Summary
Lymphatic filariasis leads to profound impairment of parasite‐specific T helper type 1 (Th1) and Th2 immune responses and significantly increases the expression of regulatory networks and regulatory effectors like transforming growth factor‐β, CD25, cytotoxic T‐lymphocyte antigen 4, glucocorticoid‐induced tumour necrosis factor receptor (GITR) and regulatory T (Treg) cells, which together play an important role in immunosuppression. While Treg cells suppress the activity of effector cells, monocyte dysfunction, characterized by an alternatively activated immunoregulatory phenotype, is one hypothesis that explains the lack of an antigen‐specific T‐cell response in infected individuals. In the present study, we administered neutralizing antibodies against the Treg cell‐associated markers CD25 and GITR and observed its effects on filaria‐induced immunosuppression. Our results show that administration of anti‐CD25 and anti‐GITR in infected animals not only arrested the accumulation of Treg cells and reduced arginase activity, but also led to an increase in the percentages of Th17 cells in the secondary lymphoid organs of mice. Elevated levels of interferon‐γ and decreased levels of interleukin‐10 were also noted in the culture supernatants of mouse splenocytes that were treated with neutralizing antibodies. Furthermore, treatment with neutralizing antibodies enhanced the expression of inducible nitric oxide synthase on host macrophages and CD40 on host dendritic cells with concomitant decreased expression of alternative activation markers Arg1, Ym1 and Fizz1, which together lead to reduced parasite burden in treated animals. In summary, administration of neutralizing antibodies helps in breaking the regulatory network in mice and limits parasite‐induced immunosuppression at the earliest host–parasite interface.
Keywords: anti‐CD25, anti‐glucocorticoid‐induced tumour necrosis factor receptor, immunosuppression, lymphatic filariasis, Wolbachia surface protein
Introduction
During filarial infection, parasites promote their survival through suppression of the host immune response.1, 2 Impaired immune response in chronically infected filarial patients shows down‐modulation of both T helper type 1 (Th1) and Th2 pathways to parasite antigens with significant increase in the expression of regulatory T (Treg) cell‐associated markers, namely CD25, cytotoxic T‐lymphocyte antigen 4 (CTLA‐4) and glucocorticoid‐induced tumour necrosis factor receptor (GITR).3, 4 Therefore, strategies to induce long‐term protective immunity are needed to counteract parasite‐induced immune deregulation. It is known that co‐stimulation of T cells is essential for generating efficient T‐cell responses and many reports indicate that agonistic signalling can enhance immunity through T‐cell co‐stimulatory receptors.5, 6 The GITR family‐related protein is one such receptor that has received significant attention in recent years.7, 8 GITR is expressed at low levels by various immune cells but is highly expressed on CD4+ Foxp3+ Treg cells and is also up‐regulated on conventional CD4+ and CD8+ T cells upon activation.9, 10 Agonistic antibody DTA‐1 has been shown to break immunological self‐tolerance in mice through stimulation of GITR, which abrogates Foxp3+ Treg‐mediated immune‐suppression and augments the CD4+ effector T cell response.8, 10 Besides anti‐GITR, anti‐CD25 administration also depletes natural Treg cells in vivo and augments protective immune response and enhances pathogen control.11, 12
Previous work in our laboratory has shown that Wolbachia surface protein of Brugia malayi acts synergistically with infective larvae stage 3 of B. malayi (Bm‐L3) in promoting a pro‐inflammatory response by increasing the numbers of Th17 cells and at the same time diminishes the host immunological tolerance by decreasing Treg cells and transforming growth factor‐β (TGF‐β).13 We also reported that administration of recombinant Wolbachia surface protein (r‐wsp) induces a Th1 response in BALB/c mice, which may facilitate B. malayi by activating multiple regulatory signalling pathways.13 As such, filariasis infected host is under constant and repetitive exposure to lipopolysaccharide‐like molecules either due to release of products from Wolbachia, death of parasites, or upon exposure to infected mosquitoes, which induces chronic inflammation and gives rise to immune tolerance with increasing parasite load.14
In the present study, we tested the hypothesis of whether breaking immunological tolerance in mice would increase the effector T‐cell activity, which may help in controlling the infection. Results of our study show that, in vivo administration of neutralizing antibodies against CD25 and GITR (anti‐CD25 and anti‐GITR) in mice infected with Bm‐L3 arrested the accumulation of Treg cells and reduced the activity of arginase in mouse peritoneal exudate cells (PECs). Furthermore, neutralizing antibodies increased the percentages of Th17 cells and Th1 cytokine interferon‐γ (IFN‐γ) but decreased interleukin‐10 (IL‐10) levels in the culture supernatants of splenocytes of treated mice. Moreover, anti‐CD25 and anti‐GITR administration reduced alternative activation of host macrophages and enhanced the expression of co‐stimulatory marker CD40 on host dendritic cells, which contributed to reduced parasite burden in infected animals. These results highlight that filaria‐induced immunosuppression can be managed at the earliest host–parasite interface by using neutralizing antibodies directed against markers expressed on Treg cells, which is effective in reducing parasite load in infected animals.
Materials and methods
Animals
BALB/c mice between 6 and 8 weeks old were used for all experimental studies. Animals were housed in our institute's laboratory animal facility under pathogen‐free conditions and fed a standard pellet diet and water ad libitum.
Ethics statement
All the animals used for experiments and their handling procedures were duly approved by the Animal Ethics Committee of the Central Drug Research Institute constituted under the provisions of the Committee for the Purpose of Control and Supervision on Experiments on Animals, Government of India. The study bears the approval no. IAEC/2011/145 dated 03/07/2013.
Reagents and antibodies
Freund's complete adjuvant, Freund's incomplete adjuvant and protease inhibitor cocktail were purchased from Sigma (St Louis, MO). Monoclonal antibodies FoxP3‐phycoerythrin (PE) (clone‐MF23), CD4‐FITC (clone‐H129.19), Ly6C/G‐PE‐Cy7 (clone‐RB6‐8C5), CD11c‐PE‐cy7 (clone‐HL‐3), CD40‐FITC (clone‐3/23), CD80‐PE (clone‐16‐10A1) and CD86‐allophycocyanin (clone‐GL1) were purchased from BD Biosciences (San Jose, CA). F4‐80 Pacific Blue (clone‐BM8) was purchased from Invitrogen (Carlsbad, CA). IL‐17‐PE was purchased from eBioscience (San Diego, CA) and CCR2‐PE (clone‐475301) was purchased from R&D Systems (Minneapolis, MN). In vivo neutralizing antibodies, namely anti‐GITR (clone‐DTA‐1) and anti‐mouse CD25 (clone‐PC61) and their relevant isotype controls rat IgG1 (clone‐HRPN) and rat IgG2b (clone‐LTF‐2), were purchased from BioXcell (West Lebanon, NH). Arginase activity assay kit was purchased from Sigma Aldrich (St Louis, MO), and the ELISA kit for IFN‐γ and IL‐10 was purchased from Biolegend (San Diego, CA).
Collection of Bm‐L3 and infection of mice
Bm‐L3 were recovered from infected Aedes aegypti that were maintained in the insectarium of our Institute. To elucidate the effect of neutralizing antibody treatment on parasite load and recruitment pattern of leucocytes in the secondary lymphoid organs of mice, mice were divided into six different experimental groups each having five or six animals. Mice in group 1 were left untreated (control group); whereas mice in group 2 were challenged with 50 live Bm‐L3 (L3 group). Mice in groups 3 and 4 were challenged with 50 live Bm‐L3 and given either 1 mg each of anti‐CD25 and anti‐GITR neutralizing antibodies (group 3, L3 + Ab group) or their respective isotype controls (group 4, L3 + Iso group) as described below. Mice in group 5 were first immunized with 25 μg of r‐wsp followed by infection with 50 live Bm‐L3 along with administration of neutralizing antibodies (r‐wsp + L3 + Ab group) whereas mice in group 6 served as immunization controls (r‐wsp group). Administration of r‐wsp was done via the subcutaneous route whereas L3 were administered via the intraperitoneal route. All the experiments were repeated thrice using the same number of mice in each group.
Immunization of mice
For immunization studies, mice were immunized subcutaneously on day 0 with 25 μg of r‐wsp emulsified in 100 μl of Freund's complete adjuvant. This was followed by two booster doses on weeks 2 and 3 in Freund's incomplete adjuvant. One week after the final booster dose, mice were infected with 50 live Bm‐L3 and killed 1 week later. Spleens and mesenteric lymph nodes (MLNs) were collected and immunological studies were carried out as described below.
In vivo antibody treatment
Anti‐CD25 and anti‐GITR were administered to animals in the L3 + Ab group and r‐wsp + L3 + Ab group as mentioned above. Briefly, 1 day before (day −1) and 3 days after Bm‐L3 challenge (day +3), mice received (intravenously) either 1 mg of each neutralizing antibody (total volume 300 μl, L3 + Ab group) or their respective isotype controls (111 μl, L3 + Iso group). Similarly, mice in r‐wsp + L3 + Ab group were first immunized with r‐wsp on day 0, followed by two boosters on day 15 and day 21. Thereafter mice were infected with Bm‐L3 and given neutralizing antibodies on day −1 and day +3 as described above. Mice were given neutralizing antibodies 1 day before Bm‐L3 infection so as to achieve sufficient antibody levels in the peripheral circulation. The second dose of neutralizing antibody was given 3 days after the L3 challenge because Bm‐L3 undergoes its first moulting (L3–L4) in about a week's time inside the host and day 3 was chosen to fit in between this time limit as we hypothesized that two separate doses would be sufficient to affect the larval moulting process.
Differential leucocyte counts in peritoneal lavage
Peritoneal cavities of infected animals were lavaged to collect PECs using standard protocol. Briefly, small aliquots of incomplete RPMI‐1640 medium supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, 2·5 mg/ml amphotericin B and 5 U/ml heparin was injected into the mouse peritoneum with the help of a needle attached to a syringe, and gently aspirated. The procedure was repeated until 8–10 ml of peritoneal fluid was collected from each mouse. Thereafter, peritoneal fluid was centrifuged at 300 g for 10 min at 4° and pelleted cells were washed twice with RPMI‐1640 medium and counted. A small sample of cells was taken for preparation of cytospins, air‐dried and stained with 0·1% Giemsa to assess cell differentials by standard morphology and staining characteristics under a bright‐field microscope.
Immunophenotyping of dendritic cells, inflammatory monocytes and macrophages
Immunophenotyping of dendritic cells, inflammatory monocytes and macrophages present in the secondary lymphoid organs of mice was performed as described below. Briefly, single cell suspension from spleen and MLNs was incubated with seroblock FcR on ice for 10 min to block non‐specific antibody binding. After incubation, cells were washed and stained with antibodies specific for each cell type analysed, e.g. CD11c CD40, CD80 and CD86 for dendritic cells, CD11c and F480 for macrophages and CCR2 and Ly6C/G for inflammatory monocytes. Flow cytometric data was acquired on four‐ or five‐decade log‐scale dot plots displaying forward scatter (FSC) area versus side scatter area (SSC). First, hierarchy gates were set in FSC versus SSC dot plots to exclude remaining lymphocytes and cell debris. Thereafter, cell populations were differentiated according to their respective expression profiles. Acquisition was carried out using FACS‐diva software on a FACS Aria cell sorter (BD Biosciences) or FACS‐Cell quest software on FACS Calibur (BD Biosciences) and analysis was performed using flow jo software (Tree Star Inc., Ashland, OR).
Estimation of Th17 cells
The Th17 cells were enumerated in the spleens and MLNs of mice essentially as described earlier.13 Briefly, 3 × 106 erythrocyte‐free splenocytes and MLNs were incubated with 50 ng PMA, 1 μm ionomycin and 10 μg/ml of Brefeldin A (BD Biosciences) for 6 hr in a CO2 incubator set at 37°. After incubation, cells were washed and incubated with mouse seroblock FcR (BD Biosciences) to curtail the background signal. Thereafter, anti‐CD4 antibody was added and after a brief incubation, cells were fixed and permeabilized using fixation and permeabilization buffer (BD Biosciences). Fixed and permeabilized cells were then stained with anti‐IL‐17 antibody followed by incubation at 4°. Acquisition and analysis was carried out as described above.
Estimation of Treg cells
Regulatory T cells were enumerated in the spleens and MLNs of mice as described earlier.13 Briefly, single‐cell suspensions from spleens and MLNs were first blocked using mouse seroblock FcR and incubated with anti‐mouse CD4 antibody for 20 min. Cells were then fixed, permeabilized and anti‐mouse FoxP3 antibody was added. Acquisition and analysis were carried out as described above.
Real‐time RT‐PCR
Real time RT‐PCR analysis of PECs was carried out as described earlier.13 Briefly, total RNA was extracted from PECs using Trizol reagent and reverse transcribed into cDNA using oligo(dT) primers. The cDNA fragments were then amplified using SYBR green master mix and gene‐specific primers against Arg1, Ym1, Fizz1, iNOS, T‐bet and GATA‐3. Primer sequences are listed in Table 1. Conditions for PCR were 95° for 4 min, followed by 35 cycles of 95° for 20 seconds, 58° for 15 seconds and 72° for 30 seconds. Mouse glyceraldehyde‐3‐phosphate dehydrogenase was used as an endogenous control and relative fold change was determined by comparative ΔCT method.15
Table 1.
Primer sequences used in real‐time RT‐PCR
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| Arg1 | CAGAAGAATGGAAGAGTCAG | CAGATATGCAGGGAGTCACC |
| Ym1 | TCACAGGTCTGGCAATTCTTCTG | TTTGTCCTTAGGAGGGCTTCCTC |
| Fizz1 | TCCCAGATACTGATGAGA | CCACTCTGGATCTCCCAAGA |
| iNOS | TGCACTACCAAGCCACAAAGCAG | AGTAAGAGCAGGCAGCATAGCA |
| GAPDH | ACGACCCCTTCATTGACC | CCAGTGAGCTTCCCGTTCAGC |
| T‐bet | GCCAGGGAACCGCTTATATG | GACGATCATCTGGGTCACATTGT |
| GATA‐3 | CTACGGTGCAGAGGTATCC | GATGGACGTCTTGGAGAAGG |
Arginase assay and estimation of cytokines
Arginase activity in PECs from different groups of treated mice was estimated using an Arginase assay kit following the manufacturer's instructions. Briefly, 1 × 106 PECs were lysed using lysis buffer [Tris–HCl, pH 7·4, containing protease inhibitor cocktail and 0·4% (weight/volume) Triton‐X‐100] for 10 min and centrifuged at 10,303 g for 10 min to remove insoluble material. Thereafter, supernatant was collected and arginase activity was measured by comparison with a urea standard. Similarly, levels of cytokines IFN‐γ and IL‐10 were estimated in the culture supernatants of splenocytes using commercially available ELISA kits.
Statistical analysis
Parasite load was calculated from at least five different animals/group. For all other experiments, data were collected from at least five or six animals/group and the experiment was repeated thrice. Mean ± SEM values are shown in the Supplementary material (Fig. S1). Statistical analysis was carried out using one‐way analysis of variance using Dunnett's test. P‐values of ≤ 0·05, ≤ 0·01 and ≤ 0·001 between different groups were considered as significant, highly significant and very highly significant and marked with *, ** and *** respectively.
Results
Anti‐CD25 and anti‐GITR treatment increases parasite clearance in mice
Mice were divided into six different experimental groups as described in Materials and methods. Pictorial representation of the experimental plan and treatment schedule is shown in Fig. 1(a). Our results show highest parasite burden (18 ± 0·86) in the peritoneal cavities of mice belonging to the r‐wsp group, followed by the L3 infected group (13 ± 0·70), whereas it was lowest (4 ± 0·44) in the L3 + Ab treated group (Fig. 1b; P ≤ 0·001). Mice in the r‐wsp + L3 + Ab treated group showed slightly higher parasite burden (7·3 ± 0·30) than the L3 + Ab group but it was significantly less when compared with the L3 infected group (Fig. 1b; P ≤ 0·01). These findings show that administration of neutralizing antibodies directed against CD25 and GITR is helpful in reducing the parasite burden in infected animals. We have previously shown that immunizing mice with r‐wsp promotes parasite survival,13 hence an increase in parasite burden in the r‐wsp and r‐wsp + L3 + Ab treated groups is in line with our earlier findings.13
Figure 1.
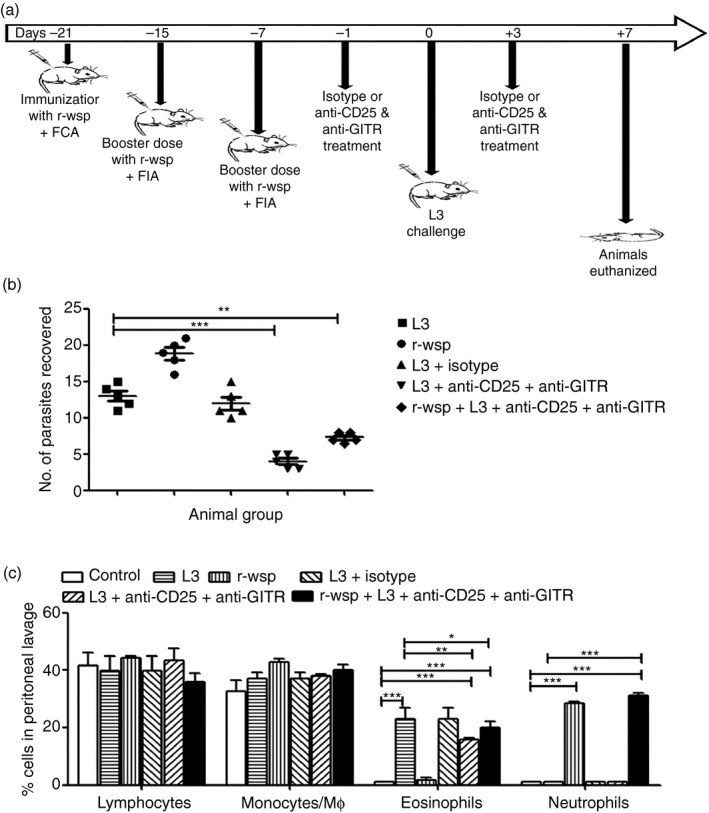
(a) Pictorial representation and treatment schedule of mice. (b) Mice were challenged intraperitoneally with 50 infective larval stage 3 of Brugia malayi (L3) and killed on day 7 post infection to recover pre‐adults. Parasite load among different groups is shown. (c) Leucocyte differentials in peritoneal exudate cells present in different groups of mice are shown. Giemsa‐stained cytospin preparations were used to assess leucocyte differentials by standard morphology and staining characteristics. Statistical significance between different groups is depicted as *P ≤ 0·05, **P ≤ 0·01 and ***P ≤ 0·001 (n = 5 or n = 6 animals/group).
Anti‐CD25 and anti‐GITR treatment affects eosinophil accumulation in the peritoneal cavity of mice
Analysis of cytospins prepared from PECs of mice from different treatment groups showed that mice in the L3 + Ab group exhibited significantly decreased percentages of eosinophils (15·6 ± 0·88) in their peritoneal cavity compared with the L3 group (23 ± 3·7), (Fig. 1c; P ≤ 0·001 between control and L3 + Ab groups and P ≤ 0·01 between L3 and L3 + Ab groups). Also, mice in the r‐wsp and rwsp + L3 + Ab groups showed significantly elevated percentages of neutrophils (28 ± 0·50 and 31 ± 1·00, respectively) compared with mice from other groups (Fig. 1c; P ≤ 0·001 between control and rwsp + L3 + Ab groups and P ≤ 0·001 between L3 and rwsp + L3 + Ab groups). However, no differences were seen with regard to the percentages of peritoneal macrophages and lymphocytes among different treatment groups (Fig. 1c). These findings are interesting because previous studies have shown that helminth infection induces infiltration of eosinophilic granulocytes to host tissues, which causes extensive tissue damage, whereas inoculation of L3 into the peritoneal cavity of mice recruits eosinophils.16 Also, studies have shown that Wolbachia delays apoptosis in neutrophils, which contributes to persistent inflammation during filarial infection and could be the reason why we observed elevated neutrophil counts in mice immunized with r‐wsp.17
Treatment with anti‐CD25 and anti‐GITR antibodies increases Th1 but decreases Th2 cytokine response
We evaluated the expression patterns of transcription factors T‐bet and GATA‐3 in the PECs of mice from different treatment groups and found that T‐bet was down‐regulated approximately 10‐fold in L3 infected mice, but increased to about sevenfold in the r‐wsp treated group and 17‐fold in the r‐wsp + L3 + Ab treated group (P ≤ 0·001; Fig. 2a). Similarly, GATA‐3 was up‐regulated approximately sevenfold in mice infected with L3, but decreased about fivefold in the r‐wsp treated group and sixfold in mice belonging to the r‐wsp + L3 + Ab treated group (P ≤ 0·001; Fig. 2a). The increase in T‐bet and concomitant decrease in GATA‐3 levels in r‐wsp and r‐wsp + L3 + Ab treated mice was also reflected in significantly elevated levels of the Th1 cytokine IFN‐γ in the r‐wsp, L3 + Ab treated and r‐wsp + L3 + Ab treated groups (Fig. 2b; P ≤ 0·05 between L3 and L3 + Ab treated groups and P ≤ 0·001 between L3 and r‐wsp + L3 + Ab treated groups). We also observed decreased levels of Th2 cytokine IL‐10 in the r‐wsp, L3 + Ab treated and r‐wsp + L3 + Ab treated groups (Fig. 2c; P ≤ 0·001 between L3 and L3 + Ab treated groups and P ≤ 0·001 between L3 and r‐wsp + L3 + Ab treated groups). These results show that infection with L3 not only promotes GATA‐3 expression, which leads to elevated Th2 cytokine production, but administration of anti‐CD25 and anti‐GITR antibodies arrests GATA‐3 expression and in turn promotes T‐bet expression leading to a dominant Th1 response.
Figure 2.
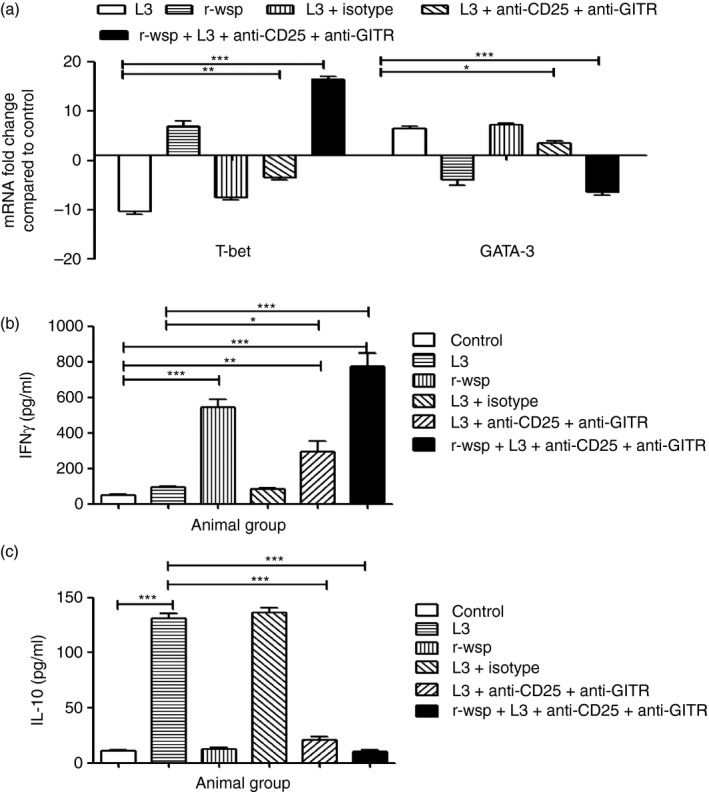
(a) Real time RT‐PCR analysis of mRNA expression levels of transcription factor T‐bet and GATA‐3 from different groups of mice. Values given are mean fold regulation compared with uninfected control. Statistical significance between different groups is depicted as *P ≤ 0·05, **P ≤ 0·01 and ***P ≤ 0·001. (b,c) Cytokine levels in culture supernatant of splenocytes from mice of different treatment groups was estimated using commercially available ELISA kits as described in Materials and methods. Values represent mean ± SEM values from three separate experiments (n = 5 or n = 6 animals/group). Statistical significance between groups is depicted as *P ≤ 0·05, **P ≤ 0·01 and ***P ≤ 0·001.
Treatment with anti‐CD25 and anti‐GITR antibodies increases the percentage of Th17 cells but decreases Treg cell accumulation in the secondary lymphoid organs of mice
Th17 cells play an important role in the establishment and maintenance of a variety of chronic inflammatory diseases, autoimmune disorders and in filarial infection.18 To study the role of Th17 cells and their interplay with Treg cells, mice were treated with anti‐CD25 and anti‐GITR antibodies as described in the Materials and methods. The results show that spleens in L3 + Ab treated mice had marginally increased percentages of CD4+ Th17 cells (0·31 ± 0·01) compared with the L3 infected group (0·26 ± 0·02), but it increased significantly in the r‐wsp + L3 + Ab treated group (0·59 ± 0·008), (Fig. 3a; P ≤ 0·001 between L3 and r‐wsp + L3 + Ab treated groups). The results were similar in MLNs where the highest percentage of CD4+ Th17 cells was found in the r‐wsp + L3 + Ab treated group (0·54 ± 0·003) compared with the L3 (0·25 ± 0·019) and L3 + Ab treated mice (0·42 ± 0·021), (Fig. 3a; P ≤ 0·01 between L3 and L3 + Ab groups and P ≤ 0·001 between L3 and r‐wsp + L3 + Ab treated groups). Since Treg cells also play an important role in promoting parasite survival by suppressing the host's effector T‐cell response, we studied the recruitment pattern of Treg cells in the secondary lymphoid organs of mice following administration of anti‐CD25 and anti‐GITR antibodies and found drastic reduction in the accumulation of Treg cells in the spleens of L3 + Ab treated mice (0·78 ± 0·043) compared with the L3 infected group (Fig. 3b; P ≤ 0·001). Interestingly, mice in the r‐wsp + L3 + Ab group showed even more significant reduction in the percentages of Treg cells (0·58 ± 0·042) compared with L3 infected mice (Fig. 3b; P ≤ 0·001). The results were similar in MLNs where reduction in Treg cell percentage was noted in the r‐wsp + L3 + Ab treated group (0·67 ± 0·010) compared with the L3 (1·9 ± 0·06) and L3 + Ab treated mice (0·81 ± 0·02), (Fig. 3b; P ≤ 0·001 between L3 and L3 + Ab and P ≤ 0·001 between L3 and r‐wsp + L3 + Ab treated group). Taken together, these results show that administration of anti‐CD25 and anti‐GITR antibodies decreases the accumulation of Treg cells, but increases the percentage of Th17 cells in the secondary lymphoid organs of mice. Interestingly, immunization of mice with r‐wsp further enhances the accumulation of Th17 cells, which might be helpful in breaking the immunological tolerance in mice.
Figure 3.
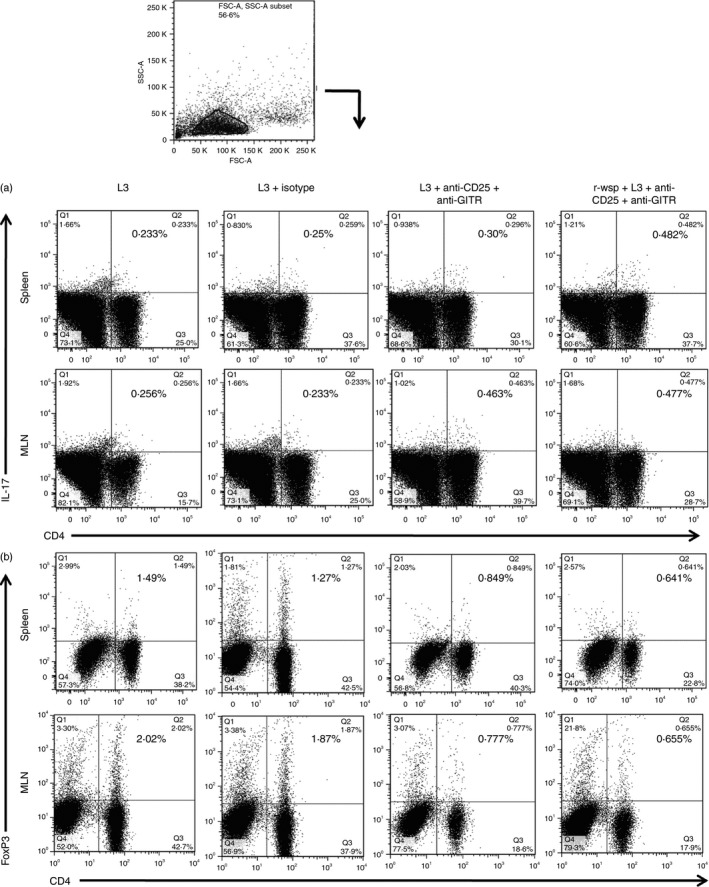
Flow cytometric identification of (a) T helper type 17 (Th17) cells and (b) regulatory T (Treg) cells present in the spleens and mesenteric lymph nodes (MLNs) of mice from different groups. Figure is a representative dot plot from one of five animals.
Anti‐CD25 and anti‐GITR treatment has no effect on recruitment of inflammatory monocytes in mice
Patients with filariasis have impaired monocyte function, which is reflected by their inability to produce cytokines/chemokines in response to activating stimuli and by alteration in their basal gene expression. To study the effect of anti‐CD25 and anti‐GITR administration on the recruitment pattern of the inflammatory monocyte subset in the secondary lymphoid organs, mice were treated as described in the Materials and methods. The results show almost no change in the percentages of inflammatory monocytes (CCR2+, Ly6C/G+) in the spleens (1·90 ± 0·09) and MLNs (3·0 ± 0·088) of L3 infected mice (Fig. 4) compared with untreated controls (1·97 ± 0·085 in control spleen and 2·8 ± 0·088 in control MLN). However, inflammatory monocytes increased significantly in spleens (3·3 ± 0·12) and MLNs (4·5 ± 0·008) of r‐wsp immunized mice compared with controls (P ≤ 0·001 in both spleen and MLN), but no difference was observed in the percentages of inflammatory monocytes between the L3 and L3 + Ab groups. Interestingly, mice in the r‐wsp + L3 + Ab treated group also showed a significant increase in the percentages of inflammatory monocytes compared with the L3 group (P ≤ 0·001 in both spleen and MLN). These findings are important, not only because they show that treatment with anti‐CD25 and anti‐GITR does not affect the recruitment pattern of inflammatory monocytes in the secondary lymphoid organs of mice, but also because they reconfirm earlier findings of our group wherein we documented the inflammatory nature of r‐wsp.
Figure 4.
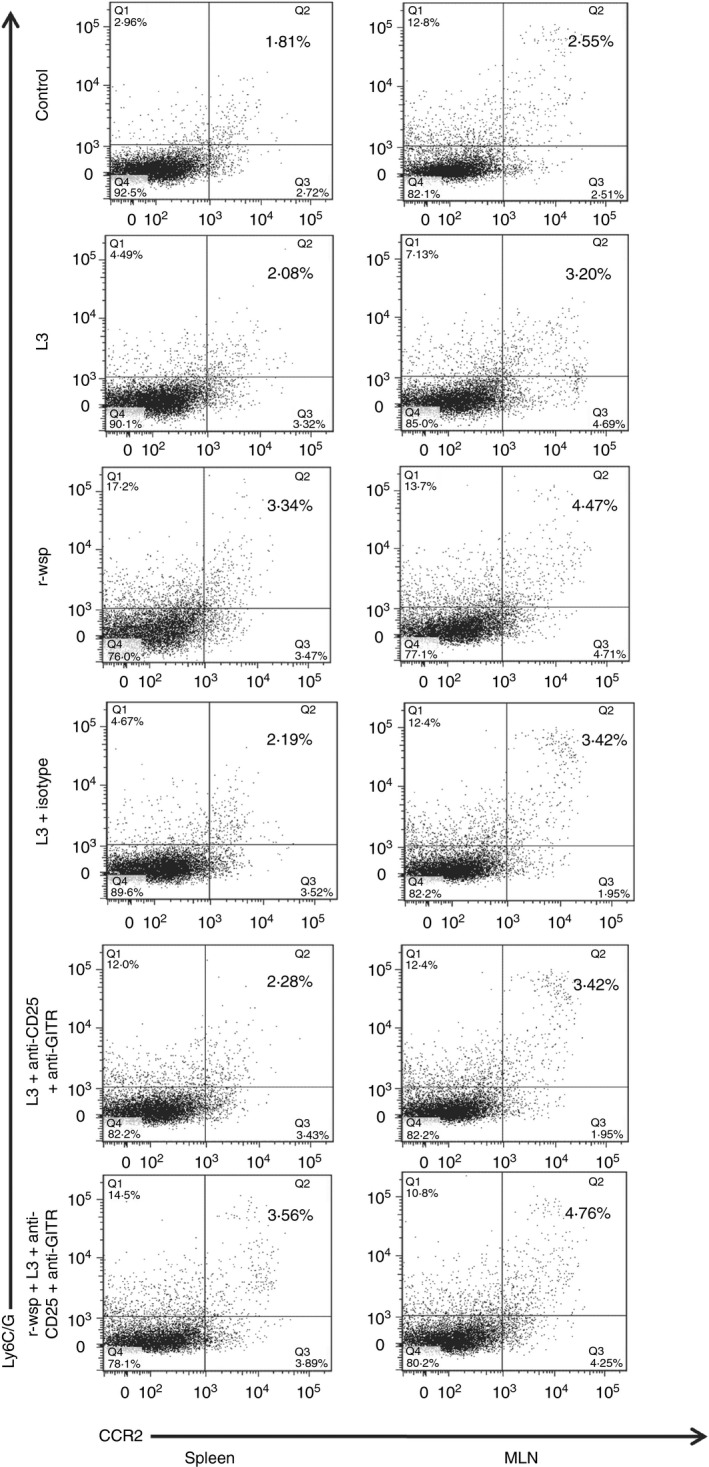
Flow cytometric identification of inflammatory monocytes present in the spleens and mesenteric lymph nodes (MLNs) of mice from different groups. Figure is a representative dot plot from one of five animals.
Anti‐CD25 and anti‐GITR treatment up‐regulates CD40 expression on host dendritic cells
Antigen‐presenting cell dysfunction is one of the mechanisms used to explain the profound parasite‐specific T‐cell hyporesponsiveness seen in chronic, patent lymphatic filariasis.19 To study the effect of anti‐CD25 and anti‐GITR administration on the maturation and activation status of dendritic cells, mice were treated as described in the Materials and methods. The results show similar percentages of splenic dendritic cells between the control and L3 infected groups, but significantly increased dendritic cell numbers were noted in the r‐wsp immunized group (4·0 ± 0·037), L3 + Ab group (4·3 ± 0·072) and was highest in the r‐wsp + L3 + Ab treated group (5·5 ± 0·006), (Fig. 5; P ≤ 0·01 between L3 and L3 + Ab and P ≤ 0·001 between L3 and r‐wsp + L3 + Ab treated groups), similarly P ≤ 0·001 between control and r‐wsp, between control and L3 + Ab and between control and r‐wsp + L3 + Ab treated groups. Similar results were obtained in MLNs where increasing percentages of dendritic cells were noted in the r‐wsp immunized group (3·4 ± 0·05), L3 + Ab group (4·06 ± 0·17) and r‐wsp + L3 + Ab treated group (5·4 ± 0·17) (Fig. 5; P ≤ 0·001 between L3 and L3 + Ab and between L3 and r‐wsp + L3 + Ab treated groups), similarly P ≤ 0·001 between control and r‐wsp, between control and L3 + Ab and between control and r‐wsp + L3 + Ab treated groups). However, surprisingly, we did not observe any significant difference in the expression patterns of CD80 and CD86 in the spleens and MLNs of mice from different treatment groups (see Supplementary material, Fig. S2). These results show that administration of neutralizing antibodies results in activation of dendritic cells, which might help in the initiation of adaptive immunity in infected hosts.
Figure 5.
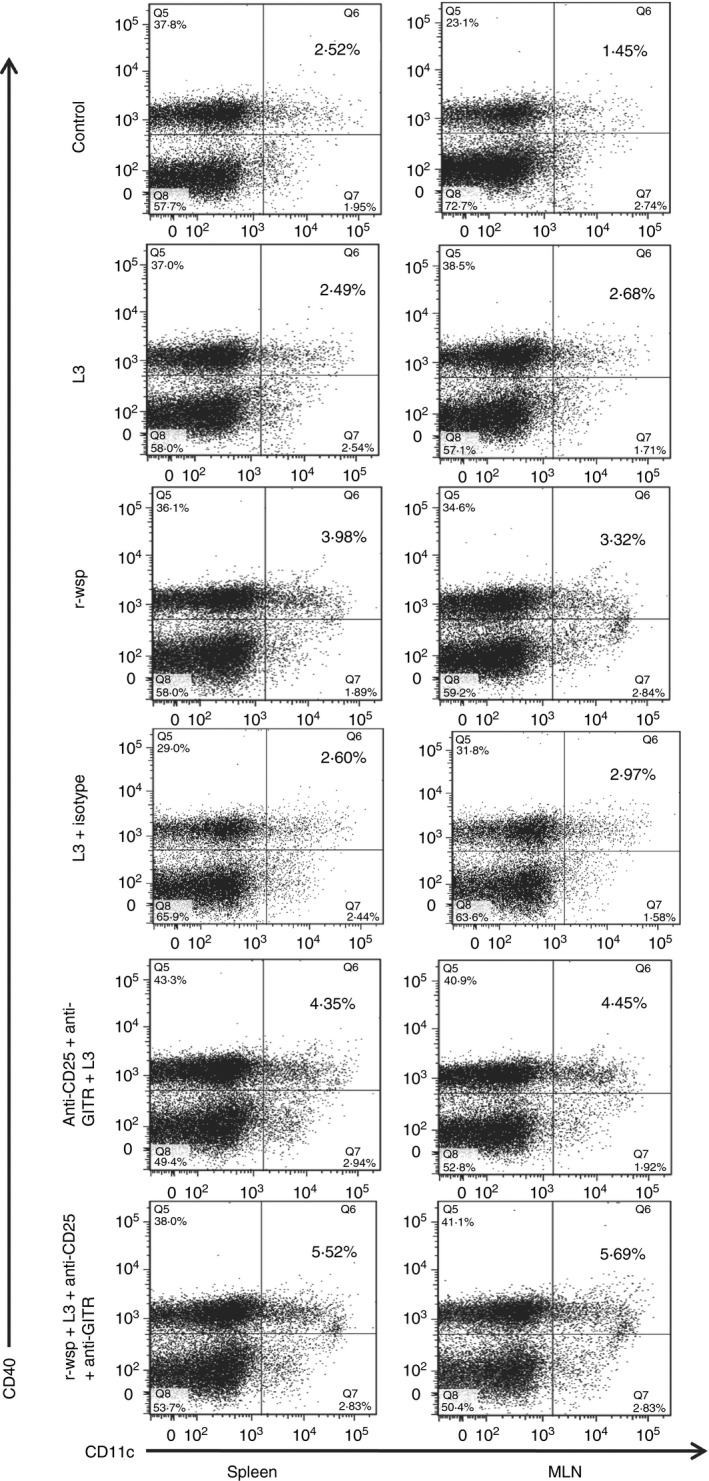
Flow cytometric identification of dendritic cells present in the spleens and mesenteric lymph nodes (MLNs) of mice from different groups. Figure is a representative dot plot from one of five animals.
Anti‐CD25 and anti‐GITR treatment arrests alternative activation of F4/80+ host macrophages
Filarial parasites have evolved mechanisms to down‐regulate the classical activation pathway of macrophages and in turn stimulate their induction towards an alternatively activated phenotype.20 Alternatively activated macrophages predominantly express Arg1, Ym1 and Fizz1 and show down‐regulation of NOS2 expression. To study the effect of anti‐CD25 and anti‐GITR administration on the macrophages present in the secondary lymphoid organs, mice were treated as described in the Materials and methods. The results show a significant increase in the percentages of macrophages in the spleens (6·9 ± 0·26) and MLNs (5·3 ± 0·09) of L3 infected mice compared with control (Fig. 6a; P ≤ 0·001). Interestingly, administration of anti‐CD25 and anti‐GITR antibodies resulted in a significant drop in the percentages of macrophages in the spleens of mice in the L3 + Ab group (4·6 ± 0·38) and r‐wsp + L3 + Ab treated group (3·5 ± 0·25) compared with the L3 infected group (Fig. 6; P ≤ 0·01 between L3 and L3 + Ab and P ≤ 0·001 between L3 and r‐wsp + L3 + Ab treated groups). Similar results were obtained in the MLNs of L3 + Ab group (3·15 ± 0·21) and r‐wsp + L3 + Ab treated group (2·7 ± 0·31) compared with the L3 infected group (Fig. 6; P ≤ 0·001 between L3 and L3 + Ab and between L3 and r‐wsp + L3 + Ab treated groups). Furthermore, administration of anti‐CD25 and anti‐GITR was successful in arresting the alternative activation of macrophages as was evident by 6‐, 2‐ and 2·5‐fold decreased expression of alternative activation markers Arg1, Ym1 and Fizz1, respectively, in the L3 + Ab group compared with the L3 infected group (Fig. 6b). Interestingly, expression of iNOS, the classical macrophage activation marker was four‐, three‐ and sixfold higher in the r‐wsp, L3 + Ab and r‐wsp + L3 + Ab treated groups, respectively compared with the L3 infected group (Fig. 6b). To further confirm these findings, we measured the activity of arginase in the culture supernatants of splenocytes of mice from different treatment groups and found the highest arginase activity (19 ± 1·0 u/l) in the L3 treated group which was reduced in r‐wsp (9·5 ± 0·50), L3 + Ab (10·50 ± 0·50) and r‐wsp + L3 + Ab treated groups (9·0 ± 1·0), (Fig. 6c; P ≤ 0·001 between L3 and L3 + Ab group and between L3 and r‐wsp + L3 + Ab treated group). These results show that infection with L3 generates an alternatively activated phenotype in the host that is rescued by the administration of anti‐CD25 and anti‐GITR antibodies.
Figure 6.
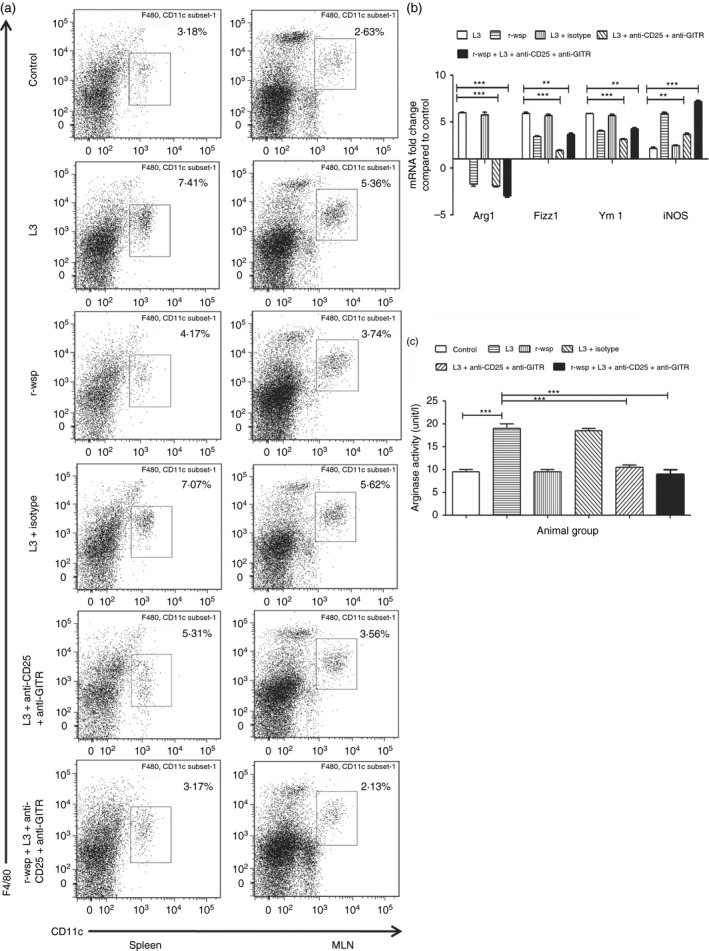
(a) Flow cytometric identification of macrophages present in the spleens and mesenteric lymph nodes (MLNs) of mice from different groups. Figure is a representative dot plot from one of five animals. (b) Real time RT‐PCR analysis of mRNA expression levels of classical (iNOS) and alternative (Arg1, Ym1, Fizz1) activation markers from different groups of mice. Values given are mean fold regulation compared with uninfected control. Statistical significance between different groups is depicted as **P ≤ 0·01 and ***P ≤ 0·001. (c) Estimation of Arginase activity present in the lysates of peritoneal exudate cells (PECs) from mice of different groups by ELISA. Data represents mean ± SEM values from three separate experiments (n = 5 or n = 6 in each group). Statistical significance between treated and control groups is depicted as ***P ≤ 0·001.
Discussion
The role of Wolbachia in filarial pathogenesis is a matter of intense investigation but its contribution to parasite survival or immune response is not completely clear. Previous work by our group showed that Wolbachia surface protein promotes a pro‐inflammatory response by increasing the accumulation of Th17 cells and decreasing the percentages of Treg cells and cytokine TGF‐β in the secondary lymphoid organs of mice. We also reported that this activity of r‐wsp was further enhanced in mice immunized with r‐wsp and challenged with Bm‐L3, thereby underlining the synergistic action of r‐wsp and L3, which not only diminished host immunological tolerance but at the same time led to an attenuated Th2 response and heightened Th1 response in infected animals.13
Previous studies have shown that functional hyporesponsiveness in filarial infection is associated with an increase in the expression of Treg cell‐associated markers CD25, CTLA‐4, and GITR and that co‐stimulation of GITR through agonistic monoclonal antibodies augments the effector T‐cell response,21, 22 overcomes Foxp3+ Treg cell‐mediated suppression,10, 23 and boosts resistance to infection with helminths, protozoa and viruses.24 Anti‐CD25 antibody administration has also been shown to reduce Foxp3+ cells by depleting natural Treg cells in vivo.25 In the present study, we hypothesized that combined administration of anti‐CD25 and anti‐GITR antibodies might be more effective in breaking self‐tolerance than either antibody alone because of their more potent synergistic action on the host Treg cell pool. Hence in the current study, mice infected with L3 were treated with neutralizing antibodies directed against CD25 and GITR and the effect of these antibodies was evaluated in terms of parasite burden and recruitment and activation pattern of different leucocyte populations present in the secondary lymphoid organs of mice.
Our results demonstrate that in vivo administration of neutralizing antibodies reduced parasite burden in L3 infected mice (L3 + Ab group) and decreased the percentage of Treg cells along with a concomitant increase in the percentages of Th17 cells in the spleens and MLNs of infected mice. Parasite burden was slightly higher in r‐wsp + L3 + Ab mice compared with L3 + Ab treated animals, which is not surprising because the inflammatory milieu generated by r‐wsp immunization provides the parasites with an alternative mechanism to survive in inflammatory conditions, which is more relevant in cases where release of Wolbachia after post‐drug treatment or worm death provides tolerance to filarial infection.13, 26, 27 We also observed increased T‐bet expression, which is an important and critical regulator of the Th1 response, and decreased GATA‐3 expression, which orchestrates the Th2 cell migratory programme in r‐wsp + L3 + Ab treated mice, which was associated with elevated levels of the Th1 cytokine IFN‐γ and decreased levels of the Th2 cytokine IL‐10. These findings lend support to our hypothesis that combined administration of anti‐CD25 and anti‐GITR antibodies dampens the protective immunity to filarial parasites. To understand if administration of neutralizing antibodies does affect the recruitment and accumulation patterns of other leucocyte subsets like inflammatory monocytes, macrophages and dendritic cells in the secondary lymphoid organs of mice we estimated the percentage of these cells in the secondary lymphoid organs of mice and our rationale behind these experiments was that previous reports had shown that filarial infection causes impairment of monocyte function by altering the basal gene expression of these cells as a result of which they fail to produce cytokines/chemokines in response to activating stimuli. Monocytes are divided into two main subsets: a short‐lived ‘inflammatory subset’ that homes to inflamed tissue, where it can trigger immune responses, and a ‘resident subset,’ with a longer half‐life, that homes to non‐inflamed tissues.28 Inflammatory monocytes express CCR2 and Ly6C/G in mice and CD14 and CD16 in humans, but on the contrary monocytes from filaria‐infected individuals have been shown to be studded with internalized filarial antigens,29 due to which they show altered lymphoproliferative responses.30, 31 In our study, significant increase in the percentages of inflammatory monocytes was noted in the r‐wsp immunized group compared with untreated controls. However, no major differences were observed between the L3 + Ab group and r‐wsp + L3 + Ab group, which shows that immunization with r‐wsp does generate an inflammatory milieu within the host tissue that remains unaffected by administration of neutralizing antibodies.
Studies in humans and animal models have demonstrated that filarially induced immune inhibition extends to many arms of the immune response, including dendritic cells,32, 33 macrophages/monocytes and T cells and that parasites secrete numerous molecules to aid in this task.34 Many in vitro studies have also shown that different life‐cycle stages of the filarial parasite induce down‐regulation of activation markers, co‐stimulatory molecules and cytokines released by monocyte‐derived macrophages and dendritic cells.20 In fact, an important feature of filarial infection is the significant expansion in F4/80+ macrophages at patency with development of a phenotype of ‘alternative activation’, which is distinct from the classical phenotype.32, 35 F4/80 antigen is a widely used marker for murine macrophages and is expressed by all kinds of macrophages present in spleen, lung, liver, peritoneal cavity and nervous system.36, 37 In the present study, F4/80+ macrophages increased significantly upon L3 infection; however, hardly any change in their numbers was reported in the r‐wsp immunized group, L3 + Ab group or r‐wsp + L3 + Ab group, which again shows that as with inflammatory monocytes, recruitment of macrophages remained unaffected by administration of neutralizing antibodies. However, despite no change in the numbers of F4/80+ macrophages, activation status of macrophages differed significantly among different groups. We found increased expression of alternative activation markers Arg1, Ym1 and Fizz1 in L3 infected mice, which decreased significantly in the L3 + Ab and r‐wsp + L3 + Ab treated groups. Interestingly, expression of the classical activation marker iNOS was highest in the r‐wsp + L3 + Ab treated group which shows that administration of anti‐CD25 and anti‐GITR was helpful in preventing alternative activation of host macrophages. Also, when we measured arginase activity among different groups of treated mice, we found the highest arginase activity in the L3 treated group and lowest in the L3 + Ab and r‐wsp + L3 + Ab group highlighting the significance of neutralizing antibodies in arresting the alternative activation of host cells. In the current study, we also evaluated the effect of anti‐CD25 and anti‐GITR administration on the maturation and activation state of dendritic cells because antigen‐presenting cell dysfunction is responsible for parasite‐specific T‐cell hyporesponsiveness during lymphatic filariasis, and genes related to antigen processing and presentation pathway exhibit diminished expression after L3 inoculation.19, 32 The results show significantly increased activation status of dendritic cells, as evident by increased expression of CD40 in spleens and MLNs of mice in the r‐wsp immunized group and L3 + Ab group, and was highest in the r‐wsp + L3 + Ab group, which shows that administration of anti‐CD25 and anti‐GITR antibodies helped in overcoming parasite‐induced suppression by increasing the expression of co‐stimulatory molecules on dendritic cells, which might have helped in the initiation of adaptive immunity in infected animals.
Taken together, our results show that anti‐CD25 and anti‐GITR antibodies help in reducing the accumulation of Treg cells in the secondary lymphoid organs of mice. Neutralizing antibodies also help in increasing the accumulation of Th17 cells, activation of host dendritic cells and arresting the alternative activation of host cells, which together result in heightened effector T‐cell activity thereby reducing parasite burden in treated animals. Such approaches are useful and must be explored further in greater detail to develop new adjuncts that have the ability to control the spread of filarial infection at the earliest host–parasite interface.
Author's contributions
MP, PS, AS, MV and MS performed the experiments. MS and SMB conceived and designed the study. MP, MS and SMB wrote the paper.
Funding
Financial support to MP, PS, AS, MV in the form of research fellowships from the Indian Council of Medical Research (ICMR), Council of Scientific and Industrial Research (CSIR) and University Grant Commision (UGC), New Delhi, India is gratefully acknowledged. The authors also wish to acknowledge funding support provided to MS and SMB via the CSIR‐Network projects ‘New approaches towards understanding of disease dynamics and to accelerate drug discovery (UNDO)’ and ‘Emerging and re‐emerging challenges in infectious diseases: systems‐based drug design for infectious diseases (SPLenDID)’. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1. Leucocytes present in the spleens and mesenteric lymph nodes of different groups of mice were estimated by flow cytometry as described in the Materials and methods. Histograms represents mean ± SEM percentages from at least three separate experiments (n = 5 or n = 6 in each group). Statistical significance between different groups is depicted as *P ≤ 0·05; **P ≤ 0·01 and ***P ≤ 0·001.
Figure S2. (a,b) Surface expression patterns of CD80 and CD86 on dendritic cells present in the spleens and mesenteric lymph nodes of mice from different groups. Figure is a representative histogram plot from one of five animals.
Acknowledgements
We are grateful to Shikha Mishra and OP Yadav for their excellent technical support in maintaining B. malayi infection in the laboratory and Mr AL Vishwakarma for assisting with flow cytometry experiments. This is communication No. 9097 of CSIR‐CDRI.
References
- 1. Maizels RM, Balic A, Gomez‐Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites – masters of regulation. Immunol Rev 2004; 201:89–116. [DOI] [PubMed] [Google Scholar]
- 2. Hoerauf A, Satoguina J, Saeftel M, Specht S. Immunomodulation by filarial nematodes. Parasite Immunol 2005; 27:417–29. [DOI] [PubMed] [Google Scholar]
- 3. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol 2006; 176:3248–56. [DOI] [PubMed] [Google Scholar]
- 4. Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune‐mediated inflammation. Int J Parasitol 2007; 37:457–64. [DOI] [PubMed] [Google Scholar]
- 5. Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol 2007; 7:532–42. [DOI] [PubMed] [Google Scholar]
- 6. Marelli‐Berg FM, Okkenhaug K, Mirenda V. A two‐signal model for T cell trafficking. Trends Immunol 2007; 28:267–73. [DOI] [PubMed] [Google Scholar]
- 7. Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol 2005; 23:23–68. [DOI] [PubMed] [Google Scholar]
- 8. Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G et al GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol 2004; 34:613–22. [DOI] [PubMed] [Google Scholar]
- 9. Muriglan SJ, Ramirez‐Montagut T, Alpdogan O, Van Huystee TW, Eng JM, Hubbard VM et al GITR activation induces an opposite effect on alloreactive CD4+ and CD8+ T cells in graft‐versus‐host disease. J Exp Med 2004; 200:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self‐tolerance. Nat Immunol 2002; 3:135–42. [DOI] [PubMed] [Google Scholar]
- 11. Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. Role for CD4+ CD25+ regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med 2004; 200:201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramirez‐Montagut T, Chow A, Hirschhorn‐Cymerman D, Terwey TH, Kochman AA, Lu S et al Glucocorticoid‐induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J Immunol 2006; 176:6434–42. [DOI] [PubMed] [Google Scholar]
- 13. Pathak M, Verma M, Srivastava M, Misra‐Bhattacharya S. Wolbachia endosymbiont of Brugia malayi elicits Th‐17 mediated proinflammatory immune response through surface protein (WSP). Immunology 2014; 144:231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor MJ. Wolbachia endosymbiotic bacteria of filarial nematodes. A new insight into disease pathogenesis and control. Arch Med Res 2002; 33:422–4. [DOI] [PubMed] [Google Scholar]
- 15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(–ΔΔCT) method. Methods 2001; 25:402–8. [DOI] [PubMed] [Google Scholar]
- 16. Falcone FH, Loke P, Zang X, MacDonald AS, Maizels RM, Allen JE. A Brugia malayi homolog of macrophage migration inhibitory factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J Immunol 2001; 167:5348–54. [DOI] [PubMed] [Google Scholar]
- 17. Bazzocchi C, Comazzi S, Santoni R, Bandi C, Genchi C, Mortarino M. Wolbachia surface protein (WSP) inhibits apoptosis in human neutrophils. Parasite Immunol 2007; 29:73–9. [DOI] [PubMed] [Google Scholar]
- 18. Babu S, Bhat SQ, Pavan Kumar N, Lipira AB, Kumar S, Karthik C et al Filarial lymphedema is characterized by antigen‐specific Th1 and Th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl Trop Dis 2009a; 3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Semnani RT, Keiser PB, Coulibaly YI, Keita F, Diallo AA, Traore D et al Filaria‐induced monocyte dysfunction and its reversal following treatment. Infect Immun 2006; 74:4409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Babu S, Kumaraswami V, Nutman TB. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis 2009b; 199:1827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nocentini G, Cuzzocrea S, Genovese T, Bianchini R, Mazzon E, Ronchetti S et al Glucocorticoid‐induced tumor necrosis factor receptor‐related (GITR)‐Fc fusion protein inhibits GITR triggering and protects from the inflammatory response after spinal cord injury. Mol Pharmacol 2008; 73:1610–21. [DOI] [PubMed] [Google Scholar]
- 22. Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: more than an effector T cell co‐stimulatory system. Eur J Immunol 2007; 37:1165–9. [DOI] [PubMed] [Google Scholar]
- 23. Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM et al Engagement of glucocorticoid‐induced TNFR family‐related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+ CD25+ T cells. J Immunol 2004; 173:5008–20. [DOI] [PubMed] [Google Scholar]
- 24. Haque A, Stanley AC, Amante FH, Rivera Fde L, Zhou Y, Kuns RD et al Therapeutic glucocorticoid‐induced TNF receptor‐mediated amplification of CD4+ T cell responses enhances antiparasitic immunity. J Immunol 2010; 184:2583–92. [DOI] [PubMed] [Google Scholar]
- 25. Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo . J Immunol 2005; 174:4924–33. [DOI] [PubMed] [Google Scholar]
- 26. Brattig NW, Bazzocchi C, Kirschning CJ, Reiling N, Buttner DW, Ceciliani F et al The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J Immunol 2004; 173:437–45. [DOI] [PubMed] [Google Scholar]
- 27. Lamb TJ, Harris A, Le Goff L, Read AF, Allen JE. Litomosoides sigmodontis: vaccine‐induced immune responses against Wolbachia surface protein can enhance the survival of filarial nematodes during primary infection. Exp Parasitol 2008; 118:285–9. [DOI] [PubMed] [Google Scholar]
- 28. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19:71–82. [DOI] [PubMed] [Google Scholar]
- 29. Semnani RT. The interaction between filarial parasites and human monocyte/macrophage populations. Adv Exp Med Biol 2013; 785:49–56. [DOI] [PubMed] [Google Scholar]
- 30. Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 2009; 27:669–92. [DOI] [PubMed] [Google Scholar]
- 31. Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 2006; 7:311–7. [DOI] [PubMed] [Google Scholar]
- 32. Semnani RT, Sabzevari H, Iyer R, Nutman TB. Filarial antigens impair the function of human dendritic cells during differentiation. Infect Immun 2001; 69:5813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode‐secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol 2000; 164:6453–60. [DOI] [PubMed] [Google Scholar]
- 34. Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 2003; 3:733–44. [DOI] [PubMed] [Google Scholar]
- 35. Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J Immunol 2006; 176:6918–27. [DOI] [PubMed] [Google Scholar]
- 36. Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 1981; 11:805–15. [DOI] [PubMed] [Google Scholar]
- 37. Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990; 39:151–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Leucocytes present in the spleens and mesenteric lymph nodes of different groups of mice were estimated by flow cytometry as described in the Materials and methods. Histograms represents mean ± SEM percentages from at least three separate experiments (n = 5 or n = 6 in each group). Statistical significance between different groups is depicted as *P ≤ 0·05; **P ≤ 0·01 and ***P ≤ 0·001.
Figure S2. (a,b) Surface expression patterns of CD80 and CD86 on dendritic cells present in the spleens and mesenteric lymph nodes of mice from different groups. Figure is a representative histogram plot from one of five animals.


