Summary
Lymphoid neogenesis is traditionally viewed as a pre‐programmed process that promotes the formation of lymphoid organs during development. Here, the spatial organization of T and B cells in lymph nodes and spleen into discrete structures regulates antigen‐specific responses and adaptive immunity following immune challenge. However, lymphoid neogenesis is also triggered by chronic or persistent inflammation. Here, ectopic (or tertiary) lymphoid organs frequently develop in inflamed tissues as a response to infection, auto‐immunity, transplantation, cancer or environmental irritants. Although these structures affect local immune responses, the contribution of these lymphoid aggregates to the underlining pathology are highly context dependent and can elicit either protective or deleterious outcomes. Here we review the cellular and molecular mechanisms responsible for ectopic lymphoid neogenesis and consider the relevance of these structures in human disease.
Keywords: arthritis, autoimmunity, cancer, infection, lymphoid neogenesis
Secondary and ectopic lymphoid organs
Secondary lymphoid organs (SLOs) are responsible for immune homeostasis and the development of adaptive immune responses to invading pathogens.1 Here, the accumulation of foreign antigens within the highly organized cellular architecture of SLOs facilitates antigen presentation to T and B cells and the establishment of adaptive immunity. Encapsulated SLOs form at predetermined locations during embryonic development and include the spleen and lymph nodes. Lymph nodes are strategically located throughout the body to monitor self and non‐self antigens displayed by antigen‐presenting cells as they are trafficking from peripheral organs and tissue. The spleen is also important for protection against pathogens carried in the blood. SLOs also include the non‐encapsulated mucosal‐associated lymphoid tissues that feature at barrier surfaces and include Peyer's patches, tonsils, nasal‐associated lymphoid tissue and bronchus‐associated lymphoid tissue (BALT). These latter types of SLOs are found in the sub‐mucosal epithelium and are responsible for preserving tissue integrity at barrier surfaces by ensuring the maintenance of immune tolerance against protective commensal microbiota and host responses to pathogenic insult.2
To generate fast and efficacious anti‐pathogen responses, lymphoid organs have evolved to maximize encounters between lymphocytes and antigen‐loaded antigen‐presenting cells. Consequently, lymphoid organs share a cellular organization that includes a germinal centre comprising antibody secreting and proliferating B cells together with follicular dendritic cells (DCs); a T‐cell zone including naive cells recruited from the blood; high endothelial venules (HEV) for lymphocyte extravasation; and a network of stromal cells that provide chemokines and extracellular matrix for cellular migration and structural integrity.1, 3
Inflammation is the consequence of our immunological response to infection, autoimmunity, cancer, injury and allograft transplantation.4 Appropriate control of inflammation ensures competent host defence and is governed by cellular communication between non‐haematopoietic stromal cells, tissue‐resident leukocytes and infiltrating immune cells.4, 5, 6, 7 However, inappropriate control, for example during autoimmunity, results in sustained immune responses causing chronic inflammation. Without therapeutic intervention, over time this inflammation drives clinical symptoms that culminate in tissue destruction and loss of function.8 Leukocyte infiltration is classically viewed as a random, diffuse accumulation of cells within affected tissues. However, there is emerging appreciation that during chronic inflammation, infiltrating immune cells can form highly organized aggregates of lymphoid cells that resemble SLOs. These ectopic lymphoid follicles (ELFs), also known as tertiary lymphoid structures, can propagate local antigen‐specific responses within tissues.9, 10 Occasionally, these ELFs are named according to their site of development (e.g. inducible bronchus‐associated lymphoid tissue; iBALT). Whereas SLOs develop during ontogeny, ELFs are ‘induced’ in response to an inflammatory insult within target tissues. This is particularly the case where there is a perceived need for sustained leukocyte extravasation due to the failure to clear antigen. Such responses often occur at sites of infection, autoimmunity, cancer, allograft rejection or continued insult from environmental irritants. Consequently, ELFs are ‘transient’ structures, and often resolve upon successful antigen clearance. So what controls the development of these structures in inflamed tissues? While the molecular signatures associated with ELFs resemble those involved in SLO formation, the development or maintenance of ELFs in these sites is significantly influenced by the nature of the local tissue microenvironment. For example, various novel immune subsets have recently been identified as inducers of ELF development that are distinct from the lymphoid tissue inducer (LTi) cells involved in secondary lymphoid organogenesis. The discovery of these subsets now provides new opportunities and therapeutic strategies for targeting ELF‐driven pathologies with biological drugs.
Here we review the cellular and molecular regulators that govern ELF development, their functional importance in disease and how ELFs impact the application of biological drug interventions in chronic disease and cancers.
Cellular initiators of ectopic lymphoneogenesis
Given that only a proportion of patients suffering any particular inflammatory condition will develop ELFs – for example, approximately 40% of patients with rheumatoid arthritis develop synovial ELFs11 – ectopic lymphoid neogenesis must be controlled by a specific set of inflammatory signals. Likewise, as some tissues and tumours are more permissive to ELF development than others, the tissue microenvironment must contribute defined signals that are conducive to lymphoid neogenesis. In this regard, the development of ELFs mimics many of the mechanisms underpinning the organogenesis of SLOs (for a comprehensive review of SLO development see refs 1, 12). Here, initiation of SLO development centres on an interaction at the lymph node anlagen between haematopoietic derived CD4+ CD45+ CD3− LTi cells and lymphoid tissue organizer (LTo) cells of mesenchymal origin. Here, LTi cells accumulate in response to the local expression of CXCL13, interleukin‐7 (IL‐7) and receptor activator of nuclear factor‐κB ligand (RANKL; also called TNFSF11), owing to the cell surface expression of CXCR5 and the IL‐7 receptor (also called CD127). In response to IL‐7 and RANKL, LTi cells secrete lymphotoxin (LT) α 1 β 2, which engages the LTβ receptor (LTβR) expressed on LTo cells. In turn, LTo cells release the homeostatic chemokines CXCL13, CCL19 and CCL21 in order to recruit haematopoietic cells and up‐regulate the expression of the adhesion molecules vascular cell adhesion molecule 1, intercellular adhesion molecule 1 and mucosal addressin cell adhesion molecule‐1 to ensure lymphocyte retention during SLO development.13, 14 LTo cells also secrete vascular growth factor‐C, fibroblast growth factor‐2 and hepatocyte growth factor, which promote the development of the lymphatic vasculature and HEVs.12, 14 Stromal LTo cells also differentiate into stromal cell lineages including follicular DCs, fibroblastic reticular cells and marginal reticular cells, which populate lymph nodes and contribute to SLO function.12, 15, 16
There is increasing evidence that immune cells recruited to inflammatory lesions initiate ELF development (Fig. 1). For example, IL‐17‐secreting CD4 T helper (Th17) cells have been extensively linked with ELF development in experimental models of chronic inflammation.17 Here, the development of iBALT as a consequence of pulmonary inflammation was dependent on the Th17 signature cytokine IL‐17, which caused an LTα‐independent induction of the lymphoid chemokine CXCL13.18 This demonstrates the ability of this effector T helper cell to initiate ELF development. Notably, the adoptive transfer of in vitro generated Th17 cells into mice is also sufficient to drive ELF development in a model of multiple sclerosis.19 The expression of the cell surface glycoprotein podoplanin (also called gp38) by Th17 cells was required for the development of these lymphoid follicles in the central nervous system. Indeed, mice deficient in podoplanin, or its receptor CLEC‐2, display a defect in the development and maintenance of lymph nodes.13, 19, 20 Our recent study of synovial ELF development in IL‐27R‐deficient mice with inflammatory arthritis identified podoplanin‐expressing T cells within synovial lymphoid aggregates and described IL‐27 as a negative regulator of podoplanin‐expressing Th17 cells.21
Figure 1.
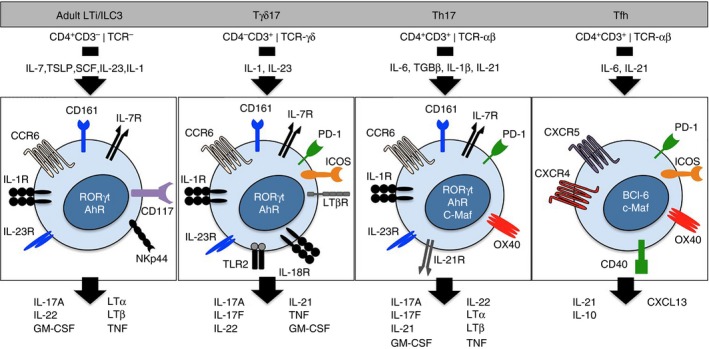
Novel immune cell subsets implicated in the regulation of ectopic lymphoid follicles (ELFs). Novel innate and adaptive immune cell subsets have recently been implicated in ELF regulation. These include the adult lymphoid tissue inducer (LTi) ‐like or innate lymphoid 3 cells, interleukin‐17 (IL‐17) ‐producing γδT (Tγδ17) cells, T helper type 17 (Th17) cells and follicular T helper (Tfh) cells. Here we highlight the similarities in their phenotype including the cytokines involved in their development, proliferation and effector function, the receptors expressed on the cell surface and the effector cytokines produced by these cells. Similarities in the effector characteristics of these cells may account for their common ability to regulate ELF development or activity. TSLP, thymic stromal lymphopoietin; SCF, stem cell factor; GM‐CSF, granulocyte–macrophage colony‐stimulating factor; TNF, tumour necrosis factor; PD‐1, programmed cell death‐1; ICOS, inducible T‐cell co‐stimulator.
Recently, other cytokines linked with the IL‐17/Th17 cell axis have also been associated with control of lymphoid neogenesis (Fig. 2). For example, IL‐23 is linked with ectopic lymphoid neogenesis in rheumatoid arthritis.22 Through control of lymphoid chemokine production in epithelial and fibroblastic stromal cells, IL‐22 also drives lymphoid neogenesis in mice following salivary gland cannulation with adenovirus.23 Podoplanin and IL‐17 have also been linked with ectopic lymphoneogenesis in human diseases.21, 24, 25
Figure 2.
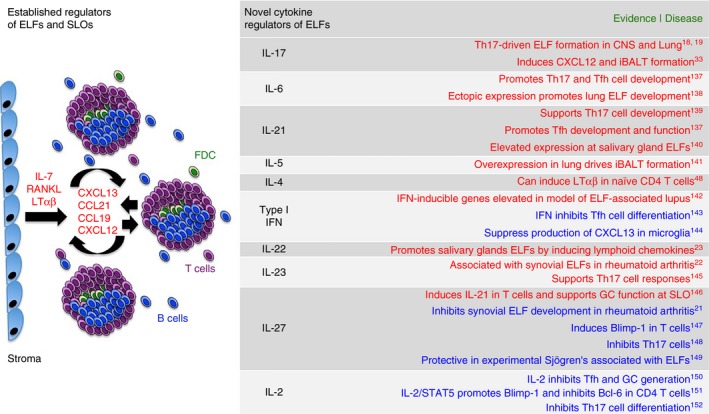
Novel cytokine regulators of ectopic lymphoid follicle (ELF) development and function. The formation of ELFs at sites of chronic inflammation mirrors the pre‐programmed development of conventional secondary lymphoid organs (SLOs). During secondary lymphoid organogenesis, the cytokines interleukin‐7 (IL‐7), receptor activator of nuclear factor‐κB ligand (RANKL) and lymphotoxin (LT) αβ initiate the chemokine‐directed positive feedback loop that drives B‐cell, T‐cell and follicular dendritic cell (DC) recruitment during lymphoid neogenesis. Recent studies have implicated novel T helper cell subsets as initiators of ELF formation. Given the role that cytokines play in the regulation of T helper cell differentiation and effector function, a number of cytokines have now been linked with the control of ELFs. For example, cytokines involved in the regulation of T helper type 17 (Th17) cell responses (IL‐6, IL‐21, IL‐23, IL‐27, IL‐2, IL‐22, IL‐17)23, 137, 139, 145, 148, 152 and follicular T helper (Tfh) cell responses [IL‐6, IL‐21, Type I interferons (IFNs), IL‐27, IL‐2]137, 139, 146, 147, 150 are emerging as regulators of lymphoid neogenesis. Here we highlight cytokines that may positively (red) and negatively (blue) control ELFs based on their ability to regulate effector T‐cell populations involved in ELF development or function. These cytokines, as well as their downstream signalling pathways and transcription factors, have the potential to serve as therapeutic targets in clinical conditions where ELFs feature.
It has recently emerged that Th17‐type responses are not solely restricted to conventional T helper cells. Adult LTi cells, a group‐3 innate lymphoid cell subset, bear many of the features of Th17 cells, which suggests an ancestral link between these cell types.26, 27 Both cells express the transcriptional regulator retinoic acid receptor‐related orphan receptor γ; are responsive to IL‐23 and aryl hydrocarbon receptor ligands; and can produce IL‐17, IL‐22 and granulocyte–macrophage colony‐stimulating factor.28, 29, 30 Like Th17 and fetal LTi cells, innate lymphoid cells have been linked with lymphoid organogenesis. The adoptive transfer of adult CD4+ CD3− LTi cells into Cxcr5 −/− mice induces the development of intestinal lymphoid tissues.31 Similarly, the increased availability of IL‐7 in transgenic mice has been associated with the LTi cell‐dependent development of additional Peyer's patches, caecal patches and de novo formation of ectopic lymphoid organs.32 A recent study has also shown that IL‐17 induces CXCL12 and iBALT development in response to Pseudomonas aeruginosa infection, where the main source of IL‐17 was γδ T cells (Tγδ17 cells).33 The similarities in effector characteristics between Th17, LTi cells and γδ T cells may therefore account for the ability of these populations to drive ELF development (Fig. 1).
T follicular helper (Tfh) cells promote B‐cell activities and support the generation of high‐affinity antibodies at germinal centres.34, 35 Plasticity among effector T helper cells may also contribute to ELF development. For example, Th17 cells are linked with ELF development in the central nervous system, lungs and inflamed joint tissue.18, 19, 21 Interestingly, in the central nervous system Th17 cells develop a ‘Tfh‐like’ phenotype that may contribute to ELF development and function.19 ELF development during inflammatory arthritis is also linked with the local expression of Th17 and Tfh effector cytokines and transcription factors.21 Similarly, Th17 cells that home to Peyer's patches can acquire Tfh‐like effector characteristics that support antigen‐specific IgA responses at germinal centres.36 Here, Th17 cells recruited to the intestine express podoplanin. Therefore, lineage plasticity may provide the ability for effector T cells to develop Tfh‐like properties that support the development, maintenance and function of ELFs. Indeed, T helper cell plasticity is not solely confined to Th17 cells, and both Th1 and Th2 cells retain the ability to acquire the IL‐21, CXCR5, Bcl‐6, programmed cell death‐1 and inducible T‐cell co‐stimulator expression that are characteristic of Tfh cells (Fig. 1).19, 36, 37, 38 Therefore, other subsets beyond Th17 cells may soon emerge as initiators of ELFs.
Inflammatory cells may substitute for LTi cells in ectopic lymphoneogenesis, but there is increasing evidence that stromal tissue cells also display LTo‐like properties39, 40, 41. In rheumatoid arthritis, synovial fibroblasts contribute to ELF formation through the secretion of homeostatic chemokines such as CXCL13, CCL21 and CXCL12.5, 23, 42, 43, 44 Interestingly, synovial fibroblasts can also contribute to other aspect of ELF activity, where they can produce B‐cell‐activating factor and a proliferation‐inducing ligand (known as APRIL).45 These factors support activation‐induced cytidine deaminase (AID) expression, which drives somatic hypermutation and antibody class‐switching in B cells.45 Here, it is important to understand the relationship between the stromal tissue compartment and the nature of the inflammatory infiltrate because ELFs are not a universal feature of synovitis in inflammatory arthritis and only occur in a certain cohort of patients.
Homeostatic chemokines in ELF development
The expression of homeostatic chemokines is increased in tissues where ELFs have emerged in response to foreign or auto antigens.1, 9, 10, 12 Chemokines such as CXCL13, CCL19, CCL21 and CXCL12 are involved not only in the initiation of ELF development, but also in the maintenance of the highly organized cellular architecture of established ELFs and SLOs. Given that the early clustering of LTi cells within the embryo is dependent on CXCL13, and that CXCL13 is detected early in the developing lymph nodes of LTα‐deficient mice,46, 47 this chemokine represents a key initiator of lymphoid organogenesis that functions upstream of LTβR signalling. At ELFs, CXCL13 and CCL21 regulate B‐cell and T‐cell infiltration and segregation at ELFs.48, 49, 50 Similarly, chemokines CCL19 and CXCL12 drive lymphocyte recruitment and the positioning of follicular DCs, B cells and plasma cells at germinal centres.48 Here, an elegant cooperation between CXCL12 and CXCL13 directs the movement of B cells from the dark zone into the light zone as they mature into antibody‐secreting plasma cells.51 Hence homeostatic and certain inflammatory chemokines contribute to both the initiation of ELF development, the cellular organization required for their function as germinal centres.
In addition to their chemotactic properties, homeostatic chemokines promote the secretion of LTα 1 β 2 by B cells and T cells, which establishes a feedback loop to perpetuate lymphocyte recruitment and positional organization.49, 50, 52 Interestingly mice lacking CXCL13, or its receptor CXCR5, fail to develop peripheral lymph nodes, underlining the importance of this chemokine in lymphoid organogenesis.50, 52, 53 Transgenic overexpression of Cxcl13 in the pancreas induces the production of LTα 1 β 2 by B cells that is required for the development of ELFs that feature T‐cell and B‐cell segregation with HEV formation.49 CCL19 and CCL21 similarly drive the expression of LTα 1 β 2 on naive CD4 T helper cells.48 Hence, homeostatic chemokines are an integral feature of ELF development, organization and function. Nevertheless, there appears to be some hierarchy among these chemokines in their ability to drive ectopic lymphoneogenesis. For example, transgenic overexpression of Ccl21 promotes the development of larger lymphoid follicles than those that emerge in response to Ccl19.48 Transgenic Ccl21 expression also promotes the development of ELFs that display higher cellular organization than Ccl19. Interestingly, ectopic Cxcl12 expression induces ELFs that contain few T cells but are enriched for follicular DCs, B cells and plasma cells.48 Therefore, as well as determining the size and degree of cellular organization within ELFs, the relative expression of homeostatic chemokines at inflammatory lesions will also determine the cellular composition of ELFs.
It is clear that some chronically inflamed tissues are more permissive to ELF development than others. For example, transgenic expression of Ccl21 in the pancreas promotes ELFs that display T‐cell and B‐cell segregation, HEV and stromal reticulum. However, this response is context dependent and Ccl21 in the skin fails to initiate lymphoneogenesis.54, 55 Histological features of ELFs are seen in various chronic inflammatory diseases and are clinically observed in the lung, joint synovium, liver, thymus, and salivary and thyroid glands (for a comprehensive review of ELFs in human disease refer to Pitzalis et al.10). Structurally mature ELFs are not, however, seen in skin conditions despite local expression of Ccl21, which suggests that the immune setting and the interaction of the stromal compartment with resident and infiltrating cells has to be conducive for ELF development to occur.56, 57 This may reflect differences in innate sensing responses or a differential stromal response to chemokines and cytokines necessary for the initiation, expansion and maintenance of tissue ELFs.
High endothelial venules at ELFs
The function of SLOs depends on HEV that express peripheral node addressin and mucosal addressin cell adhesion molecule, which regulate the entry of naive T cells into the lymph node. Similarly, HEVs are also observed in ELFs that develop in response to autoimmune disease, allograft rejection and cancers.41, 58, 59, 60, 61, 62, 63, 64 However, HEVs also develop in tissues that do not feature ELFs,65, 66, 67 which infers that HEV neogenesis occurs before the recruitment of peripheral T and B cells and the emergence of ELFs. In peripheral lymph nodes, LTβR signalling in DCs and endothelial cells promotes HEV formation and maturation.68, 69 However, the mechanisms responsible for HEV development in inflamed tissues are less clear. In human breast cancer, HEVs correspond with a heightened expression of LTβ by mature DCs.66 Here, high densities of HEVs are associated with a reduced frequency of FoxP3+ regulatory T (Treg) cells. Similar data have also been obtained in an experimental model of carcinogen‐induced fibrosarcoma, where Treg cell depletion caused reduced tumour growth and increased HEV formation.65 Indeed, HEVs in solid tumours are regarded as positive prognostic indicators and patients are often disease free for longer, show reduced evidence of tumour metastasis and display improved survival rates.9, 65, 66, 67, 70 In primary and metastatic tumours, HEVs are associated with increased infiltration of naive, central memory and activated T cells that display a Th1 effector phenotype.66, 67, 70 A high density of extra‐tumoral and intra‐tumoral Th1 cells correspond with improved survival,71 where it is proposed that their local priming, possibly within ELFs, promotes antigen‐specific tumour responses.72, 73 In murine models of melanoma and lung carcinoma, the development of lymph node‐like vasculature allows naive T‐cell entry into tumours that delay tumour outgrowth.74 Therefore modulation of HEV development or function through targeting LTα 1 β 2 to the tumour or by inhibiting Treg activity may provide opportunities for novel immunotherapies.
ELFs as inductive sites for anti‐pathogen immune responses
Chronic bacterial and viral infections can trigger inflammation that results in the development of ELFs (comprehensively reviewed elsewhere75, 76). For example, Helicobacter spp. and Mycobacterium tuberculosis bacteria, as well as influenza and hepatitis C viruses, have been linked with ELF development in mice and humans.77, 78, 79, 80, 81, 82, 83, 84 Interestingly, ELF formation in response to infection features at mucosal sites including lung, gastric and salivary gland tissues.78, 82, 85 ELFs that develop in response to microbiota are also important for maintaining intestinal homeostasis.86 There is considerable evidence linking ELFs with anti‐microbial and viral immunity. For example mice lacking spleen, lymph nodes and Peyer's patches mount robust B‐cell and T‐cell responses to influenza virus at sites of iBALT formation.82 Notably, in the absence of peripheral lymphoid organs, these mice tolerate higher virus doses than wild‐type mice, suggesting that the anti‐viral response generated at iBALT is not only protective but may also be less pathogenic than those generated in the periphery. Mice were able to show effective primary and memory responses to viral challenge via ELFs, associated with the emergence of influenza‐specific CD8 T cells and anti‐influenza nucleoprotein‐specific antibodies.81, 82 Development of iBALT in response to infection was again linked to CXCL13 and CCL21 expression locally,82 but was also dependent on CD11chi DCs.80 Here, DCs were required for the maintenance of iBALT, and DC depletion resulted in the regression of the ELFs resulting in a reduction in germinal centre reactions, the number of class‐switched plasma cells and anti‐viral serum antibodies.
Similar ELF‐associated anti‐pathogen responses are also described for M. tuberculosis and murine cytomegalovirus infection in mice. In pulmonary M. tuberculosis infection, ELF development was associated with CXCL13, CCL21 and CCL19 expression and the recruitment of functional CXCR5+ T cells.77, 79 T cells expressing CXCR5 displayed Tfh‐ and Th1‐like effector characteristics and were important for host survival, M. tuberculosis clearance, T‐cell localization within ELFs, and lymphoid follicle formation.79 In murine cytomegalovirus infection, lymphoid follicles that form in the salivary glands participate as inductive sites in oral mucosal immunity.85 Development of these salivary follicles was accompanied by a local expression of homeostatic chemokines and molecular markers such as AID and IμCα (the non‐excised rearranged DNA of IgA class‐switching), which regulate germinal centre activities including somatic hypermutation and class‐switch recombination.85
Development of ELFs is therefore an integral part of anti‐microbial and anti‐viral immunity. Although the regulation of these ELF‐driven responses can occur independent of SLOs, their presence in infected tissues supports adaptive immunity and the activities of SLOs. In humans, ELF involvement is often associated with persistent infections. Here, a failure to eradicate pathogens may lead to aberrant adaptive immune responses at ELFs that, when inappropriately controlled, drive the onset of tissue damage and chronic inflammation and transition to autoimmunity or cancer.
ELFs as regulators of anti‐tumour immunity
Current cancer immunotherapies aim to enhance the adaptive immune response to tumour antigens to overcome the immunosuppressive microenvironment of the tumour.87 In this context, it is therefore surprising that ELFs arise in tumours at all. However, ELFs have been described in numerous cancers including colorectal,60, 88 rectal,89 breast,67, 90, 91 ovarian67, 92 and germ cell93 cancers, as well as melanoma,62, 67, 94 mucosal‐associated lymphoid tissue lymphoma39 and non‐small cell lung carcinoma95, 96 (NSCLC; see Dieu‐Nosjean et al. for a comprehensive review of ELFs in cancer9).
Structures resembling lymphoid follicles are reported at all stages of cancer, including primary tumours and metastases,97, 98 and in tumours removed after chemotherapy.9, 96 A number of studies have reported a correlation between the density of ELFs and the degree of T‐cell and B‐cell infiltration in tumours.94, 95, 96, 97 This is interesting, given that the frequency of T‐cells displaying Th1 and cytotoxic effector characteristics are linked with improved patient survival in cancer.9, 71 Indeed, ELFs in melanoma and NSCLC are associated with favourable patient outcomes and represent prognostic indicators of disease progression.94, 95 In lung tumour‐associated ELFs, a high proportion of mature DCs correlate with gene signatures linked with Th1 cell activation and improved long‐term patient survival.97 Similar observations have been reported for B‐cell densities in NSCLC, where follicular B cells correlate with long‐term survival both in early‐stage disease and in advanced disease treated with chemotherapy.96 Therefore, ELF development may facilitate the recruitment of naive T and B cells from the peripheral pool via HEVs and homeostatic chemokines. Once established, T‐cell and B‐cell priming within ELFs may provide a local source of antigen‐specific effector cells to drive anti‐tumour immunity.
Of course it is also possible the ELFs develop as a consequence of cancer‐associated inflammation, and are simply spectators of the inflammatory process rather than participants in tumour‐specific immune responses. However, in experimental mouse models, effective T‐cell priming at tumour‐associated ELFs have been demonstrated, that occur independently of SLOs.72, 99 Similarly in human NSCLC tumour‐associated ELFs, the density of germinal centre B cells correlates with the number of antibody‐secreting plasma cells specific for endogenous tumour‐associated antigens.96 A recent study has also shown that Treg cells in lung adenocarcinoma suppress anti‐tumour responses within tumour‐associated ELFs.100 These studies support a role for ELFs as sentinels for the propagation of adaptive immunity against cancer antigens. Immunotherapies that support the activities of ELFs therefore represent promising anti‐cancer treatments. These may include delivery of regulatory homeostatic chemokines and cytokines or interventions that block Treg involvement at ELFs.65, 100
ELFs as perpetuators of inflammation‐driven pathology
Ectopic lymphoid follicles are associated with various autoimmune and chronic inflammatory diseases including rheumatoid arthritis,11, 101, 102 Sjögren syndrome,103 multiple sclerosis,104 experimental diabetes,105 atherosclerosis,106, 107 inflammatory bowel disease108, 109 and chronic obstructive pulmonary disease110, 111 (for a comprehensive review of ELFs in autoimmunity see refs 10, 76). In contrast to their roles in infection and cancer, ELFs in chronic inflammatory diseases are primarily associated with disease exacerbation.
Rheumatoid arthritis is an example of an autoimmune disease where ELFs impact the course of disease and the response to therapy. In rheumatoid arthritis, synovitis is classed into three distinct pathotypes based on cellular and molecular signatures of inflammation – termed pauci‐immune (or fibroblast‐rich disease), diffuse and follicular.11, 101 Synovial ELFs are a prominent feature of the follicular form of synovitis and are described in approximately 30–50% of patients with rheumatoid arthritis.11, 43, 102, 112, 113 Notably, ELFs are described at all stages of the disease and include patients with early active disease who have not received biological agents, patents with more progressive forms of rheumatoid arthritis, and those that have already received biological intervention.43, 112, 113 Although the mechanisms contributing the development of this form of pathology are largely unclear, ELFs in rheumatoid arthritis are associated with heightened synovial expression of CXCL13, CCL21, CCL19 and CXCL12, and cytokines LTα 1 β 2 and IL‐7.21, 44, 114, 115 Importantly, these structures correlate with disease severity and are associated with local T‐cell priming and autoantibody production.43, 116, 117, 118 Plasma cells in rheumatoid synovitis produce autoantibodies against citrullinated protein/peptides (ACPA/anti‐CCP).116 Using an elegant human rheumatoid arthritis–severe combined immunodeficient (HuRA–SCID) mouse chimera model, Humby et al. demonstrate that the synovial microenvironment determines the functional maintenance of ELFs and that control of this process is independent of further leukocyte infiltration.116 In this context, grafted synovial tissues containing ELFs continued to secrete ACPA and express AID.116 Although ACPA is a prognostic marker of rheumatoid arthritis in patients with early/undifferentiated disease, detection of serum ACPA is not indicative of ELF‐associated synovitis.113 The presence of synovial ELFs is however associated with severe synovitis and patients with this form of disease remain challenging to treat and display a poor response to anti‐tumour necrosis factor (anti‐TNF) therapy.112, 119 Here synovial IL‐7 receptor expression also predicts a negative response to anti‐TNF intervention.112 Although ELFs frequently correlate with the degree of synovitis and the infiltration of T cells and B cells,21, 113 it is still unclear whether their presence reflects increased clinical severity or more rapidly progressing forms of rheumatoid arthritis.113 Importantly, ELFs have been linked to an inferior clinical response to anti‐TNF therapy.112 However, in patients with ELFs that showed favourable responses to anti‐TNF treatment, regression of synovial ELFs correlated with improved clinical outcome.112 Further studies are therefore required to better define how ELFs relate to clinical severity and therapeutic response to biological agents in rheumatoid arthritis.
Ectopic lymphoid follicles often form in transplanted human tissues where chronic inflammation is associated with allograft rejection. Tissues derived from kidney transplantation demonstrate that ELFs contribute to germinal centre reactions, local B‐cell maturation and alloimmune responses (anti‐HLA antibody generation). Whereas these observations support a role for ELFs in chronic terminal rejection,120 two studies suggest that ELFs may actual elicit beneficial outcomes.121, 122 These findings in experimental models of kidney and cardiac allografts emphasize that ELF development promotes the recruitment of T cells and B cells displaying inhibitory or regulatory phenotypes.121, 122 Such activities would be predicted to suppress destructive alloimmune responses and promote graft tolerance.
Although lymphoid neogenesis is typically associated with exacerbation of chronic inflammatory processes, a recent study demonstrates a protective role for ELFs in atherosclerosis.107 ELFs develop in the aorta of aged Apoe −/− mice where they correlate with disease severity.123 These structures were typically located adjacent to atherosclerotic plaques where they control immune cell trafficking, antigen presentation and T‐cell priming and differentiation.107 Hence, aortic ELFs may play an important role in establishing local T‐cell immunity during ageing in atherosclerosis.
Concluding remarks and future perspectives on the therapeutic targeting of ELFs
Based on the current literature it is envisaged that two immunotherapeutic strategies are required to target ELFs. In infection and cancer it would be desirable to bolster the development and activity of ELFs to support antigen‐specific responses. In contrast, the neutralization of ELFs in chronic inflammatory diseases and transplantation would limit tissue damage and rejection. However, to date the number of clinical studies targeting ELF‐associated pathology remains limited.
One potential strategy is to disrupt the spatial arrangement of T and B cells in ELFs. In chronic renal allograft rejection, B‐cell depletion with rituximab (anti‐CD20 monoclonal antibody) had a limited impact on the maintenance of ELFs and biological intervention promoted expression of the B‐cell survival factor, B‐cell‐activating factor.124 In rheumatoid arthritis, patients with ELFs show an inferior clinical response to anti‐TNF therapy, which typically targets macrophage and stromal cell responses.112 Other licensed biological therapies including IL‐6 or IL‐6 receptor‐specific monoclonal antibodies (e.g. tocilizumab), T‐cell activation antagonists (e.g. abatacept) and Janus kinase inhibitors (e.g. tofacitinib) are likely to target pathways linked with ELF activity. However, although effective in attenuating inflammatory processes that reflect their broader modes of action, their efficacy in controlling ELF activity are untested.10, 125 Given their prominent roles in lymphoid neogenesis, targeting LTαβ (e.g. baminercept and pateclizumab) may prove effective in blocking ELF activity. Although clinically untested for ELF‐associated inflammation, targeting LTβ has shown promise in pre‐clinical experimental models of disease.126, 127, 128, 129 Similarly, CXCL13 blockade has shown some promise in pre‐clinical studies for the treatment of experimental inflammatory arthritis, diabetes and Sjögren syndrome.130, 131, 132 Finally, given the role of IL‐21 in regulating Tfh cells and germinal centre reactions, a monoclonal antibody against IL‐21 (NNC0114‐0006) is currently in clinical trials for rheumatoid arthritis and systemic lupus erythematosus. These trials follow encouraging evidence for amelioration of disease following blockade of IL‐21 signalling in experimental inflammatory arthritis, systemic lupus erythematosus and graft‐versus‐host disease.133, 134, 135
In cancer, promoting ELF activity may support the development of protective antigen‐specific responses. As such, targeting cytokines such as LTαβ to tumours may have therapeutic potential as demonstrated in experimental melanoma.72, 99 Recent studies also suggest that inhibition of Treg cell activities could promote endogenous anti‐tumour immune responses at ELFs.65, 100 In support of such approaches, a suppressive role for Treg cells in iBALT development has also recently been reported.136
In recent years, we have seen an increasing appreciation for the importance of ELFs as inducible sites within tissues for generating immune responses to infections, tumours, autoantigens and alloantigens. Here, experimental models of disease provide greater insight into the mechanisms that govern the development, function and maintenance of ELFs. These studies have led to the identification of novel immune cell subsets and stromal cell populations and new regulatory mechanisms that reflect potential therapeutic targets for immunomodulation of ELF activity. Significantly, molecular signatures of ELFs also have the potential to yield biomarkers capable of stratifying patients into defined classes of disease based on pathology. These are likely to facilitate clinical decisions regarding the most appropriate therapy for patients with ELF‐associated disease. Although experimental animal models have been the frontrunners in defining mechanistic aspects of ELF development, clinical studies in human conditions have validated many of these findings and shed light on the likely efficacy of current biologicals. Our capacity to stratify patients for the presence of ELFs in early disease is improving.11 The challenge is to understand when and how to target drugs against these important structures, and to adapt clinical trials in accordance with the activities of ELFs in cancer, infection, autoimmunity and transplantation.
Disclosures
The authors declare no competing interests.
Acknowledgements
GWJ and SAJ are supported by career development fellowship and programme grant funding from Arthritis Research UK (20305 #bib20770).
References
- 1. Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol 2006; 7:344–53. [DOI] [PubMed] [Google Scholar]
- 2. Kurashima Y, Goto Y, Kiyono H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur J Immunol 2013; 43:3108–15. [DOI] [PubMed] [Google Scholar]
- 3. Roozendaal R, Mebius RE. Stromal cell–immune cell interactions. Annu Rev Immunol 2011; 29:23–43. [DOI] [PubMed] [Google Scholar]
- 4. Medzhitov R. Origin and physiological roles of inflammation. Nature 2008; 454:428–35. [DOI] [PubMed] [Google Scholar]
- 5. Buckley CD. Why does chronic inflammation persist: an unexpected role for fibroblasts. Immunol Lett 2011; 138:12–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones SA. Directing transition from innate to acquired immunity: defining a role for IL‐6. J Immunol 2005; 175:3463–8. [DOI] [PubMed] [Google Scholar]
- 7. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 2007; 7:429–42. [DOI] [PubMed] [Google Scholar]
- 8. Choy EH, Kavanaugh AF, Jones SA. The problem of choice: current biologic agents and future prospects in RA. Nat Rev Rheumatol 2013; 9:154–63. [DOI] [PubMed] [Google Scholar]
- 9. Dieu‐Nosjean MC, Goc J, Giraldo NA, Sautes‐Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol 2014; 35:571–80. [DOI] [PubMed] [Google Scholar]
- 10. Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid‐like structures in infection, cancer and autoimmunity. Nat Rev Immunol 2014; 14:447–62. [DOI] [PubMed] [Google Scholar]
- 11. Pitzalis C, Kelly S, Humby F. New learnings on the pathophysiology of RA from synovial biopsies. Curr Opin Rheumatol 2013; 25:334–44. [DOI] [PubMed] [Google Scholar]
- 12. van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol 2010; 10:664–74. [DOI] [PubMed] [Google Scholar]
- 13. Peduto L, Dulauroy S, Lochner M, Spath GF, Morales MA, Cumano A et al Inflammation recapitulates the ontogeny of lymphoid stromal cells. J Immunol 2009; 182:5789–99. [DOI] [PubMed] [Google Scholar]
- 14. Vondenhoff MF, Greuter M, Goverse G, Elewaut D, Dewint P, Ware CF et al LTβR signaling induces cytokine expression and up‐regulates lymphangiogenic factors in lymph node anlagen. J Immunol 2009; 182:5439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jarjour M, Jorquera A, Mondor I, Wienert S, Narang P, Coles MC et al Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J Exp Med 2014; 211:1109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katakai T. Marginal reticular cells: a stromal subset directly descended from the lymphoid tissue organizer. Front Immunol 2012; 3:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grogan JL, Ouyang W. A role for Th17 cells in the regulation of tertiary lymphoid follicles. Eur J Immunol 2012; 42:2255–62. [DOI] [PubMed] [Google Scholar]
- 18. Rangel‐Moreno J, Carragher DM, de la Luz Garcia‐Hernandez M, Hwang JY, Kusser K, Hartson L et al The development of inducible bronchus‐associated lymphoid tissue depends on IL‐17. Nat Immunol 2011; 12:639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B et al Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 2011; 35:986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benezech C, Nayar S, Finney BA, Withers DR, Lowe K, Desanti GE et al CLEC‐2 is required for development and maintenance of lymph nodes. Blood 2014; 123:3200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones GW, Bombardieri M, Greenhill CJ, McLeod L, Nerviani A, Rocher‐Ros V et al Interleukin‐27 inhibits ectopic lymphoid‐like structure development in early inflammatory arthritis. J Exp Med 2015; 212:1793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Canete JD, Celis R, Yeremenko N, Sanmarti R, van Duivenvoorde L, Ramirez J et al Ectopic lymphoid neogenesis is strongly associated with activation of the IL‐23 pathway in rheumatoid synovitis. Arthritis Res Ther 2015; 17:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barone F, Nayar S, Campos J, Cloake T, Withers DR, Toellner KM et al IL‐22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc Natl Acad Sci USA 2015; 112:11024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaitanya GV, Omura S, Sato F, Martinez NE, Minagar A, Ramanathan M et al Inflammation induces neuro‐lymphatic protein expression in multiple sclerosis brain neurovasculature. J Neuroinflammation 2013; 10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deteix C, Attuil‐Audenis V, Duthey A, Patey N, McGregor B, Dubois V et al Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol 2010; 184:5344–51. [DOI] [PubMed] [Google Scholar]
- 26. Sawa S, Cherrier M, Lochner M, Satoh‐Takayama N, Fehling HJ, Langa F et al Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 2010; 330:665–9. [DOI] [PubMed] [Google Scholar]
- 27. Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II et al Lymphoid tissue inducer‐like cells are an innate source of IL‐17 and IL‐22. J Exp Med 2009; 206:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4+ lymphoid tissue‐inducer cells promote innate immunity in the gut. Immunity 2011; 34:122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL et al Innate lymphoid cells sustain colon cancer through production of interleukin‐22 in a mouse model. J Exp Med 2013; 210:917–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015; 517:293–301. [DOI] [PubMed] [Google Scholar]
- 31. Schmutz S, Bosco N, Chappaz S, Boyman O, Acha‐Orbea H, Ceredig R et al Cutting edge: IL‐7 regulates the peripheral pool of adult RORγ + lymphoid tissue inducer cells. J Immunol 2009; 183:2217–21. [DOI] [PubMed] [Google Scholar]
- 32. Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R et al Ectopic lymphoid‐organ development occurs through interleukin 7‐mediated enhanced survival of lymphoid‐tissue‐inducer cells. Immunity 2007; 26:643–54. [DOI] [PubMed] [Google Scholar]
- 33. Fleige H, Ravens S, Moschovakis GL, Bolter J, Willenzon S, Sutter G et al IL‐17‐induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med 2014; 211:643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M et al Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med 2000; 192:1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 2000; 192:1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM et al Plasticity of Th17 cells in Peyer's patches is responsible for the induction of T cell‐dependent IgA responses. Nat Immunol 2013; 14:372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu KT, Kanno Y, Cannons JL, Handon R, Bible P, Elkahloun AG et al Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro‐generated and in vivo‐derived follicular T helper cells. Immunity 2011; 35:622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA et al Early Th1 cell differentiation is marked by a Tfh cell‐like transition. Immunity 2011; 35:919–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barone F, Bombardieri M, Rosado MM, Morgan PR, Challacombe SJ, De Vita S et al CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren's syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol 2008; 180:5130–40. [DOI] [PubMed] [Google Scholar]
- 40. Rangel‐Moreno J, Moyron‐Quiroz JE, Hartson L, Kusser K, Randall TD. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci USA 2007; 104:10577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sato M, Hirayama S, Matsuda Y, Wagnetz D, Hwang DM, Guan Z et al Stromal activation and formation of lymphoid‐like stroma in chronic lung allograft dysfunction. Transplantation 2011; 91:1398–405. [DOI] [PubMed] [Google Scholar]
- 42. Braun A, Takemura S, Vallejo AN, Goronzy JJ, Weyand CM. Lymphotoxin β‐mediated stimulation of synoviocytes in rheumatoid arthritis. Arthritis Rheum 2004; 50:2140–50. [DOI] [PubMed] [Google Scholar]
- 43. Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O'Fallon WM et al Lymphoid neogenesis in rheumatoid synovitis. J Immunol 2001; 167:1072–80. [DOI] [PubMed] [Google Scholar]
- 44. Timmer TC, Baltus B, Vondenhoff M, Huizinga TW, Tak PP, Verweij CL et al Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin‐7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum 2007; 56:2492–502. [DOI] [PubMed] [Google Scholar]
- 45. Bombardieri M, Kam NW, Brentano F, Choi K, Filer A, Kyburz D et al A BAFF/APRIL‐dependent TLR3‐stimulated pathway enhances the capacity of rheumatoid synovial fibroblasts to induce AID expression and Ig class‐switching in B cells. Ann Rheum Dis 2011; 70:1857–65. [DOI] [PubMed] [Google Scholar]
- 46. Benezech C, White A, Mader E, Serre K, Parnell S, Pfeffer K et al Ontogeny of stromal organizer cells during lymph node development. J Immunol 2010; 184:4521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P et al Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol 2009; 10:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J et al Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol 2002; 169:424–33. [DOI] [PubMed] [Google Scholar]
- 49. Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin‐dependent lymphoid neogenesis. Immunity 2000; 12:471–81. [DOI] [PubMed] [Google Scholar]
- 50. Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD et al A chemokine‐driven positive feedback loop organizes lymphoid follicles. Nature 2000; 406:309–14. [DOI] [PubMed] [Google Scholar]
- 51. Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N et al Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol 2004; 5:943–52. [DOI] [PubMed] [Google Scholar]
- 52. Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor α, and CCR7 ligands in lymph node development. J Exp Med 2003; 197:1191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ohl L, Henning G, Krautwald S, Lipp M, Hardtke S, Bernhardt G et al Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med 2003; 197:1199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen SC, Vassileva G, Kinsley D, Holzmann S, Manfra D, Wiekowski MT et al Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node‐like structures in pancreas, but not skin, of transgenic mice. J Immunol 2002; 168:1001–8. [DOI] [PubMed] [Google Scholar]
- 55. Fan L, Reilly CR, Luo Y, Dorf ME, Lo D. Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J Immunol 2000; 164:3955–9. [DOI] [PubMed] [Google Scholar]
- 56. Christopherson KW 2nd, Hood AF, Travers JB, Ramsey H, Hromas RA. Endothelial induction of the T‐cell chemokine CCL21 in T‐cell autoimmune diseases. Blood 2003; 101:801–6. [DOI] [PubMed] [Google Scholar]
- 57. Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES et al Naive T cell recruitment to nonlymphoid tissues: a role for endothelium‐expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol 2003; 170:4638–48. [DOI] [PubMed] [Google Scholar]
- 58. Baddoura FK, Nasr IW, Wrobel B, Li Q, Ruddle NH, Lakkis FG. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant 2005; 5:510–6. [DOI] [PubMed] [Google Scholar]
- 59. Barone F, Bombardieri M, Manzo A, Blades MC, Morgan PR, Challacombe SJ et al Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid‐like structures in Sjogren's syndrome. Arthritis Rheum 2005; 52:1773–84. [DOI] [PubMed] [Google Scholar]
- 60. Bento DC, Jones E, Junaid S, Tull J, Williams GT, Godkin A et al High endothelial venules are rare in colorectal cancers but accumulate in extra‐tumoral areas with disease progression. Oncoimmunology 2015; 4:e974374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Canete JD, Santiago B, Cantaert T, Sanmarti R, Palacin A, Celis R et al Ectopic lymphoid neogenesis in psoriatic arthritis. Ann Rheum Dis 2007; 66:720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J et al Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res 2012; 72:3997–4007. [DOI] [PubMed] [Google Scholar]
- 63. Manzo A, Bugatti S, Caporali R, Prevo R, Jackson DG, Uguccioni M et al CCL21 expression pattern of human secondary lymphoid organ stroma is conserved in inflammatory lesions with lymphoid neogenesis. Am J Pathol 2007; 171:1549–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weiss JM, Cufi P, Bismuth J, Eymard B, Fadel E, Berrih‐Aknin S et al SDF‐1/CXCL12 recruits B cells and antigen‐presenting cells to the thymus of autoimmune myasthenia gravis patients. Immunobiology 2013; 218:373–81. [DOI] [PubMed] [Google Scholar]
- 65. Hindley JP, Jones E, Smart K, Bridgeman H, Lauder SN, Ondondo B et al T‐cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T‐cell depletion. Cancer Res 2012; 72:5473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Martinet L, Filleron T, Le Guellec S, Rochaix P, Garrido I, Girard JP. High endothelial venule blood vessels for tumor‐infiltrating lymphocytes are associated with lymphotoxin β‐producing dendritic cells in human breast cancer. J Immunol 2013; 191:2001–8. [DOI] [PubMed] [Google Scholar]
- 67. Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ et al Human solid tumors contain high endothelial venules: association with T‐ and B‐lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71:5678–87. [DOI] [PubMed] [Google Scholar]
- 68. Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 2011; 479:542–6. [DOI] [PubMed] [Google Scholar]
- 69. Onder L, Danuser R, Scandella E, Firner S, Chai Q, Hehlgans T et al Endothelial cell‐specific lymphotoxin‐beta receptor signaling is critical for lymph node and high endothelial venule formation. J Exp Med 2013; 210:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P et al High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor‐infiltrating lymphocytes. Oncoimmunology 2012; 1:829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fridman WH, Pages F, Sautes‐Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298–306. [DOI] [PubMed] [Google Scholar]
- 72. Schrama D, Voigt H, Eggert AO, Xiang R, Zhou H, Schumacher TN et al Immunological tumor destruction in a murine melanoma model by targeted LTα independent of secondary lymphoid tissue. Cancer Immunol Immunother 2008; 57:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schrama D, thor Straten P, Fischer WH, McLellan AD, Brocker EB, Reisfeld RA et al Targeting of lymphotoxin‐α to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid‐like tissue. Immunity 2001; 14:111–21. [DOI] [PubMed] [Google Scholar]
- 74. Peske JD, Thompson ED, Gemta L, Baylis RA, Fu YX, Engelhard VH. Effector lymphocyte‐induced lymph node‐like vasculature enables naive T‐cell entry into tumours and enhanced anti‐tumour immunity. Nat Commun 2015; 6:7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lucchesi D, Bombardieri M. The role of viruses in autoreactive B cell activation within tertiary lymphoid structures in autoimmune diseases. J Leukoc Biol 2013; 94:1191–9. [DOI] [PubMed] [Google Scholar]
- 76. Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol 2012; 33:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Khader SA, Rangel‐Moreno J, Fountain JJ, Martino CA, Reiley WW, Pearl JE et al In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J Immunol 2009; 183:8004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mazzucchelli L, Blaser A, Kappeler A, Scharli P, Laissue JA, Baggiolini M et al BCA‐1 is highly expressed in Helicobacter pylori‐induced mucosa‐associated lymphoid tissue and gastric lymphoma. J Clin Invest 1999; 104:R49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Slight SR, Rangel‐Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S et al CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin Invest 2013; 123:712–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D et al Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus‐infected mice. J Exp Med 2009; 206:2339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Moyron‐Quiroz JE, Rangel‐Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD et al Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity 2006; 25:643–54. [DOI] [PubMed] [Google Scholar]
- 82. Moyron‐Quiroz JE, Rangel‐Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S et al Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 2004; 10:927–34. [DOI] [PubMed] [Google Scholar]
- 83. Sansonno D, De Vita S, Iacobelli AR, Cornacchiulo V, Boiocchi M, Dammacco F. Clonal analysis of intrahepatic B cells from HCV‐infected patients with and without mixed cryoglobulinemia. J Immunol 1998; 160:3594–601. [PubMed] [Google Scholar]
- 84. Sansonno D, Tucci FA, Troiani L, Lauletta G, Montrone M, Conteduca V et al Increased serum levels of the chemokine CXCL13 and up‐regulation of its gene expression are distinctive features of HCV‐related cryoglobulinemia and correlate with active cutaneous vasculitis. Blood 2008; 112:1620–7. [DOI] [PubMed] [Google Scholar]
- 85. Grewal JS, Pilgrim MJ, Grewal S, Kasman L, Werner P, Bruorton ME et al Salivary glands act as mucosal inductive sites via the formation of ectopic germinal centers after site‐restricted MCMV infection. FASEB J 2011; 25:1680–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lochner M, Ohnmacht C, Presley L, Bruhns P, Si‐Tahar M, Sawa S et al Microbiota‐induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORγt and LTi cells. J Exp Med 2011; 208:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012; 12:237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bergomas F, Grizzi F, Doni A, Pesce S, Laghi L, Allavena P et al Tertiary intratumor lymphoid tissue in colo‐rectal cancer. Cancers (Basel) 2011; 4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McMullen TP, Lai R, Dabbagh L, Wallace TM, de Gara CJ. Survival in rectal cancer is predicted by T cell infiltration of tumour‐associated lymphoid nodules. Clin Exp Immunol 2010; 161:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gu‐Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A et al CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123:2873–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nzula S, Going JJ, Stott DI. Antigen‐driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res 2003; 63:3275–80. [PubMed] [Google Scholar]
- 92. Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH et al CD20+ tumor‐infiltrating lymphocytes have an atypical CD27− memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res 2012; 18:3281–92. [DOI] [PubMed] [Google Scholar]
- 93. Willis SN, Mallozzi SS, Rodig SJ, Cronk KM, McArdel SL, Caron T et al The microenvironment of germ cell tumors harbors a prominent antigen‐driven humoral response. J Immunol 2009; 182:3310–7. [DOI] [PubMed] [Google Scholar]
- 94. Ladanyi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A et al Density of DC‐LAMP+ mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother 2007; 56:1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dieu‐Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V et al Long‐term survival for patients with non‐small‐cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26:4410–7. [DOI] [PubMed] [Google Scholar]
- 96. Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J et al Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014; 189:832–44. [DOI] [PubMed] [Google Scholar]
- 97. Goc J, Germain C, Vo‐Bourgais TK, Lupo A, Klein C, Knockaert S et al Dendritic cells in tumor‐associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014; 74:705–15. [DOI] [PubMed] [Google Scholar]
- 98. Remark R, Alifano M, Cremer I, Lupo A, Dieu‐Nosjean MC, Riquet M et al Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res 2013; 19:4079–91. [DOI] [PubMed] [Google Scholar]
- 99. Kirk CJ, Hartigan‐O'Connor D, Mule JJ. The dynamics of the T‐cell antitumor response: chemokine‐secreting dendritic cells can prime tumor‐reactive T cells extranodally. Cancer Res 2001; 61:8794–802. [PubMed] [Google Scholar]
- 100. Joshi NS, Akama‐Garren EH, Lu Y, Lee DY, Chang GP, Li A et al Regulatory T cells in tumor‐associated tertiary lymphoid structures suppress anti‐tumor T cell responses. Immunity 2015; 43:579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Klimiuk PA, Goronzy JJ, Bjornsson J, Beckenbaugh RD, Weyand CM. Tissue cytokine patterns distinguish variants of rheumatoid synovitis. Am J Pathol 1997; 151:1311–9. [PMC free article] [PubMed] [Google Scholar]
- 102. Manzo A, Bombardieri M, Humby F, Pitzalis C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling. Immunol Rev 2010; 233:267–85. [DOI] [PubMed] [Google Scholar]
- 103. Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen‐driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren's syndrome. J Clin Invest 1998; 102:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M et al Meningeal B‐cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007; 130:1089–104. [DOI] [PubMed] [Google Scholar]
- 105. Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med 1998; 188:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Houtkamp MA, de Boer OJ, van der Loos CM, van der Wal AC, Becker AE. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol 2001; 193:263–9. [DOI] [PubMed] [Google Scholar]
- 107. Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P et al Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin β receptors. Immunity 2015; 42:1100–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Carlsen HS, Baekkevold ES, Johansen FE, Haraldsen G, Brandtzaeg P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut 2002; 51:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Surawicz CM, Belic L. Rectal biopsy helps to distinguish acute self‐limited colitis from idiopathic inflammatory bowel disease. Gastroenterology 1984; 86:104–13. [PubMed] [Google Scholar]
- 110. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L et al The nature of small‐airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:2645–53. [DOI] [PubMed] [Google Scholar]
- 111. Van Pottelberge GR, Bracke KR, Van den Broeck S, Reinartz SM, van Drunen CM, Wouters EF et al Plasmacytoid dendritic cells in pulmonary lymphoid follicles of patients with COPD. Eur Respir J 2010; 36:781–91. [DOI] [PubMed] [Google Scholar]
- 112. Canete JD, Celis R, Moll C, Izquierdo E, Marsal S, Sanmarti R et al Clinical significance of synovial lymphoid neogenesis and its reversal after anti‐tumour necrosis factor α therapy in rheumatoid arthritis. Ann Rheum Dis 2009; 68:751–6. [DOI] [PubMed] [Google Scholar]
- 113. Thurlings RM, Wijbrandts CA, Mebius RE, Cantaert T, Dinant HJ, van der Pouw‐Kraan TC et al Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum 2008; 58:1582–9. [DOI] [PubMed] [Google Scholar]
- 114. Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G et al Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol 2005; 35:1347–59. [DOI] [PubMed] [Google Scholar]
- 115. Shi K, Hayashida K, Kaneko M, Hashimoto J, Tomita T, Lipsky PE et al Lymphoid chemokine B cell‐attracting chemokine‐1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol 2001; 166:650–5. [DOI] [PubMed] [Google Scholar]
- 116. Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B et al Ectopic lymphoid structures support ongoing production of class‐switched autoantibodies in rheumatoid synovium. PLoS Med 2009; 6:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rosengren S, Wei N, Kalunian KC, Zvaifler NJ, Kavanaugh A, Boyle DL. Elevated autoantibody content in rheumatoid arthritis synovia with lymphoid aggregates and the effect of rituximab. Arthritis Res Ther 2008; 10:R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol 2001; 167:4710–8. [DOI] [PubMed] [Google Scholar]
- 119. van der Helm‐van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 2005; 7:R949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Thaunat O, Patey N, Caligiuri G, Gautreau C, Mamani‐Matsuda M, Mekki Y et al Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol 2010; 185:717–28. [DOI] [PubMed] [Google Scholar]
- 121. Brown K, Sacks SH, Wong W. Tertiary lymphoid organs in renal allografts can be associated with donor‐specific tolerance rather than rejection. Eur J Immunol 2011; 41:89–96. [DOI] [PubMed] [Google Scholar]
- 122. Le Texier L, Thebault P, Lavault A, Usal C, Merieau E, Quillard T et al Long‐term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant 2011; 11:429–38. [DOI] [PubMed] [Google Scholar]
- 123. Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M et al Lymphotoxin β receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med 2009; 206:233–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Thaunat O, Patey N, Gautreau C, Lechaton S, Fremeaux‐Bacchi V, Dieu‐Nosjean MC et al B cell survival in intragraft tertiary lymphoid organs after rituximab therapy. Transplantation 2008; 85:1648–53. [DOI] [PubMed] [Google Scholar]
- 125. Hunter CA, Jones SA. IL‐6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16:448–57. [DOI] [PubMed] [Google Scholar]
- 126. Fava RA, Kennedy SM, Wood SG, Bolstad AI, Bienkowska J, Papandile A et al Lymphotoxin‐β receptor blockade reduces CXCL13 in lacrimal glands and improves corneal integrity in the NOD model of Sjogren's syndrome. Arthritis Res Ther 2011; 13:R182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fava RA, Notidis E, Hunt J, Szanya V, Ratcliffe N, Ngam‐Ek A et al A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen‐induced arthritis. J Immunol 2003; 171:115–26. [DOI] [PubMed] [Google Scholar]
- 128. Gatumu MK, Skarstein K, Papandile A, Browning JL, Fava RA, Bolstad AI. Blockade of lymphotoxin‐β receptor signaling reduces aspects of Sjogren's syndrome in salivary glands of non‐obese diabetic mice. Arthritis Res Ther 2009; 11:R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J et al Recruitment and activation of naive T cells in the islets by lymphotoxin β receptor‐dependent tertiary lymphoid structure. Immunity 2006; 25:499–509. [DOI] [PubMed] [Google Scholar]
- 130. Henry RA, Kendall PL. CXCL13 blockade disrupts B lymphocyte organization in tertiary lymphoid structures without altering B cell receptor bias or preventing diabetes in nonobese diabetic mice. J Immunol 2010; 185:1460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kramer JM, Klimatcheva E, Rothstein TL. CXCL13 is elevated in Sjogren's syndrome in mice and humans and is implicated in disease pathogenesis. J Leukoc Biol 2013; 94:1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zheng B, Ozen Z, Zhang X, De Silva S, Marinova E, Guo L et al CXCL13 neutralization reduces the severity of collagen‐induced arthritis. Arthritis Rheum 2005; 52:620–6. [DOI] [PubMed] [Google Scholar]
- 133. Bucher C, Koch L, Vogtenhuber C, Goren E, Munger M, Panoskaltsis‐Mortari A et al IL‐21 blockade reduces graft‐versus‐host disease mortality by supporting inducible T regulatory cell generation. Blood 2009; 114:5375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi‐Joannopoulos K. IL‐21 has a pathogenic role in a lupus‐prone mouse model and its blockade with IL‐21R.Fc reduces disease progression. J Immunol 2007; 178:3822–30. [DOI] [PubMed] [Google Scholar]
- 135. Young DA, Hegen M, Ma HL, Whitters MJ, Albert LM, Lowe L et al Blockade of the interleukin‐21/interleukin‐21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum 2007; 56:1152–63. [DOI] [PubMed] [Google Scholar]
- 136. Foo SY, Zhang V, Lalwani A, Lynch JP, Zhuang A, Lam CE et al Regulatory T cells prevent inducible BALT formation by dampening neutrophilic inflammation. J Immunol 2015; 194:4567–76. [DOI] [PubMed] [Google Scholar]
- 137. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L et al Generation of T follicular helper cells is mediated by interleukin‐21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008; 29:138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Goya S, Matsuoka H, Mori M, Morishita H, Kida H, Kobashi Y et al Sustained interleukin‐6 signalling leads to the development of lymphoid organ‐like structures in the lung. J Pathol 2003; 200:82–7. [DOI] [PubMed] [Google Scholar]
- 139. Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB et al IL‐21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 2007; 448:484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bombardieri M, Barone F, Lucchesi D, Nayar S, van den Berg WB, Proctor G et al Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J Immunol 2012; 189:3767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE et al Interleukin‐5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med 1997; 185:2143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nacionales DC, Kelly KM, Lee PY, Zhuang H, Li Y, Weinstein JS et al Type I interferon production by tertiary lymphoid tissue developing in response to 2 #bib6 #bib10 #bib14‐tetramethyl‐pentadecane (pristane). Am J Pathol 2006; 168:1227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity 2014; 40:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Esen N, Rainey‐Barger EK, Huber AK, Blakely PK, Irani DN. Type‐I interferons suppress microglial production of the lymphoid chemokine, CXCL13. Glia 2014; 62:1452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD et al IL‐23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS et al IL‐27 supports germinal center function by enhancing IL‐21 production and the function of T follicular helper cells. J Exp Med 2010; 207:2895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Iwasaki Y, Fujio K, Okamura T, Yanai A, Sumitomo S, Shoda H et al Egr‐2 transcription factor is required for Blimp‐1‐mediated IL‐10 production in IL‐27‐stimulated CD4+ T cells. Eur J Immunol 2013; 43:1063–73. [DOI] [PubMed] [Google Scholar]
- 148. Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM et al Interleukin 27 negatively regulates the development of interleukin 17‐producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 2006; 7:937–45. [DOI] [PubMed] [Google Scholar]
- 149. Lee BH, Carcamo WC, Chiorini JA, Peck AB, Nguyen CQ. Gene therapy using IL‐27 ameliorates Sjogren's syndrome‐like autoimmune exocrinopathy. Arthritis Res Ther 2012; 14:R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ballesteros‐Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE et al Interleukin‐2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 2012; 36:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med 2012; 209:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z et al Interleukin‐2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 2007; 26:371–81. [DOI] [PubMed] [Google Scholar]


