Abstract
Degenerative mitral valve disease is the leading cause of mitral regurgitation in North America. Surgical intervention has hinged on symptoms and ventricular changes that develop as compensatory ventricular remodeling takes place. In this study, we sought to characterize the temporal response of left ventricular (LV) morphology and function to mitral valve surgery for degenerative disease, and identify preoperative factors that influence reverse remodeling. From 1986–2007, 2,778 patients with isolated degenerative mitral valve disease underwent valve repair (n=2,607/94%) or replacement (n=171/6%) and had at least 1 postoperative transthoracic echocardiogram (TTE); 5,336 TTEs were available for analysis. Multivariable longitudinal repeated-measures analysis was performed to identify factors associated with reverse remodeling. LV dimensions decreased in the first year after surgery (end-diastolic from 5.7±0.80 to 4.9±1.4 cm; end-systolic from 3.4±0.71 to 3.1±1.4 cm). LV mass index decreased from 139±44 to 112±73 g·m−2. Reduction of LV hypertrophy was less pronounced in patients with greater preoperative left heart enlargement (P<.0001) and greater preoperative LV mass (P<.0001). Postoperative LV ejection fraction initially decreased from 58±7.0 to 53±20, increased slightly over the first postoperative year, and was negatively influenced by preoperative heart failure symptoms (P<.0001) and lower preoperative LV ejection fraction (P<.0001). Risk-adjusted response of LV morphology and function to valve repair and replacement was similar (P>.2). In conclusion, a positive response toward normalization of LV morphology and function after mitral valve surgery is greatest in the first year. The best response occurs when surgery is performed before left heart dilatation, LV hypertrophy, or LV dysfunction develop.
Keywords: Degenerative mitral regurgitation, left ventricular mass index, left ventricular function
Progression of degenerative mitral valve (MV) disease is characterized by ventricular remodeling, whereby adaptive changes occur to accommodate the regurgitant volume and maintain cardiac output.1 Current surgical indications for severe degenerative mitral regurgitation (MR) are based on onset of symptoms, changes in left ventricular (LV) function or dimensions, and development of atrial fibrillation or pulmonary hypertension.2 Our group and others have documented improved clinical outcomes with early intervention and reduced long-term survival in patients with LV dysfunction undergoing MV surgery.3–5 To investigate various clinical outcomes, we focused on the ventricular remodeling process that occurs in chronic degenerative MR. In this study, we sought to 1) characterize responses of LV morphology and function to MV surgery, and 2) identify preoperative factors modulating this postoperative response.
METHODS
From January 1986 to January 2007, 3,031 patients underwent primary isolated MV surgery for degenerative MR at Cleveland Clinic. Those undergoing concomitant ablation procedures for atrial fibrillation (except full cut-and-sew Maze procedure) or tricuspid valve procedures for functional regurgitation were considered to have secondary consequences of degenerative MV disease and were included in the study. However, those with a history of prior cardiac surgery or concomitant coronary artery bypass or aortic valve procedures were excluded. Patients with epicardial coronary artery stenosis ≥50% were also excluded, as were patients with a history of coronary intervention.
To assess postoperative changes in LV morphology and function, we required at least 1 postoperative transthoracic echocardiogram (TTE). Using the Cleveland Clinic Echocardiographic Database, we determined that 2,778 (92%) of the 3,031 patients had such an examination, and they formed the final study group. Of these, 2,607 (94%) underwent MV repair and 171 (6%) MV replacement. A summary of patient demographics, and clinical and echocardiographic characteristics is provided in Table 1. Clinical data were retrieved from the prospective Cardiovascular Information Registry. This registry has been approved for use in research by the Institutional Review Board, with patient consent waived.
Table 1.
Summary of Patient Demographics, Clinical, and Echocardiographic Data
| Overall (total n = 2,778) |
MV Repair (total n = 2,607) |
MV Replacement (total n = 171) |
|||||
|---|---|---|---|---|---|---|---|
| Variable | na | No. (% of n) or Mean±SD |
na | No. (% of n) or Mean±SD |
na | No. (% of n) or Mean±SD |
P b |
| Age (years) | 2,778 | 57 ± 13 | 2,607 | 56 ± 13 | 171 | 69 ± 13 | <.0001 |
| Body surface area (m2) | 2,772 | 2.0 ± 0.24 | 2,602 | 2.0 ± 0.24 | 170 | 1.9 ± 0.27 | <.0001 |
| Female | 2,778 | 970 (35%) | 2,607 | 876 (34%) | 171 | 94 (55%) | <.0001 |
| NYHA functional class | 2,776 | 2,605 | 171 | <.0001 | |||
| I | 814 (29%) | 790 (30%) | 24 (14%) | ||||
| II | 1,558 (56%) | 1,463 (56%) | 95 (56%) | ||||
| III | 357 (13%) | 309 (12%) | 48 (28%) | ||||
| IV | 47 (1.7%) | 43 (1.7%) | 4 (2.3%) | ||||
| Mitral regurgitation grade | 2,748 | 2,579 | 169 | .04 | |||
| 2+ | 7 (0.25%) | 8 (0.31%) | 1 (0.59%) | ||||
| 3+ | 219 (8.0%) | 213 (8.3%) | 23 (14%) | ||||
| 4+ | 2,522 (92%) | 2,358 (91%) | 145 (86%) | ||||
| Leaflet prolapse | 2,778 | 2,607 | 171 | ||||
| Posterior only | 1243 (45%) | 1193 (47%) | 50 (29%) | <.0001 | |||
| Anterior only | 174 (6.3%) | 147 (5.6%) | 27 (16%) | <.0001 | |||
| Bileaflet | 1,338 (48%) | 1,258 (48%) | 81 (47%) | .8 | |||
| Ruptured chordae | 2,778 | 2,607 | 171 | <.0001 | |||
| Posterior | 1,576 (57%) | 1,511 (58%) | 65 (38%) | ||||
| Anterior | 271 (9.8%) | 231 (8.9%) | 40 (23%) | ||||
| Mitral valve calcification | 2,778 | 614 (22%) | 2,607 | 521 (20%) | 171 | 93 (54%) | <.0001 |
| Tricuspid regurgitation grade | 2,370 | 2,226 | 144 | <.0001 | |||
| 0 | 901 (38%) | 882 (40%) | 19 (13%) | ||||
| 1+ | 862 (36%) | 821 (37%) | 41 (28%) | ||||
| 2+ | 413 (17%) | 369 (17%) | 44 (31%) | ||||
| 3+ | 149 (6.3%) | 121 (5.4%) | 28 (19%) | ||||
| 4+ | 45 (1.9%) | 33 (1.5%) | 12 (8.3%) | ||||
| Echocardiographic assessment | |||||||
| LA diameter (cm) | 2,575 | 4.9 ± 0.90 | 2,422 | 4.9 ± 0.88 | 153 | 5.3 ± 1.02 | <.0001 |
| LV end-diastolic diameter (cm) | 2,698 | 5.7 ± 0.80 | 2,540 | 5.8 ± 0.79 | 158 | 5.5 ± 0.93 | <.0001 |
| LV end-systolic diameter (cm) | 2,678 | 3.4 ± 0.71 | 2,517 | 27 ± 13 | 155 | 26 ± 15 | .3 |
| LV mass index (g•m−2) | 2,623 | 139 ± 44 | 2,468 | 138 ± 43 | 155 | 147 ± 44 | .006 |
| LVEF (%) | 2,706 | 58 ± 7.4 | 2,538 | 58 ± 7.4 | 168 | 56 ± 8.0 | .0003 |
| Cardiac comorbidity | |||||||
| Heart failure | 2,778 | 634 (23%) | 2,607 | 547 (21%) | 171 | 87 (51%) | <.0001 |
| Ventricular arrhythmia | 2,655 | 357 (13%) | 2,498 | 329 (13%) | 157 | 28 (18%) | .1 |
| Atrial fibrillation/flutter | 2,778 | 343 (12%) | 2,607 | 286 (11%) | 171 | 57 (33%) | <.0001 |
| Complete heart block | 2,778 | 43 (1.5%) | 2,607 | 28 (1.1%) | 171 | 15 (8.8%) | <.0001 |
| Concomitant procedures | |||||||
| Ablation for atrial fibrillation | 2,778 | 60 (2.2 %) | 2,607 | 48 (1.8%) | 171 | 12 (7.0%) | <.0001 |
| Tricuspid valve procedure | 2,778 | 159 (5.8%) | 2,607 | 121 (4.6%) | 171 | 38 (22%) | <.0001 |
Patients with data available.
Unadjusted comparison between MV repair and replacement using the Wilcoxon Rank-Sum nonparametric test (continuous variables) or chi-squared test (categorical variables).
Key: LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; MV, mitral valve; NYHA, New York Heart Association; SD, standard deviation.
TTEs were performed routinely before discharge and at the discretion of referring physicians during follow-up. Intraoperative transesophageal echocardiograms were not used in this report. Interpretation of follow-up echocardiograms was obtained at as many postoperative time points as available for each patient. A total of 5,336 TTE records were available for the 2,778 patients. Distribution of postoperative echocardiograms permitted assessment of temporal trends up to 5 years (eFigure 1).
Chamber measurements were derived from 2-dimensional images. Morphologic data recorded and analyzed for temporal responses included left atrial (LA) diameter, from which LA volume was calculated;6 end-diastolic and end-systolic LV diameters; and septal and posterior wall thickness, from which LV mass was calculated.7 Left ventricular end-diastolic volume, endsystolic volume, and ejection fraction were measured from multiple 2-dimensional projections by planimetry and edited by visual interpretation of all available views.8
Interobserver variability in our echocardiographic laboratory is −0.1 mL (95% confidence interval [CI]: −42.8 to 42.5 mL) for LV end-diastolic volume, 5.9 mL (95% CI: −21.6 to 33.3 mL) for LV end-systolic volume, and −4% (95% CI: −16.1 to 8.0%) for LV ejection fraction. Intraobserver variability for these same 3 measurements was 7.1 mL (95% CI: −38.8 to 50 mL), 4.4 mL (95% CI: −27.9 to 36.7 mL), and 0.0% (95% CI: −13.3 to 13.3%).
To determine the temporal response to mitral surgery, trends of repeated postoperative TTE measurements were analyzed longitudinally using a generalized nonlinear mixed model regression for continuous variables (SAS PROC NLMIXED). The temporal patterns of values were characterized by temporal decomposition for each TTE variable.9
Multivariable analysis, using variables listed in eAppendix 1, was performed to identify preoperative factors that modulated each temporal phase for postoperative LV mass index and ejection fraction. We initially screened variables using ordinary multivariable linear regression (PROC REG). Bootstrap bagging methods were used to identify possible predictors with random resampling and automated stepwise selection.10 Variables or clusters of variables that entered more than 50% of 1,000 models then underwent refined generalized nonlinear mixed model regression. Specific variables of interest (including tricuspid regurgitation, MV procedure [repair or replacement], and New York Heart Association functional classification) were forced into the analysis.
Sporadic missing values were imputed using 5-fold multiple imputation (PROC MI).11 For each imputed complete data set, we estimated regression coefficients and their variance-covariance matrix. We then combined estimates from the 5 models using PROC MIANALYZE.
Continuous variables are summarized as mean ± standard deviation and as 15th, 50th (median), and 85th percentiles. They are compared using Wilcoxon rank-sum nonparametric tests. Categorical data are summarized by frequencies and percentages, and compared using chi-squared tests. All analyses were performed using SAS statistical software (SAS v9.1; SAS, Inc., Cary, NC). Uncertainty is expressed by confidence limits equivalent to ±1 standard error (68%).
RESULTS
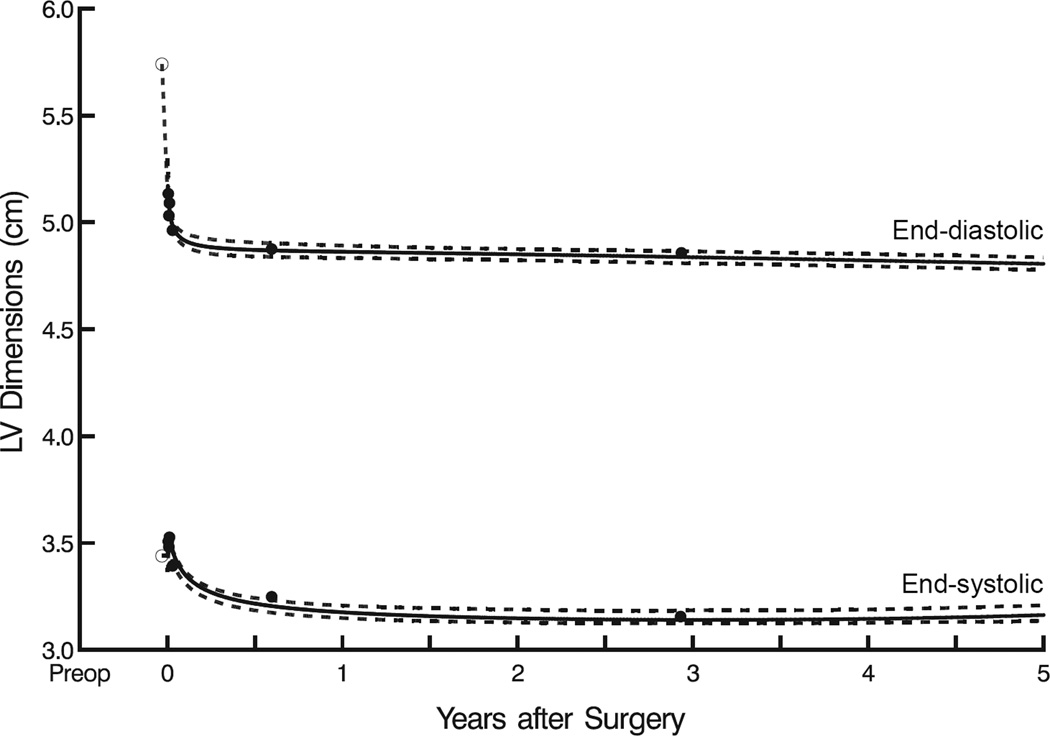
Left ventricular end-diastolic and end-systolic diameters decreased sharply during the first year after surgery, with a more rapid decline in end-diastolic than end-systolic diameter (Figure 1). Mean end-diastolic diameter decreased from 5.7±0.80 to 4.9±1.6 cm (P<.0001) within the first 6 months after surgery and remained at 4.8±1.5 cm at year 5. Mean end-systolic diameter also decreased, but more gradually from 3.4±0.71 to 3.2±1.4 cm (P<.0001) within the first 6 postoperative months, then reached a constant level of 3.2±1.8 cm at year 5.
Figure 1.
Postoperative left ventricular (LV) end-diastolic (upper curve) and end-systolic (lower curve) diameters after mitral valve surgery. Solid lines enclosed by dashed confidence limits represent parametric estimates of mean dimensions across time. Solid circles represent grouped data without regard to repeated measurements, used for crude verification. Symbols and dashed line represent mean preoperative values.
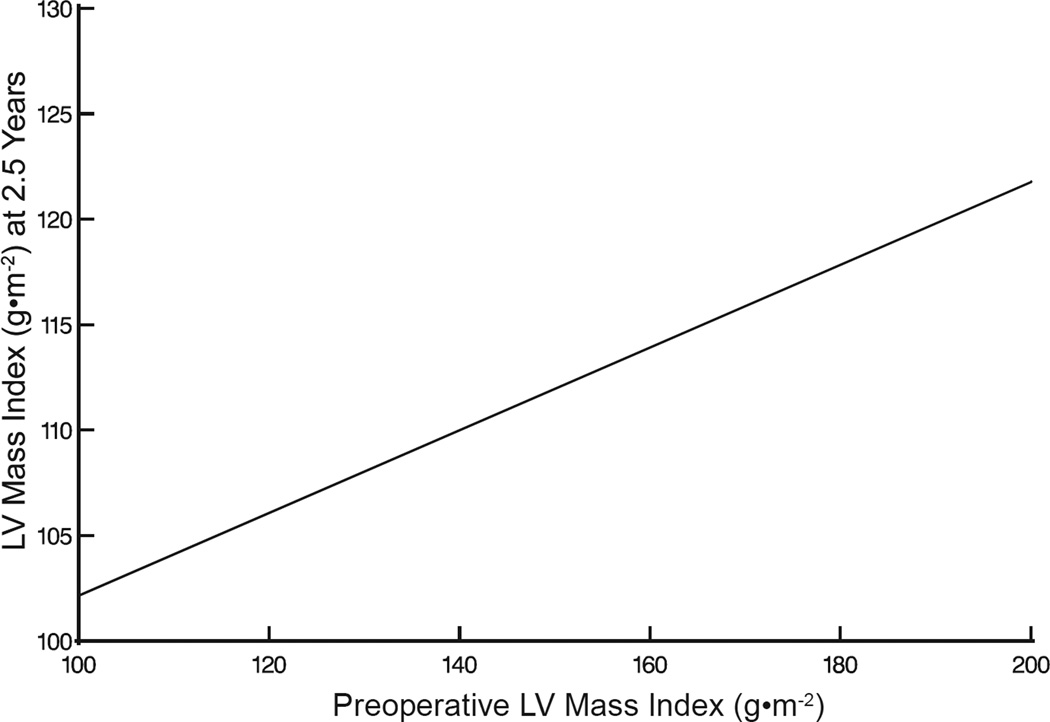
Left ventricular mass index decreased from 139±44 to 112±73 g·m−2 (P=.003) within the first postoperative year and this was maintained to 5 years (Figure 2A). Nevertheless, it remained on average greater than normal (normal values: men, 78 g·m−2; women, 61 g·m−2).12 Preoperative factors associated with a lower postoperative LV mass index included female gender, less preoperative LV hypertrophy (Figure 2B), and smaller LA volume (Figure 2C, eTable1). Preoperative tricuspid valve regurgitation, New York Heart Association classification, and mitral replacement did not significantly influence LV mass regression following mitral surgery.
Figure 2.
Left ventricular (LV) mass index after mitral valve surgery. A, LV mass regression. Black dashed line depicts 95% upper limit of normal LV mass index for sex-matched healthy adult population. Format is as in Figure 1. B, Postoperative LV mass index at 2.5 years compared with preoperative values, based on analysis presented in eTable 1. C, Postoperative LV mass index stratified by preoperative left atrial (LA) volume index, based on analysis presented in eTable 1.
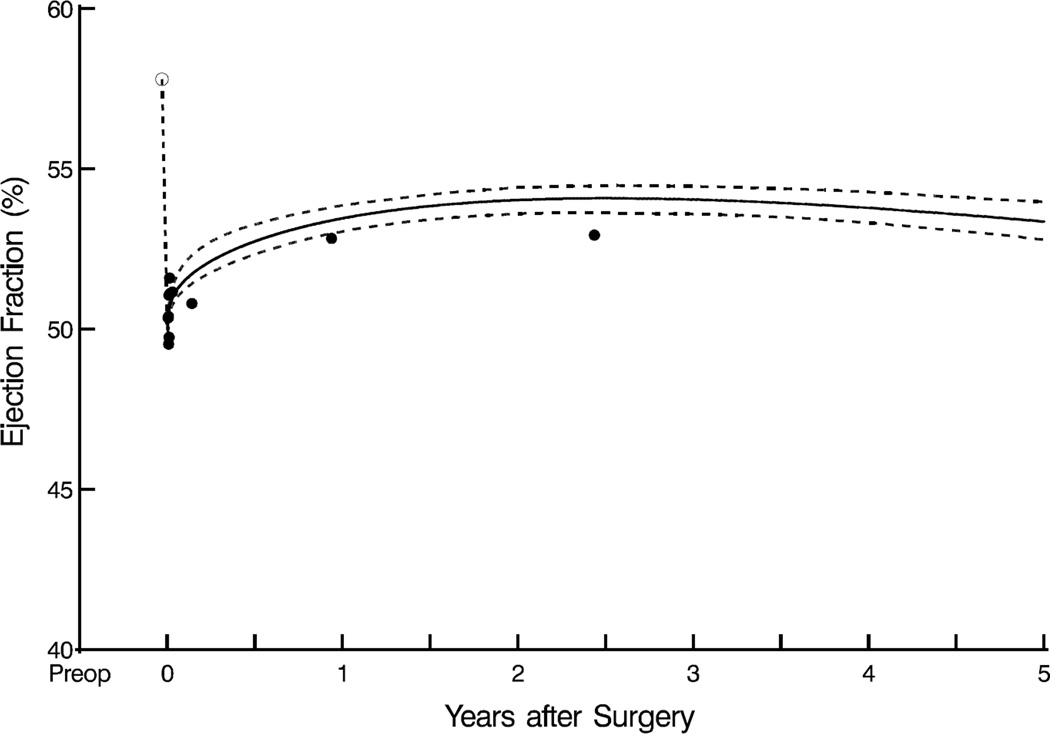
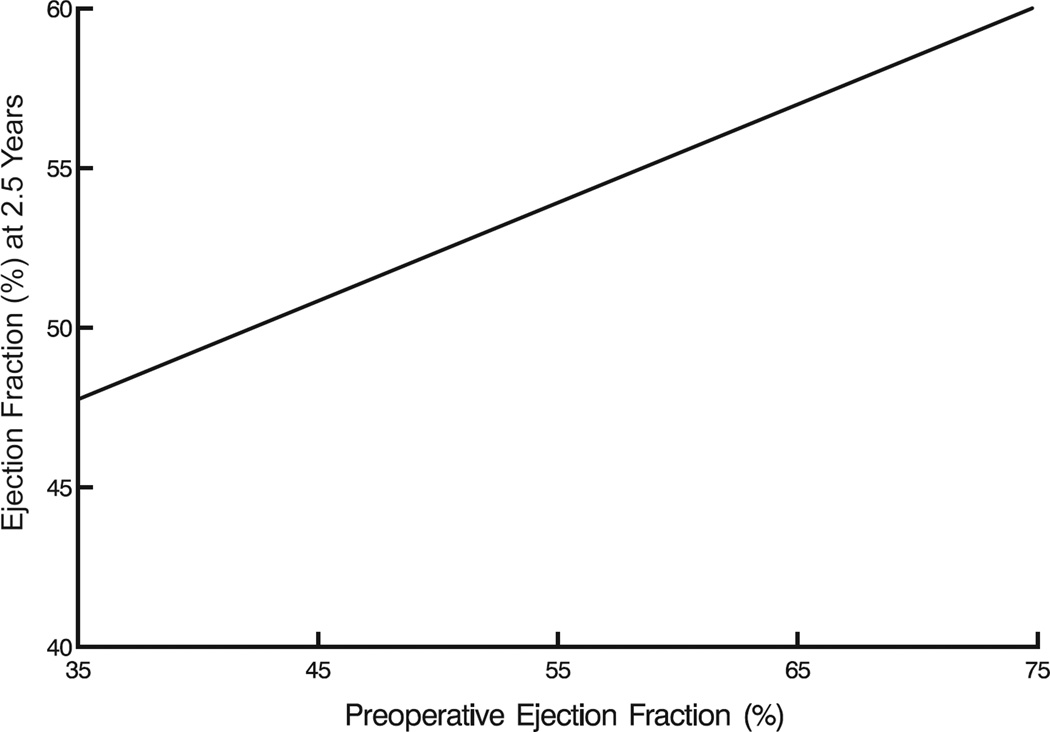
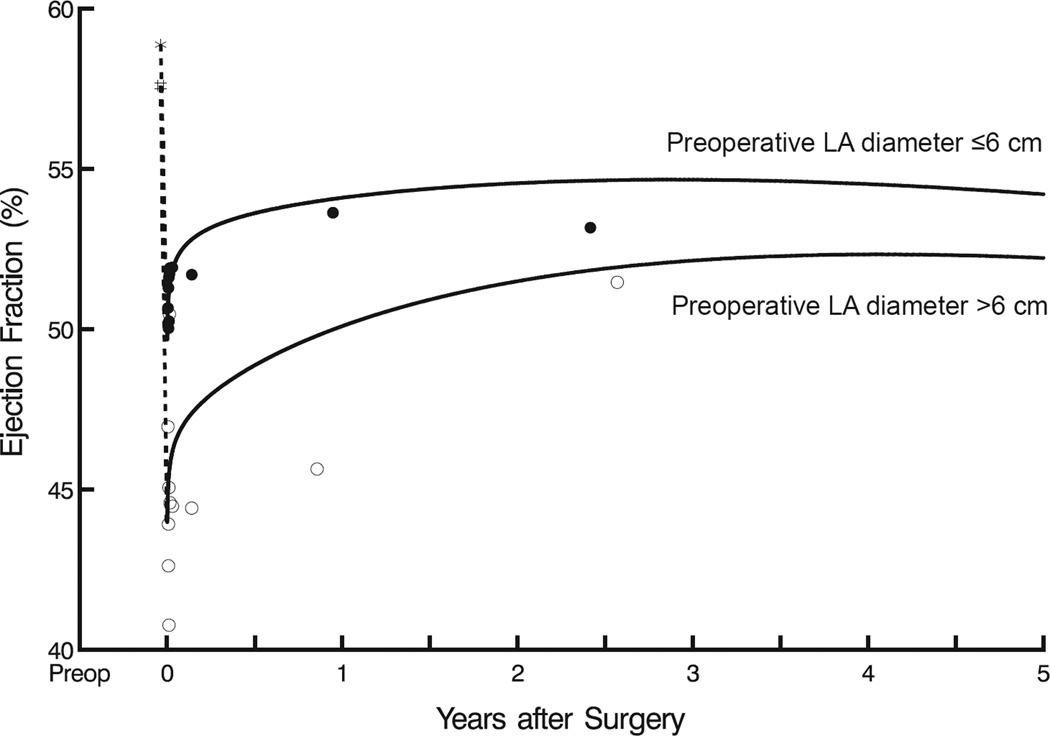
Mean LV ejection fraction initially declined, but not significantly from its preoperative value of 58%±7.4% after restoring MV competency. Thereafter, it increased slightly over the first postoperative year, from 50%±20% to 53%±20% (P=.1), and this increase was sustained to 5 years (Figure 3A). Preoperative factors associated with a higher postoperative LV ejection fraction included higher baseline LV ejection fraction (Figure 3B), no history of heart failure, LA diameter ≤6 cm (Figure 3C), and lower end-systolic volume (eTable 2). Preoperative tricuspid valve regurgitation severity, New York Heart Association classification, and choice of mitral valve procedure were not significantly associated with the response of LV ejection fraction after mitral valve surgery.
Figure 3.
Left ventricular ejection fraction (LVEF) after mitral valve surgery. A, LVEF as a function of time. Format is as in Figure 1. B, Postoperative LVEF at 2.5 years compared with preoperative LVEF, based on analysis presented in eTable 2. C, LVEF stratified by left atrial (LA) size. Format is as in Figure 1, but without confidence limits (for clarity).
DISCUSSION
This study describes the temporal response of reverse ventricular remodeling following correction of MR in degenerative mitral disease and correlates the response with preoperative factors. Our findings indicate a significant reduction of LV diastolic and systolic diameters, as well as LV mass within the first postoperative year. We also observed a modest increase in LV ejection fraction following surgery. Patients most likely to achieve favorable reverse remodeling were those who did not exhibit preoperative changes consistent with long-standing disease, such as LV dilatation, LA enlargement, and LV dysfunction.
Although our data demonstrate that reverse remodeling occurs following surgery, LV mass regression and LV ejection fraction recovery was not complete. This implies that the ventricular remodeling process imparts irreversible changes, particularly as patients develop chamber dilatation and ventricular dysfunction. These findings indirectly support a growing body of evidence suggesting an advantage to earlier intervention for severe degenerative MR.13,14 With the reliability of newer mitral repair techniques and minimally invasive approaches carried out with near zero mortality, a lower threshold for surgical intervention in degenerative MR is becoming more widely accepted. Furthermore, longitudinal studies have established superior outcomes with early surgical intervention.15,16
Early surgery in asymptomatic patients has not been universally accepted even though opposing studies supporting watchful waiting17 are limited. Awaiting onset of symptoms or measurable indices of LV dysfunction might be a reasonable approach if ventricular remodeling were completely reversible. On the contrary, we have demonstrated that ventricular reverse remodeling is incomplete in both ventricular mass regression and functional recovery. Suri and colleagues also found recovery of LV function to be dependent on early intervention before LV dysfunction or enlargement developed.18 A follow-up study of LV mass regression following MV repair showed greater residual LV mass index in patients with reduced preoperative ejection fraction and secondary tricuspid regurgitation, also suggesting incomplete reverse remodeling and advantages to early surgery.19
The effect of increased preoperative LA size on functional and morphologic ventricular recovery also supports early surgery. Considering that structural changes in the LA relate to the chronicity of exposure to abnormal filling pressures and volumes,20 LA dilatation in association with MR could represent a surrogate marker of disease duration. We found that increasing LA dilatation portends negatively on ventricular reverse remodeling. One challenge, though, is the difficulty inherent in quantifying LA size and volume. In this study, we used standard 2-dimensional echocardiographic techniques to assess LA diameter and volume. In a recent study by Marsan et al., 3-dimensional echocardiography was utilized to assess LA changes after MV repair and correlate LA reverse remodeling with LV volume reduction.21 Similarly, application of 3-dimensional echocardiography to improve determination of LA size might aid in the timing of surgical intervention for patients with chronic MR who develop LA dilatation before ventricular changes have occurred.
The ventricular response to surgery was similar when we compared repair and replacement groups. Although repair has been traditionally favored over replacement because of improved survival, preservation of LV function, and avoidance of prosthetic valve-related complications,22–24 ventricular recovery in this study was similar for repair and replacement groups. An important consideration that could explain this variance with historical results is our institution’s routine practice of chordal preservation during MV replacement. Other investigators have established the importance of preserving the subvalvar apparatus on LV mechanics.25,26 Nonetheless, for the reasons stated previously, MV repair is the procedure of choice in patients with severe MR caused by degenerative disease.
Our study has some limitations. This is a single-institution observational study of operations performed over a 20-year period. Our echocardiogram database for follow-up studies is incomplete because some patients did not return to our institution for long-term follow-up. The database does not differentiate between follow-up echocardiographic studies that were obtained for routine reasons versus those that were obtained for clinically relevant reasons. A potential bias therefore exists in the long-term follow-up studies that were obtained. Additionally, residual MR may affect the degree of reverse remodeling. Although the degree of residual MR was not accounted for in the analyses, we reported a low prevalence of important residual MR in a prior study utilizing a similar patient cohort.27 By convention, reoperation is recommended for significant residual MR, either early postoperatively or on follow-up patient surveillance.
Acknowledgments
This study was supported in part by the Judith Dion Pyle Endowed Chair in Heart Valve Research (Dr. Gillinov), the Donna and Ken Lewis Chair in Cardiothoracic Surgery and Peter Boyle Research Fund (Dr. Mihaljevic), and the Kenneth Gee and Paula Shaw, PhD, Chair in Heart Research (Dr. Blackstone).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
eFigure 1: Number of patients with transthoracic echocardiograms at and beyond various time points, and number of measurements available for analyses.
REFERENCES
- 1.Gaasch WH, Meyer TE. Left ventricular response to mitral regurgitation: implications for management. Circulation. 2008;118:2298–2303. doi: 10.1161/CIRCULATIONAHA.107.755942. [DOI] [PubMed] [Google Scholar]
- 2.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48:e1–e148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Tribouilloy CM, Enriquez-Sarano M, Schaff HV, Orszulak TA, Bailey KR, Tajik AJ, Frye RL. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation. 1999;99:400–405. doi: 10.1161/01.cir.99.3.400. [DOI] [PubMed] [Google Scholar]
- 4.de Varennes B, Haichin R. Impact of preoperative left ventricular ejection fraction on postoperative left ventricular remodeling after mitral valve repair for degenerative disease. J Heart Valve Dis. 2000;9:313–318. discussion 318–320. [PubMed] [Google Scholar]
- 5.Johnston DR, Gillinov AM, Blackstone EH, Griffin B, Stewart W, Sabik FJ, 3rd, Mihaljevic T, Svensson LG, Houghtaling PL, Lytle BW. Surgical repair of posterior mitral valve prolapse: implications for guidelines and percutaneous repair. Ann Thorac Surg. 2010;89:1385–1394. doi: 10.1016/j.athoracsur.2009.12.070. [DOI] [PubMed] [Google Scholar]
- 6.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Mason DP, Rajeswaran J, Murthy SC, McNeill AM, Budev MM, Mehta AC, Pettersson GB, Blackstone EH. Spirometry after transplantation: how much better are two lungs than one? Ann Thorac Surg. 2008;85:1193–1201. 1201, e1191–e1192. doi: 10.1016/j.athoracsur.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Breiman L. Bagging predictors. Machine Learning. 1996;24:123–140. [Google Scholar]
- 11.Rubin DB. Multiple imputation for non-response in surveys. Vol. 137. New York: Wiley; 1987. pp. 166–167. [Google Scholar]
- 12.Salton CJ, Chuang ML, O'Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, Edelman RR, Levy D, Manning WJ. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39:1055–1060. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 13.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, Schaff HV, Tajik AJ. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 14.Enriquez-Sarano M, Sundt TM., 3rd Early surgery is recommended for mitral regurgitation. Circulation. 2010;121:804–811. doi: 10.1161/CIRCULATIONAHA.109.868083. discussion 812. [DOI] [PubMed] [Google Scholar]
- 15.Kang DH, Kim JH, Rim JH, Kim MJ, Yun SC, Song JM, Song H, Choi KJ, Song JK, Lee JW. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009;119:797–804. doi: 10.1161/CIRCULATIONAHA.108.802314. [DOI] [PubMed] [Google Scholar]
- 16.Ling LH, Enriquez-Sarano M, Seward JB, Orszulak TA, Schaff HV, Bailey KR, Tajik AJ, Frye RL. Early surgery in patients with mitral regurgitation due to flail leaflets: a long-term outcome study. Circulation. 1997;96:1819–1825. doi: 10.1161/01.cir.96.6.1819. [DOI] [PubMed] [Google Scholar]
- 17.Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D, Schemper M, Maurer G, Baumgartner H. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. 2006;113:2238–2244. doi: 10.1161/CIRCULATIONAHA.105.599175. [DOI] [PubMed] [Google Scholar]
- 18.Suri RM, Schaff HV, Dearani JA, Sundt TM, Daly RC, Mullany CJ, Enriquez-Sarano M, Orszulak TA. Recovery of left ventricular function after surgical correction of mitral regurgitation caused by leaflet prolapse. J Thorac Cardiovasc Surg. 2009;137:1071–1076. doi: 10.1016/j.jtcvs.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Stulak JM, Suri RM, Dearani JA, Burkhart HM, Sundt TM, 3rd, Enriquez-Sarano M, Schaff HV. Does early surgical intervention improve left ventricular mass regression after mitral valve repair for leaflet prolapse? J Thorac Cardiovasc Surg. 2011;141:122–129. doi: 10.1016/j.jtcvs.2010.08.068. [DOI] [PubMed] [Google Scholar]
- 20.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 21.Marsan NA, Maffessanti F, Tamborini G, Gripari P, Caiani E, Fusini L, Muratori M, Zanobini M, Alamanni F, Pepi M. Left atrial reverse remodeling and functional improvement after mitral valve repair in degenerative mitral regurgitation: a real-time 3-dimensional echocardiography study. Am Heart J. 2011;161:314–321. doi: 10.1016/j.ahj.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation. 1995;91:1022–1028. doi: 10.1161/01.cir.91.4.1022. [DOI] [PubMed] [Google Scholar]
- 23.Moss RR, Humphries KH, Gao M, Thompson CR, Abel JG, Fradet G, Munt BI. Outcome of mitral valve repair or replacement: a comparison by propensity score analysis. Circulation. 2003;108(Suppl 1):II90–II97. doi: 10.1161/01.cir.0000089182.44963.bb. [DOI] [PubMed] [Google Scholar]
- 24.Gillinov AM, Blackstone EH, Nowicki ER, Slisatkorn W, Al-Dossari G, Johnston DR, George KM, Houghtaling PL, Griffin B, Sabik JF, 3rd, Svensson LG. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg. 2008;135:885–893. 893, e881–e882. doi: 10.1016/j.jtcvs.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Yun KL, Sintek CF, Miller DC, Pfeffer TA, Kochamba GS, Khonsari S, Zile MR. Randomized trial comparing partial versus complete chordal-sparing mitral valve replacement: effects on left ventricular volume and function. J Thorac Cardiovasc Surg. 2002;123:707–714. doi: 10.1067/mtc.2002.121048. [DOI] [PubMed] [Google Scholar]
- 26.Athanasiou T, Chow A, Rao C, Aziz O, Siannis F, Ali A, Darzi A, Wells F. Preservation of the mitral valve apparatus: evidence synthesis and critical reappraisal of surgical techniques. Eur J Cardiothorac Surg. 2008;33:391–401. doi: 10.1016/j.ejcts.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Gillinov AM, Mihaljevic T, Blackstone EH, George K, Svensson LG, Nowicki ER, Sabik JF, 3rd, Houghtaling PL, Griffin B. Should patients with severe degenerative mitral regurgitation delay surgery until symptoms develop? Ann Thorac Surg. 2010;90:481–488. doi: 10.1016/j.athoracsur.2010.03.101. [DOI] [PubMed] [Google Scholar]