative analyses.
In conclusion, our results indicated that preoperative IFH as determined on PET/CT was significantly associated with recurrence in patients with stage IB to IIA cervical cancer. [18F]FDG based heterogeneity may be a useful and potential predicator of patient recurrence before treatment. Further analysis in a larger patient population and with longer follow-up is needed to confirm the present findings.
Footnotes
Funding: This work was supported by the Research Resettlement Fund for new faculty of Seoul National University and by grant no. 0320140270 (2014-1040) from the Seoul National University Hospital Research Fund and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI14C1072).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Supplementary Materials
PET/CT parameters of patients who underwent PET/CT before operation for cervical cancer (n=85)
Descriptive statistics for each MTV by SUV threshold
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55:884–890. doi: 10.2967/jnumed.113.133801. [DOI] [PubMed] [Google Scholar]
- 3.Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52:369–378. doi: 10.2967/jnumed.110.082404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Naqa I, Grigsby P, Apte A, Kidd E, Donnelly E, Khullar D, et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 2009;42:1162–1171. doi: 10.1016/j.patcog.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med. 2013;54:19–26. doi: 10.2967/jnumed.112.107375. [DOI] [PubMed] [Google Scholar]
- 6.Basu S, Kwee TC, Gatenby R, Saboury B, Torigian DA, Alavi A. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging. 2011;38:987–991. doi: 10.1007/s00259-011-1787-z. [DOI] [PubMed] [Google Scholar]
- 7.Pugachev A, Ruan S, Carlin S, Larson SM, Campa J, Ling CC, et al. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys. 2005;62:545–553. doi: 10.1016/j.ijrobp.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Kidd EA, Grigsby PW. Intratumoral metabolic heterogeneity of cervical cancer. Clin Cancer Res. 2008;14:5236–5241. doi: 10.1158/1078-0432.CCR-07-5252. [DOI] [PubMed] [Google Scholar]
- 9.Francis RJ, Byrne MJ, van der Schaaf AA, Boucek JA, Nowak AK, Phillips M, et al. Early prediction of response to chemotherapy and survival in malignant pleural mesothelioma using a novel semiautomated 3-dimensional volume-based analysis of serial 18F-FDG PET scans. J Nucl Med. 2007;48:1449–1458. doi: 10.2967/jnumed.107.042333. [DOI] [PubMed] [Google Scholar]
- 10.Veit-Haibach P, Schaefer NG, Steinert HC, Soyka JD, Seifert B, Stahel RA. Combined FDG-PET/CT in response evaluation of malignant pleural mesothelioma. Lung Cancer. 2010;67:311–317. doi: 10.1016/j.lungcan.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Bundschuh RA, Dinges J, Neumann L, Seyfried M, Zsótér N, Papp L, et al. Textural parameters of tumor heterogeneity in (1)(8)F-FDG PET/CT for therapy response assessment and prognosis in patients with locally advanced rectal cancer. J Nucl Med. 2014;55:891–897. doi: 10.2967/jnumed.113.127340. [DOI] [PubMed] [Google Scholar]
- 12.Huang B, Chan T, Kwong DL, Chan WK, Khong PL. Nasopharyngeal carcinoma: investigation of intratumoral heterogeneity with FDG PET/CT. AJR Am J Roentgenol. 2012;199:169–174. doi: 10.2214/AJR.11.7336. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen M, Horsman MR, Cumming P, Munk OL, Keiding S. Effect of intratumoral heterogeneity in oxygenation status on FMISO PET, autoradiography, and electrode Po2 measurements in murine tumors. Int J Radiat Oncol Biol Phys. 2005;62:854–861. doi: 10.1016/j.ijrobp.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16. [PubMed] [Google Scholar]
- 15.Zasadny KR, Tatsumi M, Wahl RL. FDG metabolism and uptake versus blood flow in women with untreated primary breast cancers. Eur J Nucl Med Mol Imaging. 2003;30:274–280. doi: 10.1007/s00259-002-1022-z. [DOI] [PubMed] [Google Scholar]
- 16.Tateishi U, Nishihara H, Tsukamoto E, Morikawa T, Tamaki N, Miyasaka K. Lung tumors evaluated with FDG-PET and dynamic CT: the relationship between vascular density and glucose metabolism. J Comput Assist Tomogr. 2002;26:185–190. doi: 10.1097/00004728-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Zhao S, Kuge Y, Mochizuki T, Takahashi T, Nakada K, Sato M, et al. Biologic correlates of intratumoral heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase-II in experimental tumor. J Nucl Med. 2005;46:675–682. [PubMed] [Google Scholar]
- 18.van Baardwijk A, Bosmans G, van Suylen RJ, van Kroonenburgh M, Hochstenbag M, Geskes G, et al. Correlation of intra-tumour heterogeneity on 18F-FDG PET with pathologic features in non-small cell lung cancer: a feasibility study. Radiother Oncol. 2008;87:55–58. doi: 10.1016/j.radonc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Nestle U, Kremp S, Schaefer-Schuler A, Sebastian-Welsch C, Hellwig D, Rübe C, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J Nucl Med. 2005;46:1342–1348. [PubMed] [Google Scholar]
- 20.Yang F, Thomas MA, Dehdashti F, Grigsby PW. Temporal analysis of intratumoral metabolic heterogeneity characterized by textural features in cervical cancer. Eur J Nucl Med Mol Imaging. 2013;40:716–727. doi: 10.1007/s00259-012-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatt M, Majdoub M, Vallières M, Tixier F, Le Rest CC, Groheux D, et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med. 2015;56:38–44. doi: 10.2967/jnumed.114.144055. [DOI] [PubMed] [Google Scholar]
- 22.Navin N, Krasnitz A, Rodgers L, Cook K, Meth J, Kendall J, et al. Inferring tumor progression from genomic heterogeneity. Genome Res. 2010;20:68–80. doi: 10.1101/gr.099622.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging. 2013;40:133–140. doi: 10.1007/s00259-012-2247-0. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Sun Y, Zhang Y, Xue J, Wang M, Shi W, et al. Can fluorine-18 fluoroestradiol positron emission tomography-computed tomography demonstrate the heterogeneity of breast cancer in vivo? Clin Breast Cancer. 2013;13:359–363. doi: 10.1016/j.clbc.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Tateishi U, Terauchi T, Akashi-Tanaka S, Kinoshita T, Kano D, Daisaki H, et al. Comparative study of the value of dual tracer PET/CT in evaluating breast cancer. Cancer Sci. 2012;103:1701–1707. doi: 10.1111/j.1349-7006.2012.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahim MK, Kim SE, So H, Kim HJ, Cheon GJ, Lee ES, et al. Recent trends in PET image interpretations using volumetric and texture-based quantification methods in nuclear oncology. Nucl Med Mol Imaging. 2014;48:1–15. doi: 10.1007/s13139-013-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PET/CT parameters of patients who underwent PET/CT before operation for cervical cancer (n=85)
Descriptive statistics for each MTV by SUV threshold